Abstract
BACKGROUND:
Exposure to lead (Pb), arsenic (As) and copper (Cu) may cause significant health issues including harmful neurological effects, cancer or organ damage. Determination of human exposure-relevant concentrations of these metal(loids) in drinking water, therefore, is critical.
OBJECTIVE:
We sought to characterize exposure-relevant Pb, As, and Cu concentrations in drinking water collected from homes participating in the American Healthy Homes Survey II, a national survey that monitors the prevalence of Pb and related hazards in United States homes.
METHODS:
Drinking water samples were collected from a national survey of 678 U.S. homes where children may live using an exposure-based composite sampling protocol. Relationships between metal(loid) concentration, water source and house age were evaluated.
RESULTS:
18 of 678 (2.6%) of samples analyzed exceeded 5 μg Pb L−1 (Mean = 1.0 μg L−1). 1.5% of samples exceeded 10 μg As L−1 (Mean = 1.7 μg L−1) and 1,300 μg Cu L−1 (Mean = 125 μg L−1). Private well samples were more likely to exceed metal(loid) concentration thresholds than public water samples. Pb concentrations were correlated with Cu and Zn, indicative of brass as a common Pb source is samples analyzed.
SIGNIFICANCE:
Results represent the largest national-scale effort to date to inform exposure risks to Pb, As, and Cu in drinking water in U.S. homes using an exposure-based composite sampling approach.
IMPACT STATEMENT:
To date, there are no national-level estimates of Pb, As and Cu in US drinking water collected from household taps using an exposure-based sampling protocol. Therefore, assessing public health impacts from metal(loids) in drinking water remains challenging. Results presented in this study represent the largest effort to date to test for exposure-relevant concentrations of Pb, As and Cu in US household drinking water, providing a critical step toward improved understanding of metal(loid) exposure risk.
Keywords: Lead, Arsenic, Copper, Drinking water, Human exposure
INTRODUCTION
The American Healthy Homes Survey (AHHS) II is a collaboration between the United States Department of Housing and Urban Development (HUD) and the United States Environmental Protection Agency (USEPA). AHHS II’s primary focus is to monitor changes in the prevalence of lead-based paint and associated hazards in homes and to identify changes in findings from AHHS I (conducted 2005–2006) [1] and the National Survey of Lead and Allergens in Housing (conducted 1998–1999) [2]. AHHS II also included assessments of lead (Pb) in soil and dust collected from homes, along with pesticide, formaldehyde and mold levels. New to AHHS II, and the focus of this study, was the collection and analysis of drinking water samples for Pb, arsenic (As), and copper (Cu), along with additional elements of interest, using an exposure-based composite sampling protocol. Exposure to these elements may result in severe health effects including neurological effects in developing infants and children (Pb) [3], various types of cancer (As) [4], and gastrointestinal, nervous system, liver or kidney damage (Cu) [4, 5]. Therefore, investigation of Pb, As, and Cu concentrations in drinking water and how these may be related to source materials is critical.
Pb, As, and Cu levels in household drinking water are driven by multiple factors including source water quality, water chemistry, drinking water treatment effectiveness, plumbing materials, plumbing configuration and water usage patterns [6, 7]. Where Pb service lines (LSLs) are present, corrosion or dissolution of leaded plumbing materials present the largest contributors of Pb to household drinking water [8, 9]. Other Pb plumbing materials include brass fixtures and fittings [10, 11], leaded solder [12], Pb goosenecks [13], and galvanized steel pipes [9]. The USEPA has estimated that Pb in drinking water may contribute as much as 60% of total Pb exposure in infants [14]. The relative contribution of Pb from drinking water to total Pb exposure may be increasing over time as other Pb sources are reduced or removed from homes [15]. Similar to Pb, sources of Cu in drinking water include service lines and premise plumbing materials including Cu pipes and brass components via corrosion and release from associated pipe scales [9, 16, 17].
Arsenic concentrations vary in groundwaters and surface waters as a result of both natural and anthropogenic sources [18]. In natural environments, high concentrations of As are generally caused by weathering of As-containing minerals [18, 19]. Anthropogenic sources of As contamination include discharge from mining, petroleum refining, glass and ceramics manufacturing, and legacy pesticide application [19]. Geogenic and anthropogenic As sources can accumulate in drinking water distribution systems and, under some circumstances, be released back to tap water at elevated concentrations [20, 21].
Pb, As and Cu concentrations in drinking water of public health interest are informed by governmental regulatory efforts and public health organization guidelines. In the United States, these have included passage and subsequent amendments to the Safe Drinking Water Act (SDWA) and promulgation of the Lead and Copper Rule (LCR) [22]. The SDWA authorized USEPA to set nonenforceable Maximum Contaminant Level Goals (MCLGs), defined as the level at which no known or expected health risks occur, and enforceable Maximum Contaminant Levels (MCLs), defined as the highest level of contaminant allowed in drinking water considering available treatment technologies and costs. The MCLG and MCL for As, measured at the point of entry to the drinking water distribution system, are 0 and 0.010 mg L−1 respectively [22]. The MCLG for Pb and Cu are 0 and 1.3 mg L−1 respectively [22]. Enforceable levels of Pb and Cu were established under the LCR based on treatment technique in lieu of an MCL, where 90 percent of monitoring samples must fall below 0.015 mg L−1 and 1.3 mg L−1, respectively [23]. Amendments to the SDWA in 1986 and 1996 and passage of the Reduction of Lead in Drinking Water Act in 2011 established and updated requirements involving the use of Pb-free plumbing pipes, fittings and fixtures to contain no more than 0.25% Pb by weighted average across the wetted surface and solder and flux to contain no more than 0.2% Pb [24]. Human health-based targets for Pb, As and Cu in drinking water are also informed by other government and public health agency guidelines. Notably, Health Canada has established a maximum acceptable concentration, defined as the level to have a known or suspected adverse health effect, for Pb, As and Cu in drinking water of 5 ug L−1, 10 ug L−1 and 2000 ug L−1 respectively [25], with additional guidance to keep As and Pb levels as low as reasonably achievable. The World Health Organization has established guidelines of health significance for Pb, As and Cu in drinking water of 10 ug L−1, 10 ug L−1, and 2000 ug L−1 respectively, where the Pb guideline is considered provisional.
This study summarizes AHHS II Pb, As and Cu in drinking water results from 678 homes where children may reside. Samples were collected between March 2018 and June 2019. Results are presented in context of metal(loid) concentrations in drinking water of public health interest. Metal(loid) concentrations between homes on public water supply systems versus private wells are compared, as are correlations between Pb, Cu, and Zinc (Zn) that may suggest brass fittings as a Pb source. Additional elements of secondary interest are also reported. Results provide a first-of-itskind assessment of Pb, As, and Cu concentrations in drinking water across a national survey of US homes where children may reside using an exposure-based composite sampling approach.
METHODS
Sample collection
AHHS II was conducted in a nationally representative sample of permanently occupied, non-institutional homes in the U.S. where children may live. Field operations took place from March 2018 to June 2019. Additional information regarding selection of homes is provided in Supporting Information (SI).
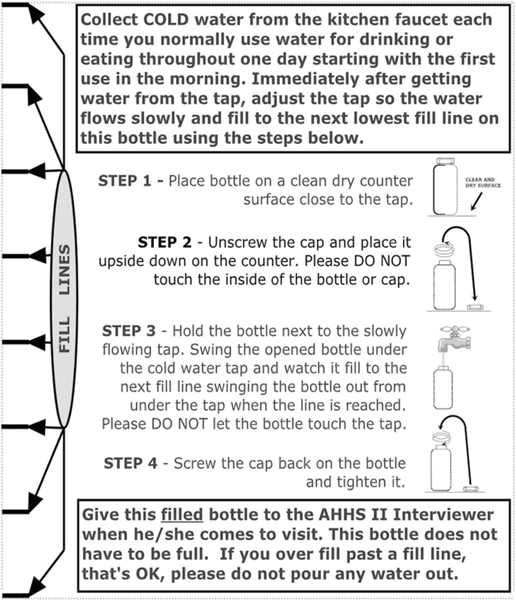
A manual composite water sampling approach that captured residential exposure to metals in drinking water over the course of one day was used to estimate human exposure from multiple household Pb sources [26–29]. Composite samples are collected by diverting a fraction of tap water to a single sample bottle at a specified frequency [26–31]. This sampling approach is more representative of metal(loid) exposure via drinking water than other common sampling procedures such as first draw, fully flushed, or random grab sampling, as it is intended to collect water under normal household usage conditions, making the resulting data more powerful from a public health standpoint [8, 32, 33]. It also enabled collection of exposure-relevant water samples easily and cost-effectively, which were important considerations of this study. For sample and data collection from homes, a trained interviewer and a State- or EPA-certified Lead-Based Paint Inspector/Risk Assessor were dispatched to each Primary Sampling Unit (PSU), defined as a Metropolitan Statistical Area, single county, or group of contiguous counties that together comprise a minimum population of 15,000 (based on the 2000 Census) and maximum end-toend distance of 100 miles. Residents were provided with a labeled collection bottle (Thermo Scientific™ Nalgene™ Certified Wide-Mouth HDPE Bottle) with instructions for collecting a composite sample of the household’s consumptive water use over the course of one day (Fig. 1). The sampling team instructed the resident to collect only cold water from their kitchen faucet each time the faucet was used for water consumption (drinking, food preparation etc.) the day before the sample was scheduled to be picked up. Once the resident had drawn the water for their consumptive purpose, they were instructed to decrease the flow from the tap and place the sample collection bottle under the faucet, filling the bottle to the next fill line marked on the bottle. Additional details on water sample collection and associated questionnaire data are summarized in the SI.
Fig. 1.
Sample collection bottle instructions.
Sample analysis
Upon completion of field collection, sealed samples were shipped by the field sampling team under chain-of-custody to the USEPA’s Office of Research and Development (ORD) in Durham, NC for analysis. Upon receipt, bottle IDs were matched to chain-of-custody reports included with each shipment. Water samples were acidified to 2% nitric acid (by mass) within two weeks of receipt and stored at 4 °C for subsequent analysis.
Prior to analysis, samples were digested at 85 °C for 2 h by hotblock (SCP Science; Quebec, Canada). Quality control (QC) samples associated with each hotblock digestion batch included a 2% nitric acid in DI water blank, blank spike, NIST SRM (1640a or 1643f or both), field blank spike, matrix spike (every 10 samples) and sample duplicate (every 10 samples) (Table S3 and S4). Samples were analyzed for primary (Pb, As and Cu) and secondary (Zn, Cd, Ni, P, Sb, and Si) elements of interest by inductively coupled plasma-mass spectrometry (Thermo Fisher Scientific; Bremen, Germany) in accordance with a standard operating procedure approved by USEPA (see SI).
Statistical analyses
Statistical analyses were performed using R version 4.0.1 (R Development Core Team 2020). All findings were considered statistically-significant at α= 0.05. Because metal(loid) concentrations in water samples were not normally distributed, non-parametric statistical tests were used.
Relationships, if any, between the age of the home and Pb, As, and Cu water concentrations were explored by Spearman’s rank correlation. Mann-Whitney U test was used to explore differences in Pb concentrations in water samples collected from homes built before versus in or after 1988, following implementation of the ban on use of Pb service lines.
Pb, As, and Cu concentrations in water samples collected from homes reported by the resident to be on public water supply systems versus private wells were evaluated by permutation tests. Differences in probabilities of public water supply or private well samples exceeding Pb, As and Cu thresholds of human-health interest were evaluated using 2 × 2 contingency tables [34, 35].
To explore brass fittings as a potential Pb source, correlations between Pb, Zn and Cu were evaluated using Spearman’s rank correlation. Brass is an alloy of Cu (60–80%) and Zn (4–32%) that may contain other elements in smaller quantities, including Pb (2–8%), tin, nickel, aluminum, iron and/or cadmium [17]. This approach has been used to explore brass as a Pb source in drinking water in other studies [6, 9, 36].
RESULTS
Pb, As, and Cu in drinking water samples
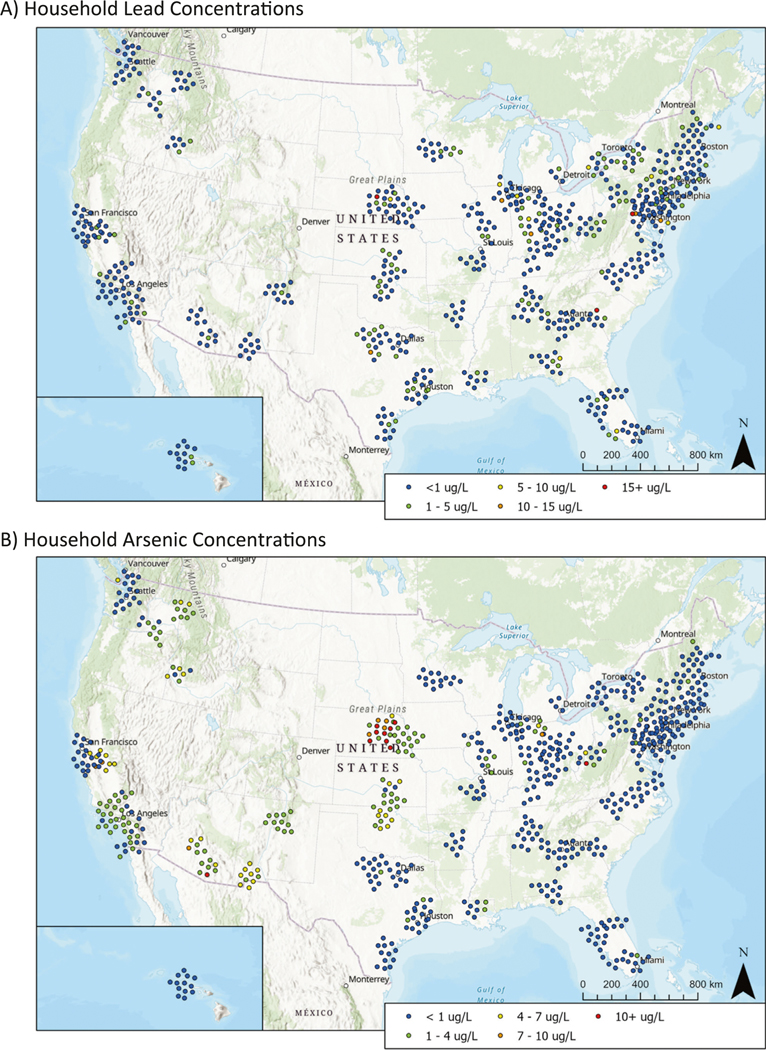
Of the 24 to 36 homes targeted for sampling per PSU, participation ranged from 3 to 20 homes (mean = 8.7, median = 9), for a total of 678 water samples collected and analyzed across the 78 PSUs. Mean Pb, As and Cu concentrations were 1.0, 1.7, and 125 μg L−1 respectively. Median Pb, As and Cu concentrations were 0.4, 0.4, and 33.9 μg L−1 respectively (Table 1). Ninety-nine percent (99%) of samples analyzed for Pb and Cu were above method detection limits (MDLs) of 0.021 and 0.551 μg L−1, respectively. Fifty-seven percent (57%) of samples analyzed for As were above the MDL of 0.308 μg L−1. The proportion of water samples that exceeded specific concentration thresholds of human health interest are also summarized in Table 1. Pb, As, and Cu concentrations are presented geographically by overlaying results by ZIP code on a map of the United States (Fig. 2) [37–39]. Cd, Ni, P, Sb, Si, and Zn results measured in water samples, while not a primary focus of this study, are presented in SI (Table S7).
Table 1.
Descriptive statistics for Pb, As and Cu concentrations in the 678 drinking water samples analyzed.
| Statistic | Pb (μg/L) | As (μg/L) | Cu (μg/L) | |||
|---|---|---|---|---|---|---|
| Mean* | 1.0 ± 0.3 | 1.7 ± 0.6 | 124.8 ± 34.1 | |||
| Median | 0.4 | 0.4 | 33.9 | |||
| Max | 67.8 | 146.7 | 6783 | |||
| # (%) samples: | >MDL** | 674 (99%) | >MDL | 386 (57%) | >MDL | 675 (99%) |
| >LLOQ | 118 (17%) | >LLOQ | 169 (24.9%) | >LLOQ | 526 (78%) | |
| >5 μg/L | 18 (2.6%) | >5 μg/L | 26 (3.8%) | >650 μg /L | 15 (2.2%) | |
| >10 μg/L | 8 (1.2%) | >10 μg/L | 10 (1.5%) | >1300 μg /L | 10 (1.5%) | |
| >15 μg/L | 3 (0.4%) |
± 95% Confidence Interval.
Method Detection Limit (MDL) and Lower Limit of Quantitation (LLOQ) determinations for Pb, As and Cu are presented in Table S2.
Fig. 2.
Household Pb (A) and As (B) results overlaid on a map of the United States. Maps were constructed using the R packages “ggplot”, “sf”, and “ggspatial”. Cu results are similarly presented in Figure S2.
Spearman’s rank correlation coefficient (rs), used to evaluate potential relationships between home age and Pb, As, and Cu water concentrations, was 0.08 for Pb (p= 0.04) and was not statistically significant for As or Cu. Mean and median Pb concentrations in homes built prior to the 1988 effective date of the Pb service line ban (mean = 0.95 μg L−1, median = 0.39 μg L−1) were similar to those built in or after 1988 (mean = 1.06 μg L−1, median = 0.34 μg L−1), and Mann-Whitney U test results did not indicate a statistically significant difference between the two groups.
Public water supply versus private wells
Pb, As and Cu concentrations were compared between homes reported by the resident to be on a public water supply system (n= 595) versus private well (n= 65). Permutation test results showed mean Pb, As and Cu concentrations were significantly higher (2.4, 5.6, and 202 μg L−1 higher for Pb, As and Cu respectively) in water samples collected from homes on private wells than public water supplies (Table 2). Median Pb concentrations were also higher in private well samples (p= 0.01). Median As and Cu concentrations between public and private water samples were not statistically different.
Table 2.
Descriptive statistics for Pb, As and Cu in public (n= 595) versus private well water (n= 65) samples.
| Statistic | Pb μg L−1 | As μg L−1 | Cu μg L−1 | ||||
|---|---|---|---|---|---|---|---|
| public | private | public | private | public | private | ||
| Mean | 0.7 | 3.1 | 1.1 | 6.7 | 107 | 309 | |
| Median | 0.4 | 0.5 | 0.4 | 0.4 | 37 | 22 | |
| Max | 15.6 | 67.8 | 2.4 | 146.7 | 6783 | 4689 | |
| #(%) | >5 (Pb, As) or 650 (Cu) μg L−1 | 10 (1.7%) | 8 (12.3%) | 14 (2.4%) | 9 (13.8%) | 9 (1.5%) | 6 (9.2%) |
| >10 (Pb, As) or 1300 (Cu) μg L−1 | 5 (0.8%) | 3 (4.6%) | 5 (0.8%) | 5 (7.7%) | 6 (1.0%) | 4 (6.2%) | |
| >15 μg L−1 | 1 (0.2%) | 2 (3.1%) |
For Pb, water samples collected from homes on private wells were more likely to have a Pb concentration > 5 μg L−1 (12% of well samples tested) than samples collected from public water supplies (2% of public water supply samples tested) (p= 0.01). Proportions of samples > 10 or 15 μg Pb L−1 in private well versus public water supply samples were not statistically different. For As, proportions of samples from homes on private wells that exceeded 5 and 10 ug As L−1 (14% and 8% of samples, respectively) were higher than those from homes on public water supplies (2% and 1% of samples, respectively) (p= 0.01 and 0.02 for 5 and 10 μg As L−1 thresholds, respectively). Observationally, a majority (4 out of 5) of samples from homes on public water supplies that had As concentrations > 10 μg L−1 were from one ZIP code. 9% and 6% of samples from homes served by private wells exceeded 650 and 1300 μg Cu L−1 respectively, versus 2% and 1% of samples collected from homes on public water supplies (p= 0.02 and 0.04 for 650 and 1300 μg Cu L−1 thresholds, respectively).
Pb and brass fittings: Relationships among Pb, Cu and Zn
Spearman’s rank correlation coefficient (rs) values between Pb and Zn across all 678 water samples was 0.43. Private wells had a higher rs (0.73) than public water supplies (0.40). rs values between Pb and Cu across all 678 water samples was 0.46. Private wells had a higher rs (0.79) than public water supplies (0.42). P-values for all rs values relating Pb, Cu and Zn were < 0.01.
DISCUSSION
Our results summarize drinking water sampling efforts of Pb, As, and Cu collected from a U.S. survey of 678 homes participating in AHHS II. New and innovative to this large-scale national effort was the use of an exposure-based composite drinking water sampling protocol. This sampling approach is important in representing Pb exposure given the inherent variability associated with Pb measurements in drinking water, which are dependent on many factors including water chemistry, various Pb sources in the plumbing and service lines, and household water usage patterns [8, 29, 33, 40]. Pb concentrations from a single tap can change over time, along with variations among different taps in a single residence [29, 32, 33, 41–44]. Therefore, sampling approaches such as first draw stagnated samples and sequential or profile samples may not reflect mean daily metal(loid) exposure to residents from drinking water [29, 33, 42, 44]. Instead, the sampling method used in this study is designed to better capture variability that occurs in drinking water metal(loid) concentration within a home, ultimately providing a more appropriate estimate of metal(loid) exposure [26, 29, 33, 44]. Results of this work demonstrate that such a sampling approach can be implemented at a large scale.
LSLs represent the largest Pb source in drinking water when present [8, 9, 29]. The installation of LSLs was banned in 1986, with an effective date of 1988, although most water systems discontinued LSL use decades earlier [8, 41]. Similarly, leaded solder was banned in 1986 and reductions in Pb content in brasses and other plumbing followed [10, 11, 17]. Therefore, older homes are expected to have an increased likelihood to contain higher drinking water Pb concentrations. However, mean and median Pb concentrations in homes built before versus on or after 1988 were not statistically different, and only a limited relationship between overall household age and Pb was observed (SI Figure S1). Several potential causes for the discrepancy include homes with LSLs were not specifically targeted and use of corrosion control treatment was unknown. Previous studies reporting limitations of exposure assessment sampling support findings of this study that gross surrogate estimators of Pb concentrations in drinking water (e.g., age of housing, etc.) are poor predictors of measured Pb concentrations [14, 29].
18 of 678 samples tested (2.6%) were > 5 μg Pb L−1. Recent studies of contributions of waterborne Pb to blood Pb levels (BLL) have found that even low levels of Pb in drinking water may impact children’s BLLs, potentially disrupting childhood brain development and causing other detrimental health effects [45–51]. Three of 678 samples tested (0.4%) exceeded 15 μg Pb L−1, and these residents were sent a letter with the drinking water Pb concentration, an EPA guide on how to reduce exposure to lead in drinking water, and contact information for local, state, and National Lead Information Center resources. Two of these samples were reported to be from private well water. Additionally, mean and median Pb levels in homes on private wells were statistically higher than those on public water supplies. Further testing of water samples from homes on private wells versus public water supply using equivalent sampling approaches is needed to affirm this finding as nationally representative. Differences in Pb levels in private wells versus public water supply systems observed in this study are consistent with a recent study in North Carolina reporting higher blood Pb levels in children drinking private well versus city water [52–54]. Collectively, these observations may reflect the influence of the EPA’s LCR, which regulates Pb and Cu in community water systems [23, 55]. Under the LCR, public water supplies are required to assess Pb and Cu concentrations in these systems and apply corrosion control treatment when necessary. Without community regulatory oversight, such assessment is less likely in homes with private wells.
Spearman Rank Correlation coefficients (rs) values characterizing correlations among Pb, Zn and Cu observed in this and other studies suggest brass may be a common source of elevated Pb in drinking water. Pb:Zn rs values were observed to be higher in private well samples than those from public water supplies, consistent with generally higher Pb concentrations observed in well water samples. The correlation between Pb and Cu is more complicated. Like Zn, Cu is a component of brass. However, Cu is generally common in plumbing components. Pb:Cu correlations, therefore, may also suggest a link to overall corrosiveness of the source waters. Lead-containing brass taps and fittings have been linked to Pb and Cu contamination of drinking water [56]. Observed rs values between Pb and Zn and Pb and Cu in private well samples in our study are similar to those reported by others [36].
Drinking water Cu concentrations exceeded 1.3 mg L−1 in 10 of 678 (1.5%) samples tested. As with Pb, Cu concentrations were significantly greater in homes on wells, likely reflecting the influence of LCR corrosion treatment requirements, although individual background water quality is also important. Cu concentrations in water were not statistically related to house age. Research has shown that in the absence of phosphates in the water, Cu levels considerably decrease with time as Cu-containing scales age [57]. Cu concentrations are also a function of water chemistry, particularly pH and alkalinity [16, 58].
Arsenic exceeded 10 μg As L−1 in 10 of 678 (1.5%) samples tested. A higher proportion was observed in well water (5 of 65) compared to public water supply system samples (5 of 613). The fraction of private wells with elevated As is unsurprising given the wide distribution of naturally occurring As in groundwaters across the United States [19]. Geographic clusters of elevated As, like those observed in this study in portions of the central U.S., are also indicative of groundwater as the primary source. A 2017 statistical analysis of As concentrations from 20,450 US domestic wells predicted that 2.1 million of the 44.1 million people using well water (4.8%) received water containing over 10 μg As L−1 [59]. A lower proportion (1%) of drinking water samples collected from homes on public water supplies exceeded 10 μg As L−1, with 4 of 5 observations in this study being from a single ZIP code. While water samples collected as part of this study were not associated with a specific community water supply (CWS) — samples were identified only by city/state and zip code — a review of the Safe Drinking Water Information System Federal Reporting Services database indicated a similar rate of exceedance of 10 μg As L−1 in CWS’s between 2018 and 2020 of 0.5 to 0.6% by year [60]. It should be noted that As compliance sampling is conducted at the entry point of the distribution system at a public water supply, whereas sampling in this study was conducted at the household tap, as build-up and release of As in water supply plumbing between the point of distribution and the household tap may contribute to different levels of As when the water reaches a home [20, 21, 58]. Differences in As concentrations in public water supply versus private well samples likely reflect the implementation of the EPA’s Arsenic Rule to community water systems [61], and application of treatment strategies such as iron coagulation and adsorption media approaches. As noted for Pb, further testing of water samples from homes on private wells versus public water supply using equivalent sampling approaches is needed to affirm this finding as nationally representative.
The overarching AHHS II study was designed to maximize national representation of results to the greatest extent feasible given available resources. However, several limitations should be considered when evaluating or extrapolating water data presented in this study. As previously noted, the sampling protocol used in this study was selected in part due to its ease of implementation and cost-effectiveness relative to other exposurebased sampling methods, notably use of automated proportional sampling devices [29, 31, 33, 44]. Because samples were collected immediately after the resident’s consumptive use, some Pb in drinking water may not have been captured that would have been represented via use of automated proportional sampling devices. However, when comparing the two sampling methods, it is not possible to estimate measurement bias relevant to this study in part because important contributory factors, including the concentration of Pb from premise plumbing sources in sampled homes, are unknown. Additionally, non-parametric statistical methods were used in this study to enable direct comparisons to similar studies of metal(loid) concentrations in U.S. drinking water using equivalent statistical methods. While application of parametric statistical methods may result in small changes in statistical significance, use of such methods are not expected to impact reported findings. Important factors that may impact metal(loid) concentration in drinking water, such as presence of LSLs, types and relative location of plumbing materials, use or type of corrosion control, or water usage were not assessed in this study. Inclusion/exclusion of communities located near geogenic As sources may have a significant influence on overall As results. Sampling was also limited to a single day in each sampled home. Future investigations considering these factors may improve our understanding of metal(loid) exposure from drinking water.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Bill Thayer for his assistance with statistical methods. We thank Alicia Kirby and Kelsey Miller for reviewing the manuscript. The manuscript has been reviewed in accordance with EPA policy and approved for publication. Approval does not signify that contents necessarily reflect views and policies of the Agency, nor does mention of trade names or commercial products constitute endorsement or recommendation for use.
FUNDING
The Department of Housing and Urban Development funded the AHHS II field study collection of the drinking water samples. EPA did not receive financial assistance in support of this study.
Footnotes
COMPETING INTERESTS
The authors declare no competing interests.
ETHICAL APPROVAL
EPA’s Office of Research and Development (ORD), Center for Environmental Measurement and Modeling was not directly engaged in the collection of information from human subjects. HUD’s contractor, QuanTech, conducted the field study and collected the drinking water samples. QuanTech received IRB Approval CR00077983 for HUD OHHLHC - AHHS II, American Healthy Homes Survey (AHHS) II (Pro00019737). According to the requirements of EPA Order 1000.17 A (Policy and Procedures on Protection of Human Research Subjects) and EPA Regulation 40 CFR 26 (Protection of Human Subjects), it was determined that the EPA investigators were not engaged in human subjects research (HSR-001225).
ADDITIONAL INFORMATION
Supplementary information The online version contains supplementary material available at https://doi.org/10.1038/s41370-022-00461-6.
Reprints and permission information is available at http://www.nature.com/reprints
DATA AVAILABILITY
Data presented in this publication are available from the corresponding author on reasonable request.
REFERENCES
- 1.Dewalt FG, Cox DC, O’Haver R, Salatino B, Holmes D, Ashley PJ, et al. Prevalence of lead hazards and soil arsenic in US housing. J Environ Health. 2015;78:22–29. [PubMed] [Google Scholar]
- 2.Jacobs DE, Clickner RP, Zhou JY, Viet SM, Marker DA, Rogers JW, et al. Theprevalence of lead-based paint hazards in US housing. Environ Health Perspect. 2002;110:A599–A606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rees N, Fuller R. The toxic truth: children’s exposure to lead pollution undermines a generation of future potential. UNICEF, 2020. https://www.unicef.org/sites/default/files/2020-07/The-toxic-truth-children%E2%80%99s-exposure-to-leadpollution-2020.pdf [Google Scholar]
- 4.Tsai S-M, Wang T-N, Ko Y-C. Mortality for certain diseases in areas with high levelsof arsenic in drinking water. Arch Environ Health: Int J. 1999;54:186–93. [DOI] [PubMed] [Google Scholar]
- 5.Hsu H-W, Bondy SC, Kitazawa M. Environmental and dietary exposure to copperand its cellular mechanisms linking to Alzheimer’s disease. Toxicological Sci. 2018;163:338–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carter JA, Erhardt RJ, Jones BT, Donati GL. Survey of Lead in Drinking Water from Schools and Child Care Centers Operating as Public Water Suppliers in North Carolina, USA: Implications for Future Legislation. Environmental Sci Technol. 2020; 54:14152–60. [DOI] [PubMed] [Google Scholar]
- 7.Frank JJ, Poulakos AG, Tornero-Velez R, Xue J. Systematic review and metaanalyses of lead (Pb) concentrations in environmental media (soil, dust, water, food, and air) reported in the United States from 1996 to 2016. Sci Total Environ. 2019;694:133489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lytle DA, Schock MR, Wait K, Cahalan K, Bosscher V, Porter A, et al. Sequential drinking water sampling as a tool for evaluating lead in flint, Michigan. Water Res. 2019;157:40–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sandvig A, Kwan P, Kirmeyer G, Maynard B, Mast D, Trussell RR, et al. Contributionof service line and plumbing fixtures to lead and copper rule compliance issues. Water Environment Research Foundation Alexandria, VA, 2009. [Google Scholar]
- 10.Kimbrough DE. Brass corrosion and the LCR monitoring program. J-Am Water Works Assoc. 2001;93:81–91. [Google Scholar]
- 11.Schock MR, Neff CH. Trace metal contamination from brass fittings. J-Am Water Works Assoc. 1988;80:47–56. [Google Scholar]
- 12.Subramanian K, Sastri V, Elboujdaini M, Connor J, Davey A. Water contamination: Impact of tin-lead solder. Water Res. 1995;29:1827–36. [Google Scholar]
- 13.Ramaley BL. Monitoring and control experience under the lead and copper rule.J-Am Water Works Assoc. 1993;85:64–7. [Google Scholar]
- 14.Stanek LW, Xue J, Lay CR, Helm EC, Schock M, Lytle DA, et al. Modeled impacts ofdrinking water Pb reduction scenarios on children’s exposures and blood lead levels. Environ Sci Technol. 2020;54:9474–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Levin R, Brown MJ, Kashtock ME, Jacobs DE, Whelan EA, Rodman J, et al. Leadexposures in US children, 2008: implications for prevention. Environ health Perspect. 2008;116:1285–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Edwards M, Schock MR, Meyer TE. Alkalinity, pH, and copper corrosion by-product release. J-Am Water Works Assoc. 1996;88:81–94. [Google Scholar]
- 17.Lytle DA, Schock MR. Stagnation time, composition, pH, and orthophosphateeffects on metal leaching from brass. National Risk Management Research Laboratory, Office of Research; and…, 1996. [Google Scholar]
- 18.Smedley P, Kinniburgh DG. Arsenic in groundwater and the environment. In: Essentials of medical geology. Springer, 2013, 279–310. https://link.springer.com/chapter/10.1007/978-94-007-4375-5_12 [Google Scholar]
- 19.Smedley PL, Kinniburgh DG. A review of the source, behaviour and distribution ofarsenic in natural waters. Appl Geochem. 2002;17:517–68. [Google Scholar]
- 20.Lytle DA, Sorg TJ, Frietch C. Accumulation of arsenic in drinking water distributionsystems. Environ Sci Technol. 2004;38:5365–72. [DOI] [PubMed] [Google Scholar]
- 21.Triantafyllidou S, Lytle DA, Chen AS, Wang L, Muhlen C, Sorg TJ. Patterns ofarsenic release in drinking water distribution systems. AWWA water Sci.2019;1:e1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.USEPA. National Primary Drinking Water Regulations. In, 2022. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7077812/
- 23.Kastury F, Smith E, Doelsch E, Lombi E, Donnelley M, Cmielewski PL, et al. In vitro,in vivo, and spectroscopic assessment of lead exposure reduction via ingestion and inhalation pathways using phosphate and iron amendments. Environ Sci Technol. 2019;53:10329–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.USEPA. Use of Lead Free Pipes, Fittings, Fixtures, Solder, and Flux for DrinkingWater. In, 2021. [Google Scholar]
- 25.Canada H. Guidelines for Canadian Drinking Water Quality—Summary Table. In: Water and Air Quality Bureau HE, and Consumer Safety Branch; HC, Ottawa, Ontario, (eds). 2020. https://www.canada.ca/en/health-canada/services/environmental-workplace-health/reports-publications/water-quality/guidelinescanadian-drinking-water-quality-summary-table.html [Google Scholar]
- 26.Greathouse D, Craun G, Worth D. Epidemiologic study of the relationship between lead in drinking water and blood lead levels. In: Trace Substances in Environmental Health, vol. 10. University of Missouri Columbia, 1976, 9–24. https://www.google.com/search?rlz=1C1GCEA_enUS990US990&source=univ&tbm=isch&q=Greathouse+D,+Craun+G,+Worth+D.+Epidemiologic+study+of+the+relationship+between+lead+in+drinking+water+and+blood+lead+levels.+In:+Trace+Substances+in+Environmental+Health,+vol.+10.+University+of+Missouri+Columbia,+1976,+pp+9-24.&fir=HTVntCoG2U6IyM%252CyL_JZ72iaYfU_M%252C_%253BHwzofZ9a43SKXM%252CemUaX4qbCp_hPM%252C_%253BP9tTEZaj-5cA_M%252CyL_JZ72iaYfU_M%252C_%253BlBJe4lNcFOWMSM%252CyL_JZ72iaYfU_M%252C_%253BY99ozP7kTLS4QM%252CyL_JZ72iaYfU_M%252C_%253BCDLc4mKa1PQiM%252CyL_JZ72iaYfU_M%252C_%253BvBiR6UzVjfnt3M%252CW5LWU55vBcoXZM%252C_%253BOFWtY15TqaFjxM%252CPJvvYXVkXSC7M%252C_%253B5EZyny9EUv3qYM%252CGHlZR010rUigAM%252C_%253BMANjqn-qMO0d7M%252C-PJvvYXVkXSC7M%252C_&usg=AI4_kQhZ353V11_ptaKb8qgIOjg9-a1gA&sa=X&ved=2ahUKEwiK89bE_sv5AhWyM1kFHW34A7kQjJkEegQIHxAC&cshid=1660674548860343&biw=854&bih=540&dpr=1.5 [Google Scholar]
- 27.Jarvis P, Quy K, Macadam J, Edwards M, Smith M. Intake of lead (Pb) from tap water of homes with leaded and low lead plumbing systems. Sci Total Environ. 2018;644:1346–56. [DOI] [PubMed] [Google Scholar]
- 28.Lacey R, Moore M, Richards W. Lead in water, infant diet and blood: The Glasgow duplicate diet study. Sci Total Environ. 1985;41:235–57. [DOI] [PubMed] [Google Scholar]
- 29.Triantafyllidou S, Burkhardt J, Tully J, Cahalan K, DeSantis M, Lytle D, et al. Variability and sampling of lead (Pb) in drinking water: Assessing potential human exposure depends on the sampling protocol. Environ Int.2021;146:. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Clement M, Seux R, Rabarot S. A practical model for estimating total lead intake from drinking water. Water Res. 2000;34:1533–42. [Google Scholar]
- 31.Van de Hoven T, Buijs P, Jackson P, Gardner M, Leroy P, Baron J, et al. Developing a new protocol for the monitoring of lead in drinking water. Brussels: European Comission; 1999. [Google Scholar]
- 32.Lytle DA, Formal C, Cahalan K, Muhlen C, Triantafyllidou S. The impact of sampling approach and daily water usage on lead levels measured at the tap. Water Res. 2021;197:117071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Diagnostic sampling tools for lead in drinking water. Proceedings of the Proc AWWA Annual Conference and Exposition, 2019. [Google Scholar]
- 34.Conover WJ. Practical nonparametric statistics. John Wiley & Sons, 1998. [Google Scholar]
- 35.Snedecor GW, Cochran WG. Statistical methods, 8thEdn. Ames: Iowa State UnivPress Iowa; 1989;54:71–82. [Google Scholar]
- 36.Pieper KJ, Krometis L-AH, Gallagher DL, Benham BL, Edwards M. Incidence ofwaterborne lead in private drinking water systems in Virginia. J Water Health. 2015;13:897–908. [DOI] [PubMed] [Google Scholar]
- 37.Dunnington D, Thorne B. ggspatial: Spatial Data Framework for ggplot2. Rpackage version1 2020; 1. https://scholar.google.ca/citations?view_op=view_citation&hl=en&user=Ik_72RsAAAAJ&citation_for_view=Ik_72RsAAAAJ:4TOpqqG69KYC
- 38.Pebesma EJ. Simple features for R: Standardized support for spatial vector data. RJ. 2018;10:439. [Google Scholar]
- 39.Wickham H. Elegant graphics for data analysis. Media 2009;35:10.1007. [Google Scholar]
- 40.Schock MR, Lemieux FG. Challenges in addressing variability of lead in domesticplumbing. Water Sci Technol: Water Supply. 2010;10:793–9. [Google Scholar]
- 41.Schock MR. Causes of temporal variability of lead in domestic plumbing systems. Environ Monit Assess. 1990;15:59–82. [DOI] [PubMed] [Google Scholar]
- 42.Triantafyllidou S, Edwards M. Lead (Pb) in tap water and in blood: implications forlead exposure in the United States. Crit Rev Environ Sci Technol.2012;42:1297–352. [Google Scholar]
- 43.Redmon JH, Levine KE, Aceituno AM, Litzenberger K, Gibson JM. Lead in drinking water at North Carolina childcare centers: Piloting a citizen science-based testing strategy. Environ Res. 2020;183:109126. [DOI] [PubMed] [Google Scholar]
- 44.Riblet C, Deshommes E, Laroche L, Prévost M. True exposure to lead at the tap: insights from proportional sampling, regulated sampling and water use monitoring. Water Res. 2019;156:327–36. [DOI] [PubMed] [Google Scholar]
- 45.Bellinger DC. Childhood lead exposure and adult outcomes. Jama.2017;317:1219–20. [DOI] [PubMed] [Google Scholar]
- 46.Gilbert SG, Weiss B. A rationale for lowering the blood lead action level from 10 to2 μg/dL. Neurotoxicology. 2006;27:693–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ngueta G, Gonthier C, Levallois P. Colder-to-warmer changes in children’s blood lead concentrations are related to previous blood lead status: Results from a systematic review of prospective studies. J Trace Elem Med Biol.2015;29:39–46. [DOI] [PubMed] [Google Scholar]
- 48.Deshommes E, Prévost M, Levallois P, Lemieux F, Nour S. Application of leadmonitoring results to predict 0–7 year old children’s exposure at the tap. Water Res. 2013;47:2409–20. [DOI] [PubMed] [Google Scholar]
- 49.Ngueta G, Abdous B, Tardif R, St-Laurent J, Levallois P. Use of a cumulative exposure index to estimate the impact of tap water lead concentration on blood lead levels in 1-to 5-year-old children (Montréal, Canada). Environ Health Perspect. 2016;124:388–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Levallois P, St-Laurent J, Gauvin D, Courteau M, Prévost M, Campagna C, et al. Theimpact of drinking water, indoor dust and paint on blood lead levels of children aged 1–5 years in Montréal (Québec, Canada). J exposure Sci Environ Epidemiol. 2014;24:185–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Levallois P, Barn P, Valcke M, Gauvin D, Kosatsky T. Public health consequences oflead in drinking water. Curr Environ health Rep. 2018;5:255–62. [DOI] [PubMed] [Google Scholar]
- 52.Gibson JM, Fisher M, Clonch A, MacDonald JM, Cook PJ. Children drinking privatewell water have higher blood lead than those with city water. Proc Natl Acad Sci. 2020;117:16898–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Maas RP, Patch SC, Pope J, Thornton L. Lead-leaching characteristics of sub-mersible residential water pumps. J Environ health. 1998;60:8. [Google Scholar]
- 54.Sidle W, Li P. Impact of submersible pumps on Pb constituents in residential wells. Environ Geochem health. 2008;30:1–9. [DOI] [PubMed] [Google Scholar]
- 55.Pontius FW. Appendix H: Outline of 40 CFR 141, 142, and 143. Drinking Water Regulation and Health 2003.971–7. https://www.google.com/search?rlz=1C1GCEA_enUS990US990&source=univ&tbm=isch&q=Pontius+FW+Appendix+H:+Outline+of+40+CFR+141,+142,+and+143.+Drinking+Water+Regulation+and+Health+2003.+971-977.&fir=GkEzIay8cBD0zM%252CCp4d2TkoKdsGtM%252C_%253B98DDpzuulJOaWM%252CHRWU-g2YM362CM%252C_%253BazHuO6G83DH5hM%252CJeYr91AUvfri_M%252C_%253BazSgoiUXxRgqmM%252C244nVIg_9laDsM%252C_%253BKIZ1y15N90D1QM%252CCfDNIgX4ctKy-M%252C_%253BsEIhQmw-BWX5WM%252CQDZsDGoFRTACDM%252C_%253BFWInDZFZ30J9dM%252CsYR-lwpbzWEJKM%252C_%253BGbYc7-Zx2Gk6QM%252CsYR-lwpbzWEJKM%252C_%253BgUCCEh9zYIt9qM%252CtPfkTkqgneyG9M%252C_%253BkM64pzCO6ZrwfM%252CNEwofWcIDV13ZM%252C_&usg=AI4_kQbfL9qIUwzkP7c8bJqAGKYXy6YbA&sa=X&ved=2ahUKEwihyZKw_8v5AhX8FlkFHYC1CrQQjJkEegQIKBAC&biw=854&bih=540&dpr=1.5 [Google Scholar]
- 56.Harvey P, Handley H, Taylor M. Widespread copper and lead contamination of household drinking water, New South Wales, Australia. Environ Res. 2016;151:275–85. [DOI] [PubMed] [Google Scholar]
- 57.Lagos GE, Cuadrado CA, Letelier MV. Aging of copper pipes by drinking water. J-Am Water Works Assoc. 2001;93:94–103. [Google Scholar]
- 58.Schock MR, Lytle DA. Internal corrosion and deposition control. McGraw-Hill, Inc: New York, 2011. [Google Scholar]
- 59.Ayotte JD, Medalie L, Qi SL, Backer LC, Nolan BT. Estimating the high-arsenic domestic-well population in the conterminous United States. Environ Sci Technol. 2017;51:12443–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.EPA. Safe Drinking Water Information System (SDWIS) Database. 2021. [Google Scholar]
- 61.EPA U. Implementation guidance for the arsenic rule. In: Office of Water, US EPA; Washington, DC, USA, 2002. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data presented in this publication are available from the corresponding author on reasonable request.