Abstract
Milk is a widely consumed nutrient-rich food containing protein variants such as casein A2 and A1. A1 differs from A2 in an amino acid at position 67 (Pro67 to His67). The breakdown of β-casein yields β-casomorphins (BCM), among which BCM-7 is extensively studied for its effects on the human body. Animal studies have shown that A1 β-casein milk increases digestive transit time and enhances myeloperoxidase activity. Individuals with lactose intolerance prefer A2 milk to conventional A1 milk, as BCM-7 in A1 milk can lead to inflammation and discomfort in sensitive individuals. A2 milk, which contains A2 β-casein, is believed to be more easily digestible than A1 β-casein. Its popularity has grown owing to reports linking A1 casein to diseases such as type 1 diabetes, heart disease, and autism. A2 milk has gained popularity as an alternative to A1 milk, primarily because of its potential benefits for individuals with certain diseases. This review aims to provide an updated understanding of A2 milk consumption and its health benefits. This review aims to provide an updated understanding of A2 milk consumption and its health benefits.
Keywords: A2 milk, β-casomorphin-7, Lactose intolerance, Inflammation, Health benefit
Introduction
Milk is considered a nutrient-dense food, as it contains various nutrients such as carbohydrates, fats, proteins, vitamins, and minerals. However, it does not provide sufficient iron (Fe) and folate, especially to meet the nutritional needs of growing infants (Kaskous, 2020). Caseins contribute to approximately 80% of the total protein in bovine milk and to one-third of the protein in human milk (Swaisgood, 2003). Bovine milk consists of four caseins, ⍺s1-casein (26.9%), ⍺s2-casein (7.3%), β-casein (22.0%), and κ-casein (7.3%), and two major whey proteins, ⍺-lactoglobulin and ⍺-lactalbumin (Lucey and Horne, 2018). The whey proteins, including lactoglobulin (7.8%), serum albumin (1.0%), lactoferrin (7.3%), immunoglobulin (17.1%), and enzymes (0.2%) (Franzoi et al., 2019) make up the remaining 20% of the total protein content in milk (Fig. 1). β-Casein consists of 209 amino acids.
Fig. 1.
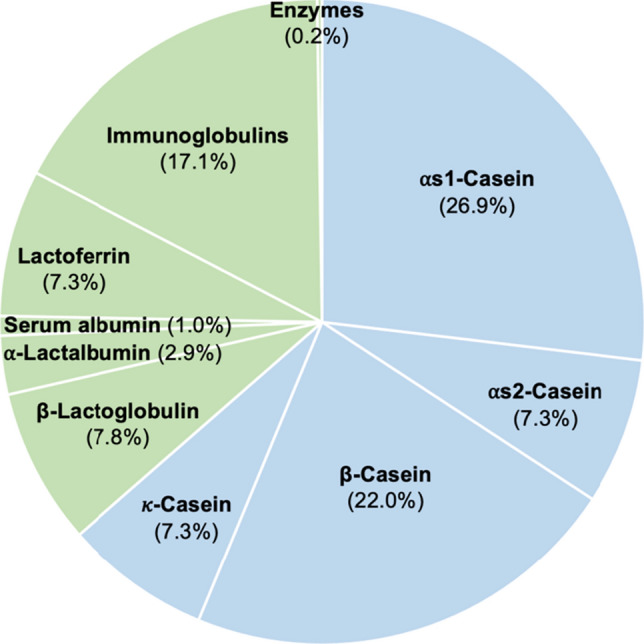
Protein composition of bovine milk. Sky blue and yellowish-green areas indicate caseins and whey proteins, respectively. The numbers show the percentage of each protein in the milk based on protein content of 100%. The data are extracted from Franzoi et al. (2019)
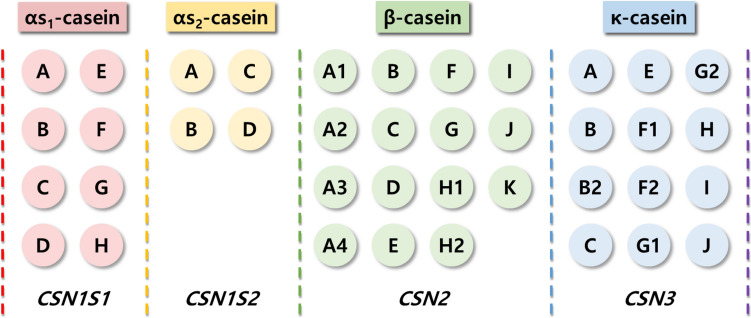
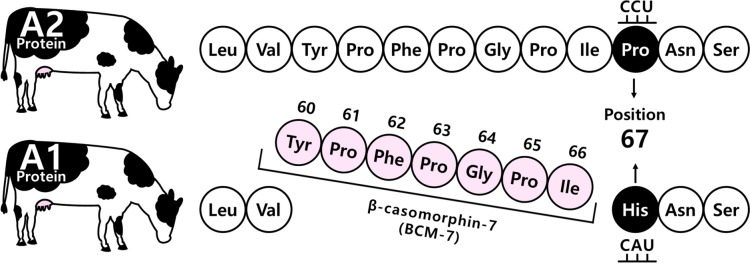
Currently, bovine milk contains 13 genetic variants of casein: A1, A2, A3, B, C, D, E, F, G, H1/H2, I, and J (Fig. 2). The A1 and A2 variants are the most common. Notably, the A1 and A2 variants differed in the 67th amino acid in the β-casein polypeptide chain. The A1 type contains histidine (His), whereas the A2 type harbors proline (Pro) at this position. Studies have shown that humans can digest His67 in casein using digestive and microbial enzymes; this results in the release of a heptapeptide, β-casomorphin-7 (BCM-7), during digestion as well as during the ripening of cheese. BCM-7 belongs to a group of peptides with opioid properties, commonly called casomorphins (De Noni and Cattaneo, 2010).
Fig. 2.
Different casein forms and their genetic variants and genes
Among BCMs, only BCM-7 has been studied extensively. Recent epidemiological evidence has suggested that the consumption of milk containing the A1 variant is associated with higher risk of developing type 1 diabetes (T1D) and heart disease (Laugesen and Elliott, 2003; McLachlan, 2001). The formed BCMs can bind to opioid receptors in the central nervous system, as well as in the gastrointestinal tract. In contrast, A2 milk with Pro67 substitution hampers protein digestion in humans. Researchers have focused on the potential effects of BCM-7 and its associated peptides on human health. In 2009, the European Food Safety Authority (EFSA) concluded that BCM-7 is not directly related to non-communicable diseases such as cardiovascular diseases (CVD), autism, and insulin-dependent diabetes (De Noni et al., 2009). Nevertheless, BCM-7-like peptides have been identified in raw milk (Cieslinska et al., 2022), which requires further research because of their possible link to an elevated risk of developing chronic diseases. Whether BCMs are formed and the amount of BCMs formed at different steps of milk processing or fermentation remain highly intriguing.
Systematic reviews on the effects of A1 or A2 β-casein intake on human health are limited. Four reviews have mentioned that the intake of A2 milk may favor digestion compared to that of A1 milk (Brooke-Taylor et al., 2017; Kaskous, 2020; Kay et al., 2021; Kullenberg de Gaudry et al., 2019). However, evidence regarding its health benefits is limited. Information regarding the differences in the structure and physicochemical properties of β-casein mutants, which can be used in technology and cheese manufacturing, is also scarce. Apparently, the substitution of Pro by His affected both the structure and hydrophobicity of β-casein. Similarly, these two forms appear to affect the stability and emulsifying and coagulation properties of casein.
This review summarizes the current knowledge regarding BCMs, their content in dairy products, and the possible health benefits of bovine milk containing A2 β-casein (Table 1).
Table 1.
Summary of health effects of A1 and A2 β-casein consumption
| Health Condition | Effects of A1 β-casein consumption | Effects of A2 β-casein consumption | References |
|---|---|---|---|
| Type 1 diabetes (T1D) | Associated with increased risk of T1D | – | Chia et al. (2018), Elliott et al. (1999), and Laugesen and Elliott (2003) |
|
Cardiovascular diseases (CVD) |
Associated with increased risk of CVD, atherosclerosis, and IHD | – | De Noni et al. (2009), Heinecke (1999), Laugesen and Elliott (2003), McLachlan (2001), Tailford et al. (2003), and Torreilles and Guerin (1995) |
| Gastrointestinal disease | Associated with chronic constipation, altered gastrointestinal transit, inflammation, and lactose intolerance symptoms |
A2 milk is easier to digest and better absorbed than A1 milk Milk containing only A2 β-casein induces fewer symptoms of lactose intolerance than milk containing both A1 and A2 β-caseins |
Andiran et al. (2003), Brooke-Taylor et al. (2017), Defilippi et al. (1995), Park and Haenlein (2021), Ramakrishnan et al. (2020), and Summer et al. (2020) |
| Autism and neurological disorders | BCM-7 is implicated in the pathogenesis of autism and other neurological disorders | – | Cade et al. (2000), Cieslinska et al. (2015), Dohan (1980), Jarmolowska et al. (2019), Kost et al. (2009), Lindstrom et al. (1984), Osman et al. (2021), and Sokolov et al. (2014) |
|
Sudden infant death syndrome (SIDS) |
BCM-7 may inhibit the respiratory center in infants leading to apnea and SIDS | – | Kost et al. (2009), Sun et al. (2003), and Wasilewska et al. (2011) |
| Allergy | BCM-7 may influence inflammation and allergies | – | Fiedorowicz et al. (2011), Fiedorowicz et al. (2014), Fiedorowicz et al. (2016), and Trivedi et al. (2015) |
| Glutathione production | Consumption of milk with both A1 and A2 β-casein led to a decrease in plasma glutathione concentration | Consumption of milk with only A2 β-casein led to an increase in plasma glutathione concentration | Deth et al. (2016), Sheng et al. (2019), and Trivedi et al. (2014) |
Methodology used
We systematically searched the bibliographic data in different electronic databases (Scopus, MEDLINE, PubMed, and Google Scholar) from March 13 to June 11, 2023. The search terms were used in the Title/Abstract/Keywords, MeSH, Title/Abstract, or Topic fields. For the MeSH search, casein and A2 milk, BCMs/BCM-7, A2/A2 milk, and A2 variants were used for searching Title/Keywords/Abstracts. The resulting data were filtered for relevance. Some full-text papers were subjected to full-text analysis when the data were ambiguous in terms of compatibility. The analyzed data were further screened to identify the studies included in the meta-analysis. Letters to the editor, conference papers, or studies that had not been peer-reviewed were excluded. All cited references were carefully examined for compatibility prior to citation. This manuscript was shared with the authors using EndNote 20 program (Clarivate, London, UK).
Structure of casein micelle in milk
Caseins, a family of related phosphoproteins, are the predominant proteins in mammalian milk, constituting approximately 80% of the proteins in cow’s milk and between 20 and 60% of the proteins in human milk (Kunz and Lonnerdal, 1990). The structure of milk casein is primarily determined by its ability to assemble into colloidal particles called casein micelles, which are spherical entities containing all four casein species (αS1, αS2, β, and κ). These micelles are held together and stabilized by their unique physicochemical properties, including the ability of phosphorylated serine residues to bind to calcium (Lucey, 2002).
Several models have been proposed to explain the formation and organization of casein micelles. One model suggests that the micellar core is formed by submicelles, clusters of αS1 and β-caseins linked by calcium phosphate bridges, whereas the periphery of the micelle consists of casein microvilli that prevent aggregation via steric and electrostatic repulsion (Slattery, 1976). Bovine casein, the most hydrophobic casein, plays a critical role in the binding and self-assembly of casein micelles because of its amphipathic nature resulting from the phosphorylation of all five phosphoserines (Daniloski et al., 2021). Another model, known as the dual-binding model, proposes that casein micelles are formed via two mechanisms: hydrophobic attraction between casein proteins and colloidal calcium phosphate bridging between phosphoserine residues and calcium phosphate (Horne, 2020). The nanocluster model proposes that casein micelles are formed by the aggregation of calcium phosphate nanoclusters linked by casein proteins. Nanoclusters provide a structural framework, whereas caseins contribute to stability via their interactions with each other and with the nanoclusters (Holt, 2016). According to the water-channel model, casein micelles have porous structures that contain water channels. Caseins form a network that allows the movement of water and small solutes (Dalgleish and Corredig, 2012). The network model describes casein micelles as a porous network of nonspherical primary casein particles linked by calcium phosphate nanoclusters. This emphasizes the importance of the nonspherical particle shape in determining the micelle structure (Huppertz et al., 2017). These models provide different perspectives on the structure and assembly of casein micelles. Possibly, elements from multiple models coexist within a single micelle, thereby contributing to its complexity.
Milk protein-coding genes, such as CSN1S1, CSN1S2, CSN2, and CSN3 are responsible for the production of different casein variants. These genetic variants can influence the physicochemical properties and functionality of bovine milk, ultimately affecting the structure and stability of casein micelles (Gai et al., 2021). To date, 39 genetic variants of bovine casein have been reported (Gazi et al., 2022) (Fig. 2). Casein micelles are complex structures that play a critical role in determining the properties and functionality of milk. Understanding the structures and mechanisms regulating their formation is essential for optimizing dairy processing and enhancing the nutritional value of milk.
Formation of BCM-7 and other BCMs
A major difference between the A1 and A2 variants was a point mutation at the 67th amino acid in the β-casein polypeptide chain, wherein the A1 type contained His, while the A2 type contained Pro in its sequence (Fig. 3). Casein with His67 is readily digestible by gastrointestinal and microbial enzymes; therefore, BCM-7 can be released during the digestive process or ripening of cheese.
Fig. 3.
Difference in the amino acid sequences of A1 and A2 variants and formation of β-casomorphin-7
In contrast, A2 milk containing Pro67 hampers protein digestion in humans. Studies have confirmed that the BCMs formed can bind to the μ-opioid receptors which are commonly found in the central nervous tissue and digestive tract. For decades, many researchers have been interested in BCM-7, its associated peptides, and their potential effects on the human body. These molecules may activate lymphocyte proliferation and antibody production.
Gastrointestinal transit time associated with A1 or A2 milk
An animal experiment in rodents reported that A1 β-casein milk increased the transit time in the digestive tract and significantly enhanced myeloperoxidase activity, an inflammation marker (Pal et al., 2015). Despite limited evidence in clinical trials for humans, two independent clinical studies suggested that proinflammatory factors affect the transit time in the gastrointestinal tract (Ho et al., 2014). Four systematic reviews published between 2017 and 2021 suggested tools for analyzing the variable evidence provided by in vitro animal experiments (Brooke-Taylor et al., 2017; Kaskous, 2020; Kay et al., 2021; Kullenberg de Gaudry et al., 2019). Using the PRISMA method, the results obtained from animal studies were carefully examined to determine how consumption of A1 β-casein milk and A2 β-casein milk can affect human health. These reviews revealed that A1 β-casein milk is highly related to increased gastrointestinal transit time. According to Kullenberg de Gaudry et al. (2019), A1 β-casein milk shows negligible association with CVD, diabetes, or neurological diseases. Daniloski et al. (2021) confirmed these claims using a series of clinical and animal experiments.
Acid gelation and rennet coagulation
The composition and technological properties of milk, including its ability to undergo acid gelation and rennet coagulation, are strongly influenced by milk proteins, particularly β-casein variants (Bisutti et al., 2022). These processes play a fundamental role in the production of dairy products such as cheese and yogurt. The β-casein variants, A1 and A2, have been extensively studied because of their significant impact on the coagulation properties and protein profile of milk (Nguyen et al., 2018).
Acid gelation refers to the coagulation of milk proteins under acidic conditions, and is commonly used in the production of yogurt and certain types of cheese. Research has shown that the polymorphic structure of β-casein, specifically the A1 and A2 variants, considerably affects the acid gelation behavior of milk (Daniloski et al., 2022). In particular, milk containing the A2/A2 casein variant had lower elastic modulus, water-holding capacity, and gel permeability than milk containing the A1/A1 and A1/A2 variants. In addition, milk with the A2/A2 casein phenotype contains higher proportion of free ionic calcium and improved foaming capacity, although it requires longer time to gel than milk with the A1/A1 composition (Nguyen et al., 2018).
In contrast, rennet coagulation, used in cheese production, involves the addition of the enzyme, rennet, to milk to form curds. The A1 and A2 variants have been associated with significant differences in rennet coagulation properties. Milk with higher concentration of casein B, which includes the A1 and B variants, positively affected rennetting time, curd firmness, and protein, casein, and fat content (Vigolo et al., 2022). Conversely, milk containing the A2/A2 casein variant shows poorer performance in terms of cheese-making ability, particularly in terms of rennet coagulation time and curd firmness (Bisutti et al., 2022).
These variations in acid gelling and rennet coagulation properties of the β-casein variants have significant implications for the dairy industry. Selection of milk with specific casein variants has the potential to improve the quality of raw milk and increase cheese yield (Poulsen et al., 2013). However, the high prevalence of certain β-casein variants, such as variant F, in native breeds, which are associated with poor coagulation properties, poses a challenge for dairy producers (Poulsen et al., 2016). Therefore, further research to completely understand the impact of β-casein variants on milk coagulation properties and to develop strategies for optimizing milk selection in dairy production is required.
In conclusion, β-casein variants, especially A1 and A2, significantly influence the acid gelation and rennet coagulation properties of milk. Understanding these effects is crucial for optimizing milk production processes and improving the quality of dairy products. In future, the complex relationships between β-casein variants and milk coagulation properties should be investigated, with a focus on their practical applications in dairy production.
Which diseases can occur after consumption of A1 or A2 bovine milk?
Most studies on the possible effects of milk containing A1 and A2 β-casein on human health have been conducted without any systematic review strategy. Individual papers have reported the possible health effects following the consumption of A1 milk. Previous publications have associated consumption of casein with an increase in the incidence of specific disorders, although contradictory results were obtained in some cases. Truswell (2005) reported that the consumption of A1 β-casein was negligibly related to T1D and CVD. In contrast, according to Kaminski et al. (2007), the consumption of A1 β-casein milk appeared to be related to CVD and T1D. Moreover, they suggested that increase in the levels of urinary BCM-7 was related to sudden infant death syndrome (SIDS) and neurological disorders such as autism and schizophrenia. Based on the available evidence, the European Food Safety Authority (EFSA) concluded that orally consumed BCM-7 or similar peptides were not related to nonconductive diseases (De Noni et al., 2009).
Interestingly, Raikos and Dassios (2014) reported that BCM-7 was released from an infant formula after simulated gastrointestinal digestion; however, they could not explain how the released BCM-7 affected the human body. Chia et al. (2017) also reported evidence from animal experiments. A1 β-casein and BCM-7 were the major causative agents of T1D for people with genetic risk factors. This claim is consistent with the findings of Kalra and Dhingra (2018) who reported higher incidence of T1D in patients exposed to A1/A1 β-casein. Kohil et al. (2021) concluded that specific food habits can directly cause T1D by inducing epigenetic mutations. Moreover, they proposed that BCM-7 might methylate the genes responsible for T1D and thereby act as an epigenetic regulator.
In contrast, Aslam et al. (2020) recently reported the absence of any clear relationship between BCM-7 consumption and disorders such as T1D, CVD, and neurological diseases. In addition, the consumption of BCM-7 should not be linked to obesity or indigestion because of insufficient experimental evidence. They insisted that conclusions based on experimental data from epidemiological or animal studies should not be extrapolated to humans. A review of synthetic BCMs, with an emphasis on their effects on health, revealed that they affected lymphocyte proliferation, antibody production, and modulation of leukemia cell production. The antileukemic properties of BCM-7 have been reported in a series of studies on prostate cancer, breast cancer T47 cells, and HL-60 cells (Ledesma-Martinez et al., 2019; Mori et al., 2021).
It has been investigated whether BCM-7 can cross the intestinal barrier and exert immunological effects (Fukui, 2016; Haq et al., 2014a). This may help address the relationship between BCM-7 and other potential diseases, such as T1D, infant mental disorders, SIDS, and atopic syndrome. More recently, BCM-7 has been strongly associated with indigestion (Haq et al., 2014b). Indigestion occurs via the same mechanism as inflammation in rodents. It has been suggested that BCM-7 first activates the opioid receptors, followed by damage to the integral intestinal barrier and bile salt metabolism (Wada and Lonnerdal, 2014). During this process, the intestinal microbiota changes accordingly.
According to Woodford (2021), opioid receptors for various peptides are ubiquitous in different organs, which may provide insights for understanding the functions of β-casein-derived BCM-7 found in the organs of the human body. Many studies support this hypothesis. Interestingly, several studies have demonstrated the proinflammatory role of the A1 subvariant of casein and its impact on various aspects of health, including the gastrointestinal, endocrine, neurological, and cardiovascular systems. (Kay et al., 2021). A large body of experimental evidence shows that food-borne opioid molecules can delay gut passage time, affect intestinal permeability, and change the gut microbiota (Rueda-Ruzafa et al., 2020; Vaarala et al., 2008).
Four review papers published between 2017 and 2021 reported that A1 β-casein milk was responsible for delaying the intestinal passage time (Brooke-Taylor et al., 2017; Kaskous, 2020; Kay et al., 2021; Kullenberg de Gaudry et al., 2019). However, according to Kuellenberg de Gaudry et al. (2022), the experimental certainty of milk being the causative agent for other diseases, such as CVD, diabetes, and neurological disorders, is lacking. These results were confirmed by Daniloski et al. (2021) in an animal study.
In this context, a long-term study is required to confirm the physiological roles of specific β-casein genetic mutants and BCMs in the development of non-communicable diseases. Toward this, different geographical locations, sex, and ages of the subjects should be carefully considered. In addition, the physicochemical and technological properties of A2 β-casein should be investigated. However, most studies have not investigated the technological and qualitative aspects of A1/A2 milk, dairy products, or cheese processing.
T1D
The relationship between T1D and A1 milk is a subject of ongoing research and debate. T1D is an autoimmune disease that results from the destruction of insulin-producing cells in the pancreas, leading to insulin deficiency (Pozzilli, 1999). The incidence of T1D is increasing worldwide, and environmental factors, including diet, play a significant role in its etiology (Rewers and Ludvigsson, 2016). The consumption of cow's milk, specifically A1 β-casein, is one of the dietary factors implicated in the development of T1D (Elliott et al., 1999). A1 β-casein has been shown to be diabetogenic in animal studies (Chia et al., 2018), and epidemiological studies have suggested a correlation between A1 milk consumption and the incidence of T1D (Laugesen and Elliott, 2003). Interestingly, geographical variations have been observed in the incidence of T1D, which may be related to differences in A1 milk consumption. For example, Iceland has lower incidence of T1D than Scandinavia, which may be due to the lower consumption of A1 β-casein in Iceland (Thorsdottir et al., 2000). This suggests that the type of cow's milk consumed during infancy may influence the risk of developing T1D. In addition, recent research has shown that individuals with T1D have increased small intestinal permeability, also known as “leaky gut syndrome” (Vaarala, 2008). This condition, which is characterized by the passage of harmful substances from the gut into the bloodstream, has been linked to autoimmune diseases, including T1D. It has been hypothesized that A1 β-casein may contribute to increased intestinal permeability, thereby promoting the development of T1D (Chia et al., 2017). However, the relationship between A1 milk and T1D is complex and may be influenced by other factors. For example, genetic predisposition plays a critical role in the development of T1D (Pociot and Lernmark, 2016). Additionally, other environmental triggers such as viral infections may interact with dietary factors and influence the risk of developing T1D (Lempainen et al., 2012).
In conclusion, despite evidence regarding a potential association between A1 milk consumption and T1D, further research is required to completely understand this relationship and its implications in disease prevention. Considering the increasing incidence of T1D, particularly in children and adolescents, it is critical to identify modifiable risk factors such as diet that can be targeted in strategies to prevent this disease (Wu et al., 2016).
CVD
The relationship between A1 β-casein consumption and CVD has been extensively investigated for several decades. Compelling findings from various key research papers emphasize the necessity for comprehensively understanding this association owing to its potential implications for public health, particularly in regions where A1 β-casein is predominant. Tailford et al. (2003) investigated the direct effect of consuming A1 β-casein versus A2 β-casein on atherosclerosis progression and observed that rabbits fed a diet containing A1 β-casein exhibited significantly elevated levels of serum cholesterol, low-density lipoprotein (LDL), high-density lipoprotein (HDL), and triglycerides compared to rabbits fed A2 β-casein or whey protein. Additionally, the consumption of A1 β-casein results in increase in the surface area of aorta covered with fatty streaks, an early indication of atherosclerosis, and thicker lesions in the aortic arch. In another investigation, Torreilles and Guerin (1995) discovered that casein-derived peptides may promote the oxidation of human LDL cholesterol, a crucial process in atherosclerotic plaque formation. This oxidation of LDL occurs via peroxidase-dependent and metal-independent processes, suggesting a distinct and direct pathway via which casein-derived peptides, including those from A1 β-casein, contribute to atherosclerosis. Moreover, Heinecke (1999) found that LDL oxidation in the human arterial wall leads to an increase in the production of specific oxidized amino acids in proteins. These findings suggested that oxidized LDL, which is potentially influenced by A1 β-casein consumption, contributes to the progression of atherosclerosis.
To broaden the research scope, De Noni et al. (2009) examined the potential health effects of BCM-7 peptides. Their research suggests that these peptides may affect cardiovascular health by adding complexity to the relationship between A1 β-casein and CVD. In a broader epidemiological context, McLachlan (2001) presented substantial evidence linking A1 β-casein consumption to mortality associated with ischemic heart disease (IHD). This study revealed that the regions with higher A1 β-casein consumption had higher IHD mortality rates. Similarly, Laugesen and Elliott (2003) identified an association between A1 β-casein consumption and IHD. However, cautionary notes by Allison and Clarke (2006) and Elliott et al. (1999) emphasize the necessity for further research to completely comprehend this complex relationship. In fact, Elliott et al. (1999) suggested a potentially common causative risk factor for T1D and CVD, adding another dimension to the health implications of A1 β-casein consumption.
In summary, an emerging body of research on the association between A1 β-casein and CVD has provided evidence that this milk protein variant may contribute to the development of atherosclerosis and other forms of CVD. Considering the widespread consumption of dairy products across many populations, these findings raise concerns regarding its public health implications. Consequently, further research regarding the health implications of A1 β-casein is of critical importance.
Gastrointestinal disease
Gastrointestinal disorders, such as chronic constipation, anal fissures, and lactose intolerance, pose a significant burden on global health. Interest in elucidating the potential effects of A1 β-casein on gastrointestinal health is increasing. Several studies have suggested a possible association between A1 β-casein consumption and the incidence of chronic constipation in infants and children. In particular, Andiran et al. (2003) found higher intake of cow's milk, specifically A1 β-casein, in children diagnosed with chronic constipation and anal fissures. Accordingly, shorter breastfeeding duration, coupled with an early transition to bottle feeding with cow's milk, was associated with the onset of constipation, suggesting a possible role for A1 β-casein in the etiology of constipation. Experimental research by Brooke-Taylor et al. (2017) showed that A1 β-casein may affect gastrointestinal transit time and function. Unlike A2 β-casein, A1 β-casein has been shown to prolong the gastrointestinal transit time and alter the gut microbiota composition in rat models. Results from human studies corroborate these findings, suggesting that A1 β-casein intake correlates with prolonged intestinal transit and less firm stool consistency, which could lead to digestive discomfort. It has been reported that the A2 milk is easier to digest and better absorb than A1 or other types of milk (Park and Haenlein, 2021). Numerous studies have examined the interplay between A1 β-casein and opioid receptors in the gastrointestinal tract. BCMs, the metabolic by-products of A1 β-casein digestion, have been shown to bind to peripheral opiate receptors and thereby alter gastrointestinal motility in animal models (Defilippi et al., 1995). These interactions may explain the observed effects on gastrointestinal transit and discomfort. In addition, Summer et al. (2020) found a possible correlation between A1 β-casein intake and gastrointestinal inflammation, suggesting the involvement of A1 β-casein in inducing intestinal inflammation. For certain lactose-intolerant individuals, consumption of A1 β-casein may exacerbate their symptoms. According to Ramakrishnan et al. (2020), milk containing only A2 β-casein induces fewer symptoms of lactose intolerance than milk containing both A1 and A2 β-caseins. This highlights the possibility that A1 β-casein exacerbates lactose intolerance symptoms, possibly via its interaction with gastrointestinal function and inflammation.
The concept of the microbiota-gut-brain axis has emerged as a compelling area of research in gastrointestinal studies. Cieslinska et al. (2017) suggested that a diet free of gluten and casein, and thus free of A1 β-casein, could provide potential benefits to individuals with autism spectrum disorder (ASD). This highlights a potential link between A1 β-casein consumption and gut-brain interactions and underscores the need to further investigate this phenomenon. Taken together, the scientific literature reviewed here provides invaluable insights regarding the potential impact of A1 β-casein on gastrointestinal health. Cumulative evidence suggests that A1 β-casein ingestion may be associated with chronic constipation, altered gastrointestinal transit, increased inflammatory response, and exacerbation of lactose intolerance symptoms. The intricate interplay between A1 β-casein and opioid receptors, as well as their potential influence on the microbiota-gut-brain axis, remains fertile ground for future research.
Autism and neurological disorders
ASD is a multifaceted neurodevelopmental disorder characterized by diverse etiologies and pathophysiologies. Numerous studies have highlighted a significant role of BCM-7 in the pathogenesis of ASD and other neurological disorders. Dohan (1980) was one of the first to propose a correlation between autism and food-derived opioids. They postulated that schizophrenia and autism could be associated with the absorption of exorphins, such as BCMs, from gluten and casein. This hypothesis was supported by Cade et al. (2000), who discovered elevated levels of IgG antibodies against gliadin (a gluten component) and bovine casein in patients with autism and schizophrenia compared to in control subjects. Their study also revealed improvements in schizophrenia and autism symptoms following a gluten- and casein-free dietary regimen. Cieslinska et al. (2015) investigated the impact of polymorphisms in the dipeptidyl peptidase IV (DPPIV) and-opioid receptor (MOR) genes, which play crucial roles in the modulation of BCM-7 activity. Although they did not find any connection between specific single-nucleotide polymorphisms in these genes and heightened incidence of autism, they did discern differences in the expression of DPPIV and MOR when exposed to BCM-7 between healthy children and those with ASD, implying an interaction between BCM-7 and these genes. Further research bolstered the proposed role of BCMs in autism. Jarmolowska et al. (2019) observed higher serum concentrations of BCM-7 and DPPIV in children with ASD than in healthy controls. Similarly, Sokolov et al. (2014) identified a correlation between the severity of autism symptoms and urinary concentrations of BCM-7.
BCMs have also been implicated in other neurological and behavioral disorders. Lindstrom et al. (1984) detected elevated levels of BCM-7-like opioid peptides in the cerebrospinal fluid (CSF) and plasma of women with postpartum psychosis. Kost et al. (2009) reported higher levels of bovine BCM-7 in formula-fed infants, who exhibited delayed psychomotor development and increased muscle tone. Moreover, a study by Osman et al. (2021) revealed that the intake of A1 β-casein milk, a significant source of BCM-7, beyond the weaning age resulted in depressive-like behavior in rats and alterations in brain-opioid and oxytocin receptors. This suggests a potential effect of A1 β-casein (and hence BCM-7) on mood, potentially via the gut-brain axis.
In conclusion, although the precise mechanisms remain to be completely elucidated and require additional investigation, the current body of evidence supports the hypothesis that BCMs, specifically BCM-7, contribute to the pathogenesis of autism, as well as other neurological and behavioral disorders.
SIDS
SIDS, commonly known as cognitive death, is a major cause of infant mortality worldwide. Despite extensive research, the exact causes of SIDS remain unclear. However, recent studies have begun to investigate a possible link between SIDS and A1 milk consumption. A key finding in this area of research is the potential effect of BCM-7 on the immature central nervous system of infants. In a comprehensive review, Sun et al. (2003) proposed that BCM-7 could potentially inhibit the respiratory center in the brainstem of infants, leading to apnea (temporary cessation of breathing) and ultimately death. This hypothesis is supported by empirical evidence from a study conducted by Wasilewska et al. (2011) on an association between elevated levels of bovine BCM-7, infant apnea, and cow milk consumption. Researchers have found that infants who experienced an apneic event had higher serum levels of BCM-7 than healthy infants. In addition, decreased activity of DPPIV, an enzyme responsible for degrading BCMs, was observed in all infants after an apneic event. These findings suggest that the consumption of A1 milk may contribute to apneic events in infants, which is a known risk factor for SIDS.
In addition, Kost et al. (2009) conducted a study showing that breastfed infants had higher levels of human β-casomorphin-7 (irHCM), while formula-fed infants consuming cow's milk-based formula had higher levels of bovine β-casomorphin-7 (irBCM). Furthermore, elevated irBCM levels were associated with delayed psychomotor development and increased muscle tone in formula-fed infants. This suggests that the consumption of A1 milk may contribute to developmental delays in infants, further supporting the potential link between A1 milk and SIDS.
Allergy
The potential impact of BCM-7 on human health and its role in triggering allergic reactions have been studied extensively. BCM-7, an opioid peptide, has the ability to cross the intestinal-blood barrier and influence various functions in humans. Fiedorowicz et al. (2011) investigated the effects of BCM-7 on proliferation and cytokine secretion by human peripheral blood mononuclear cells (PBMCs). The results suggested that BCM-7 may induce changes in PBMC proliferation and cytokine secretion, thereby shedding light on the immunomodulatory effects of food-derived opioid peptides. These findings have significant implications, particularly in the context of allergic diseases in newborns. Fiedorowicz et al. (2014) investigated the effects of BCM-7 in children diagnosed with severe atopic dermatitis (AD). Their results showed that incubation of PBMCs with peptide extracts from cow milk resulted in the upregulation of opioid receptor (MOR) gene expression and concomitant downregulation of DPPIV expression. This study advances our understanding regarding the role of BCM-7 in the pathogenesis of AD and provides a valuable diagnostic tool. The effects of BCM-7 are not limited to allergies. According to Fiedorowicz et al. (2016), the presence of BCM-7 in human milk and infant formula leads to changes in intestinal epithelial proliferation and increased interleukin-8 secretion. These findings improve our understanding regarding the pathogenesis of inflammation and food allergy in infants. In another study, Trivedi et al. (2015) found that BCM-7 reduced cysteine uptake, decreased the levels of the antioxidant glutathione (GSH), and decreased the levels of the methyl donor S-adenosylmethionine (SAM) in SH-SY5Y human neuroblastoma cells. The epigenetic effects of milk-derived opioid peptides may contribute to gastrointestinal dysfunction and inflammation in susceptible individuals. In conclusion, milk-derived opioid peptides, particularly BCM-7, influence inflammation and allergies, possibly via their effects on PBMC proliferation, cytokine secretion, and gene expression. Further studies are required to completely understand the mechanisms underlying these effects and their clinical significance.
Glutathione production
The pivotal role of β-casein variants, particularly A1 and A2, in modulating the biosynthesis of glutathione, a vital antioxidant in the human physiological context, has been highlighted in recent scientific literature. Glutathione has been implicated in several neurological and psychiatric disorders. For example, a significant decrease in glutathione levels in the CSF and prefrontal cortex has been reported in patients with schizophrenia. This deficit may contribute to degenerative processes in the brain and to the symptoms of schizophrenia (Cascella et al., 2011). In addition, individuals with schizophrenia have higher levels of antibodies associated with celiac disease and gluten sensitivity, which may be related to glutathione (Do et al., 2000). In the context of ASD, a decrease in the optimal ratio of glutathione/oxidized glutathione (GSH/GSSG) leads to increased oxidative stress (Frustaci et al., 2012). This stress can trigger chronic inflammation, increase hydrogen peroxide production in the mitochondria, and cause oxidative damage to proteins and DNA. In addition, cells from patients with autism have reduced glutathione capacity, which impairs their defense against oxidative stress (Melnyk et al., 2012). A meticulously designed double-blind randomized controlled crossover study was conducted by Deth et al. (2016) to investigate plasma glutathione concentrations in healthy subjects after consumption of milk containing different subtypes of casein.
Empirical evidence suggests a significant increase in plasma glutathione concentration following the consumption of milk containing only A2 β-casein, contrary to that observed after the consumption of milk containing a combination of A1 and A2 β-casein types. This indicates a potential advantage of A2 β-casein-specific milk in promoting the production of antioxidant glutathione in humans. Simultaneously, a randomized trial was conducted by Sheng et al. (2019) on Chinese preschool children diagnosed with mild to moderate milk intolerance. They postulated that replacing conventional milk, characterized by the presence of both A1 and A2 β-casein proteins, with milk containing only A2 β-casein would lead to reduction in gastrointestinal symptoms and improvement in cognitive performance. Interestingly, their findings also indicated a decrease in glutathione levels following the consumption of conventional milk, as opposed to the consumption of milk containing only A2 β-casein. To further expand the scope of this research area, Trivedi et al. (2014) investigated the effects of food-derived opioid peptides on cysteine uptake, a critical determinant of glutathione production. Their investigation revealed that peptides derived from the hydrolytic digestion of casein and gliadin exerted a modulatory effect on cysteine uptake in human neuronal and gastrointestinal cells. This modulation was associated with changes in the levels of glutathione and SAM. These findings underscore the profound influence of β-casein variants in milk on glutathione biosynthesis in humans.
The A2 milk market
The global A2 milk market has experienced steady growth in recent years, driven primarily by increased consumer interest in health and wellness. A2 milk has emerged as a preferred alternative, particularly in fast-growing markets such as Asia, where dairy consumption is on the rise. Key regions such as Europe and North America are also experiencing strong market growth. A key player in this market is The A2 Milk Company Ltd. in New Zealand, which specializes in the production and distribution of A2 milk products. In addition, well-known dairy companies such as Nestlé, Danone and Provilac have ventured into the A2 milk market, strengthening their positions through rigorous research and strategic marketing initiatives.
Significantly, reports from Grand View Research, Fortune Business Insights, and IMARC Group have provided insight into the market's potential. These reports estimate the global A2 milk market size at $2.44 billion in 2021, $1.84 billion in 2022, and an impressive $11.4 billion in 2022 (Fortune Business Insights, 2022; Grand View Research, 2023, IMARC Group, 2023). The projected compound annual growth rates further underscore the market's expansive potential in the coming years. Driving this market expansion is the versatility of A2 milk in products ranging from baked goods to beverages. Its ability to improve the quality and nutritional value of these products has increased demand. In addition, the digestibility of A2 milk, especially for people with lactose intolerance, has popularized it as a dairy alternative. More recently, A2 milk has become increasingly important in infant formula production. Its perceived health benefits have made it a top choice among parents. In addition, online food delivery platforms have played a key role in promoting A2 milk-based products by making them more accessible. Although the A2 milk market faced challenges during the COVID-19 pandemic, it demonstrated resilience and continued growth, underscoring its importance in the broader food and beverage industry (Fortune Business Insights, 2022; Grand View Research, 2023, IMARC Group, 2023). Its health benefits, particularly in aiding digestion and for those with lactose intolerance, have been key to its market growth.
In conclusion, the global A2 milk market is expected to witness remarkable growth in coming years. The key market players have established strong positions via effective marketing strategies, robust production capabilities, and extensive distribution networks. The ongoing research and development efforts will continue to drive industry growth and innovation to meet the evolving consumer demand for healthier and more easily digestible dairy alternatives. The A2 milk market is a testament to the growing consumer focus on health and wellness that has driven the adoption of A2 milk as the preferred choice worldwide.
Bovine milk contains four caseins, αs1, αs2, β, and κ-casein, and two major whey proteins, β-lactoglobulin and lactalbumin. Currently, bovine milk is known to contain 13 genetic variants of β-casein, with A1 and A2 being the most common among them. The A1 and A2 variants differ in the 67th amino acid in the β-casein polypeptide chain. The A1 type contains His, whereas the A2 type contains Pro at this position. It is well documented that His67 in casein can be digested in the human gastrointestinal tract by digestive and microbial enzymes, resulting in the possible release of BCM-7 during digestion, as well as during the ripening of cheese.
BCM-7 is a heptapeptide dietary molecule derived from the digestion of β-casein in dairy products, particularly A1 milk. It is an opioid peptide that can cross the intestinal-blood barrier and can influence various functions in the human body. BCM-7 has been extensively studied to understand its potential effects on the human body. Although some claims are inconclusive and require further research, certain studies have reported correlations between BCM-7 and human health conditions, such as CVD, autism, and T1D.
Furthermore, A2 milk β-casein is mostly found in some breeds of cows and other mammals (such as goat, sheep, and buffalo). Despite insufficient scientific evidence, A2 milk protein is believed to be easily digestible and to possess various health benefits compared with A1 milk protein. Human breast milk also contains β-casein that is mostly of the A2 type. Some researchers believe that A2 milk can be easily digested by the human body as it does not contain A1 β-casein. The A1 β-casein protein is associated with BCM-7, which may cause inflammation and discomfort upon consumption.
In 2016, a double-blind randomized controlled crossover study was conducted to investigate the plasma glutathione concentrations in healthy subjects. The results revealed a significant increase in plasma glutathione concentrations following the consumption of milk containing only A2 β-casein, contrary to that observed after the consumption of milk containing a combination of A1 and A2 β-casein types. This observation suggests a potential advantage of the A2 β-casein milk in promoting the production of the antioxidant, glutathione, in humans.
The global A2 milk market has grown steadily in recent years, especially in the fast-growing Asian market where dairy consumption is on the rise. Consumer awareness regarding the health benefits associated with A2 milk consumption was a key driver of market growth. The use of A2 milk in various products such as baked goods and baby formulas has become a successful trend, thanks to promotions and online shipping. The key market players have established strong positions via effective marketing strategies, robust production capabilities, and extensive distribution networks. Further research on A1 and A2 β-casein is required to drive industry growth and innovation, which will lead to the satisfaction of the evolving consumer demand for healthier and easily digestible dairy alternatives.
Acknowledgements
The authors thank Matthew J. Yoon from the North Carolina State of Veterinary Medicine, USA, for revising and proofreading this research paper.
Declarations
Conflict of interest
The authors declare no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Young-Seo Park and Sung-Sik Yoon have contributed equally to this work.
Contributor Information
Young-Seo Park, Email: ypark@gachon.ac.kr.
Sung-Sik Yoon, Email: sungsik@yonsei.ac.kr.
References
- Allison AJ, Clarke AJ. Further research for consideration in 'the A2 milk case'. European Journal of Clinical Nutrition. 60: 921-924; reply 924-925 (2006) [DOI] [PubMed]
- Andiran F, Dayi S, Mete E. Cows milk consumption in constipation and anal fissure in infants and young children. Journal of Paediatrics and Child Health. 2003;39:329–331. doi: 10.1046/j.1440-1754.2003.00152.x. [DOI] [PubMed] [Google Scholar]
- Aslam H, Ruusunen A, Berk M, Loughman A, Rivera L, Pasco JA, Jacka FN. Unravelled facets of milk derived opioid peptides: A focus on gut physiology, fractures and obesity. International Journal of Food Sciences and Nutrition. 2020;71:36–49. doi: 10.1080/09637486.2019.1614540. [DOI] [PubMed] [Google Scholar]
- Bisutti V, Pegolo S, Giannuzzi D, Mota LFM, Vanzin A, Toscano A, Trevisi E, Ajmone Marsan P, Brasca M, Cecchinato A. The beta-casein (CSN2) A2 allelic variant alters milk protein profile and slightly worsens coagulation properties in Holstein cows. Journal of Dairy Science. 2022;105:3794–3809. doi: 10.3168/jds.2021-21537. [DOI] [PubMed] [Google Scholar]
- Brooke-Taylor S, Dwyer K, Woodford K, Kost N. Systematic review of the gastrointestinal effects of A1 compared with A2 beta-casein. Advances in Nutrition. 2017;8:739–748. doi: 10.3945/an.116.013953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cade R, Privette M, Fregly M, Rowland N, Sun Z, Zele V, Wagemaker H, Edelstein C. Autism and schizophrenia: intestinal disorders. Nutritional Neuroscience. 2000;3:57–72. doi: 10.1080/1028415X.2000.11747303. [DOI] [PubMed] [Google Scholar]
- Cascella NG, Kryszak D, Bhatti B, Gregory P, Kelly DL, Mc Evoy JP, Fasano A, Eaton WW. Prevalence of celiac disease and gluten sensitivity in the United States clinical antipsychotic trials of intervention effectiveness study population. Schizophrenia Bulletin. 2011;37:94–100. doi: 10.1093/schbul/sbp055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chia JSJ, McRae JL, Enjapoori AK, Lefevre CM, Kukuljan S, Dwyer KM. Dietary cows' milk protein A1 beta-casein increases the incidence of T1D in NOD mice. Nutrients. 2018;10:1291. doi: 10.3390/nu10091291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chia JSJ, McRae JL, Kukuljan S, Woodford K, Elliott RB, Swinburn B, Dwyer KM. A1 beta-casein milk protein and other environmental pre-disposing factors for type 1 diabetes. Nutrition & Diabetes. 2017;7:e274. doi: 10.1038/nutd.2017.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cieslinska A, Fiedorowicz E, Rozmus D, Sienkiewicz-Szlapka E, Jarmolowska B, Kaminski S. Does a Little Difference Make a Big Difference? Bovine beta-casein A1 and A2 variants and human health-an update. International Journal of Molecular Sciences. 2022;23:15637. doi: 10.3390/ijms232415637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cieslinska A, Kostyra E, Savelkoul HFJ. Treating autism spectrum disorder with gluten-free and casein-free diet: the underlying microbiota-gut-brain axis mechanisms. HSOA Journal of Clinical Immunology and Immunotherapy. 3: (2017)
- Cieslinska A, Sienkiewicz-Szlapka E, Wasilewska J, Fiedorowicz E, Chwala B, Moszynska-Dumara M, Cieslinski T, Bukalo M, Kostyra E. Influence of candidate polymorphisms on the dipeptidyl peptidase IV and mu-opioid receptor genes expression in aspect of the beta-casomorphin-7 modulation functions in autism. Peptides. 2015;65:6–11. doi: 10.1016/j.peptides.2014.11.012. [DOI] [PubMed] [Google Scholar]
- Dalgleish DG, Corredig M. The structure of the casein micelle of milk and its changes during processing. Annual Review of Food Science and Technology. 2012;3:449–467. doi: 10.1146/annurev-food-022811-101214. [DOI] [PubMed] [Google Scholar]
- Daniloski D, Cunha NM, McCarthy NA, O'Callaghan TF, McParland S, Vasiljevic T. Health-related outcomes of genetic polymorphism of bovine β-casein variants: A systematic review of randomised controlled trials. Trends in Food Science & Technology. 2021;111:233–248. doi: 10.1016/j.tifs.2021.02.073. [DOI] [Google Scholar]
- Daniloski D, McCarthy NA, Gazi I, Vasiljevic T. Rheological and structural properties of acid-induced milk gels as a function of β-casein phenotype. Food Hydrocolloids. 2022;131:107846. doi: 10.1016/j.foodhyd.2022.107846. [DOI] [Google Scholar]
- De Noni I, Cattaneo S. Occurrence of β-casomorphins 5 and 7 in commercial dairy products and in their digests following in vitro simulated gastro-intestinal digestion. Food Chemistry. 2010;119:560–566. doi: 10.1016/j.foodchem.2009.06.058. [DOI] [Google Scholar]
- De Noni I, FitzGerald RJ, Korhonen HJ, Le Roux Y, Livesey CT, Thorsdottir I, Tomé D, Witkamp R. Review of the potential health impact of β-casomorphins and related peptides. EFSA Journal. 2009;231:1–107. [Google Scholar]
- Defilippi C, Gomez E, Charlin V, Silva C. Inhibition of small intestinal motility by casein: A role of beta casomorphins? Nutrition. 1995;11:751–754. [PubMed] [Google Scholar]
- Deth R, Clarke A, Ni J, Trivedi M. Clinical evaluation of glutathione concentrations after consumption of milk containing different subtypes of beta-casein: Results from a randomized, cross-over clinical trial. Nutrition Journal. 2016;15:82. doi: 10.1186/s12937-016-0201-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Do KQ, Trabesinger AH, Kirsten-Kruger M, Lauer CJ, Dydak U, Hell D, Holsboer F, Boesiger P, Cuenod M. Schizophrenia: Glutathione deficit in cerebrospinal fluid and prefrontal cortex in vivo. European Journal of Neuroscience. 2000;12:3721–3728. doi: 10.1046/j.1460-9568.2000.00229.x. [DOI] [PubMed] [Google Scholar]
- Dohan F. Hypothesis: Genes and neuroactive peptides from food as cause of schizophrenia. Advances in Biochemical Psychopharmacology. 1980;22:535–548. [PubMed] [Google Scholar]
- Elliott RB, Harris DP, Hill JP, Bibby NJ, Wasmuth HE. Type I (insulin-dependent) diabetes mellitus and cow milk: Casein variant consumption. Diabetologia. 1999;42:292–296. doi: 10.1007/s001250051153. [DOI] [PubMed] [Google Scholar]
- Fiedorowicz E, Jarmolowska B, Iwan M, Kostyra E, Obuchowicz R, Obuchowicz M. The influence of mu-opioid receptor agonist and antagonist peptides on peripheral blood mononuclear cells (PBMCs) Peptides. 2011;32:707–712. doi: 10.1016/j.peptides.2010.12.003. [DOI] [PubMed] [Google Scholar]
- Fiedorowicz E, Kaczmarski M, Cieslinska A, Sienkiewicz-Szlapka E, Jarmolowska B, Chwala B, Kostyra E. Beta-casomorphin-7 alters mu-opioid receptor and dipeptidyl peptidase IV genes expression in children with atopic dermatitis. Peptides. 2014;62:144–149. doi: 10.1016/j.peptides.2014.09.020. [DOI] [PubMed] [Google Scholar]
- Fiedorowicz E, Markiewicz LH, Sidor K, Swiatecka D, Cieslinska A, Matysiewicz M, Piskorz-Ogorek K, Sienkiewicz-Szlapka E, Teodorowicz M, Swiatecki A, Kostyra E. The influence of breast milk and infant formulae hydrolysates on bacterial adhesion and Caco-2 cells functioning. Food Research International. 2016;89:679–688. doi: 10.1016/j.foodres.2016.09.022. [DOI] [PubMed] [Google Scholar]
- Fortune Business Insights. A2 milk market size, share & covid-19 impact analysis, by form (liquid and powder), by distribution channel (supermarkets and hypermarkets, convenience stores, online retail, and others), and regional forecast, 2022–2029. Available at: https://www.fortunebusinessinsights.com/a2-milk-market-103212. Accessed Jun. 12, 2023.
- Franzoi M, Niero G, Visentin G, Penasa M, Cassandro M, De Marchi M. Variation of detailed protein composition of cow milk predicted from a large database of mid-infrared spectra. Animals. 2019;9:176. doi: 10.3390/ani9040176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frustaci A, Neri M, Cesario A, Adams JB, Domenici E, Dalla Bernardina B, Bonassi S. Oxidative stress-related biomarkers in autism: Systematic review and meta-analyses. Free Radical Biology and Medicine. 2012;52:2128–2141. doi: 10.1016/j.freeradbiomed.2012.03.011. [DOI] [PubMed] [Google Scholar]
- Fukui H. Increased intestinal permeability and decreased barrier function: Does it really influence the risk of inflammation? Inflammatory Intestinal Diseases. 2016;1:135–145. doi: 10.1159/000447252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gai N, Uniacke-Lowe T, O'Regan J, Faulkner H, Kelly AL. Effect of protein genotypes on physicochemical properties and protein functionality of bovine milk: A review. Foods. 2021;10:2409. doi: 10.3390/foods10102409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazi I, Johansen LB, Huppertz T. Heterogeneity, fractionation, and isolation. Encyclopedia of Dairy Sciences. 2022 doi: 10.1016/B978-0-12-818766-1.00278-6. [DOI] [Google Scholar]
- Grand View Research. A2 milk market size, share & trends analysis report by product (liquid, powder), by packaging (cartons, bottles), by distribution channel (supermarket & hypermarket, convenience stores, online), by region, and segment forecasts, 2022 - 2028. Available at: https://www.grandviewresearch.com/industry-analysis/a2-milk-market-report. Accessed Jun. 12, 2023.
- Haq MRU, Kapila R, Saliganti V. Consumption of β-casomorphins-7/5 induce inflammatory immune response in mice gut through Th2 pathway. Journal of Functional Foods. 2014;8:150–160. doi: 10.1016/j.jff.2014.03.018. [DOI] [Google Scholar]
- Haq MRU, Kapila R, Sharma R, Saliganti V, Kapila S. Comparative evaluation of cow β-casein variants (A1/A2) consumption on Th 2-mediated inflammatory response in mouse gut. European Journal of Nutrition. 2014;53:1039–1049. doi: 10.1007/s00394-013-0606-7. [DOI] [PubMed] [Google Scholar]
- Heinecke JW. Mass spectrometric quantification of amino acid oxidation products in proteins: insights into pathways that promote LDL oxidation in the human artery wall. FASEB Journal. 1999;13:1113–1120. doi: 10.1096/fasebj.13.10.1113. [DOI] [PubMed] [Google Scholar]
- Ho S, Woodford K, Kukuljan S, Pal S. Comparative effects of A1 versus A2 beta-casein on gastrointestinal measures: a blinded randomised cross-over pilot study. European Journal of Clinical Nutrition. 2014;68:994–1000. doi: 10.1038/ejcn.2014.127. [DOI] [PubMed] [Google Scholar]
- Holt C. Casein and casein micelle structures, functions and diversity in 20 species. International Dairy Journal. 2016;60:2–13. doi: 10.1016/j.idairyj.2016.01.004. [DOI] [Google Scholar]
- Horne DS. Casein micelle structure and stability. pp. 213-250. In: Milk proteins. Elsevier (2020)
- Huppertz T, Gazi I, Luyten H, Nieuwenhuijse H, Alting A, Schokker E. Hydration of casein micelles and caseinates: Implications for casein micelle structure. International Dairy Journal. 2017;74:1–11. doi: 10.1016/j.idairyj.2017.03.006. [DOI] [Google Scholar]
- IMARC Group. A2 milk market: global industry trends, share, size, growth, opportunity and forecast 2023–2028. Available at: https://www.imarcgroup.com/a2-milk-market. Accessed Jun. 12, 2023.
- Jarmolowska B, Bukalo M, Fiedorowicz E, Cieslinska A, Kordulewska NK, Moszynska M, Swiatecki A, Kostyra E. Role of milk-derived opioid peptides and proline dipeptidyl peptidase-4 in autism spectrum disorders. Nutrients. 2019;11:87. doi: 10.3390/nu11010087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalra S, Dhingra M. Childhood diabetes in India. Annals of Pediatric Endocrinology & Metabolism. 2018;23:126–130. doi: 10.6065/apem.2018.23.3.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaminski S, Cieslinska A, Kostyra E. Polymorphism of bovine beta-casein and its potential effect on human health. Journal of Applied Genetics. 2007;48:189–198. doi: 10.1007/BF03195213. [DOI] [PubMed] [Google Scholar]
- Kaskous S. A1- and A2- milk and their effect on human health. Journal of Food Engineering and Technology. 2020;9:15–21. doi: 10.32732/jfet.2020.9.1.15. [DOI] [Google Scholar]
- Kay SS, Delgado S, Mittal J, Eshraghi RS, Mittal R, Eshraghi AA. Beneficial effects of milk having a2 beta-casein protein: Myth or reality? Journal of Nutrition. 2021;151:1061–1072. doi: 10.1093/jn/nxaa454. [DOI] [PubMed] [Google Scholar]
- Kohil A, Al-Asmakh M, Al-Shafai M, Terranegra A. The interplay between diet and the epigenome in the pathogenesis of type-1 diabetes. Frontiers in Nutrition. 2021;7:612115. doi: 10.3389/fnut.2020.612115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kost NV, Sokolov OY, Kurasova OB, Dmitriev AD, Tarakanova JN, Gabaeva MV, Zolotarev YA, Dadayan AK, Grachev SA, Korneeva EV, Mikheeva IG, Zozulya AA. Beta-casomorphins-7 in infants on different type of feeding and different levels of psychomotor development. Peptides. 2009;30:1854–1860. doi: 10.1016/j.peptides.2009.06.025. [DOI] [PubMed] [Google Scholar]
- Kuellenberg de Gaudry D, Lohner S, Bischoff K, Schmucker C, Hoerrlein S, Roeger C, Schwingshackl L, Meerpohl JJ. A1- and A2 beta-casein on health-related outcomes: A scoping review of animal studies. European Journal of Nutrition. 2022;61:1–21. doi: 10.1007/s00394-021-02551-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kullenberg de Gaudry D, Lohner S, Schmucker C, Kapp P, Motschall E, Horrlein S, Roger C, Meerpohl JJ. Milk A1 beta-casein and health-related outcomes in humans: A systematic review. Nutrition Reviews. 2019;77:278–306. doi: 10.1093/nutrit/nuy063. [DOI] [PubMed] [Google Scholar]
- Kunz C, Lonnerdal B. Casein and casein subunits in preterm milk, colostrum, and mature human milk. Journal of Pediatric Gastroenterology and Nutrition. 1990;10:454–461. doi: 10.1097/00005176-199005000-00007. [DOI] [PubMed] [Google Scholar]
- Laugesen M, Elliott R. Ischaemic heart disease, Type 1 diabetes, and cow milk A1 beta-casein. New Zealand Medical Journal. 2003;116:U295. [PubMed] [Google Scholar]
- Ledesma-Martinez E, Aguiniga-Sanchez I, Weiss-Steider B, Rivera-Martinez AR, Santiago-Osorio E. Casein and peptides derived from casein as antileukaemic agents. Journal of Oncology. 2019;2019:8150967. doi: 10.1155/2019/8150967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lempainen J, Tauriainen S, Vaarala O, Makela M, Honkanen H, Marttila J, Veijola R, Simell O, Hyoty H, Knip M, Ilonen J. Interaction of enterovirus infection and cow's milk-based formula nutrition in type 1 diabetes-associated autoimmunity. Diabetes/Metabolism Research and Reviews. 2012;28:177–185. doi: 10.1002/dmrr.1294. [DOI] [PubMed] [Google Scholar]
- Lindstrom LH, Nyberg F, Terenius L, Bauer K, Besev G, Gunne LM, Lyrenas S, Willdeck-Lund G, Lindberg B. CSF and plasma beta-casomorphin-like opioid peptides in postpartum psychosis. American Journal of Psychiatry. 1984;141:1059–1066. doi: 10.1176/ajp.141.9.1059. [DOI] [PubMed] [Google Scholar]
- Lucey JA. ADSA Foundation Scholar. Award Formation and physical properties of milk protein gels. Journal of Dairy Science. 2002;85:281–94. doi: 10.3168/jds.S0022-0302(02)74078-2. [DOI] [PubMed] [Google Scholar]
- Lucey JA, Horne DS. Perspectives on casein interactions. International Dairy Journal. 2018;85:56–65. doi: 10.1016/j.idairyj.2018.04.010. [DOI] [Google Scholar]
- McLachlan CN. Beta-casein A1, ischaemic heart disease mortality, and other illnesses. Medical Hypotheses. 2001;56:262–272. doi: 10.1054/mehy.2000.1265. [DOI] [PubMed] [Google Scholar]
- Melnyk S, Fuchs GJ, Schulz E, Lopez M, Kahler SG, Fussell JJ, Bellando J, Pavliv O, Rose S, Seidel L, Gaylor DW, James SJ. Metabolic imbalance associated with methylation dysregulation and oxidative damage in children with autism. Journal of Autism and Developmental Disorders. 2012;42:367–377. doi: 10.1007/s10803-011-1260-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori S, Fujiwara-Tani R, Kishi S, Sasaki T, Ohmori H, Goto K, Nakashima C, Nishiguchi Y, Kawahara I, Luo Y, Kuniyasu H. Enhancement of anti-tumoral immunity by beta-casomorphin-7 inhibits cancer development and metastasis of colorectal cancer. International Journal of Molecular Sciences. 2021;22:8232. doi: 10.3390/ijms22158232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen HTH, Schwendel H, Harland D, Day L. Differences in the yoghurt gel microstructure and physicochemical properties of bovine milk containing A(1)A(1) and A(2)A(2) beta-casein phenotypes. Food Research International. 2018;112:217–224. doi: 10.1016/j.foodres.2018.06.043. [DOI] [PubMed] [Google Scholar]
- Osman A, Zuffa S, Walton G, Fagbodun E, Zanos P, Georgiou P, Kitchen I, Swann J, Bailey A. Post-weaning A1/A2 beta-casein milk intake modulates depressive-like behavior, brain mu-opioid receptors, and the metabolome of rats. iScience. 2021;24:103048. doi: 10.1016/j.isci.2021.103048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal S, Woodford K, Kukuljan S, Ho S. Milk intolerance, beta-casein and lactose. Nutrients. 2015;7:7285–7297. doi: 10.3390/nu7095339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park YW, Haenlein GFW. A2 bovine milk and caprine milk as a means of remedy for milk protein allergy. Dairy. 2021;2:191–201. doi: 10.3390/dairy2020017. [DOI] [Google Scholar]
- Pociot F, Lernmark A. Genetic risk factors for type 1 diabetes. Lancet. 2016;387:2331–2339. doi: 10.1016/S0140-6736(16)30582-7. [DOI] [PubMed] [Google Scholar]
- Poulsen N, Rosengaard A, Szekeres B, Gregersen V, Jensen H, Larsen L. Protein heterogeneity of bovine β-casein in Danish dairy breeds and association of rare β-casein F with milk coagulation properties. Acta Agriculturae Scandinavica, Section A—Animal Science. 2016;66:190–198. [Google Scholar]
- Poulsen NA, Bertelsen HP, Jensen HB, Gustavsson F, Glantz M, Mansson HL, Andren A, Paulsson M, Bendixen C, Buitenhuis AJ, Larsen LB. The occurrence of noncoagulating milk and the association of bovine milk coagulation properties with genetic variants of the caseins in 3 Scandinavian dairy breeds. Journal of Dairy Science. 2013;96:4830–4842. doi: 10.3168/jds.2012-6422. [DOI] [PubMed] [Google Scholar]
- Pozzilli P. Beta-casein in cow's milk: A major antigenic determinant for type 1 diabetes? Journal of Endocrinological Investigation. 1999;22:562–567. doi: 10.1007/BF03343610. [DOI] [PubMed] [Google Scholar]
- Raikos V, Dassios T. Health-promoting properties of bioactive peptides derived from milk proteins in infant food: a review. Dairy Science & Technology. 2014;94:91–101. doi: 10.1007/s13594-013-0152-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramakrishnan M, Eaton TK, Sermet OM, Savaiano DA. Milk containing A2 β-casein only, as a single meal, causes fewer symptoms of lactose intolerance than milk containing A1 and A2 β-caseins in subjects with lactose maldigestion and intolerance: A randomized, double-blind, crossover trial. Nutrients. 2020;12:3855. doi: 10.3390/nu12123855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rewers M, Ludvigsson J. Environmental risk factors for type 1 diabetes. Lancet. 2016;387:2340–2348. doi: 10.1016/S0140-6736(16)30507-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rueda-Ruzafa L, Cruz F, Cardona D, Hone AJ, Molina-Torres G, Sanchez-Labraca N, Roman P. Opioid system influences gut-brain axis: Dysbiosis and related alterations. Pharmacological Research. 2020;159:104928. doi: 10.1016/j.phrs.2020.104928. [DOI] [PubMed] [Google Scholar]
- Sheng X, Li Z, Ni J, Yelland G. Effects of conventional milk versus milk containing only a2 beta-casein on digestion in Chinese children: a randomized study. Journal of Pediatric Gastroenterology and Nutrition. 2019;69:375–382. doi: 10.1097/MPG.0000000000002437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slattery CW. Review of casein micelle structure: examination of models. Journal of Dairy Science. 1976;59:1547–1556. doi: 10.3168/jds.S0022-0302(76)84403-7. [DOI] [PubMed] [Google Scholar]
- Sokolov O, Kost N, Andreeva O, Korneeva E, Meshavkin V, Tarakanova Y, Dadayan A, Zolotarev Y, Grachev S, Mikheeva I, Varlamov O, Zozulya A. Autistic children display elevated urine levels of bovine casomorphin-7 immunoreactivity. Peptides. 2014;56:68–71. doi: 10.1016/j.peptides.2014.03.007. [DOI] [PubMed] [Google Scholar]
- Summer A, Di Frangia F, Ajmone Marsan P, De Noni I, Malacarne M. Occurrence, biological properties and potential effects on human health of beta-casomorphin 7: Current knowledge and concerns. Critical Reviews in Food Science and Nutrition. 2020;60:3705–3723. doi: 10.1080/10408398.2019.1707157. [DOI] [PubMed] [Google Scholar]
- Sun Z, Zhang Z, Wang X, Cade R, Elmir Z, Fregly M. Relation of beta-casomorphin to apnea in sudden infant death syndrome. Peptides. 2003;24:937–943. doi: 10.1016/S0196-9781(03)00156-6. [DOI] [PubMed] [Google Scholar]
- Swaisgood HE. Chemistry of the caseins. Advanced Dairy Chemistry—1 Proteins: Part A/Part B: 139-201 (2003)
- Tailford KA, Berry CL, Thomas AC, Campbell JH. A casein variant in cow's milk is atherogenic. Atherosclerosis. 2003;170:13–19. doi: 10.1016/S0021-9150(03)00131-X. [DOI] [PubMed] [Google Scholar]
- Thorsdottir I, Birgisdottir BE, Johannsdottir IM, Harris DP, Hill J, Steingrimsdottir L, Thorsson AV. Different beta-casein fractions in Icelandic versus Scandinavian cow's milk may influence diabetogenicity of cow's milk in infancy and explain low incidence of insulin-dependent diabetes mellitus in Iceland. Pediatrics. 2000;106:719–724. doi: 10.1542/peds.106.4.719. [DOI] [PubMed] [Google Scholar]
- Torreilles J, Guerin M. Casein-derived peptides can promote human LDL oxidation by a peroxidase-dependent and metal-independent process. Comptes Rendus des Séances de la Société de Biologie et de Ses Filiales. 1995;189:933–942. [PubMed] [Google Scholar]
- Trivedi MS, Hodgson NW, Walker SJ, Trooskens G, Nair V, Deth RC. Epigenetic effects of casein-derived opioid peptides in SH-SY5Y human neuroblastoma cells. Nutrition & Metabolism. 2015;12:54. doi: 10.1186/s12986-015-0050-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trivedi MS, Shah JS, Al-Mughairy S, Hodgson NW, Simms B, Trooskens GA, Van Criekinge W, Deth RC. Food-derived opioid peptides inhibit cysteine uptake with redox and epigenetic consequences. The Journal of Nutritional Biochemistry. 2014;25:1011–1018. doi: 10.1016/j.jnutbio.2014.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truswell AS. The A2 milk case: a critical review. European Journal of Clinical Nutrition. 2005;59:623–631. doi: 10.1038/sj.ejcn.1602104. [DOI] [PubMed] [Google Scholar]
- Vaarala O. Leaking gut in type 1 diabetes. Current Opinion in Gastroenterology. 2008;24:701–706. doi: 10.1097/MOG.0b013e32830e6d98. [DOI] [PubMed] [Google Scholar]
- Vaarala O, Atkinson MA, Neu J. The, "perfect storm" for type 1 diabetes - the complex interplay between intestinal microbiota, gut permeability, and mucosal immunity. Diabetes. 2008;57:2555–2562. doi: 10.2337/db08-0331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigolo V, Franzoi M, Penasa M, De Marchi M. β-Casein variants differently affect bulk milk mineral content, protein composition, and technological traits. International Dairy Journal. 2022;124:105221. doi: 10.1016/j.idairyj.2021.105221. [DOI] [Google Scholar]
- Wada Y, Lonnerdal B. Bioactive peptides derived from human milk proteins–mechanisms of action. The Journal of Nutritional Biochemistry. 2014;25:503–514. doi: 10.1016/j.jnutbio.2013.10.012. [DOI] [PubMed] [Google Scholar]
- Wasilewska J, Sienkiewicz-Szlapka E, Kuzbida E, Jarmolowska B, Kaczmarski M, Kostyra E. The exogenous opioid peptides and DPPIV serum activity in infants with apnoea expressed as apparent life threatening events (ALTE) Neuropeptides. 2011;45:189–195. doi: 10.1016/j.npep.2011.01.005. [DOI] [PubMed] [Google Scholar]
- Woodford KB. Casomorphins and gliadorphins have diverse systemic effects spanning gut, brain and internal organs. International Journal of Environmental Research and Public Health. 2021;18:7911. doi: 10.3390/ijerph18157911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu HB, Zhong JM, Hu RY, Wang H, Gong WW, Pan J, Fei FR, Wang M, Guo LH, Yang L, Yu M. Rapidly rising incidence of type 1 diabetes in children and adolescents aged 0–19 years in Zhejiang, China, 2007 to 2013. Diabetic Medicine. 2016;33:1339–1346. doi: 10.1111/dme.13010. [DOI] [PubMed] [Google Scholar]