Abstract
The induction of secretory and inflammatory responses in calves by Salmonella typhimurium and Salmonella dublin strains was compared, and the effects of mutations in the invH and stn genes were assessed. S. typhimurium induced greater secretory and inflammatory responses than S. dublin in bovine ileal loops, despite the fact that these serotypes were recovered from bovine ileal mucosa in comparable numbers (P. R. Watson, S. M. Paulin, A. P. Bland, P. W. Jones, and T. S. Wallis, Infect. Immun. 63:2743–2754, 1995). These results implicate serotype-specific factors other than, or in addition to, intestinal invasion in the induction of enteritis. The secretory and inflammatory responses induced by S. typhimurium and S. dublin in bovine ligated ileal loops were not significantly altered by mutation of stn, which suggests that stn does not have a major role in Salmonella-induced enteritis. The invH mutation significantly reduced the secretory and inflammatory responses induced in bovine ileal loops, and this correlated with a reduction in the severity of enteritis following oral inoculation of calves. The attenuation associated with the invH mutation did not appear to be due to an increased susceptibility to the innate host defense mechanisms, because the resistance of S. typhimurium to the bactericidal action of either bovine polymorphonuclear leukocytes or bovine serum was not significantly altered. However, lysis of macrophages following infection with S. typhimurium was significantly reduced by the invH mutation. The invH mutation prevented the normal secretion of several proteins, including SipC, by S. typhimurium, indicating that the function of the inv-spa-encoded type III protein secretion system was disrupted. Taken together, these observations implicate inv-spa-dependent effectors in mediation of Salmonella-induced enteritis in cattle. Clearly, however, other undefined serotype-specific virulence factors are also involved in Salmonella-induced enteritis.
Salmonella-induced enteritis is a common disease of several mammalian species (20), but its study has been restricted by the lack of convenient and biologically relevant animal models. The pathology of enteric salmonellosis is similar in several susceptible animal species, including calves (8, 35), humans (9, 22), and rhesus monkeys (31). Pathological changes include shortening of, and edema within, intestinal villi and an abnormal extrusion of enterocytes. An intestinal inflammatory response is induced, as evidenced by a rapid and large influx of polymorphonuclear leukocytes (PMNs) into the intestinal mucosa and lumen. The normal movement of electrolytes and water in the intestines is disrupted, and there is net fluid secretion into the intestinal lumen. These changes result in the main symptom of Salmonella-induced enteritis, namely, watery diarrhea.
The mechanism by which Salmonella serotypes disrupt the normal intestinal physiology is unclear. Several Salmonella serotypes readily invade the intestinal mucosa, resulting in the delivery of endotoxin into the submucosa. It is probable that this invasion event will either induce or amplify the intestinal inflammatory response associated with infection. For example, intestinal invasion may trigger the production of proinflammatory cytokines or other mediators of inflammation in infected cells (19, 27). Several components of the inflammatory response are potentially disruptive to normal intestinal absorptive functions. Therefore, the inflammatory response associated with bacterial invasion may contribute to pathogenesis. In addition, the interaction of Salmonella serotypes and epithelial cells, independent of bacterial invasion, can result in the recruitment of PMNs in vitro. This process is dependent on the inv-spa-encoded type III protein secretion system (21). Recently, we have identified a sip-dependent effector protein of Salmonella dublin, SopB, which influences the recruitment of inflammatory cells and the induction of fluid secretion, but not bacterial invasion, in bovine ileal loops (12). Several studies have shown that a functional inv-spa system represents an important virulence factor in mice (reviewed in reference 11), which is a model widely used to study systemic salmonellosis. To date, the relative contribution of inv-spa-dependent effector proteins to the induction of enteritis has not been clarified.
A role for enterotoxin production in Salmonella-induced enteritis has frequently been postulated but never proven. There are numerous reports of cytotonic and enterotoxic activities associated with Salmonella products (reviewed in reference 20), but the role of such toxins remains unclear because the reports are often conflicting and nonreproducible. The stn gene, which confers biological activity typical of an enterotoxin on cell lysates of Escherichia coli K-12, has been cloned from Salmonella typhimurium (6, 30). However, to date, the role of stn in the induction of enteritis has not been directly assessed.
The aim of this study was to define more clearly the contribution of bacterial factors to Salmonella-induced enteritis in cattle. S. typhimurium and S. dublin were chosen for study because both serotypes can cause enteritis in 2- to 6-week-old calves (38). The effects of a defined mutation in invH, which has previously been shown to reduce the intestinal invasion of S. typhimurium in bovine ligated ileal loops (35), and of a defined mutation in stn, were assessed. It is anticipated that the results of this study could be extrapolated to enteric salmonellosis in other serotype-host combinations.
MATERIALS AND METHODS
Bacterial strains.
Two strains each of S. typhimurium (ST4/74 and ST12/75) and S. dublin (SD2229 and SD3246), which are all bovine isolates, were routinely handled as previously described (35). The invH mutation in S. typhimurium ST4/74 and ST12/75, consisting of a TnphoA insertion in invH, has been described previously (35). Kanamycin and nalidixic acid were added to growth media at concentrations of 75 and 30 μg ml−1, respectively, when required. A mutant of S. typhimurium resistant to nalidixic acid (termed ST12/75NalR) was prepared by subculturing the wild-type strain on Luria-Bertani (LB) agar containing nalidixic acid and incubating for 24 to 48 h at 37°C. One colony was selected and streaked to single colonies on fresh LB agar containing nalidixic acid. E. coli K-12 (36) was used as a control in the in vitro assays.
Construction of an insertion mutation in stn.
A genetic construct, consisting of a kanamycin resistance (Kmr) cassette (Pharmacia Biotech, St. Albans, Hertfordshire, United Kingdom) flanked on each side by approximately 300 bp of DNA complementary to stn, was prepared and used to introduce the Kmr cassette into stn of S. typhimurium by homologous recombination. The construct was cloned into plasmid pDM4 (24), which is a suicide vector requiring λpir for replication and which therefore will not survive in Salmonella species. Plasmid pDM4 encodes chloramphenicol resistance and also contains the sacB gene, whose product is lethal for bacterial cells grown in the presence of sucrose.
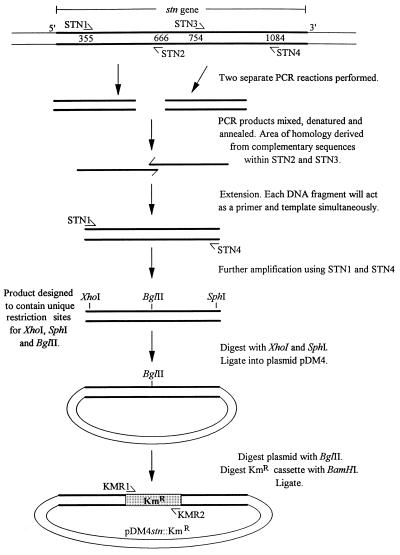
The preparation of the genetic construct in pDM4 is summarized in Fig. 1. Four oligonucleotide primers, STN1 (5′-CTGTTGTCTCGAGATGACTGGCAACC-3′), STN2 (5′-CAGGTGCTGTTAGATCTGTACCTGAAC-3′), STN3 (5′-CAGATCTAACAGCACCTGACCAGATTCAGGGAGT-3′), and STN4 (5′-T TTTTGGCATGCGCGTTATCAGCGCT-3′), were designed to be complementary to the DNA sequence of the stn gene of S. typhimurium (6) and to incorporate restriction cleavage sites for the enzymes XhoI, BglII, and SphI (shown in boldface). The underlined nucleotides in STN2 and STN3 are complementary to each other. Two PCRs were performed with chromosomal DNA from S. typhimurium ST12/75 as the template and with STN1 and STN2 or STN3 and STN4 as the primers. The resulting PCR products corresponded to nucleotides 355 to 666 and 754 to 1084 of stn, respectively. These PCR products were mixed together and used in a third PCR with STN1 and STN4 as the primers. The complementary regions in the PCR products caused them to anneal to each other, which resulted in their acting simultaneously as templates and primers in the initial PCR cycles. This generated a product of approximately 650 bp complementary to the central region of stn, but with a deletion of 100 bp, which was then amplified in the later cycles of PCR. This product was ligated into plasmid pDM4 following digestion of both the PCR product and pDM4 with XhoI and SphI. This recombinant plasmid was transformed into E. coli SY327 λpir (23). The Kmr cassette, which had been digested with BamHI, was ligated into the BglII site of the cloned PCR fragment. The resulting recombinant plasmid was designated pDM4stn::KmR and was transformed into E. coli SY327 λpir.
FIG. 1.
Procedure for the preparation of a genetic construct consisting of a Kmr cassette flanked on each side by approximately 300 bp of DNA complementary to stn. STN1, STN2, STN3, and STN4 are oligonucleotide primers designed to be complementary to the DNA sequence of stn. A more detailed description of this procedure is given in Materials and Methods. The oligonucleotide primers KMR1 and KMR2 were used to check the stn mutation by PCR.
Plasmid pDM4stn::KmR was introduced into E. coli S17-1 λpir (32) and then conjugated into S. typhimurium ST12/75NalR. A transconjugant, designated ST12/75NalRstn-652::KmR, was selected on LB agar containing kanamycin, nalidixic acid, and 5% sucrose. The phenotype of ST12/75NalRstn-652::KmR resulted from the transfer of the Kmr cassette from plasmid pDM4stn::KmR to the chromosome of S. typhimurium by a double homologous recombination event between stn on the chromosome and the complementary sequences flanking the Kmr cassette. The loss of plasmid pDM4 was confirmed by the inability of ST12/75NalRstn-652::KmR to grow in the presence of chloramphenicol. The stn mutation was transferred from ST12/75NalRstn-652::KmR to S. typhimurium ST4/74 and ST12/75 and S. dublin SD2229 and SD3246 by P22 transduction. The stn mutation and the transductants were checked by PCR using primers STN1 and STN4 and KMR1 and KMR2, which are shown in Fig. 1.
Bovine ligated ileal loop assay.
The experimental techniques used in this assay have been described in detail elsewhere (34). All experiments were performed within the midilea of 28-day-old Friesian bull calves. The inocula were prepared by culturing bacteria overnight in brain heart infusion broth at 37°C with shaking at 150 rpm. The cultures were diluted 1:100 into fresh broth and incubated as before for 4 h. Bacteria were harvested by centrifugation (at 3,345 × g for 10 min at 4°C), resuspended in fresh broth, and injected into bovine ligated ileal loops 6 cm long. The lumen of the midileum was gently flushed with 0.9% NaCl prior to the construction of the loops in order to remove the intestinal contents. The inoculum injected into each loop was in the range of 1 × 109 to 2 × 109 CFU. Approximately 50 ml of blood was removed from the calves 4 h after injection of the loops. PMNs were isolated, labelled with 111In, and reinjected intravenously. The secretory response (volume of fluid within a loop/length of loop [ml cm−1]) and the influx of PMNs, as assessed by the magnitude of γ-irradiation emitted from 111In-labelled PMNs within each loop, were recorded 12 h after injection of the loops. The PMN influx ratio was defined as the PMN influx in the test loops/the PMN influx in the negative-control loops. The PMN influx ratio in the negative-control loops was therefore equal to 1.00.
Oral inoculation of calves with S. typhimurium.
Twenty-eight-day-old Friesian bull calves were housed in single cubicles in a disease-secure animal unit and fed on a diet of powdered milk. None of the calves excreted salmonellas either 1 week before inoculation or immediately before inoculation, as assessed by enrichment of fecal samples in Rappaport broth (at 37°C for 18 h) and Selenite brilliant green broth (at 43°C for 18 h) followed by incubation of the enrichment cultures on modified brilliant green agar (Difco, West Molesey, Surrey, United Kingdom).
Bacterial cultures were prepared by inoculating Bacto Tryptose broth (17) with several bacterial colonies and incubating at 37°C statically for 18 h. Bacteria were harvested by centrifugation (at 2,500 × g, at 4°C for 10 min) and resuspended in fresh Bacto Tryptose broth to give a concentration in the range of 0.6 × 109 to 1 × 109 CFU ml−1. The bacterial suspension (1 ml) was mixed with 20 ml of sterile double-distilled water containing 5% (wt/vol) Mg(SiO3)3, 5% (wt/vol) NaHCO3, and 5% (wt/vol) MgCO3. The mixture was administered orally to each calf with a syringe immediately before its morning feed.
The rectal temperature and the severity of diarrhea (termed scouring) were recorded every 12 h. A semiquantitative scoring system (16, 34) was used to record the severity of diarrhea. A scour score of 0 to 3 was assigned to the feces depending on their water content, with 0 denoting normalcy and 3 denoting the consistency of water. The presence of blood in the feces was given an additional score of 1, and the presence of sloughed intestinal mucosa or pseudomembrane formation was given an additional score of 2. A calf was killed for humane reasons if any of the following occurred during infection: anorexia, dehydration, or the inability to stand unaided. At postmortem the numbers of bacteria in the liver, spleen, lung, ileum, cecum, and colon, and in the lymph nodes associated with these sites, were determined by viable counts as described previously (34). All tissue samples were taken in triplicate, and the intestinal samples were washed gently in water to remove nonadherent bacteria from the luminal surface. One gram of tissue was homogenized in 9 ml of phosphate-buffered saline (PBS) containing 1% Triton X-100. Serial dilutions were performed, and 100 μl of each dilution was spread in triplicate onto modified brilliant green agar plates. The plates were incubated overnight at 37°C. The limit of detection for salmonellas was 2.0 log10 CFU g−1 of tissue, and samples which contained numbers of salmonellas below this limit were excluded from the calculation of mean CFU per gram.
The stability of the invH mutation in vivo was confirmed by replica plating 400 colonies, which had been isolated from four different types of tissues in two different calves, onto LB agar with and without kanamycin. All the colonies retained the kanamycin resistance phenotype, which is associated with carriage of TnphoA. In addition, it was confirmed that bacteria derived from 15 of these colonies (of a total of 15 colonies tested) had retained the phenotype of reduced invasion for Int 407 cells (data not shown), which is associated with the invH mutation, in a standard gentamicin protection assay (35).
Susceptibility of bacteria to killing by bovine PMNs and serum.
Bovine PMNs were prepared by a modification of the method of Carlson and Kaneko (4) as follows. Approximately 60 ml of blood (each) was collected from 21-day-old Friesian bull calves in 1/10 its volume of 0.0132 M phosphate buffer (pH 6.8) containing 1.5% (wt/vol) EDTA and 0.7% (wt/vol) NaCl. The blood was centrifuged (at 1,000 × g, at 4°C for 15 min), and the plasma and buffy coat were discarded. The cell pellet was weighed, and 4 ml of sterile double-distilled water per g was added and mixed for 30 to 45 s to lyse the erythrocytes. Isotonicity was restored by adding 2 ml of 0.0132 M phosphate buffer (pH 6.8) containing 2.7% (wt/vol) NaCl per g of cell pellet. The PMNs were collected by centrifugation (at 200 × g, at 4°C for 10 min) and washed twice in 0.0132 M phosphate buffer (pH 6.8) containing 0.8% (wt/vol) NaCl. The concentration of the PMNs was adjusted to 107 cells ml−1. Bovine serum was prepared from each calf and was used in combination with PMNs from the same animal. Heat-inactivated serum was prepared by incubating normal serum at 65°C for 1 h. All sera were stored at −70°C until use.
The inocula were prepared by culturing bacteria overnight in LB broth at 37°C with shaking at 150 rpm. The cultures were diluted 1:100 into fresh broth and incubated as before for 4 h. Bacteria were harvested by centrifugation (at 2,500 × g, at 4°C for 10 min) and resuspended in PBS at a concentration of approximately 107 CFU ml−1. The following reaction mixtures were prepared in triplicate for each strain: 300 μl of normal or heat-inactivated serum, 100 μl of PMNs or phosphate buffer (pH 6.8) containing 0.8% (wt/vol) NaCl, and 100 μl of bacterial suspension. The reaction mixtures were incubated at 37°C for 90 min on a rolling platform at 120 rpm. PMNs were lysed by the addition of 55 μl of PBS containing 1% sodium deoxycholate, and bacteria were enumerated by viable count on MacConkey agar.
Quantification of Salmonella-induced macrophage lysis.
Bovine alveolar macrophages were prepared and incubated by methods described elsewhere (13). The magnitude of macrophage lysis during infection with salmonellas was estimated by using the cytoTox 96 assay (Promega, Southampton, United Kingdom) as described previously (13). Briefly, overnight cultures of bacteria in LB broth were diluted 1:100 into fresh LB broth and incubated for 4 h at 37°C with shaking. The bacteria were diluted in prewarmed medium to give a ratio of 3 to 5 bacteria per macrophage at the time of infection. Bacteria were added to each macrophage monolayer, and the monolayers were incubated for 1, 2, or 3 h. Lysis buffer was added to three uninfected monolayers at 45 min before each time point. At each time point, the amount of lactate dehydrogenase released by damaged macrophages was determined by an enzymatic colorimetric reaction and measured by optical density. The percentage lysis of macrophages was calculated as follows: [A492(test strain) − A492(medium)]/[A492(macrophages + lysis buffer) − A492(medium + lysis buffer)].
Secretion of Sip proteins by S. typhimurium.
The secretion of proteins by S. typhimurium was assessed by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis as described previously (37). Salmonella strains were grown overnight in LB broth at 25°C, diluted 1:10 into 50 ml of fresh LB broth, and incubated for 4 h at 37°C with shaking at 150 rpm. The numbers of bacteria in each culture were similar as assessed by spectrophotometry and by performing viable counts. Bacteria were removed by centrifugation (at 10,000 × g, for 20 min at 4°C), and the culture supernatants were filter sterilized with a 0.45-μm-pore-size filter. Proteins present in the supernatants were concentrated by precipitation with 10% (vol/vol) trichloroacetic acid and were resuspended in 50 μl of sample buffer. The resuspended proteins (10 μl) were separated on an SDS-polyacrylamide gel and were either stained with Coomassie brilliant blue or transferred to a nitrocellulose membrane. The presence of SipC on the membrane was detected by using an anti-SipC monoclonal antibody (26) as described previously (37).
Statistical analyses.
Data from the bovine ligated ileal loop assays and the in vitro assays were examined by analysis of variance. In the oral inoculation study, there was an insufficient number of animals to allow a meaningful statistical comparison of the recovery of the two strains. Even when the data were pooled over the three different time points and the strain-time interaction was investigated, the analysis yielded insufficient degrees of freedom for estimating error. Where appropriate, all data are presented with the standard errors of the mean.
RESULTS
Characterization of stn mutants.
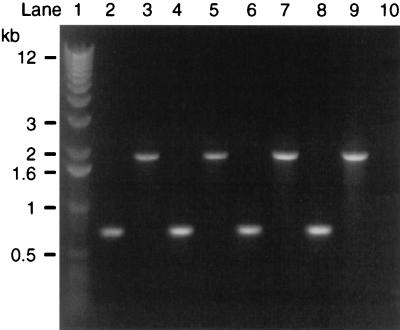
The stn gene in S. typhimurium ST4/74 and ST12/75 and in S. dublin SD2229 and SD3246 was disrupted by the insertion of a Kmr cassette. Correct insertion of the Kmr cassette into stn was confirmed by two different sets of PCRs. In the first set of reactions, primers complementary to the sequence of stn flanking the predicted site of insertion of the Kmr cassette (STN1 and STN4 [Fig. 1]) were used. The amplified PCR products of the mutants were approximately 1.2 kb larger than the products of the wild-type strains (Fig. 2). This increase corresponds to the insertion of the Kmr cassette (1.3 kb) and the deletion of approximately 100 bp of stn. In the second set of reactions, different combinations of STN1 and STN4 with two primers complementary to either end of the Kmr cassette (designated KMR1 and KMR2 [Fig. 1]) were used. The reactions were performed by using template DNA from only one strain (S. typhimurium ST4/74) and the corresponding mutant. A PCR product was obtained with the mutant but not with the wild-type strain, and only with two of the four combinations of primers (data not shown). This indicates that the Kmr cassette was correctly inserted in stn.
FIG. 2.
PCR products generated with primers designed to be complementary to the sequence of stn flanking the predicted site of insertion of Kmr (STN1 and STN4) and with template DNA from wild-type Salmonella strains (lanes 2, 4, 6, and 8) or from stn mutants (lanes 3, 5, 7, and 9). Lane 1, standard markers; lanes 2 and 3, S. typhimurium ST4/74; lanes 4 and 5, S. typhimurium ST12/75; lanes 6 and 7, S. dublin SD2229; lanes 8 and 9, S. dublin SD3246; lane 10, negative control. Sizes of standard markers are shown on the left.
Intestinal secretory and inflammatory responses are reduced by mutation of invH, but not by mutation of stn.
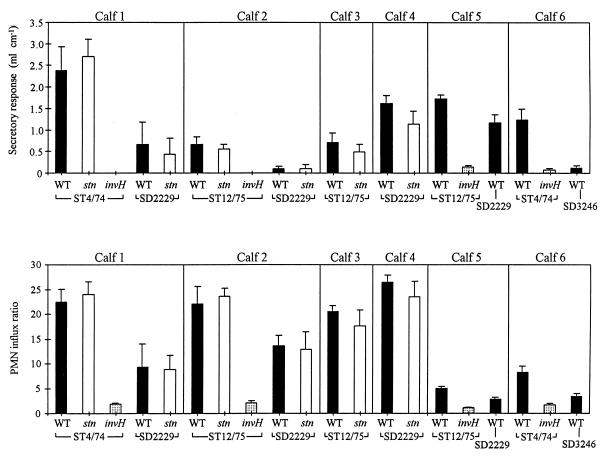
The induction of fluid secretion and PMN influxes by S. typhimurium and S. dublin within ligated ileal loops, and the effects of mutations in invH and stn on these responses, were assessed. Data from a total of 78 separate loops within six different calves are presented (Fig. 3). In the first four calves, the secretory and inflammatory responses induced by either strain of S. typhimurium or either strain of S. dublin were not significantly reduced by the stn mutation (P > 0.1). The secretory and inflammatory responses induced by both strains of S. typhimurium were significantly reduced by the invH mutation (P < 0.05) in all four animals in which the strains were tested. The two S. dublin strains induced lower secretory and inflammatory responses than the two S. typhimurium strains (P < 0.1) in all four calves in which the different serotypes were compared. Loops injected with sterile brain heart infusion broth did not induce a secretory response in any of the animals.
FIG. 3.
Secretory and inflammatory responses in bovine ileal loops 12 h after inoculation with S. typhimurium ST4/74 or ST12/75 or with S. dublin SD2229 or SD3246. The secretory response is expressed as the volume of fluid within a loop/length of the loop. The PMN influx ratio is expressed as the PMN influx within a test loop/PMN influx in the control loops. The results from six calves are presented; each strain was tested in triplicate in each animal. Symbols: ▪, wild type; □, stn mutant; ░⃞, invH mutant.
S. typhimurium-induced enteritis is reduced by the invH mutation.
The effect of the invH mutation on the severity of enteritis induced by S. typhimurium ST4/74 following oral inoculation was assessed in 11 calves. Five calves were inoculated with the wild-type strain, and six were inoculated with the invH mutant. Two calves from each group were killed at 18 h postinoculation. Two of the three remaining calves inoculated with the wild-type strain were killed at 54 h postinoculation, and the remaining calf was killed at 96 h postinoculation, because their symptoms had reached the level of severity described in Materials and Methods. Two calves inoculated with the invH mutant were also killed at each of these times in order to allow direct comparisons of tissue counts to be made between the strains, although none of these animals were exhibiting severe symptoms.
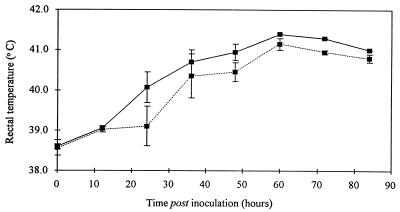
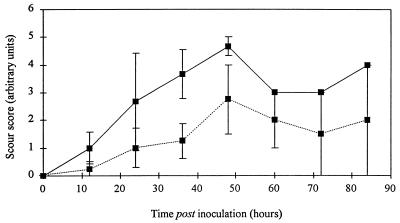
All the calves developed pyrexia following oral inoculation (Fig. 4), but the onset of pyrexia was more rapid in the calves inoculated with the wild-type strain. There was some variation between animals in the severity of scouring following oral inoculation, but on average, the wild-type strain induced a more rapid onset and greater severity of scouring than the invH mutant (Fig. 5).
FIG. 4.
Mean rectal temperatures of calves following oral inoculation with either S. typhimurium ST4/74 (wild type) (solid line) or its derivative invH mutant (dotted line). Each datum point up to and including that for 48 h is derived from three calves for the wild-type strain and four calves for the invH mutant. After this time, the data for the wild-type strain are from only one calf and the data for the invH mutant are from two calves.
FIG. 5.
Mean scour scores of calves following oral inoculation with either S. typhimurium ST4/74 (wild type) (solid line) or its derivative invH mutant (dotted line). Each datum point up to and including that for 48 h is derived from three calves for the wild-type strain and four calves for the invH mutant. After this time, the data for the wild-type strain are from only one calf and the data for the invH mutant are from two calves.
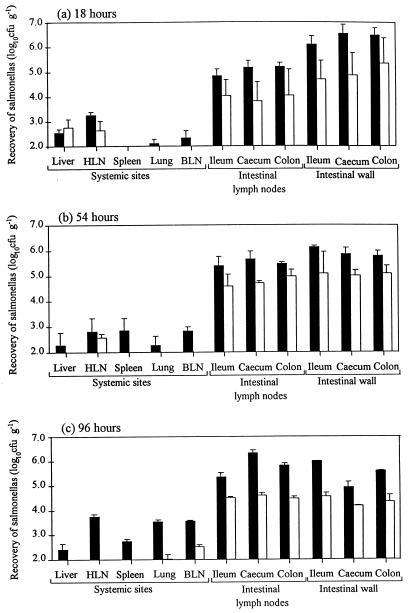
The number of salmonellas in selected intestinal and systemic sites at postmortem was determined (Fig. 6). It should be noted that these results are derived from only two calves per time point per strain (except for that for the wild-type strain at 96 h, which is from only one calf) and so should be interpreted accordingly. Despite this limitation, there are two general points to note. First, the wild-type strain is generally recovered in higher numbers from both intestinal and systemic sites than the invH mutant. Second, as early as 18 h postinoculation, both the wild-type strain and the invH mutant are recovered in high numbers from the intestinal wall and intestinal nodes and in low numbers from some of the systemic sites.
FIG. 6.
Recovery of salmonellas from systemic sites, intestinal lymph nodes, and intestinal walls of calves at 18 (a), 54 (b), and 96 (c) h after oral inoculation with S. typhimurium ST4/74 (wild type) (▪) or its derivative invH mutant (□). Each bar represents the mean of triplicate samples from two animals, with the exception of that for the wild-type strain at 96 h, which is the mean from only one animal.
InvH is required for lysis of bovine macrophages, but not for resistance to killing by PMNs or serum.
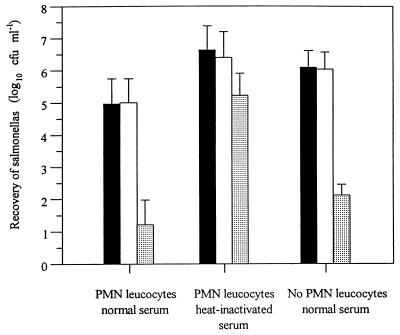
The effect of the invH mutation on the interaction of S. typhimurium ST4/74 with the bovine innate immune system was assessed. The recovery of S. typhimurium following incubation with PMNs in the presence of normal or heat-inactivated serum, or after incubation with normal serum alone, was not significantly affected (P > 0.1) by the invH mutation (Fig. 7). E. coli K-12 was used as a control to confirm that the PMNs and serum were bactericidal.
FIG. 7.
Recovery of bacteria following incubation with PMNs in the presence or either normal or heat-inactivated bovine serum and after incubation with normal serum alone. Each bar represents the mean from three experiments, each performed in triplicate. Symbols: ▪, wild type S. typhimurium ST4/74; □, invH mutant of S. typhimurium ST4/74; ░⃞, E. coli K-12.
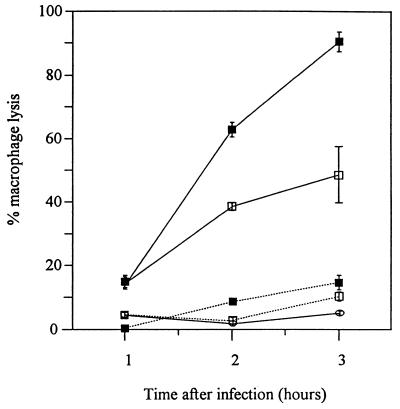
The magnitude of macrophage lysis was significantly reduced (0.1 > P > 0.05 at 1 h after infection; P < 0.001 at 2 and 3 h after infection) by the invH mutation (Fig. 8). E. coli K-12 did not induce lysis of macrophages. Quantification of the effect of the invH mutation on the uptake and persistence of S. typhimurium within macrophages was not attempted because this difference in macrophage lysis would make the results from a gentamicin assay difficult to interpret.
FIG. 8.
Lysis of macrophages following incubation with S. typhimurium ST4/74 (▪), S. typhimurium ST12/75 (□), or E. coli K-12 (○) for 1, 2, and 3 h after infection. This is a representative experiment from a total of three experiments and was performed in triplicate. Solid lines, wild type; dotted lines, invH mutant.
The mutation in invH affects the expression or secretion of Salmonella proteins.
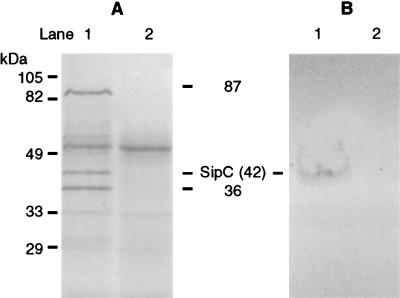
The invH gene is located adjacent to, but is transcribed in the opposite direction from, the inv-spa loci of Salmonella species, which encode a type III protein secretion system (11). The involvement of the invH gene in this system was assessed by examining the secretion of proteins by the invH mutant. Several proteins were absent in the concentrated culture supernatant of the S. typhimurium ST12/75 invH mutant compared to the wild-type strain (Fig. 9A). Three of the most prominent of these proteins had molecular sizes similar to those reported for the secreted proteins SipA, SipC, and SipD (87, 42, and 36 kDa, respectively) (15). A band corresponding to the 42-kDa protein was identified by Western blotting with an anti-SipC monoclonal antibody (Fig. 9B). Similar results were obtained with S. typhimurium ST4/74 (data not shown).
FIG. 9.
Analysis of proteins secreted by wild-type S. typhimurium ST12/75 (lanes 1) and its derivative invH mutant (lanes 2) by electrophoresis (A) and by Western blotting (B). Proteins from culture supernatants were concentrated and separated on an SDS-polyacrylamide gel and stained with Coomassie brilliant blue. Three proteins present only in the supernatant of the wild-type strain were of molecular sizes similar to those of SipA, SipC, and SipD (87, 42, and 36 kDa, respectively). A band corresponding to the 42-kDa protein was identified by Western blotting with an anti-SipC monoclonal antibody. The sizes of the molecular mass standards are given on the left.
DISCUSSION
The aim of this study was to investigate the mechanisms by which S. typhimurium and S. dublin induce enteritis in calves by using defined mutations in putative virulence genes. The invH gene was selected for study because it affects bacterial invasion (1, 35), and the stn gene was studied because it bestows enterotoxic activity on E. coli K-12 (30). Calves were selected as the animal model because, unlike more convenient laboratory animals such as mice, they exhibit typical symptoms of Salmonella-induced enteritis. In addition, cattle are a natural host animal for both S. typhimurium and S. dublin.
Wild-type S. typhimurium induced significantly greater secretory and inflammatory responses than wild-type S. dublin in the bovine ligated ileal loop assay, despite the fact that both serotypes are highly invasive for bovine ileal mucosa (35). This suggests that factors independent of intestinal invasion influence the magnitude of the secretory and inflammatory responses and that these factors are serotype specific.
The magnitudes of the secretory and inflammatory responses induced by either S. typhimurium or S. dublin were not altered by the mutation in stn. This result suggests that stn is not required for the induction of these intestinal responses by either serotype. It is possible that, despite carrying the stn gene, neither strain of S. typhimurium or S. dublin expressed the Stn protein and so mutation of stn would have no effect on the bacterial phenotype. It is likely that Stn production is tightly regulated, and so the detection of Stn expression in vitro (7) would give no indication of its level of expression in vivo. However, both S. typhimurium strains used in this study induce potent secretory and inflammatory responses in bovine ligated ileal loops within 12 h, and all four Salmonella strains used in this study induce severe salmonellosis in calves (this study and references 18 and 34). Therefore, if Stn is not expressed in these strains in vivo, then clearly S. typhimurium and S. dublin are able to cause intestinal secretory and inflammatory responses in its absence. It is concluded that Stn is unlikely to be a major virulence factor in enteric salmonellosis in calves.
Mutations in invH reduce invasion by Salmonella strains into eukaryotic cells (1, 35), but the effect of these mutations on virulence is not well established. A TnphoA mutation in invH did not affect the virulence of Salmonella choleraesuis following oral inoculation of mice (1, 10). Furthermore, an insertion mutation in invH had either a small effect or no effect (depending on the strain background) on the virulence of S. typhimurium following oral inoculation of chickens and did not affect the recovery of S. typhimurium from the ileum and spleen (29). However, a TnphoA mutation in invH reduced the recovery of Salmonella enteritidis from chicken ileum and spleen following oral inoculation (3, 14). It is probable that the differences in these results are due to differences in the strains and animal models used. In the present study, the secretory and inflammatory responses induced by S. typhimurium in bovine ileal loops were almost completely eliminated by mutation of invH. This correlated to a reduction in the severity of enteritis following oral inoculation of calves associated with the invH mutation, although the invH mutant did induce pyrexia and mild diarrhea. It was not possible to complement the invH mutation by introducing the intact gene on a plasmid into the mutant (data not shown). This difficulty in complementing invH has been reported previously and was attributed to inappropriate expression of invH on the plasmid rather than to polar effects of the mutation on downstream genes (1). The effect of the invH mutation in S. dublin was not studied because although S. dublin contains the invH gene, it was not possible to transfer this mutation into the S. dublin background (35).
The biological basis of the attenuation associated with the invH mutation was assessed further. It has previously been demonstrated that the recovery of S. typhimurium from bovine mucosa was reduced significantly by the invH mutation (35). In this study, the effects of the invH mutation on other parameters, which could also potentially affect the induction of enteritis, were studied. The first parameter studied was resistance to the bactericidal activity of PMNs. Infection of the intestines results in a rapid and large PMN influx (33, 34); therefore, an increased sensitivity to PMN killing could limit the ability of a strain to induce enteritis. The resistance to the bactericidal activity of bovine serum was also studied, since it is likely that the intestinal inflammatory response will allow leakage of serum proteins into the intestinal mucosa. The invH mutation did not affect the resistance of S. typhimurium to the bactericidal activity of either PMNs, serum, or a combination of both.
Bacteria have frequently been observed within lamina propria macrophages of intestines infected with salmonellas (28, 35), and so the effect of the invH mutation on the interaction of S. typhimurium with bovine alveolar macrophages was assessed. Intestinal macrophages were the cells of choice to use for this experiment, but it was not possible to recover either a pure cell population or sufficient numbers for these experiments (data not shown). Alveolar macrophages can be isolated in high numbers and with high purity. They share some properties with intestinal macrophages in that they are resident tissue macrophages residing in a nonsterile environment. In addition, pneumonia often occurs during bovine salmonellosis (16, 38), which suggests that salmonellas will interact with alveolar macrophages during natural infections. The invH mutation significantly reduced the magnitude of macrophage lysis induced during infection with S. typhimurium. Lysis of lamina propria macrophages in infected intestines could contribute to the induction of enteritis, for example, by increasing the survival or spread of bacteria, or by altering the nature or magnitude of the intestinal inflammatory response, as has been proposed for Shigella species (39). It is possible that the reduction in macrophage lysis associated with the invH mutant may contribute to its reduced ability to induce enteritis. However, previous results suggest that this may not be the case, since the virulence plasmid from S. dublin SD2229 has been implicated in the lysis of infected macrophages, but not in the induction of enteritis in cattle (13, 34). In bovine ileal loops, the invH mutants do not invade the mucosa sufficiently to reach lamina propria macrophages in large numbers (35). Therefore, it is unlikely that the reduction in macrophage lysis associated with the invH mutation is responsible for the reduction in secretory and inflammatory responses.
It has previously been reported that mutations which alter the normal function of the inv-spa-encoded type III protein secretion system of Salmonella strains also alter the bacterial phenotypes of invasion of eukaryotic cells and lysis of murine macrophages (5, 25). In the present study the invH mutation affected the secretion of the Sip proteins, which are dependent on the inv-spa-encoded type III protein secretion system. Therefore, it is likely that the invH mutation exerts its effects on intestinal invasion, macrophage lysis, and the induction of secretory and inflammatory responses by altering the normal function of the type III protein secretion system. However, what is not clear is whether the secreted proteins are directly involved in the induction of enteritis, or whether they have an indirect effect by mediating intestinal invasion. The relationship between intestinal invasion and the induction of enteritis has been directly assessed in only a few studies, and the results of such studies are contradictory. For example, intestinal invasion correlated to the induction of secretory and inflammatory responses by different strains of S. typhimurium in rabbit ileal mucosa (2), whereas no correlation was found between the invasion of cultured human cell monolayers and the induction of PMN migration across the monolayers or the induction of enteritis in humans by different Salmonella serotypes (21). Furthermore, a PMN influx into Salmonella-infected ileal mucosa does not per se result in fluid secretion (33), which implies that additional factors, other than the host-derived inflammatory mediators, are required for Salmonella-induced fluid secretion. Recent work in our laboratory has identified an effector protein, SopB, which is translocated into eukaryotic cells via a sip-dependent pathway by S. dublin. Mutation of sopB significantly reduced the secretory and inflammatory responses induced in bovine ligated ileal loops by S. dublin, but it did not affect the recovery of S. dublin from bovine ileal mucosa, demonstrating that this attenuated mutant is still highly invasive (12). Clearly, SopB is an important virulence factor in Salmonella-induced enteritis. However, disruption of invH, and the associated blocking of the secretion of Sip’s, failed to completely attenuate S. typhimurium in orally inoculated calves. Taken together, these observations implicate serotype-specific virulence factors, independent of a functional inv-spa locus, in the induction of enteritis. However, stn does not appear to be one such factor.
ACKNOWLEDGMENT
This work was supported by the Ministry for Agriculture, Fisheries, and Food.
REFERENCES
- 1.Altmeyer R M, McNern J K, Bossio J C, Rosenshine I, Finlay B B, Galán J E. Cloning and molecular characterization of a gene involved in Salmonellaadherence and invasion of cultured epithelial cells. Mol Microbiol. 1993;7:89–98. doi: 10.1111/j.1365-2958.1993.tb01100.x. [DOI] [PubMed] [Google Scholar]
- 2.Amin I I, Douce G R, Osborne M P, Stephen J. Quantitative studies of invasion of rabbit ileal mucosa by Salmonella typhimuriumstrains which differ in virulence in a model of gastroenteritis. Infect Immun. 1994;62:569–578. doi: 10.1128/iai.62.2.569-578.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barrow, P. (Institute for Animal Health). 1997. Personal communication.
- 4.Carlson G P, Kaneko J J. Isolation of leukocytes from bovine peripheral blood. Proc Soc Exp Biol Med. 1973;142:853–855. doi: 10.3181/00379727-142-37131. [DOI] [PubMed] [Google Scholar]
- 5.Chen L M, Kaniga K, Galán J E. Salmonellaspp. are cytotoxic for cultured macrophages. Mol Microbiol. 1996;21:1101–1115. doi: 10.1046/j.1365-2958.1996.471410.x. [DOI] [PubMed] [Google Scholar]
- 6.Chopra A K, Peterson J W, Chary P, Prasad R. Molecular characterization of an enterotoxin from Salmonella typhimurium. Microb Pathog. 1994;16:85–98. doi: 10.1006/mpat.1994.1010. [DOI] [PubMed] [Google Scholar]
- 7.Chopra A K, Xu X-J, Peterson J W. Salmonella typhimuriumenterotoxin epitopes shared among bacteria. FEMS Microbiol Lett. 1994;118:237–242. doi: 10.1111/j.1574-6968.1994.tb06834.x. [DOI] [PubMed] [Google Scholar]
- 8.Clarke R C, Gyles C L. Virulence of wild and mutant strains of Salmonella typhimuriumin ligated intestinal segments of calves, pigs, and rabbits. Am J Vet Res. 1987;48:504–510. [PubMed] [Google Scholar]
- 9.Day D W, Mandal B K, Morson B C. The rectal biopsy appearances in Salmonella colitis. Histopathology. 1978;2:117–131. doi: 10.1111/j.1365-2559.1978.tb01700.x. [DOI] [PubMed] [Google Scholar]
- 10.Finlay B B, Starnbach M N, Francis C L, Stocker B A D, Chatfield S, Dougan G, Falkow S. Identification and characterization of TnphoA mutants of Salmonellathat are unable to pass through a polarized MDCK epithelial cell monolayer. Mol Microbiol. 1988;2:757–766. doi: 10.1111/j.1365-2958.1988.tb00087.x. [DOI] [PubMed] [Google Scholar]
- 11.Galán J. Molecular genetic bases of Salmonellaentry into host cells. Mol Microbiol. 1996;20:263–271. doi: 10.1111/j.1365-2958.1996.tb02615.x. [DOI] [PubMed] [Google Scholar]
- 12.Galyov E E, Wood M W, Rosqvist R, Mullan P B, Watson P R, Hedges S, Wallis T S. A secreted effector protein of Salmonella dublinis translocated into eukaryotic cells and mediates inflammation and fluid secretion in infected ileal mucosa. Mol Microbiol. 1997;25:903–912. doi: 10.1111/j.1365-2958.1997.mmi525.x. [DOI] [PubMed] [Google Scholar]
- 13.Guilloteau L A, Wallis T S, Gautier A V, MacIntyre S, Platt D J, Lax A J. The Salmonella virulence plasmid enhances Salmonella-induced lysis of macrophages and influences inflammatory responses. Infect Immun. 1996;64:3385–3393. doi: 10.1128/iai.64.8.3385-3393.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Halavatkar H, Barrow P. Preliminary characterisation of TnphoA mutants of Salmonella enteritidis with reduced invasiveness in vivo. In: Cabello F, Hormaeche C, Mastroeni P, Bonina L, editors. Biology of Salmonella. London, United Kingdom: Plenum; 1993. pp. 395–396. [Google Scholar]
- 15.Hueck C J, Hantman M J, Bajaj V, Johnston C, Lee C A, Miller S I. Salmonella typhimurium secreted invasion determinants are homologous to ShigellaIpa proteins. Mol Microbiol. 1995;18:479–490. doi: 10.1111/j.1365-2958.1995.mmi_18030479.x. [DOI] [PubMed] [Google Scholar]
- 16.Jones P W, Dougan G, Hayward C, Mackensie N, Collins P, Chatfield S N. Oral vaccination of calves against experimental salmonellosis using a double aro mutant of S. typhimurium. Vaccine. 1991;9:29–34. doi: 10.1016/0264-410x(91)90313-u. [DOI] [PubMed] [Google Scholar]
- 17.Jones P W. Effect of the storage in slurry on the virulence of Salmonella dublin. J Hyg. 1975;74:65–70. doi: 10.1017/s0022172400046726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jones, P. W. Unpublished data.
- 19.Jung H C, Eckmann L, Yang S-K, Panja A, Fierer J, Morzycka-Wroblewska E, Kagnoff M F. A distinct array of proinflammatory cytokines is expressed in human colon epithelial cells in response to bacterial invasion. J Clin Invest. 1995;95:55–65. doi: 10.1172/JCI117676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lax A J, Barrow P A, Jones P W, Wallis T S. Current perspectives in salmonellosis. Br Vet J. 1995;151:351–376. doi: 10.1016/s0007-1935(95)80126-x. [DOI] [PubMed] [Google Scholar]
- 21.McCormick B A, Miller S I, Carnes D, Madara J L. Transepithelial signaling to neutrophils by salmonellae: a novel virulence mechanism for gastroenteritis. Infect Immun. 1995;63:2302–2309. doi: 10.1128/iai.63.6.2302-2309.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McGovern V J, Slavutin L J. Pathology of salmonella colitis. Am J Surg Pathol. 1979;3:483–490. doi: 10.1097/00000478-197912000-00001. [DOI] [PubMed] [Google Scholar]
- 23.Miller V L, Mekalanos J J. A novel suicide vector and its use in construction of insertion mutations: osmoregulation of outer membrane proteins and virulence determinants in Vibrio cholerae requires toxR. J Bacteriol. 1988;170:2575–2583. doi: 10.1128/jb.170.6.2575-2583.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Milton D L, O’Toole R, Hörstedt P, Wolf-Watz H. Flagellin A is essential for the virulence of Vibrio anguillarum. J Bacteriol. 1996;178:1310–1319. doi: 10.1128/jb.178.5.1310-1319.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Monack D M, Raupach B, Hromockyj A E, Falkow S. Salmonella typhimuriuminvasion induces apoptosis in infected macrophages. Proc Natl Acad Sci USA. 1996;93:9833–9838. doi: 10.1073/pnas.93.18.9833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mullan, P., and E. E. Galyov. Unpublished data.
- 27.Pace J, Hayman M J, Galán J E. Signal transduction and invasion of epithelial cells by S. typhimurium. Cell. 1993;72:505–514. doi: 10.1016/0092-8674(93)90070-7. [DOI] [PubMed] [Google Scholar]
- 28.Popiel I, Turnbull P C B. Passage of Salmonella enteritidis and Salmonella thompsonthrough chick ileocecal mucosa. Infect Immun. 1985;47:786–792. doi: 10.1128/iai.47.3.786-792.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Porter S B, Curtiss R., III Effect of inv mutations on Salmonella virulence and colonization in 1-day-old White Leghorn chicks. Avian Dis. 1997;41:45–57. [PubMed] [Google Scholar]
- 30.Prasad R, Chopra A K, Peterson J W, Pericas R, Houston C W. Biological and immunological characterization of a cloned cholera toxin-like enterotoxin from Salmonella typhimurium. Microb Pathog. 1990;9:315–329. doi: 10.1016/0882-4010(90)90066-y. [DOI] [PubMed] [Google Scholar]
- 31.Rout W R, Formal S B, Dammin G J, Giannella R A. Pathophysiology of Salmonelladiarrhea in the rhesus monkey: intestinal transport, morphological and bacteriological studies. Gastroenterology. 1974;67:59–70. [PubMed] [Google Scholar]
- 32.Simon R, Preifer U, Puhler A. A broad host range mobilisation system for in vivogenetic engineering: transposon mutagenesis in Gram-negative bacteria. Bio/Technology. 1983;1:784–791. [Google Scholar]
- 33.Wallis T S, Hawker R J H, Candy D C A, Qi G-M, Clarke G J, Worton K J, Osborne M P, Stephen J. Quantification of the leukocyte influx into rabbit ileal loops induced by strains of Salmonella typhimuriumof different virulence. J Med Microbiol. 1989;30:149–156. doi: 10.1099/00222615-30-2-149. [DOI] [PubMed] [Google Scholar]
- 34.Wallis T S, Paulin S M, Plested J S, Watson P R, Jones P W. The Salmonella dublinvirulence plasmid mediates systemic but not enteric phases of salmonellosis in cattle. Infect Immun. 1995;63:2755–2761. doi: 10.1128/iai.63.7.2755-2761.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Watson P R, Paulin S M, Bland A P, Jones P W, Wallis T S. Characterization of intestinal invasion by Salmonella typhimurium and Salmonella dublin and effect of a mutation in the invHgene. Infect Immun. 1995;63:2743–2754. doi: 10.1128/iai.63.7.2743-2754.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Williams Smith H. Is it safe to use Escherichia coli K12 in recombinant DNA experiments? J Infect Dis. 1978;137:655–660. doi: 10.1093/infdis/137.5.655. [DOI] [PubMed] [Google Scholar]
- 37.Wood M W, Rosqvist R, Mullan P B, Edwards M H, Galyov E E. SopE, a secreted protein of Salmonella dublin, is translocated into the target eukaryotic cell via a sip-dependent mechanism and promotes bacterial entry. Mol Microbiol. 1996;22:327–333. doi: 10.1046/j.1365-2958.1996.00116.x. [DOI] [PubMed] [Google Scholar]
- 38.Wray C. Salmonellosis in cattle. In Pract. 1991;13:13–15. [Google Scholar]
- 39.Zychlinsky A, Sansonetti P J. Apoptosis as a proinflammatory event: what can we learn from bacteria-induced cell death? Trends Microbiol. 1997;5:201–204. doi: 10.1016/S0966-842X(97)01044-5. [DOI] [PubMed] [Google Scholar]