Abstract
Every year, 10 million people fall ill with tuberculosis (TB). Despite being a preventable and curable disease, 1.5 million people die from TB each year -making it the world’s top infectious disease. TB is the leading cause of death of people with HIV and also a major contributor to antimicrobial resistance. Its presumed that TB was the cause of 1% of the total deaths among inpatients in Sudan in 2017. The current study is aimed to provide pooled prevalence of Mycobacterium tuberculosis among Sudanese as well as to determine any socio-cultural risk factors associated. A systematic review of the literature was conducted and regulated in accordance with the PRISMA Statement. After abstract and full text screening only twenty-six articles met our inclusion criteria and passed the quality assessment procedure. Pulmonary tuberculosis prevalence was assessed in sixteen included studies among participants from Khartoum, Gezira, Kassala, Blue Nile, River Nile, White Nile, Gadarif, Red sea, North Kordofan, Northern State, Sennar and West Darfur States, representing a total sample size of 11,253 participants of suspected individuals such as febrile outpatients, TB patients’ contacts and other groups such as HIV/AIDS patients, hemodialysis patients, School adolescents as well as pregnant women. The pooled prevalence was 30.72% [CI: 30.64, 30.81]. Moreover, Khartoum State recorded the highest pooled prevalence as 41.86% [CI: 14.69, 69.02] based on a total sample size of 2,737 participants. Furthermore, male gender and rural residence were found to be significantly associated with TB infection. Further research with larger sample sizes targeting prevalence and risk factors of TB among Sudanese population is needed to be conducted.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12890-024-02865-6.
Keywords: Africa, Developing countries, Respiratory infections, Risk factors
Introduction
Bearing in mind the political issues that have plagued Sudan with war and hostility for the last 25 years, health care has become an afterthought and basically lost in the midst of what the government might believe to be more pressing matters. The country faces escalating humanitarian catastrophe, with 7.8 million people facing critical problems related to mental and physical wellbeing, including 1.6 million internally displaced people and 1.1 million refugees. Resources are scarce, economic output is collapsed by two-thirds between 2017 and 2018 and the country’s health system is ill-equipped to respond to growing and neglected needs. Adding insult to injury, Sudan still has a long way to go to achieve the Sustainable Development Goals (SDGs). According to the WHO as well as the Sudan Health Observatory in the federal ministry of health, the major communicable diseases contributing to morbidity are Malaria, Tubercelosis, Schistosomiasis, Pneumonia and Diarrheal diseases [1, 2].
Every year, 10 million people fall ill with tuberculosis (TB). Despite being a preventable and curable disease, 1.5 million people die from TB each year -making it the world’s top infectious disease. TB is the leading cause of death of people with HIV and also a major contributor to antimicrobial resistance. Its presumed that TB was the cause of 1% of the total deaths among inpatients in Sudan in 2017 [3].The current study is aimed to provide pooled prevalence of Mycobacterium tuberculosis among Sudanese as well as to determine any socio-cultural risk factors associated.
Materials and methods
Search strategy
To identify relevant studies; a systematic review of the literature was conducted in the 1st of December 2022. The review was regulated in accordance with the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) Statement [4] (Table S1). A comprehensive search was operated in PubMed, Embase, Google scholar, Scopus, Index Copernicus, DOAJ, EBSCO-CINAHL, Cochrane databases without language limits (studies written in languages other than English were later excluded). To obtain a current situation evidence; only studies published in or after 2010 were included. Furthermore, all studies where the data collection process took place before 2010 were also excluded, the only exception was if the collection process started in or before 2010 and ended in 2010 or afterwards. All studies were checked independently by each author, votes were given for each study and any discrepancy was resolved through discussion.
As medical literature in Sudan is generally scarce in international databases as well as socio-cultural factors may be differently reported, socio-cultural factors were not used in keyword formulation and their related results were later extracted from included studies, the keywords used in PubMed was as follow:
Tuberculosis[Mesh] OR Tuberculosis[tiab] AND Sudan*. As previously described [5].
Moreover, to optimize our search, hand searches of reference lists of included articles were also performed.
Study selection and data extraction
Titles and abstracts were assessed for preliminary eligibility. A copy of the full text was obtained for all research articles that were available and approved in principle to be included. Abstraction of data was in accordance with the task separation method; method and result sections in each study were separately abstracted in different occasions to reduce bias. Moreover, data abstraction was conducted with no consideration of author’s qualifications or expertise as described in details previously [6]. Each research article was screened for all relevant information and recorded in the data extraction file (Microsoft Excel), data from each method section was extracted using a predefined set of variables; study characteristics, type of participants, study population size, geographical region, methodology used in prevalence or risk assessment and the period of the study conduction.
After inclusion, studies were further classified into studies determining prevalence, studies determining socio-cultural risk factors and studies determining both prevalence and socio-cultural risk factors. Furthermore, as risk factors-related keywords were not formulated in the search strategy, each study was fully screened to check the nature of the risk investigated by authors, studies determining risk factors in which socio-cultural risks have not been assessed, were later excluded.
Although age grouping is available alongside their corresponding included study in (Table 1), it was not possible to be included in the Meta analysis due to the complexity and diversity of categorization of ‘’age’’ variable among studies included.
Table 1.
Characteristics of tuberculosis related studies
| Study ID | Year of publication | Study design | State | study population/s | Assessment of | sample size | Gender | Participants’ Age |
|---|---|---|---|---|---|---|---|---|
| Abdallah, 2015 [8] | 2015 | Cross sectional | Kassala | Suspected Adults | Prevalence (Nuclic acid) | 985 | Both | Mean 34 years |
| Abdallah, 2012 (9) | 2012 | Cross sectional | Kassala | TB patients | Risk factors | 670 | Both | Not determined |
| Abdelhadi, 2015 [12] | 2015 | Case control | Kassala | TB patients | Risk factors | 306 | Both | 15–85 years |
| Agab Eldour, 2014 [19] | 2014 | Retrospective | Kordofan | Suspected TB patients | Prevalence (Histopathological methods) | 103 | Both | 13–65 years |
| Mustafa, 2021 [33] | 2021 | Cross sectional | Kassala | TB patients | Risk factors | 251 | Both | 4–80 years |
| Ali, A. A., 2012 [21] | 2012 | Cross sectional | Kassala | Suspected TB patients | Prevalence & Risk factors | 2,778 | Female | Not determined |
| Ali, A. 2016 [22] | 2016 | Case control | Khartoum | TB patients | Risk factors | 315 | Both | Mean 33 years |
| Aman, 2017 [23] | 2017 | Case control | Gezira | TB patients’ contacts | Prevalence (Bacteriological and radiological methods) | 657 | Both | Mean 33 years |
| Awadalla, 2010 [24] | 2010 | Case control | Khartoum | HIV/AIDS patients | Prevalence | 291 | Both | Mean 36 years |
| Banaga, 2016 [25] | 2016 | Cross sectional | khartoum | hemodialysis patients | Prevalence & Risk factors | 1,328 | Both | Mean 36 years |
| Bottieau, 2022 [26] | 2022 | Cross sectional | Gadarif | Febrile patients | Prevalence (Bacteriological and Nuclic acid methods) | 667 | Both | Media 35 years |
| Elamin, 2017 [20] | 2017 | Cross sectional | Blue Nile | Suspected TB patients | Prevalence & Risk factors | 208 | Both | 5–54 years |
| Elhassan, 2011 [28] | 2011 | Cross sectional | Khartoum | Suspected TB patients | Prevalence (Nuclic acid) | 90 | Not determined | Not determined |
| Elhassan, 2016 [27] | 2016 | Cross sectional | Khartoum | Suspected TB patients | Prevalence (Bacteriological methods) | 197 | Both | < 15 years |
| Elmadhooun, 2017 [29] | 2017 | Secondary analysis | River Nile | TB patients | Prevalence (Bacteriological methods) | 187 | Both | < 15 years |
| El-Muttalut & Elnimeiri, 2017 [30] | 2017 | Cross sectional | Kassala | TB patients | Risk factors | 366 | Both | 10 - > 60 years |
| Ismail, 2016 [31] | 2016 | Retrospective | Gezira | TB patients | Risk factors | 839 | Both | < 18 - > 45 years |
| Khalid, 2020 [32] | 2020 | Cross sectional | Kassala | School adolescents | Prevalence (Bacteriological methods) | 2,568 | Both | 5–15 years |
| Osman, 2014 [10] | 2014 | Cross sectional | Khartoum | Suspected TB children | Prevalence (Bacteriological methods & Nuclic acid) | 179 | Both | Median 8 years |
| Osman, 2017 [11] | 2017 | Cross sectional | Eastern Sudan* | TB patients’ contacts | Prevalence (Bacteriological methods & ELISA | 768 | Both | Mean 33 years |
| Saeed, 2021 [13] | 2021 | Cross sectional | River Nile | TB patients | Risk factors | 212 | Both | 15 - > 45 years |
| Shakak, 2013 [14] | 2013 | Cross sectional | Khartoum | TB patients’ contacts & General population | Prevalence (Bacteriological methods & ELISA | 284 | Both | Not determined |
| Shigidi, M., 2012 [15] | 2012 | Retrospective | Khartoum | hemodialysis patients | Prevalence (Bacteriological methods & Nuclic acid) & Risk factors | 350 | Both | Mean 37 years |
| Shuaib, 2018 [16] | 2018 | Cross sectional | Kassala, Red Sea & Gadarif | Suspected TB patients | Prevalence (Bacteriological methods & Nuclic acid) | 385 | Both | Median 35 years |
| Sirelkhatim, 2016 [17] | 2016 | Cross sectional | Khartoum, Red Sea & North Kordofan | Suspected TB patients | Prevalence (Bacteriological methods) & Risk factors | 243 | Both | < 15–70 years |
| Yassin, 2019 [18] | 2019 | Cross sectional | Kassala | Pregnant women | Prevalence (Bacteriological methods) & Risk factors | 149 | Female | Mean 30 years |
*Further details are not available
Assessment of quality
Each included article was evaluated based on a framework for making a summary assessment of the quality. The related published literature was crossed, then a framework was structured specifically to determine the level of representativeness of the studied population and to judge the strength of the estimates provided. Five questions were to be answered in each article, each answer represent either 1 score for yes, 0 score for No or 0 score for not available; a total score for risk of bias and quality was calculated by adding up the scores in all five domains, resulting in a score of between 0 and 5. The highest score indicates the highest quality, only studies with a score for quality greater or equal to 3 (higher quality) were included.
The five domains were: is the study objective clearly defined?, is the study sample completely determined?, is the study population clearly defined and specified?, is the methodology rigorous? And is the data analysis rigorous?
Secondary analysis
Among all included studies reporting either prevalence or risk factor estimates, articles were crossed whether Standard Error (SE) is reported. In studies where the SE is not reported; the following formula was used to calculate it: SE = √p (1-p)/ n, where p stands for Prevalence. Regarding risk factors, as each included study may have different objective influencing thereby their result demonstration (i.e. adjusted OR, unadjusted OR, frequencies), each individual category in a given socio-cultural variable investigated the Odd Ratio (OR) was calculated (whenever possible) to provide univariate analysis for the given category among investigated population.
Categorizations of variables was designed to increase the population size of a given estimate, i.e. when the majority of studies investigating Tuberculosis socio-cultural risks categorize education level as below secondary and secondary/above; primary, secondary and university categorization in the minority of studies was re-categorized as (Primary = below secondary, secondary and university = secondary/above).
Quantitative analysis
Meta-analysis was performed—whenever possible using Review Manager Software (Version 5.3). The software automatically provided the Confidence Interval (CI) according to the calculated SE, if the CI is provided in a study; it was introduced accordingly. The heterogeneity of each meta-analysis was assessed as well, the random effect was favored over the fixed effect model in all meta-analysis established as variations between studies is predicted to be probable due to the diversity of the study populations. Sensitivity analysis was also approached to determine the effect of studies conducted in populations proposed to behave in indifference manners or proposed to have low risk on the overall pooled data. Moreover, subgroup analysis was also conducted -whenever suitable to determine prevalence or risk level in specific State or population. An outcome to take part in the meta-analysis has to be included in at least two studies.
Trim and Fill method was used to assess the risk of publication bias in each Meta analysis conducted [7].
Results
Studies included
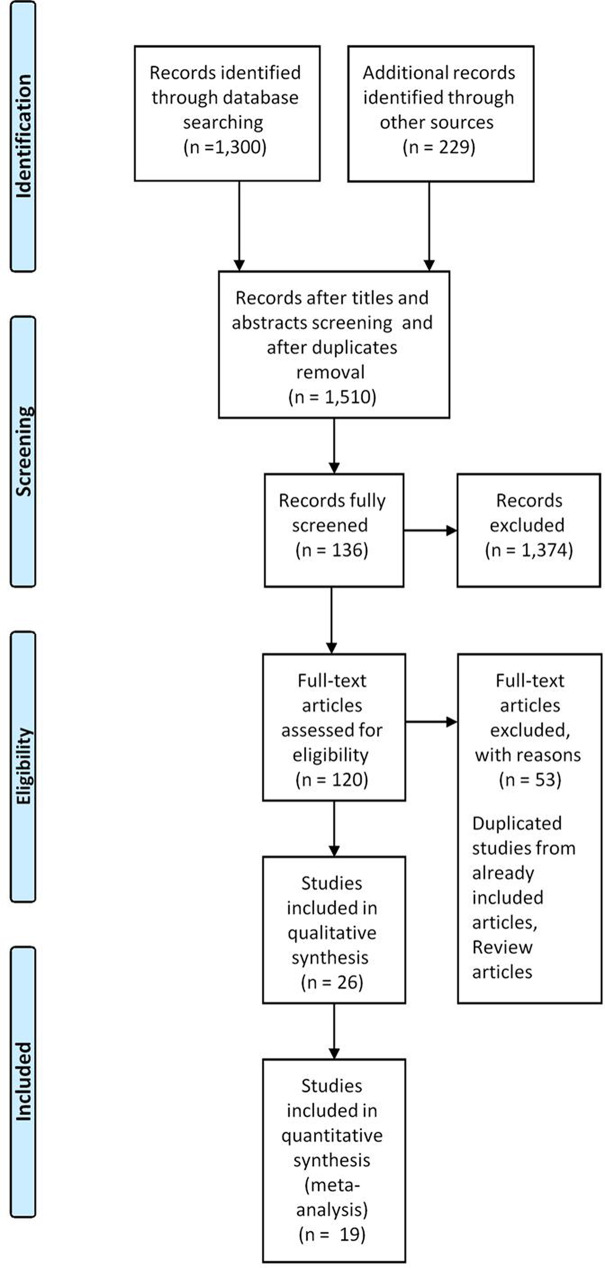
A total of 1,510 articles were identified from the search strategy including hand searches of reference lists of included original research articles and reviews. From these, 1,371 articles were excluded. After abstract and full text screening only twenty-six articles met our inclusion criteria and passed the quality assessment procedure. The articles reported prevalence among specific population and/or risk factors. Figure 1 illustrates the PRISMA flow diagram. The included articles are depicted in (Table 1). Assessment of the quality of included studies is depicted in (Table S2).
Fig. 1.
PRISMA flow diagram
Study characteristics
The characteristics of the included studies are depicted in (Table 1), twenty-six studies were recruited to the study [8, 9, 10–19, 20, 21–26, 27–33], among which sixteen studies determined prevalence of pulmonary TB among Sudanese participants from different populations; participants were from Khartoum in seven included studies, from Kassala in three included studies, Blue Nile, River Nile, White Nile, Gadarif, Red sea, North Kordofan, Northern State, Sennar and West Darfur States were also participated, three studies only provided data related to prevalence of pulmonary and extra-pulmonary TB rather than general TB prevalence. Moreover, majority of studies were conducted among both genders, two studies were conducted among females, while one study did not determine the gender of their participants. Participant’s age varies among studies. All characteristics of included studies are depicted in (Table 1). Publication bias assessment indicated no major asymmetry.
Tuberculosis prevalence
Prevalence estimates were synthesized to represent the overall burden of the disease as well as to estimate subgroup burden related to disease type, study population and geographic location–whenever possible as illustrated below, summary of prevalence estimates synthesized from Tuberculosis related included studies is available in (Table 2).
Table 2.
Summary of prevalence estimates synthesized from tuberculosis related included studies
| Prevalence | Assessed in (State) | Assessed among | Total sample size | References | Pooled prevalence | [95% CI] |
|---|---|---|---|---|---|---|
| Prevalence among different populations | Khartoum, Gezira, Kassala, Blue Nile, River Nile, White Nile, Gadarif, Red sea, North Kordofan, Northern State, Sennar & West Darfur | Suspected patients, HIV/AIDS patients, Hemodialysis patients, school adolescents, TB patient contacts, general population & pregnant women | 11,153 |
Agab Eldour, 2014 Ali, 2012 Aman, 2017 Awadalla, 2010 Banaga, 2016 Bottieau, 2022 Elhassan, 2011 Elhassan, 2016 Khalid, 2020 Osman, 2014 Osman, 2017 Shakak, 2013 Shigidi, 2012 Shuaib, 2018 Sirelkhatim, 2016 Yassin, 2019 |
30.72% | [30.64, 30.81] |
| Prevalence of pulmonary tuberculosis | Khartoum, Gezira, Kassala, Blue Nile, River Nile, White Nile, Gadarif, Red sea, North Kordofan, Northern State, Sennar & West Darfur | Suspected patients, TB patients’ contacts, HIV/AIDS patients, hemodialysis patients, School adolescents & pregnant women | 11,253 |
Abdallah, 2015 Banaga, 2016 Elamin, 2017 Elmadhooun, 2017 Shigidi, 2012 |
56.74% | [11.25, 102.23] |
| Prevalence of extra-pulmonary TB | Kassala, Gadarif, Blue Nile & Khartoum | Hemodialysis patients & suspected patients | 2,871 |
Abdallah, 2015 Banaga, 2016 Elamin, 2017 Shigidi, 2012 |
13.67% | [1.08, 26.27] |
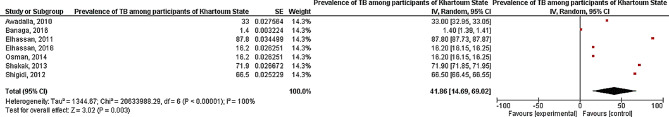
| Prevalence of TB among Khartoum State residents | Khartoum | TB patients’ contacts, HIV/AIDS patients, hemodialysis patients & suspected patients | 2,737 |
Awadalla, 2010 Banaga, 2016 Elhassan, 2011 Elhassan, 2016 Osman, 2014 Shakak, 2013 Shigidi, 2012 |
41.86% | [14.69, 69.02] |
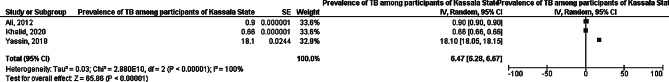
| Prevalence of TB among Kassala State residents | Kassala | General population, school adolescents, pregnant women & suspected patients | 8,163 |
Ali, 2012 Khalid, 2020 Yassin, 2019 |
6.47% | [6.28, 6.67] |
| Prevalence of TB among suspected patients | Khartoum Kassala, Gadarif, North Kordofan & Red Sea | Suspected patients | 4,658 |
Agab Eldour, 2014 Ali, 2012 Bottieau, 2022 Elhassan, 2011 Elhassan, 2016 Osman, 2014 Shuaib, 2018 Sirelkhatim, 2016 |
33.76% | [25.47, 42.06] |
| Prevalence of TB among TB patients’ contacts | Eastern Sudan, Khartoum & Gezira | TB patients’ contacts | 1,709 |
Aman, 2017 Osman, 2017 Shakak, 2013 |
25.40% | [5.67, 45.13] |
Tuberculosis prevalence among different populations
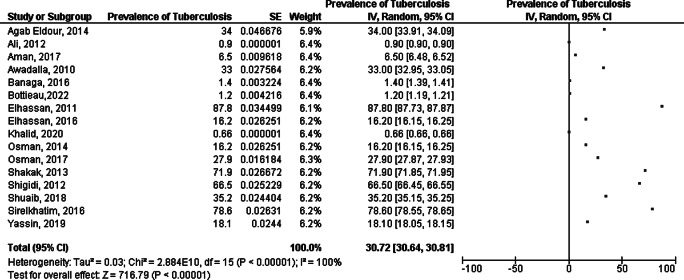
Nineteen included studies determined prevalence of TB among Sudanese participants from different populations, among which three studies only provided data related to prevalence of pulmonary and extra-pulmonary TB rather than general TB prevalence. Pulmonary tuberculosis prevalence was assessed in sixteen included studies; studies were conducted in Khartoum, Gezira, Kassala, Blue Nile, River Nile, White Nile, Gadarif, Red sea, North Kordofan, Northern State, Sennar and West Darfur States, representing a total sample size of 11,253 participants of suspected individuals such as febrile outpatients, TB patients’ contacts and other groups such as HIV/AIDS patients, hemodialysis patients, School adolescents as well as pregnant women. All characteristics are depicted in (Table 1). The pooled prevalence was 30.72% [CI: 30.64, 30.81]. After conducting sensitivity analysis the pooled prevalence was 28.74% [CI: 25.76, 31.72]. However, heterogeneity was high (I2 = 100%) (Fig. 2).
Fig. 2.
Meta analysis of prevalence of TB among participants
Extra-pulmonary TB prevalence
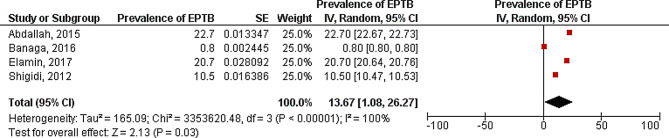
Extra-pulmonary TB prevalence was assessed among participants in four included studies. Studies were conducted among suspected adults in Kassala, Gadarif as well as Blue Nile States and among hemodialysis patients in Khartoum State [26], representing a total sample size of 2,871 participants from both genders with majority of participants being in their thirties. The pooled prevalence was 13.97% [CI: 1.08, 26.27]. Heterogeneity was high (I2 = 100%) (Fig. 3).
Fig. 3.
Meta analysis of prevalence of Extra-Pulmonary TB among participants
TB prevalence among specific populations
TB prevalence among patients’ contacts
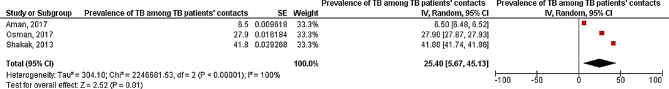
TB prevalence among patients’ contacts was assessed among participants in three included studies. Studies were conducted in Eastern Sudan without State specification in one included study as well as Khartoum and Gezira States, representing a total sample size of 1,709 participants from both genders with participants’ age mean as 33 years in two studies while it was not determined among participants of the third study. The pooled prevalence was 25.40% [CI: 5.67, 45.13]. Heterogeneity was high (I2 = 100%) (Fig. 4).
Fig. 4.
Meta analysis of prevalence of TB among TB patients’ contacts
TB prevalence according to geographical region
TB prevalence in Khartoum State
TB prevalence among residents of Khartoum State was assessed among participants in seven included studies. Studies were conducted among TB patients’ contacts, HIV/AIDS patients, hemodialysis patients and other suspected participants, representing a total sample size of 2,737 participants from both genders in the majority of studies. Participants’ were in their thirties in the majority of studies, while two studies were concerned with children as well as adolescents. The pooled prevalence was 41.86% [CI: 14.69, 69.02]. Heterogeneity was high (I2 = 100%) (Fig. 5).
Fig. 5.
Meta analysis of prevalence of TB among TB patients’ contacts
TB prevalence in Kassala State
TB prevalence among residents of Kassala State was assessed among participants in three included studies. Studies were conducted among general population as school adolescents and other at risk groups such as pregnant women or suspected patients, representing a total sample size of 5,595 participants as female only in two studies while the third study recruited 2,568 school adolescents from both genders [12]. Participants’ age was described as from 5 to 15 years in a study, mean as 30 years in a study while it was not determined in the third study. The pooled prevalence was 6.47% [CI: 6.28, 6.67]. Heterogeneity was high (I2 = 100%) (Fig. 6).
Fig. 6.
Meta analysis of prevalence of TB among participants from Kassala State
Socio-cultural risk factors of TB
Gender
Gender was investigated as a possible risk factor toward Tuberculosis in 5 included studies; participants were from Kassala, Khartoum and River Nile States, with 1525 total sample size of male TB and HIV/AIDS patients. The pooled odd ratio of them being infected was 1.81 [1.46, 2.24] with significant p value z = 5.45 (P < 0.00001). On the other hand, 822 female were investigated among the same population. The pooled odd ratio of them being infected was 0.55 [0.42, 0.72] with significant p value z = 4.33 (P < 0.0001). All results are depicted in (Table 3).
Table 3.
Summary of socio-cultural risk factors estimates synthesized from Tuberculosis related included studies
| Risk | Assessed in (State) | Assessed among | Total sample size | References | Pooled OR [95% CI] | Test for overall effect (z score) |
|---|---|---|---|---|---|---|
| Male gender | Kassala, Khartoum & River Nile | TB & HIV/AIDS patients | 1525 |
Abdallah, 2012 Ahmed, 2021 Awadalla, 2015 El-Muttalut & Elnimeiri, 2017 Saeed, 2021 |
1.81 [1.46, 2.24] | 5.45 (P < 0.00001) |
| Female gender | Kassala, Khartoum & River Nile | TB & HIV/AIDS patients | 822 |
Abdallah, 2012 Ahmed, 2021 Awadalla, 2015 El-Muttalut & Elnimeiri, 2017 Saeed, 2021 |
0.55 [0.42, 0.72] | 4.33 (P < 0.0001) |
| Secondary education and below | Kassala & River Nile | Suspected TB, HIV/AIDS patients & TB patients | 1643 |
Abdallah, 2012 Ali, 2012 Awadalla, 2015 El-Muttalut & Elnimeiri, 2017 Saeed, 2021 |
0.66 [0.01, 32.48] | 0.21 (P = 0.84) |
| Education above secondary | Kassala & River Nile | Suspected TB, HIV/AIDS patients & TB patients | 638 |
Abdallah, 2012 Awadalla, 2015 El-Muttalut & Elnimeiri, 2017 Saeed, 2021 |
0.00 [0.00, 2519.91] | 0.85 (P = 0.40) |
| Single | Kassala and River Nile | TB & HIV/AIDS patients | 340 |
Ahmed, 2021 Awadalla, 2015 El-Muttalut & Elnimeiri, 2017 Saeed, 2021 |
1.33 [0.34, 5.29] | 0.41 (P = 0.68) |
| Married | Kassala and River Nile | TB & HIV/AIDS patients | 660 |
Ahmed, 2021 Awadalla, 2015 El-Muttalut & Elnimeiri, 2017 Saeed, 2021 |
0.75 [0.14, 4.09] | 0.34 (P = 0.74) |
| Urban residence | Kassala, River Nile, Khartoum & Gezira | TB patients | 812 |
Abdallah, 2012 Ahmed, 2021 El-Muttalut & Elnimeiri, 2017 Saeed, 2021 |
0.66 [0.34, 1.26] | 1.27 (P = 0.20) |
| Rural residence | Kassala, River Nile, Khartoum & Gezira | TB patients | 1338 |
Abdallah, 2012 Ahmed, 2021 Ali, 2012 El-Muttalut & Elnimeiri, 2017 Saeed, 2021 |
1.82 [1.01, 3.27] | 2.01 (P = 0.04) |
| Good knowledge about TB | Kassala & River Nile | TB patients | 100 |
Ahmed, 2021 Saeed, 2021 |
1.15 [0.66, 1.98] | 0.49 (P = 0.62) |
| Poor knowledge about TB | Kassala & River Nile | TB patients | 363 |
Ahmed, 2021 Saeed, 2021 |
0.85 [0.49, 1.48] | 0.57 (P = 0.57) |
| Illiteracy | Kassala | TB patients | 159 | Ahmed, 2021 | ||
| Education below secondary | Kassala | TB patients | 239 | Abdelhadi, 2015 | ||
| Secondary education and above | Kassala | TB patients | 62 | Abdelhadi, 2015 | ||
| Not working | River Nile | TB patients | 37 | Saeed, 2021 | ||
| TB treatment Default | ||||||
| Male gender | Kassala, Khartoum & Gezira | TB patients | 857 |
Abdelhadi, 2015 Ali, 2016 Ismail, 2016 |
1.00 [0.69, 1.46] | 0.03 (P = 0.98) |
| Female gender | Kassala, Khartoum & Gezira | TB patients | 466 |
Abdelhadi, 2015 Ali, 2016 Ismail, 2016 |
1.40 [0.50, 3.94] | 0.63 (P = 0.53) |
Education
Education was investigated as a possible socio-cultural risk factor toward Tuberculosis infection in 5 included studies; participants were suspected TB, HIV or TB patients from Kassala and River Nile States. Among 1643 participants described as secondary educated or below, the pooled odd ratio of their infection was 0.66 [0.01, 32.48] with insignificant p value z = 0.21 (P = 0.84). On the other hand, among 638 participants described as above secondary educated in the same populations, the pooled odd ratio of them being infected was 0.00 [0.00, 2519.91] with insignificant p value as well, z = 0.85 (P = 0.40). All results are depicted in (Table 3).
Marital status
Marital status was investigated as a possible socio-cultural risk factor toward Tuberculosis infection in 4 included studies; participants were HIV or TB patients from Kassala and River Nile States. Based on a total sample size of 340 single participants, the pooled odd ratio of them being infected was 1.33 [0.34, 5.29] with insignificant p value z = 0.41 (P = 0.68). On the other hand, 660 married participant were assessed among the same populations, the pooled odd ratio of them being infected was 0.75 [0.14, 4.09] with insignificant p value as well, z = 0.34 (P = 0.74). All results are depicted in (Table 3).
Residence
Residence was investigated as a possible socio-cultural risk factor toward Tuberculosis infection in 4 included studies; participants were TB patients from Kassala, River Nile, Khartoum and Gezira States. Among which 812 participants were urban residents, the pooled odd ratio of them being infected was 0.66 [0.34, 1.26] with insignificant p value z = 1.27 (P = 0.20). On the other hand, 1338 participants were rural residents among the same population, the odd ratio of them being infected was 1.82 [1.01, 3.27] with significant p value z = 2.01 (P = 0.04). All results are depicted in (Table 3).
Knowledge about TB
TB knowledge was investigated as a possible socio-cultural risk factor toward Tuberculosis infection in 2 included studies; participants were TB patients from Kassala and River Nile States, 100 participants were described as having good TB knowledge, the pooled odd ratio of them being infected was 1.15 [0.66, 1.98] with insignificant p value z = 0.49 (P = 0.62). On the other hand, 363 participants among the same population was described as having poor TB knowledge, the pooled odd ratio of them being infected was 0.85 [0.49, 1.48]with insignificant p value z = 0.57 (P = 0.57). All results are depicted in (Table 3).
Socio-cultural risk factors of TB
Gender
Gender was investigated as a possible socio-cultural risk factor toward Tuberculosis treatment default in 3 included studies; participants were TB patients from Kassala, Khartoum and Gezira States. Among which 857 were males, the pooled odd ratio of them being defaulted from TB treatment was 1.00 [0.69, 1.46] with insignificant p value z = 0.03 (P = 0.98). On the other hand, 466 females were investigated among the same population, the pooled odd ratio of them being defaulted from TB treatment was 1.40 [0.50, 3.94] with insignificant p value as well, z = 0.63 (P = 0.53). All results are depicted in (Table 3).
Discussion
To our knowledge, this review is the first attempt to find out the magnitude of information on pooled prevalence of TB as well as its associated socio-cultural risk factors in Sudan. A widespread search from several published databases and stringent methodology to screen and include every potential study was approached in the present study.
Tuberculosis (TB) is a major health problem, with an estimated 10 million people (range 9 to 11.1 million) developing TB disease in 2018, of which 5.8 million, 3.2 million, and 1 million were men, women and children, respectively [34].
The pooled prevalence of TB in the current study was 30.72% and 28.74% after conducting sensitivity analysis. While Extra-Pulmonary TB was 13.97%. This finding is much lower than what has been reported in China. China has one of the highest burdens of TB in the world, according to the World Health Organization (WHO), the number of new TB cases was about 833,000 in China in 2019 [35]. However, a retrospective study conducted in Indonesia where total of 67,944 records were reviewed, the prevalence of TB was as low as 0.8% among general population [36]. Moreover, prevalence of TB in neighboring Ethiopia- one of the TB endemic areas is reported to be 16.7% among random populations [37]. Furthermore, much higher prevalence estimates have been reported in Egypt where it was reported as 70.87% for females and 29.13% for males in a study conducted in Assiut, Egypt [38]. However, the fact that the pooled prevalence synthesized in the current study is based on different study populations of varying infection risk is to be considered when interpreting the current finding.
Moreover, in concern with Extra-Pulmonary TB and agreeing to some extent with the current finding; WHO stated that among 6.3 million new TB cases recognized in 2017, 16% were extra-pulmonary TB cases; prevalence ranged from 8% in the Western Pacific Region up to 24% in the Eastern Mediterranean Region [39].
Regarding TB prevalence among TB patients’ contacts; the current study indicated a pooled prevalence of 25.40% [CI: 5.67, 45.13] based on a total sample size of 1,709 participants. However, a related survey was conducted targeting 69,054 populations from 43 villages in Tiruvallure district, India during 2015–2018. This survey indicated a low incidence of 307 per 100,000 [40].
The pooled odd ratio of male being TB infected was 1.81 [1.46, 2.24] with significant association in the current study. However, a large study conducted in Morocco reported that the top three contributing risk factors were malnutrition, smoking and HIV infection [41]. Nevertheless, aligning with the current study; several studies indicated that male gender is significantly associated with TB infection [42–44].
The strengths of this review are that we systematically identified and included related studies from 2010 to 2022. Moreover; we have conducted meta-analysis to derive pooled prevalence estimates of studies related. Furthermore, we carried out a quality assessment of the included studies based on criteria specifically developed to determine the quality of included studies.
Nevertheless, several limitations are to be considered when interpreting study results; grey literature evidence was not assessed. Moreover, African journals that are not indexed in the screened databases was not considered for inclusion as well, although all included studies are of good quality, several good studies might have been missed. Moreover, publication bias cannot be completely rolled out due to the relatively small amount of published data. Lastly, as a result of the inclusion of publications describing different patient cohorts, the heterogeneity was high among the Meta analysis conducted.
Conclusion
The current study findings indicate that the pooled prevalence of TB is around 30%. Moreover, male gender and rural residence were found to be significantly associated with TB infection. Further research with larger sample sizes targeting prevalence and risk factors of TB among Sudanese population is needed to be conducted.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Material 1: Table (S1): PRISMA checklist of included studies
Acknowledgements
Not applicable.
Author contributions
Conceptualization, BMM, methodology, SMA, IAB, IEB, MSG and BMM; Validation, BMM, SMA; formal analysis SMA; writing—original draft preparation, SMA, IAB, IEB, MSG writing—review and editing, BMM; visualization, SMA, IAB, IEB, MSG; supervision, BMM, SMA; project administration, BMM. All authors have read and agreed to the published version of the manuscript.
Funding
Not applicable.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Republic of the. Sudan FM of H. Annual Health Statistical Report. 2021.
- 2.A-Rahman NHA, Jacquet GA. The state of emergency care in the Republic of the Sudan. Afr J Emerg Med. 2014;4(2):55–60. doi: 10.1016/j.afjem.2013.12.002. [DOI] [Google Scholar]
- 3.Federal Ministry of Health, Department of Health Information, Research & Evidence, Sudan Health Observatory [Internet]. 2021. Available from: http://www.sho.gov.sd/.
- 4.Moher D, Liberati A, Tetzlaff J, Altman DGTPG. Preferred reporting items for systematic reviews and Meta-analyses: the PRISMA Statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peetluk LS, Ridolfi FM, Rebeiro PF, Liu D, Rolla VCST. Systematic review of prediction models for pulmonary tuberculosis treatment outcomes in adults. BMJ Open. 2021;11(3):e044687. doi: 10.1136/bmjopen-2020-044687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Badawi MM, Atif MSMY. Systematic review and meta-analysis of HIV, HBV and HCV infection prevalence in Sudan. 2018;15:148. Virol Journal. 2018;15(148). [DOI] [PMC free article] [PubMed]
- 7.R. DS and T. Trim and fill: a simple funnel-plot–based method of testing and adjusting for Publication Bias in Meta-Analysis. Biometrics. 2000;56:455–63. [DOI] [PubMed]
- 8.Abdallah TEM, Toum FEM, Bashir OH, Mansoor TI, Yuosif MM, Elkhawad MAE, et al. Epidemiology of extra pulmonary tuberculosis in Eastern Sudan. Asian Pac J Trop Biomed. 2015;5(6):505–8. doi: 10.1016/j.apjtb.2015.02.004. [DOI] [Google Scholar]
- 9.Abdallah TM, Ali AAA. Epidemiology of tuberculosis in Eastern Sudan. Asian Pac J Trop Biomed. 2012;2(12):999–1001. doi: 10.1016/S2221-1691(13)60013-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elamin DHE. Socio-economic factors Associated with Tuberculosis in Blue Nile State, Sudan (2014–2016). University of Gezira; 2017.
- 11.Elhassan MM, Elmekki MA, Osman AL, Hamid ME. Challenges in diagnosing tuberculosis in children: a comparative study from Sudan. Int J Infect Dis. 2016;43:25–9. doi: 10.1016/j.ijid.2015.12.006. [DOI] [PubMed] [Google Scholar]
- 12.Elhassan M, Elhassan O, Dirar A, Abbas E, Elmekki M. Validity of Antimycolic Acids Antibodies in the diagnosis of pulmonary tuberculosis in TB/HIV co-infected patients in Khartoum State, Sudan. Egypt Acad J Biol Sci G Microbiol. 2011;3(1):27–32. [Google Scholar]
- 13.Elmadhooun WM, Salah ET, Noor SK, Bushara SO, Ahmed EO, Mustafa H, et al. Prevalence of tuberculosis among children in North Sudan: are we only seeing the tip of the iceberg? J Nat Sci Biol Med. 2017;8(1):114–8. doi: 10.4103/0976-9668.198359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.El-muttalut M, Khidirelnimeiri M. Factors contributing to non-compliance with treatment among Tuberculosis Patients-Kassala. Int Sch J. 2017;6(3):332–8. [Google Scholar]
- 15.Ismail SA, Salmiah MS, Hayati KSOM. Determinants of default from Tuberculosis Treatment among Tuberculosis patients at a Hospital Chest Clinic in Gezira State, Sudan. Int J Public Heal Clin Sci. 2016;3(6):183–92. [Google Scholar]
- 16.Khalid F, Eldirdery M, El-Gasim M, Mukhtar M. Tuberculin reactivity in schoolchildren, Kassala State, Sudan. Int J Mycobacteriology. 2020;9(2):200–4. doi: 10.4103/ijmy.ijmy_16_20. [DOI] [PubMed] [Google Scholar]
- 17.Mustafa GMA, Yassin ME, Shami A, Rahim SA. Screening of human immunodeficiency virus (HIV) among newly diagnosed tuberculosis patients in eastern Sudan. Pol J Microbiol. 2021;70(2):201–6. doi: 10.33073/pjm-2021-017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Osman AL, Saeed NS, Elhassan MM. Polymerase chain reaction targeting insertion sequence IS6110 for the diagnosis of pulmonary tuberculosis among Sudanese children and young adults. Int J Mycobacteriology. 2014;3(4):252–8. doi: 10.1016/j.ijmyco.2014.09.014. [DOI] [PubMed] [Google Scholar]
- 19.Osman SA, Saeed WSE, Musa AM, Younis BM, Bashir AEA, Idris FEM, et al. Prevalence of latent tuberculosis infection (LTBI) among House hold contacts of Sudanese patients with pulmonary tuberculosis in Eastern Sudan: revisiting the tuberculin skin test. J Tuberc Res. 2017;05(01):69–76. doi: 10.4236/jtr.2017.51007. [DOI] [Google Scholar]
- 20.Abdelhadi MA, Mahdi TE, Soghaier MA, Awadalla HM, Ahmed AE, Khalid FA. Factors associated with default from treatment among tuberculosis patients in Kassala State, Sudan 2013. J Public Heal Epidemiol. 2015;7(6):183–8. doi: 10.5897/JPHE2015.0740. [DOI] [Google Scholar]
- 21.Saeed IKMA, Mahdi TE, Elagib AE, Bilal S, Alamin HSH, Babeker EE, et al. Factors associated with patient Delay in diagnosis of tuberculosis at Tuberculosis Management Units, River Nile State, Sudan, 2019. Merit Res J Med Med Sci. 2021;9(2):163–75. [Google Scholar]
- 22.Shakak AO, Khalil EAG, Musa AM, Salih KAEM, Bashir AEA, Ahmed AH, et al. Prevalence of latent tuberculosis infection in Sudan: a case-control study comparing interferon-γ release assay and tuberculin skin test. BMC Public Health. 2013;13(1):1128. doi: 10.1186/1471-2458-13-1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shigidi M, Farouk N, Abulikailik RI, Tag Alsir R, Abu-Aisha H. Active tuberculous infection among adult Sudanese patients on long term peritoneal dialysis. Arab J Nephrol Transplant. 2012;5(3):135–40. [PubMed] [Google Scholar]
- 24.Shuaib YA, Khalil EAG, Schaible UE, Wieler LH, Bakheit MAM, Mohamed-Noor SE, et al. Smear Microscopy for diagnosis of Pulmonary Tuberculosis in Eastern Sudan. Tuberc Res Treat. 2018;2018:1–8. doi: 10.1155/2018/8038137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sirelkhatim M, Eldin GS, Almula I, Babiker A, Omer R. Tuberculosis in Sudan: efficiency of diagnostic techniques commonly used and investigation of some sociodemographic factors which may influence the disease prevalence. Sudan Med Monit. 2016;11(3):71. doi: 10.4103/1858-5000.189559. [DOI] [Google Scholar]
- 26.Yassin K, Ahmed EGE, Musa AO, Hamdan HZ, Abuzied N, Fagear AA, et al. Prevalence of latent tuberculosis (LTB) among pregnant women in a high Burden setting in Sudan using Interferon Gamma (IFN- γ) releasing assay (IGRA) Curr Women s Heal Rev. 2019;15(3):214–7. doi: 10.2174/1573404814666180906125843. [DOI] [Google Scholar]
- 27.Agab Eldour AA, Mohmed Salih EN, Ahmed HG. Incidence of tuberculosis and amyloidosis among Sudanese patients presented with enlarged nodes. J Trop Med. 2014;2014:832029. doi: 10.1155/2014/832029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ali AA, Abdallah TM. Clinical presentation and epidemiology of female genital tuberculosis in eastern Sudan. Int J Gynecol Obstet. 2012;118(3):236–8. doi: 10.1016/j.ijgo.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 29.Ali AOA, Prins MH. Patient non adherence to tuberculosis treatment in Sudan: Socio demographic factors influencing non adherence to tuberculosis therapy in Khartoum State. Pan Afr Med J. 2016;25:80. doi: 10.11604/pamj.2016.25.80.9447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aman AM, Zeidan ZA. Latent tuberculosis infection among Household contacts of pulmonary tuberculosis cases in Central State, Sudan: Prevalence and Associated factors. J Tuberc Res. 2017;05(04):265–75. doi: 10.4236/jtr.2017.54028. [DOI] [Google Scholar]
- 31.Awadalla H, El-Samani F, Soghaier A, Makki M. Risk factors Associated with the development of Tuberculosis among HIV-Infected patients in Khartoum in 2010. AIMS Public Heal. 2015;2(4):784–92. doi: 10.3934/publichealth.2015.4.784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Banaga ASI, Siddiq NK, Alsayed RT, Babiker R, Elmusharaf K. Prevalence and presentation of tuberculosis among hemodialysis patients in Khartoum, Sudan. Saudi J Kidney Dis Transpl. 2016;27(5):992–6. doi: 10.4103/1319-2442.190873. [DOI] [PubMed] [Google Scholar]
- 33.Bottieau E, Van Duffel L, El Safi S, Koirala KD, Khanal B, Rijal S, et al. Etiological spectrum of persistent fever in the tropics and predictors of ubiquitous infections: a prospective four-country study with pooled analysis. BMC Med. 2022;20(1):144. doi: 10.1186/s12916-022-02347-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barry M. Prevalence of Latent Tuberculosis Infection in the Middle East and North Africa: A Systematic Review. Pulmonary Medicine. 2021;2021. [DOI] [PMC free article] [PubMed]
- 35.Chen X, Wu R, Xu J, Wang J, Gao M, Chen Y, et al. Prevalence and associated factors of psychological distress in tuberculosis patients in Northeast China: a cross-sectional study. BMC Infect Dis. 2021;21(1):563. doi: 10.1186/s12879-021-06284-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Noviyani A, Nopsopon T, Pongpirul K. Variation of tuberculosis prevalence across diagnostic approaches and geographical areas of Indonesia. PLoS ONE. 2021;16:e0258809. doi: 10.1371/journal.pone.0258809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mohammed H, Oljira L, Roba KT, Ngadaya E, Manyazewal T, Ajeme T et al. Tuberculosis prevalence and predictors among Health Care-seeking people screened for Cough of any duration in Ethiopia: a Multicenter cross-sectional study. Front Public Heal. 2022;9. [DOI] [PMC free article] [PubMed]
- 38.Mohamed A, Mohamed GAF. A study of tuberculous cases admitted at Assiute Chest Hospital during period (2005–2009). South Vally Univ. 2010.
- 39.Pang Y, An J, Shu W, Huo F, Chu N, Gao M, et al. Epidemiology of extrapulmonary tuberculosis among inpatients, China, 2008–2017. Emerg Infect Dis. 2019;25(3):457–64. doi: 10.3201/eid2503.180572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dolla CK, Dhanaraj B, Chandrasekaran P, Selvaraj S, Menon PA, Thiruvengadam K et al. Prevalence of bacteriologically confirmed pulmonary tuberculosis and associated risk factors: a community survey in Thirvallur District, south India. PLoS ONE. 2021;16(10 October). [DOI] [PMC free article] [PubMed]
- 41.Sbayi A, Arfaoui A, Janah H, Koraichi SEL, Quyou A. Epidemiological characteristics and some risk factors of extrapulmonary tuberculosis in larache, Morocco. Pan Afr Med J. 2020;36(36):1–9. doi: 10.11604/pamj.2020.36.381.24870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shimeles E, Enquselassie F, Aseffa A, Tilahun M, Mekonen A, Wondimagegn GHT. Risk factors for tuberculosis: a case-control study in Addis Ababa, Ethiopia. PLoS ONE. 2019;14(4):e0214235. doi: 10.1371/journal.pone.0214235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fernandes P, Ma Y, Gaeddert M, Tsacogianis T, Marques-Rodrigues P, Fregona G, et al. Sex and age differences in Mycobacterium tuberculosis infection in Brazil. Epidemiol Infect. 2018;146(12):1503–10. doi: 10.1017/S0950268818001450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cheng J, Sun YN, Zhang CY, Yu YL, Tang LH, Peng H et al. Incidence and risk factors of tuberculosis among the elderly population in China: a prospective cohort study. Infect Dis Poverty. 2020;9(1). [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material 1: Table (S1): PRISMA checklist of included studies
Data Availability Statement
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.