Abstract
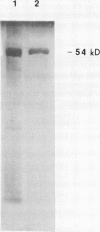
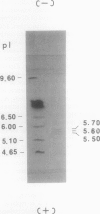
A ferric leghemoglobin reductase from the cytosol of soybean (Glycine max) root nodules was purified to homogeneity and partially characterized. The enzyme is a flavoprotein with flavin adenine dinucleotide as the prosthetic group and consists of two identical subunits, each having a molecular mass of 54 kilodaltons. The pure enzyme shows a high activity for ferric leghemoglobin reduction with NADH as the reductant in the absence of any exogenous mediators. The enzyme also exhibits NADH-dependent 2,6-dichloroindophenol reductase activity. A sequence of the first 50 N-terminal amino acids of the purified protein was obtained. Comparisons with known protein sequences have shown that the sequence of the ferric leghemoglobin reductase is highly related to those of the flavin-nucleotide disulfide oxido-reductases, especially dihydrolipoamide dehydrogenase of the pyruvate dehydrogenase complex.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bergersen F. J., Turner G. L. Leghaemoglobin and the supply of O2 to nitrogen-fixing root nodule bacteroids: presence of two oxidase systems and ATP production at low free O2 concentration. J Gen Microbiol. 1975 Dec;91(2):345–354. doi: 10.1099/00221287-91-2-345. [DOI] [PubMed] [Google Scholar]
- Browning K. S., Uhlinger D. J., Reed L. J. Nucleotide sequence for yeast dihydrolipoamide dehydrogenase. Proc Natl Acad Sci U S A. 1988 Mar;85(6):1831–1834. doi: 10.1073/pnas.85.6.1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carothers D. J., Pons G., Patel M. S. Dihydrolipoamide dehydrogenase: functional similarities and divergent evolution of the pyridine nucleotide-disulfide oxidoreductases. Arch Biochem Biophys. 1989 Feb 1;268(2):409–425. doi: 10.1016/0003-9861(89)90309-3. [DOI] [PubMed] [Google Scholar]
- Danson M. J. Dihydrolipoamide dehydrogenase: a 'new' function for an old enzyme? Biochem Soc Trans. 1988 Apr;16(2):87–89. doi: 10.1042/bst0160087. [DOI] [PubMed] [Google Scholar]
- Ide S., Hayakawa T., Okabe K., Koike M. Lipoamide dehydrogenase from human liver. J Biol Chem. 1967 Jan 10;242(1):54–60. [PubMed] [Google Scholar]
- Lee K. K., Klucas R. V. Reduction of ferric leghemoglobin in soybean root nodules. Plant Physiol. 1984 Apr;74(4):984–988. doi: 10.1104/pp.74.4.984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Light D. R., Walsh C., Marletta M. A. Analytical and preparative high-performance liquid chromatography separation of flavin and flavin analog coenzymes. Anal Biochem. 1980 Nov 15;109(1):87–93. doi: 10.1016/0003-2697(80)90014-7. [DOI] [PubMed] [Google Scholar]
- Otulakowski G., Robinson B. H. Isolation and sequence determination of cDNA clones for porcine and human lipoamide dehydrogenase. Homology to other disulfide oxidoreductases. J Biol Chem. 1987 Dec 25;262(36):17313–17318. [PubMed] [Google Scholar]
- Saari L. L., Klucas R. V. Ferric leghemoglobin reductase from soybean root nodules. Arch Biochem Biophys. 1984 May 15;231(1):102–113. doi: 10.1016/0003-9861(84)90367-9. [DOI] [PubMed] [Google Scholar]
- Stephens P. E., Lewis H. M., Darlison M. G., Guest J. R. Nucleotide sequence of the lipoamide dehydrogenase gene of Escherichia coli K12. Eur J Biochem. 1983 Oct 3;135(3):519–527. doi: 10.1111/j.1432-1033.1983.tb07683.x. [DOI] [PubMed] [Google Scholar]
- Tsai C. S. Nitroreductase activity of heart lipoamide dehydrogenase. Biochem J. 1987 Mar 1;242(2):447–452. doi: 10.1042/bj2420447. [DOI] [PMC free article] [PubMed] [Google Scholar]