Abstract
Background
This systematic review and meta-analysis aimed to evaluate the safety and efficacy of sclerotherapy methods for hemorrhoidal disease (HD) over the past 40 years.
Methods
The review followed the 2020 Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines. A comprehensive literature search was conducted, including studies reporting the use of sclerotherapy in patients with HD. Study eligibility criteria were defined, and data were extracted independently by the authors. Random-effects meta-analyses were performed to assess outcomes of interest.
Results
Out of 1965 records identified, 44 studies met the inclusion criteria, involving 9729 patients. The majority of studies were conducted in Japan, followed by the UK, Italy, and Portugal. The median age of participants was 52 years, and the majority were male. The Goligher grade distribution indicated varying degrees of HD severity. Sclerotherapy was predominantly administered through anoscopy, with polidocanol being the most commonly used agent. The procedure was generally performed without pre-injection analgesia. The meta-analysis of 14 randomized controlled trials (RCTs) revealed that sclerotherapy was not inferior to control interventions in terms of success rate (risk ratio [RR] 1.00, 95% CI 0.71–1.41) and recurrence rate (RR 1.11, 95% CI 0.69–1.77), while resulting in fewer complications (RR 0.46, 95% CI 0.23–0.92).
Conclusions
This systematic review highlights the safety and efficacy of sclerotherapy for HD, which yields similar success rates and fewer complications compared to other conservative or surgical approaches. Further research is warranted to optimize sclerotherapy techniques and evaluate long-term outcomes.
Registration
PROSPERO 2023 CRD42023396910.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10151-023-02908-w.
Keywords: Hemorrhoidal disease, Sclerotherapy, Polidocanol foam, Systematic review, Meta-analysis
Introduction
Hemorrhoidal disease (HD) is one of the most common proctological diseases affecting the general population from mid-teens onward, with significant implications for national health services both in terms of surgeons’ workload and economic impact [1, 2].
Conventional surgical excisional or non-excisional treatments have shown high success rates but are also associated with increased pain and longer recovery periods compared to office-based procedures [3–5].
Sclerotherapy is a procedure indicated for grade I–II and grade III HD that is unresponsive to medical treatment. Additionally, it is effective in the symptomatic treatment of bleeding HD in elderly patients or those with severe comorbidities who are not suitable for traditional surgical interventions [6, 7]. While various methods and sclerosing preparations have been described in this context, most studies report the efficacy of liquid agents such as aluminum potassium sulfate and tannic acid [8], phenol in almond oil [9], and polidocanol [6]. Currently, polidocanol foam is one of the most widely used products, with apparently lower risk of complications compared to the liquid agents [10, 11]. It consists in the injection of sclerosing agents above the dentate line, directly into the internal hemorrhoidal plexus with consequent fibrosis and scarring of the hemorrhoids [5, 12].
The use of polidocanol in foam form has gained attention owing to its practical advantages, including potential cost-effectiveness, potential for fewer required office-based sessions, and possibly lower incidence of complications, which may contribute to good patient compliance [13, 14]. The foam formulation allows for a reduction in the injected dose of the sclerosing agent, potentially increasing the area of contact with the endothelium [15, 16].
The aim of this systematic review is to investigate methods of sclerotherapy for HD over the last 40 years and evaluate their safety and efficacy.
Methods
The authors developed the protocol for review, detailing pre-specified methods of analysis and eligibility of the studies in line with the 2020 Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidance [17]. The protocol was registered with PROSPERO on 5 March 2023 (CRD42023396910).
Study characteristics
Search term definitions were inclusive, promoting a wide search of studies reporting the use of sclerotherapy in patients with HD. Studies were eligible regardless of whether they were retrospective or prospective in design, controlled or uncontrolled.
Studies were ineligible for inclusion if they described superseded series, out-of-scope procedures (e.g., sclerotherapy associated with other intervention(s) in the same patient [e.g., mucopexy or rubber band ligation]). Similarly, studies were excluded if outcomes could not be segregated for the index population (i.e., multiple or combined interventions for HD, where data were not stratified).
A minimum population sample of 15 adult subjects (index population) was imposed for eligibility. This pragmatic threshold excluded case reports and small case series that often reported on early experience with the techniques.
Report characteristics
Any publication date was eligible from 1 January 1983 to the date of the final search performed on 1 December 2022. As a result of the large number of studies retrieved, it was decided to include only studies with full-text publications written in English. Only peer-reviewed publications reporting primary data were eligible. Thus reviews, editorials, and letters were excluded at the screening stage. Conference abstracts and proceedings were also excluded.
Information sources and study selection
The authors performed a comprehensive search of the literature using Medline (PubMed), Web of Sciences, Scopus, and EMBASE and hand-searching using all common search terms encompassing sclerotherapy with synonymous variants (i.e., [hemorrhoids] AND [sclerotherapy or injection or foam or polidocanol or sclerofoam]). Reference lists of all full-texts were hand-selected for any additional studies.
Data extraction
Screening was independently performed at the title and abstract levels by three coauthors (CA, EL, and GT), excluding studies not meeting eligibility criteria where these could be readily determined from the title/abstract alone. Full-text copies of all remaining studies were also obtained and assessed by the junior authors, who were un-blinded to the names of studies, authors, institutions, or publications. Disagreement regarding inclusion was resolved by consensus. Study characteristics and outcome data were extracted into a Microsoft Excel spreadsheet (XP professional edition; Microsoft Corp, Redmond, Washington, USA).
First author, publication year, country of origin, reason for exclusion, and type of study were extracted for each study, and the following data for each arm: Goligher grade, route of administration of sclerotherapy (i.e., anoscopic or endoscopic), type of sclerosing agent, type of needle, method of formation of the injected product, site(s) of injection, injected volume (total and per pile), patient position, anesthesia, study length (months), number of patients, number of male patients, mean or median age, mean injection time, intra- and postoperative complications, pain, success rate (overall and after the first injection), recurrence rate, follow-up (months), and scoring system(s).
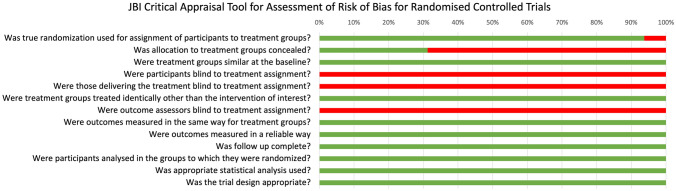
We assessed the risk of bias in randomized controlled trials (RCTs) using the JBI Critical Appraisal Tool for Assessment of Risk of Bias [18], which provided a structured approach to evaluating study quality and potential sources of bias.
Statistical analysis
A narrative synthesis of the studies was reported for the included studies. Meta-analyses were limited to RCTs based on the type of sclerotherapy for HD and for each outcome of interest (overall morbidity, postoperative pain, success, and recurrence rates). Acknowledging heterogeneity across studies we fitted random-effects models using the Sidik-Jonkman-Hartung-Knapp estimate of the heterogeneity. Statistical heterogeneity was assessed by formal test of homogeneity and evaluating the proportion of variability attributable to heterogeneity rather than sampling error (I2). Small study effects were assessed by evaluation of the funnel plot and via regression-based Egger test. Statistical analyses were performed using STATA V.17 (StataCorp, College Station, Texas, USA).
Results
Study selection
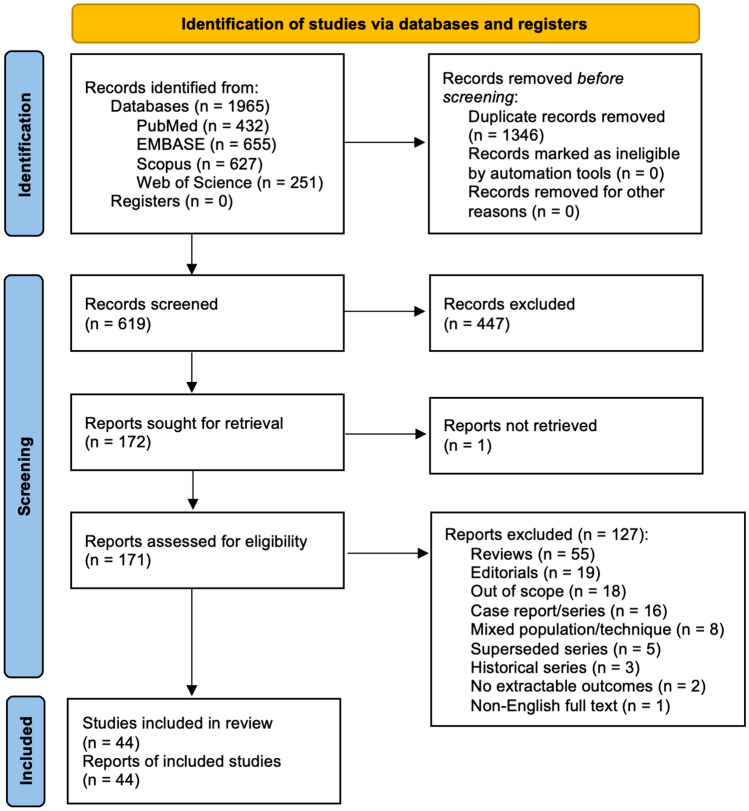
After removal of duplicates from a total of 1965 identified records, 619 were screened. Among these, 447 (72.2%) were excluded (Fig. 1). One report could not be retrieved [19].
Fig. 1.
PRISMA diagram
Overall, 44 studies published between 1985 and 2022 met the inclusion criteria and reported data on 9729 patients. These studies included 17 RCTs [3, 14, 20–34], 1 case–control study [35], and 26 cohort studies (Table 1). The majority of the studies were conducted in Japan (n = 9) [8, 35–42], followed by the UK (n = 6) [20–23, 43, 44], Italy (n = 6) [12, 45–49], and Portugal (n = 4) [3, 33, 50, 51]. Six (14%) of the studies were multicenter [14, 35, 41, 47, 51, 52].
Table 1.
Characteristics of the included studies
| First author, reference | Year | Country | Study type | No. patients | Recruitment time (months) | Index injected agent |
|---|---|---|---|---|---|---|
| Ambrose [20] | 1985 | UK | RCT | 62 | NR | 5% phenol in oil |
| Khoury [21] | 1985 | UK | RCT | 62 | 35 | 5% phenol in oil |
| Gartell [22] | 1985 | UK | RCT | 109 | 72 | 5% phenol in oil |
| Senapati [23] | 1988 | UK | RCT | 23 | NR | 5% phenol in oil |
| Mann [43] | 1988 | UK | PCS | 100 | 8 | 5% phenol in oil |
| Jamjoom [58] | 1991 | Saudi Arabia | PCS | 280 | 36 | 5% phenol in oil |
| Ponsky [54] | 1991 | USA | RCS | 18 | NR | 23.4% saline |
| Varma [24] | 1991 | Hong Kong | RCT | 28 | NR | 5% phenol in oil |
| Santos [44] | 1993 | UK | RCS | 189 | 12 | 5% phenol in oil |
| Jaspersen [25] | 1993 | Germany | RCT | 40 | NR | 5% phenol in oil |
| Kanellos [62] | 2000 | Greece | RCS | 240 | 72 | 5% phenol in oil |
| Kanellos [26] | 2003 | Greece | RCT | 80 | 48 | 5% phenol in oil |
| Khan [27] | 2006 | Pakistan | RCT | 52 | 8 | 5% phenol in oil |
| Takano [35] | 2006 | Japana | CC | 80 | 36 | OC-108 |
| Benin [45] | 2007 | Italy | RCS | 250 | NR | STS foam |
| Yuksel [28] | 2008 | Turkey | RCT | 62 | 24 | 3% polidocanol liquid |
| Hachiro [8] | 2011 | Japan | RCS | 448 | 50 | ALTA |
| Awad [29] | 2012 | Egypt | RCT | 60 | 24 | 5% EO or N-BC |
| Moser [14] | 2013 | Germanya | RCT | 66 | 25 | 3% polidocanol foam |
| Tokunaga [36] | 2013 | Japan | PCS | 940 | 38 | ALTA |
| An [52] | 2014 | China/Japana | PCS | 760 | 72 | An’s Shaobei |
| Yano [38] | 2014 | Japan | PCS | 57 | 60 | ALTA |
| Zhang [55] | 2015 | China | PCS | 30 | NR | Polidocanol |
| Yano [37] | 2015 | Japan | RCS | 55 | 12 | 5% phenol in oil |
| Tomiki [40] | 2015 | Japan | PCS | 83 | 36 | ALTA |
| Miyamoto [41] | 2016 | Japana | RCS | 169b | 73 | ALTA |
| Akindiose [59] | 2016 | Nigeria | PCS | 40 | 18 | 5% phenol in oil |
| Shah [30] | 2018 | India | RCT | 25 | NR | 3% polidocanol liquid |
| Tomiki [39] | 2019 | Japan | PCS | 33 | 36 | ALTA |
| Fernandes [50] | 2019 | Portugal | PCS | 2000 | 68 | 2% polidocanol foam |
| Ronconi [46] | 2019 | Italy | RCS | 615 | 132 | 3% polidocanol foam |
| Abiodun [31] | 2020 | Nigeria | RCT | 30 | 12 | 50% dextrose water |
| Makanjuola [53] | 2020 | Nigeria | PCS | 37 | 12 | 3% polidocanol liquid |
| Mishra [34] | 2020 | India | RCT | 75 | 15 | 3% polidocanol liquid |
| Shafi [32] | 2021 | Pakistan | RCT | 60 | 12 | 3% polidocanol liquid |
| Lobascio [12] | 2021 | Italy | RCS | 66 | 17 | 3% polidocanol foam |
| Neves [33] | 2022 | Portugal | RCT | 24 | 6 | 3% polidocanol foam |
| Gallo [47] | 2022 | Italya | PCS | 183 | 6 | 3% polidocanol foam |
| Xie [56] | 2022 | China | RCS | 201 | 45 | 1% polidocanol liquid |
| Abe [42] | 2022 | Japan | RCS | 1180 | 96 | ALTA |
| Salgueiro [51] | 2022 | Portugala | PCS | 228 | 19 | 3% polidocanol foam |
| Salgueiro [3] | 2022 | Portugal | RCT | 60 | NR | 3% polidocanol foam |
| Goglia [48] | 2022 | Italy | PCS | 50 | 3 | 3% polidocanol foam |
| Lisi [49] | 2022 | Italy | PCS | 19 | 14 | 3% polidocanol foam |
RCT randomized controlled trial; RCS retrospective cohort study; PCS prospective cohort study; CC case–control study; ALTA aluminum potassium sulfate and tannic acid; EO ethanolamine oleate; N-BC N-butyl cyanoacrylate; STS sodium tetradecyl sulfate; NR not reported; An’s Shaobei is a Chinese herbal remedy containing extracts of herbs, i.e., citric acid, gallic acid, paeoniflorin; adjuvant material was aseptic sterile water for injection
aMulticenter
bGrade III
Demographic and clinical characteristics
In 39 (89%) of the studies, the median age of the participants was 52 years (interquartile range 47–56). Thirty-eight (86%) studies reported the gender of the patients, comprising a total of 9170 (94.3%) subjects, of which 5535 (62.1%) were male. The median recruitment time was 25 months (range 3–132) in 36 (82%) of the studies.
Data on Goligher grade were available in 33 (75%) studies, including 7480 (77%) patients. Among them, 632 (8.4%) had grade I, 3259 (43.6%) had grade II, 3309 (44.2%) had grade III, and 280 (3.7%) had grade IV HD. Only 7 (16%) studies (all published between 2020 and 2022) incorporated validated scores for HD [3, 12, 33, 47, 48, 51, 53].
Procedure
Only eight studies described endoscopic administration [29, 31, 39, 40, 46, 54–56], while anoscopy was the adopted route in the remaining studies. The most frequently injected agent was polidocanol (n = 17 [39%] studies) in different states (liquid or foam; Table 1) and at different concentrations (1–3%), followed by 5% phenol oil (n = 15 [34%] studies). The needle’s caliber ranged between 20 and 25 gauge in 14 (32%) studies. Tessari’s method [57] was used to prepare the foam of polidocanol in nine studies [3, 12, 14, 33, 46, 47, 49–51], and the Varixio system in one study [48].
The site of injection was submucosal intra-hemorrhoidal in 25 (57%) studies, at the anorectal junction in seven studies [3, 12, 24, 31, 41, 46, 58], around the three afferent branches of the superior rectal artery in one study [25]. In another study, each pile was injected once, in several directions, and at variable depths [50]. The median injected volume per pile and total volume were 3 ml (interquartile range 3–4.5) and 9 ml (9–15), respectively, for 5% phenol oil (n = 12 studies); 2 ml (1.5–2) and 5 ml (4.5–5.5) for polidocanol liquid (n = 5 studies); and 2 ml (2–2) and 6 ml (6–7) for polidocanol foam (n = 10 studies).
Patient positioning was described in 24 (54%) studies. The majority of the injections were performed in the Sims position (n = 20 studies), while a few authors described the jack-knife [37, 42] and the lithotomy position [34, 41] as alternatives.
The procedure was performed without the use of pre-injection analgesia in most studies. Some authors preferred local analgesia [31, 36, 39, 41, 59], while lumbar analgesia was limited to Japanese authors [8, 35, 37, 42].
The median procedural time was 8.5 min (6.5–12) in 11 studies. Pain (mostly mild) was the most frequent perioperative issue,[14, 27, 48, 59] and typically occurred in 8% (3–13%) of cases in 30 (68%) studies. Bleeding affected a mean of 3.4% of patients in 18 (41%) studies. Other minor complications were rarely reported, such as urinary retention, local edema, tenesmus/discomfort, pruritus, paraesthesia, and external thrombosis. The median follow-up was 12 months (3–12), with 11 (25%) studies reporting a follow-up exceeding 12 months. Overall, 87% (67–98%) of patients were satisfied with the treatment in 9 (20%) studies.
Meta-analyses of RCTs
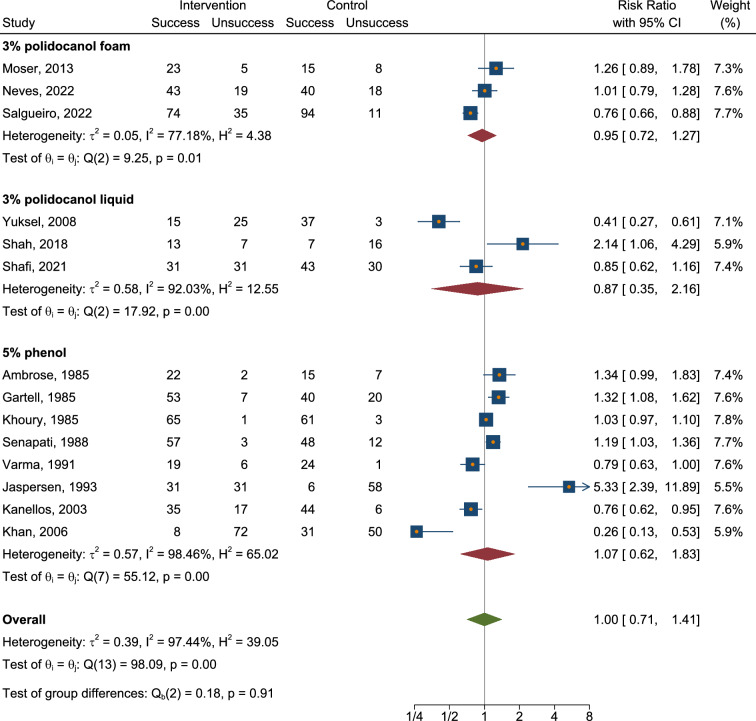
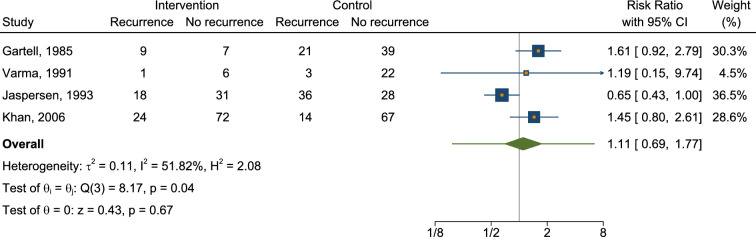
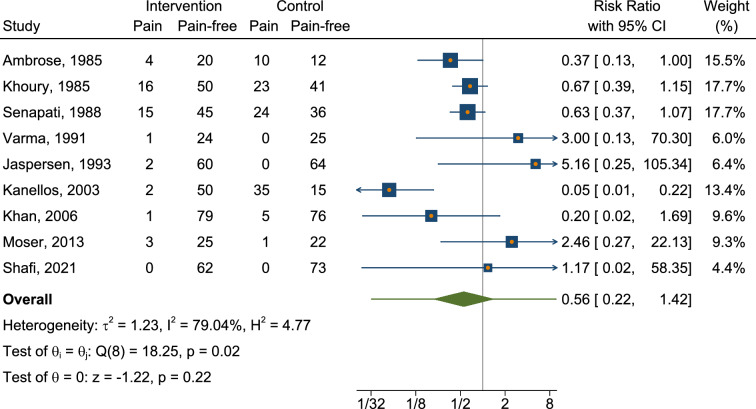
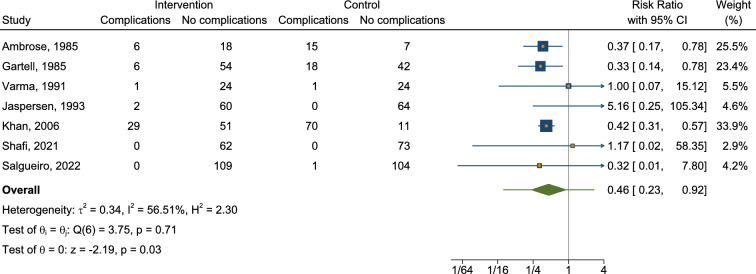
We conducted a meta-analysis of available RCTs to provide a quantitative assessment of sclerotherapy outcomes. Fourteen RCTs were identified that assessed the outcomes of sclerotherapy administered through the anoscope (Table 2). Notably, two additional RCTs describing sclerotherapy administered endoscopically were excluded from the quantitative meta-analysis because of methodological differences (i.e., study population consisting of patients with liver cirrhosis [29] or injection of an uncommon product [31]). One further RCT was excluded because of unconventional patients’ position (lithotomy rather than left lateral) [34]. The control treatments in the RCT included various interventions such as photocoagulation, rubber band ligation (RBL), bulking laxative, electrocoagulation, venotonic flavonoid, polidocanol liquid, alkaline of Achyranthes aspera Linn., 5% phenol almond oil, and HAL-RAR under local anesthesia, with some treatments administered in multiple sessions and under Doppler guidance. In terms of success rates, our meta-analysis revealed that sclerotherapy was not inferior to control interventions, with a risk ratio of 1.00 (95% CI 0.71–1.41) (Fig. 2, Appendix 1). The analysis showed substantial heterogeneity (I2 = 97.44%), and the test of group differences indicated no statistically significant variation (Qb(2) = 0.18, p = 0.91). We also analyzed the recurrence rate based on data from a total of four available studies (Fig. 3, Appendix 2). The risk ratio was 1.11 (95% CI 0.69–1.77), with moderate heterogeneity (I2 = 51.82%). A pooled risk ratio of 0.56 was estimated for the association between pain and intervention, but the evidence of less pain in the sclerotherapy group compared to control was not statistically significant (95% CI 0.22–1.42) based on data from nine available studies (Fig. 4, Appendix 3). The analysis revealed substantial heterogeneity (I2 = 79.04%). Our meta-analysis showed a significant reduction in overall complications following sclerotherapy compared to control interventions, with a risk ratio of 0.46 (95% CI 0.23–0.92) based on data from seven available studies (Fig. 5, Appendix 4). The analysis indicated moderate heterogeneity (I2 = 56.51%). Upon review, the primary flaw identified across the included studies predominantly pertained to blinding issues. Both participants and operators involved in performing the procedures or assessing outcomes often lacked adequate blinding, which could potentially introduce bias into the results (Fig. 6).
Table 2.
Randomized controlled trials assessing the anoscopic injection of sclerotherapy
| First author, reference | Type of treatments | |
|---|---|---|
| Index | Control | |
| Ambrose [20] | 5% phenol | Photocoagulation |
| Khoury [21] | 5% phenol (one session) | 5% phenol (multiple sessions) |
| Gartell [22] | 5% phenol | RBL |
| Senapati [23] | 5% phenol | Bulking laxative |
| Varma [24] | 5% phenol | Electrocoagulation |
| Jaspersen [25] | 5% phenol | 5% phenol under Doppler guidance |
| Kanellos [26] | 5% phenol | RBL or sclerobanding |
| Khan [27] | 5% phenol | Electrocoagulation |
| Yuksel [28] | 3% polidocanol liquid | Venotonic flavonoid |
| Moser [14] | 3% polidocanol foam | Polidocanol liquid |
| Shah [30] | 3% polidocanol liquid | Alkaline of Achyranthes aspera Linn. |
| Shafi [32] | 3% polidocanol liquid | 5% phenol almond oil |
| Neves [33] | 3% polidocanol foam | HAL-RAR under local anesthesia |
| Salgueiro [3] | 3% polidocanol foam | RBL |
RBL rubber band ligation, HAL-RAR hemorrhoidal artery ligation and recto anal repair
3 RCTs were not included in the quantitative meta-analysis because of different patient populations (patients with coexistent liver cirrhosis [29] or injection of an uncommon product [31]) or unconventional patients’ position (lithotomy rather than left lateral) [34]
Fig. 2.
Forest plot showing rates of success
Fig. 3.
Forest plot showing rates of recurrence
Fig. 4.
Forest plot showing rates of pain
Fig. 5.
Forest plot showing rates of complications (overall)
Fig. 6.
JBI Critical Appraisal Tool for Assessment of Risk of Bias for Randomized Controlled Trials
Discussion
In this systematic review, we examined the safety and efficacy of various sclerotherapy methods for HD over the past four decades. We conducted a comprehensive analysis of 44 selected studies encompassing 9729 patients, including 17 RCTs. The data analysis revealed that sclerotherapy administered through anoscopy was compared against various control interventions, including photocoagulation, RBL, bulking laxative, electrocoagulation, venotonic flavonoid, polidocanol liquid, alkaline of Achyranthes aspera Linn., 5% phenol almond oil, and HAL-RAR under local anesthesia in RCTs. The meta-analysis demonstrated that sclerotherapy yielded comparable success rates to these control interventions, with trends toward reduced pain and overall complications, despite issues related to blinding in the included studies.
It is noteworthy that a temporal trend has emerged in recent years, with a notable shift towards the use of polidocanol over 5% phenol oil. This shift is particularly evident when comparing studies from the last two decades to earlier research. From 2006 onwards, only one study has described the use of 5% phenol oil, in contrast to a more frequent mention of this agent in older studies dating back to 1985. This temporal evolution suggests a growing preference for polidocanol as a sclerosing agent in the treatment of HD, possibly due to its perceived increased safety.
The use of polidocanol in foam form offers several advantages over liquid agents. The foam formulation allows for a reduced injected dose of the sclerosing agent as a result of its larger volume, which increases the area of contact with the endothelium [16, 47]. This may contribute to potentially improved sclerotherapy outcomes and enhanced patient satisfaction. Our results align with the increasing evidence supporting the use of polidocanol foam, which has become a frequently utilized agent in recent RCTs [3, 33].
Our study also revealed that most of the included studies were conducted in Japan, suggesting a preference for sclerotherapy in this region [8, 35–42]. This observation may be attributed to cultural factors, variations in healthcare practices, or the availability of specific sclerosing agents. Further research from diverse geographical regions is needed to evaluate the generalizability of our findings and to explore potential regional variations in sclerotherapy practices.
It is worth noting that the variable “procedure duration” has only recently started to be consistently recorded in studies, reflecting an evolving trend in research methodology. Similarly, the utilization of validated scoring systems specific to HD has been a relatively recent development, with only seven studies incorporating these systems in the last 2 years. These advancements in data collection and standardized assessment tools are promising steps towards enhancing the comprehensiveness and comparability of future research in this field.
The comprehensive analysis of procedure-related aspects in this review, such as the route of administration, injected agent, needle caliber, site of injection, and patient positioning, underscores the need for a consensus to standardize clinical practice and reduce heterogeneity in sclerotherapy techniques.
Pain and bleeding were the most commonly reported perioperative issues, with pain occurring in approximately 8% of cases [14, 27, 48, 59]. These complications were generally mild and manageable. The overall satisfaction rates among patients undergoing sclerotherapy were high, emphasizing the positive impact of this procedure on patient outcomes. Long-term follow-up is necessary to assess the durability of treatment outcomes and to determine the recurrence rates associated with different sclerotherapy methods.
It is important to acknowledge the limitations of our study. The majority of included studies were retrospective or non-randomized, which may introduce selection bias and confounding factors. Additionally, the heterogeneity among the studies regarding patient characteristics, treatment protocols, and outcome measures limits the ability to perform a quantitative meta-analysis for all outcomes of interest. Heterogeneity exists even among the studies specifically focused on polidocanol foam regarding the various methods of foam preparation, including but not limited to the Tessari technique, the Easy Foam Kit, and Varixio. Standardization of foam preparation protocols in future studies could help reduce this source of heterogeneity and provide more consistent evidence on the effectiveness of polidocanol foam sclerotherapy. In addition to the aforementioned limitations, it is noteworthy that in our meta-analysis of the 14 RCTs, we had to group various “control treatments” under a single term, even though these control groups represented different conservative and non-excisional approaches to HD management. While all control treatments were non-surgical and aimed at conservative management of HD, the specific interventions within these groups varied. This grouping was required to allow analysis of the available data for analysis and should be taken into account when interpreting the results of our meta-analysis. Furthermore, we recognize the importance of considering individual patient circumstances and preferences, as supported by existing guidelines [60, 61]. Future well-designed studies are needed to address these limitations and provide a more comprehensive understanding of the role of sclerotherapy in the management of HD. Future well-designed RCTs are needed to provide more robust evidence on the safety and efficacy of sclerotherapy techniques.
Conclusion
This systematic review and meta-analysis contributes valuable insights into the safety and efficacy of sclerotherapy for HD, highlighting its comparable success rates, potential for reduced pain, and lower overall complication rates when compared to control interventions.
These findings support the consideration of sclerotherapy as a valid alternative to conventional surgical interventions, particularly in patients with lower-grade hemorrhoids or those who are not suitable candidates for surgery. Further research is warranted to optimize sclerotherapy techniques, standardize protocols, and evaluate long-term outcomes.
Supplementary Information
Below is the link to the electronic supplementary material.
Appendix 1 Funnel plot showing rates of success. Supplementary file1 (PDF 55 KB)
Appendix 2 Funnel plot showing rates of recurrence. Supplementary file2 (PDF 54 KB)
Appendix 3 Funnel plot showing rates of pain. Supplementary file3 (PDF 54 KB)
Appendix 4 Funnel plot showing rates of complications (overall). Supplementary file4 (PDF 54 KB)
Funding
Open access funding provided by Università degli Studi di Padova within the CRUI-CARE Agreement.
Data availability
The data that support the findings of this study are available from the corresponding authors upon reasonable request.
Declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
Ethics approval was not required at our institution for systematic reviews.
Informed consent
For this type of study, formal consent is not required.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
G. Gallo, Email: ga.gallo@uniroma1.it
U. Grossi, Email: ugo.grossi@unipd.it
References
- 1.Johanson JF, Sonnenberg A (1990) The prevalence of hemorrhoids and chronic constipation. An epidemiologic study. Gastroenterology 98:380–386 10.1016/0016-5085(90)90828-O [DOI] [PubMed] [Google Scholar]
- 2.Riss S, Weiser FA, Schwameis K et al (2012) The prevalence of hemorrhoids in adults. Int J Colorectal Dis 27:215–220 10.1007/s00384-011-1316-3 [DOI] [PubMed] [Google Scholar]
- 3.Salgueiro P, Garrido M, Santos RG, Pedroto I, Castro-Pocas FM (2022) Polidocanol foam sclerotherapy versus rubber band ligation in hemorrhoidal disease grades I/II/III: randomized trial. Dis Colon Rectum 65:e718–e727 10.1097/DCR.0000000000002117 [DOI] [PubMed] [Google Scholar]
- 4.Davis BR, Lee-Kong SA, Migaly J, Feingold DL, Steele SR (2018) The American Society of Colon and Rectal Surgeons clinical practice guidelines for the management of hemorrhoids. Dis Colon Rectum 61:284–292 10.1097/DCR.0000000000001030 [DOI] [PubMed] [Google Scholar]
- 5.Gallo G, Martellucci J, Sturiale A et al (2020) Consensus statement of the Italian Society of Colorectal Surgery (SICCR): management and treatment of hemorrhoidal disease. Tech Coloproctol 24:145–164 10.1007/s10151-020-02149-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gallo G, Ronconi M, Trompetto M (2021) Sclerotherapy with 3% polidocanol foam: revolutionizing outpatient treatment in patients with haemorrhoidal disease. Updates Surg 73:2029–2030 10.1007/s13304-021-01008-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tarasconi A, Perrone G, Davies J et al (2021) Anorectal emergencies: WSES-AAST guidelines. World J Emerg Surg 16:48 10.1186/s13017-021-00384-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hachiro Y, Kunimoto M, Abe T, Kitada M, Ebisawa Y (2011) Aluminum potassium sulfate and tannic acid (ALTA) injection as the mainstay of treatment for internal hemorrhoids. Surg Today 41:806–809 10.1007/s00595-010-4386-x [DOI] [PubMed] [Google Scholar]
- 9.Yamana T (2017) Japanese practice guidelines for anal disorders I. Hemorrhoids. J Anus Rectum Colon 1:89–99 10.23922/jarc.2017-018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McCloud JM, Jameson JS, Scott AN (2006) Life-threatening sepsis following treatment for haemorrhoids: a systematic review. Colorectal Dis 8:748–755 10.1111/j.1463-1318.2006.01028.x [DOI] [PubMed] [Google Scholar]
- 11.Barwell J, Watkins RM, Lloyd-Davies E, Wilkins DC (1999) Life-threatening retroperitoneal sepsis after hemorrhoid injection sclerotherapy: report of a case. Dis Colon Rectum 42:421–423 10.1007/BF02236364 [DOI] [PubMed] [Google Scholar]
- 12.Lobascio P, Laforgia R, Novelli E et al (2021) Short-term results of sclerotherapy with 3% polidocanol foam for symptomatic second- and third-degree hemorrhoidal disease. J Invest Surg 34:1059–1065 10.1080/08941939.2020.1745964 [DOI] [PubMed] [Google Scholar]
- 13.Rosa B (2019) Polidocanol foam: a breath of fresh air for the treatment of internal hemorrhoids. GE Port J Gastroenterol 26:153–154 10.1159/000493440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moser KH, Mosch C, Walgenbach M et al (2013) Efficacy and safety of sclerotherapy with polidocanol foam in comparison with fluid sclerosant in the treatment of first-grade haemorrhoidal disease: a randomised, controlled, single-blind, multicentre trial. Int J Colorectal Dis 28:1439–1447 10.1007/s00384-013-1729-2 [DOI] [PubMed] [Google Scholar]
- 15.Nastasa V, Samaras K, Ampatzidis C et al (2015) Properties of polidocanol foam in view of its use in sclerotherapy. Int J Pharm 478:588–596 10.1016/j.ijpharm.2014.11.056 [DOI] [PubMed] [Google Scholar]
- 16.Gallo G, Picciariello A, Pietroletti R et al (2022) Sclerotherapy with 3% polidocanol foam to treat second-degree haemorrhoidal disease: three-year follow-up of a multicentre, single arm, IDEAL phase 2b trial. Colorectal Dis. 10.1111/codi.16380 10.1111/codi.16380 [DOI] [PubMed] [Google Scholar]
- 17.Page MJ, McKenzie JE, Bossuyt PM et al (2021) The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 372:n71 10.1136/bmj.n71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barker TH, Stone JC, Sears K et al (2023) The revised JBI critical appraisal tool for the assessment of risk of bias for randomized controlled trials. JBI Evid Synth 21:494–506 10.11124/JBIES-22-00430 [DOI] [PubMed] [Google Scholar]
- 19.Moser KH (2007) Evaluation of the efficacy and safety of polidocanol foam in the sclerotherapy of first degree bleeding haemorrhoids. Phlebol Rev (Przeglad Flebologiczny) 15:103–106 [Google Scholar]
- 20.Ambrose NS, Morris D, Alexander-Williams J, Keighley MR (1985) A randomized trial of photocoagulation or injection sclerotherapy for the treatment of first- and second-degree hemorrhoids. Dis Colon Rectum 28:238–240 10.1007/BF02554043 [DOI] [PubMed] [Google Scholar]
- 21.Khoury GA, Lake SP, Lewis MC, Lewis AA (1985) A randomized trial to compare single with multiple phenol injection treatment for haemorrhoids. Br J Surg 72:741–742 10.1002/bjs.1800720924 [DOI] [PubMed] [Google Scholar]
- 22.Gartell PC, Sheridan RJ, McGinn FP (1985) Out-patient treatment of haemorrhoids: a randomized clinical trial to compare rubber band ligation with phenol injection. Br J Surg 72:478–479 10.1002/bjs.1800720624 [DOI] [PubMed] [Google Scholar]
- 23.Senapati A, Nicholls RJ (1988) A randomised trial to compare the results of injection sclerotherapy with a bulk laxative alone in the treatment of bleeding haemorrhoids. Int J Colorectal Dis 3:124–126 10.1007/BF01645317 [DOI] [PubMed] [Google Scholar]
- 24.Varma JS, Chung SC, Li AK (1991) Prospective randomised comparison of current coagulation and injection sclerotherapy for the outpatient treatment of haemorrhoids. Int J Colorectal Dis 6:42–45 10.1007/BF00703960 [DOI] [PubMed] [Google Scholar]
- 25.Jaspersen D, Koerner T, Schorr W, Hammar CH (1993) Proctoscopic doppler ultrasound in diagnostics and treatment of bleeding hemorrhoids. Dis Colon Rectum 36:942–945 10.1007/BF02050630 [DOI] [PubMed] [Google Scholar]
- 26.Kanellos I, Goulimaris I, Christoforidis E, Kelpis T, Betsis D (2003) A comparison of the simultaneous application of sclerotherapy and rubber band ligation, with sclerotherapy and rubber band ligation applied separately, for the treatment of haemorrhoids: a prospective randomized trial. Colorectal Dis 5:133–138 10.1046/j.1463-1318.2003.00395.x [DOI] [PubMed] [Google Scholar]
- 27.Khan N, Malik MA (2006) Injection sclerotherapy versus electrocoagulation in the management outcome of early haemorrhoids. J Pak Med Assoc 56:579–582 [PubMed] [Google Scholar]
- 28.Yuksel BC, Armagan H, Berkem H, Yildiz Y, Ozel H, Hengirmen S (2008) Conservative management of hemorrhoids: a comparison of venotonic flavonoid micronized purified flavonoid fraction (MPFF) and sclerotherapy. Surg Today 38:123–129 10.1007/s00595-007-3582-9 [DOI] [PubMed] [Google Scholar]
- 29.Awad AE, Soliman HH, Saif SA, Darwish AM, Mosaad S, Elfert AA (2012) A prospective randomised comparative study of endoscopic band ligation versus injection sclerotherapy of bleeding internal haemorrhoids in patients with liver cirrhosis. Arab J Gastroenterol 13:77–81 10.1016/j.ajg.2012.03.008 [DOI] [PubMed] [Google Scholar]
- 30.Shah B, Dudhamal TS (2018) Efficacy of Apamarga Kshara application and sclerotherapy in the management of Arsha (1(st) and 2(nd) degree piles) - an open-labeled, randomized, controlled clinical trial. Ayu 39:213–219 10.4103/ayu.AYU_147_18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Abiodun AA, Alatise OI, Okereke CE, Adesunkanmi AK, Eletta EA, Gomna A (2020) Comparative study of endoscopic band ligation versus injection sclerotherapy with 50% dextrose in water, in symptomatic internal haemorrhoids. Niger Postgrad Med J 27:13–20 10.4103/npmj.npmj_128_19 [DOI] [PubMed] [Google Scholar]
- 32.Shafi ASHF, Hamayunkhan M, Ahmad T, Mahmood K (2021) Compare the effectiveness of 5% phenol almond oil versus polidocanol sclerotherapy in patients with second degree hemorrhoids. Pak J Med Health Sci 15(2):730–732 [Google Scholar]
- 33.Neves S, Falcao D, Povo A, Castro-Pocas F, Oliveira J, Salgueiro P (2023) 3% polidocanol foam sclerotherapy versus hemorrhoidal artery ligation with recto anal repair in hemorrhoidal disease grades II-III: a randomized, pilot trial. Rev Esp Enferm Dig 115:115–120 [DOI] [PubMed] [Google Scholar]
- 34.Mishra S, Sahoo AK, Elamurugan TP, Jagdish S (2020) Polidocanol versus phenol in oil injection sclerotherapy in treatment of internal hemorrhoids: a randomized controlled trial. Turk J Gastroenterol 31:378–383 10.5152/tjg.2020.19276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Takano M, Iwadare J, Ohba H et al (2006) Sclerosing therapy of internal hemorrhoids with a novel sclerosing agent. Comparison with ligation and excision. Int J Colorectal Dis 21:44–51 10.1007/s00384-005-0771-0 [DOI] [PubMed] [Google Scholar]
- 36.Tokunaga Y, Sasaki H (2013) Impact of less invasive treatments including sclerotherapy with a new agent and hemorrhoidopexy for prolapsing internal hemorrhoids. Int Surg 98:210–213 10.9738/INTSURG-D-13-00030.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yano T, Asano M, Tanaka S, Oda N, Matsuda Y (2014) Prospective study comparing the new sclerotherapy and hemorrhoidectomy in terms of therapeutic outcomes at 4 years after the treatment. Surg Today 44:449–453 10.1007/s00595-013-0564-y [DOI] [PubMed] [Google Scholar]
- 38.Yano T, Yano K (2015) Comparison of injection sclerotherapy between 5% phenol in almond oil and aluminum potassium sulfate and tannic acid for grade 3 hemorrhoids. Ann Coloproctol 31:103–105 10.3393/ac.2015.31.3.103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tomiki Y, Aoki J, Motegi S et al (2019) Effectiveness of endoscopic sclerotherapy with aluminum potassium sulfate and tannic acid as a non-surgical treatment for internal hemorrhoids. Clin Endosc 52:581–587 10.5946/ce.2019.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tomiki Y, Ono S, Aoki J et al (2015) Treatment of Internal hemorrhoids by endoscopic sclerotherapy with aluminum potassium sulfate and tannic acid. Diagn Ther Endosc 2015:517690 10.1155/2015/517690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Miyamoto H, Hada T, Ishiyama G, Ono Y, Watanabe H (2016) Aluminum potassium sulfate and tannic acid sclerotherapy for Goligher grades II and III hemorrhoids: results from a multicenter study. World J Hepatol 8:844–849 10.4254/wjh.v8.i20.844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Abe T, Kunimoto M, Hachiro Y, Ohara K, Inagaki M (2022) Long-term outcomes of aluminum potassium sulfate and tannic acid sclerotherapy for prolapsed hemorrhoids: a single-center, observational study. Dis Colon Rectum 65:271–275 10.1097/DCR.0000000000002284 [DOI] [PubMed] [Google Scholar]
- 43.Mann CV, Motson R, Clifton M (1988) The immediate response to injection therapy for first-degree haemorrhoids. J R Soc Med 81:146–148 10.1177/014107688808100309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Santos G, Novell JR, Khoury G, Winslet MC, Lewis AA (1993) Long-term results of large-dose, single-session phenol injection sclerotherapy for hemorrhoids. Dis Colon Rectum 36:958–961 10.1007/BF02050633 [DOI] [PubMed] [Google Scholar]
- 45.Benin P, D’Amico C (2007) Foam sclerotherapy with fibrovein (STD) for the treatment of hemorrhoids, using a flexible endoscope. Minerva Chir 62:235–240 [PubMed] [Google Scholar]
- 46.Ronconi M, Casiraghi S, Schieppati M (2019) EndoTHeF: endoluminal treatment of hemorrhoids with foam. Ann Colorectal Res. 10.5812/acr.86297 10.5812/acr.86297 [DOI] [Google Scholar]
- 47.Gallo G, Pietroletti R, Novelli E et al (2022) A multicentre, open-label, single-arm phase II trial of the efficacy and safety of sclerotherapy using 3% polidocanol foam to treat second-degree haemorrhoids (SCLEROFOAM). Tech Coloproctol 26:627–636 10.1007/s10151-022-02609-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Goglia M, Nigro C, Aurello P, Diaco E, Trompetto M, Gallo G (2022) Preliminary results of the first 50 patients undergoing sclerotherapy for II-degree hemorrhoidal disease using an automated device. Front Surg 9:882030 10.3389/fsurg.2022.882030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lisi G, Gentileschi P, Spoletini D, Passaro U, Orlandi S, Campanelli M (2022) Sclerotherapy for III- and IV-degree hemorrhoids: results of a prospective study. Front Surg 9:978574 10.3389/fsurg.2022.978574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fernandes V, Fonseca J (2019) Polidocanol foam injected at high doses with intravenous needle: the (almost) perfect treatment of symptomatic internal hemorrhoids. GE Port J Gastroenterol 26:169–175 10.1159/000492202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Salgueiro P, Rei A, Garrido M et al (2022) Polidocanol foam sclerotherapy in the treatment of hemorrhoidal disease in patients with bleeding disorders: a multicenter, prospective, cohort study. Tech Coloproctol 26:615–625 10.1007/s10151-022-02600-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.An AY, Feng DY, Wang CH et al (2014) Comparing the effect of An’s Shaobei injection ([symbols; see text]) with Xiaozhiling injection ([symbols; see text]) in patients with internal hemorrhoids of grade I-III: a prospective cohort study. Chin J Integr Med 20:555–560 10.1007/s11655-014-1870-y [DOI] [PubMed] [Google Scholar]
- 53.Makanjuola A, Balogun OS, Osinowo AO, Adesanya AA, da Rocha JT (2020) Comparison of rubber band ligation with 3% polidocanol injection sclerotherapy for the treatment of internal haemorrhoids at a Nigerian tertiary hospital. Niger Postgrad Med J 27:311–316 10.4103/npmj.npmj_232_20 [DOI] [PubMed] [Google Scholar]
- 54.Ponsky JL, Mellinger JD, Simon IB (1991) Endoscopic retrograde hemorrhoidal sclerotherapy using 23.4% saline: a preliminary report. Gastrointest Endosc 37:155–158 10.1016/S0016-5107(91)70675-5 [DOI] [PubMed] [Google Scholar]
- 55.Zhang T, Xu LJ, Xiang J et al (2015) Cap-assisted endoscopic sclerotherapy for hemorrhoids: methods, feasibility and efficacy. World J Gastrointest Endosc 7:1334–1340 10.4253/wjge.v7.i19.1334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xie YT, Yuan Y, Zhou HM, Liu T, Wu LH, He XX (2022) Long-term efficacy and safety of cap-assisted endoscopic sclerotherapy with long injection needle for internal hemorrhoids. World J Gastrointest Surg 14:1120–1130 10.4240/wjgs.v14.i10.1120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tessari L, Cavezzi A, Frullini A (2001) Preliminary experience with a new sclerosing foam in the treatment of varicose veins. Dermatol Surg 27:58–60 [PubMed] [Google Scholar]
- 58.Jamjoom AM, Jamal YS (1991) A comparative study of different treatments of hemorrhoids. Ann Saudi Med 11:73–79 10.5144/0256-4947.1991.73 [DOI] [PubMed] [Google Scholar]
- 59.Akindiose C, Alatise OI, Arowolo OA, Agbakwuru AE (2016) Evaluation of two injection sclerosants in the treatment of symptomatic haemorrhoids in Nigerians. Niger Postgrad Med J 23:110–115 10.4103/1117-1936.190347 [DOI] [PubMed] [Google Scholar]
- 60.Muldoon R (2020) Review of American Society of Colon and Rectal Surgeons clinical practice guidelines for the management of hemorrhoids. JAMA Surg 155:773–774 10.1001/jamasurg.2020.0788 [DOI] [PubMed] [Google Scholar]
- 61.van Tol RR, Kleijnen J, Watson AJM et al (2020) European Society of Coloproctology: guideline for haemorrhoidal disease. Colorectal Dis 22:650–662 10.1111/codi.14975 [DOI] [PubMed] [Google Scholar]
- 62.Kanellos I, Goulimaris I, Vakalis I, Dadoukis I (2000) Long-term evaluation of sclerotherapy for haemorrhoids. A prospective study. Int J Surg Investig 2:295–298 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 1 Funnel plot showing rates of success. Supplementary file1 (PDF 55 KB)
Appendix 2 Funnel plot showing rates of recurrence. Supplementary file2 (PDF 54 KB)
Appendix 3 Funnel plot showing rates of pain. Supplementary file3 (PDF 54 KB)
Appendix 4 Funnel plot showing rates of complications (overall). Supplementary file4 (PDF 54 KB)
Data Availability Statement
The data that support the findings of this study are available from the corresponding authors upon reasonable request.