Key Points
Question
Among patients treated for localized prostate cancer, what are the associations between specific treatments and functional outcomes such as urinary incontinence and sexual dysfunction?
Findings
In this observational study of 2445 people treated for localized prostate cancer and followed up for 10 years, compared with radiotherapy or surveillance, radical prostatectomy was associated with worse urinary incontinence but not worse sexual function among 1877 patients with favorable prognosis at baseline. Compared with radiotherapy with androgen deprivation therapy, radical prostatectomy was associated with worse urinary incontinence but not worse sexual function among the 568 patients with unfavorable prognosis.
Meaning
In localized prostate cancer, radical prostatectomy was associated with worse urinary incontinence, but not worse sexual function, at 10-year follow-up.
Abstract
Importance
Adverse outcomes associated with treatments for localized prostate cancer remain unclear.
Objective
To compare rates of adverse functional outcomes between specific treatments for localized prostate cancer.
Design, Setting, and Participants
An observational cohort study using data from 5 US Surveillance, Epidemiology, and End Results Program registries. Participants were treated for localized prostate cancer between 2011 and 2012. At baseline, 1877 had favorable-prognosis prostate cancer (defined as cT1-cT2bN0M0, prostate-specific antigen level <20 ng/mL, and grade group 1-2) and 568 had unfavorable-prognosis prostate cancer (defined as cT2cN0M0, prostate-specific antigen level of 20-50 ng/mL, or grade group 3-5). Follow-up data were collected by questionnaire through February 1, 2022.
Exposures
Radical prostatectomy (n = 1043), external beam radiotherapy (n = 359), brachytherapy (n = 96), or active surveillance (n = 379) for favorable-prognosis disease and radical prostatectomy (n = 362) or external beam radiotherapy with androgen deprivation therapy (n = 206) for unfavorable-prognosis disease.
Main Outcomes and Measures
Outcomes were patient-reported sexual, urinary, bowel, and hormone function measured using the 26-item Expanded Prostate Cancer Index Composite (range, 0-100; 100 = best). Associations of specific therapies with each outcome were estimated and compared at 10 years after treatment, adjusting for corresponding baseline scores, and patient and tumor characteristics. Minimum clinically important differences were 10 to 12 for sexual function, 6 to 9 for urinary incontinence, 5 to 7 for urinary irritation, and 4 to 6 for bowel and hormone function.
Results
A total of 2445 patients with localized prostate cancer (median age, 64 years; 14% Black, 8% Hispanic) were included and followed up for a median of 9.5 years. Among 1877 patients with favorable prognosis, radical prostatectomy was associated with worse urinary incontinence (adjusted mean difference, −12.1 [95% CI, −16.2 to −8.0]), but not worse sexual function (adjusted mean difference, −7.2 [95% CI, −12.3 to −2.0]), compared with active surveillance. Among 568 patients with unfavorable prognosis, radical prostatectomy was associated with worse urinary incontinence (adjusted mean difference, −26.6 [95% CI, −35.0 to −18.2]), but not worse sexual function (adjusted mean difference, −1.4 [95% CI, −11.1 to 8.3), compared with external beam radiotherapy with androgen deprivation therapy. Among patients with unfavorable prognosis, external beam radiotherapy with androgen deprivation therapy was associated with worse bowel (adjusted mean difference, −4.9 [95% CI, −9.2 to −0.7]) and hormone (adjusted mean difference, −4.9 [95% CI, −9.5 to −0.3]) function compared with radical prostatectomy.
Conclusions and Relevance
Among patients treated for localized prostate cancer, radical prostatectomy was associated with worse urinary incontinence but not worse sexual function at 10-year follow-up compared with radiotherapy or surveillance among people with more favorable prognosis and compared with radiotherapy for those with unfavorable prognosis. Among men with unfavorable-prognosis disease, external beam radiotherapy with androgen deprivation therapy was associated with worse bowel and hormone function at 10-year follow-up compared with radical prostatectomy.
This observational study uses registry data to compare rates of adverse functional outcomes between specific treatments for localized prostate cancer (radical prostatectomy, external beam radiotherapy, brachytherapy, or active surveillance for favorable-prognosis disease and radical prostatectomy or external beam radiotherapy with androgen deprivation therapy for unfavorable-prognosis disease).
Introduction
Treatment options for localized prostate cancer (PC) include radical prostatectomy, radiotherapy with or without androgen deprivation therapy (ADT), or observation with active surveillance (AS) and are selected based on risk of PC recurrence, life expectancy, and patient preference.1 Most men survive at least 15 years after diagnosis of localized PC.2 Therefore, understanding associations of each treatment with functional outcomes, such as sexual, urinary, and bowel function, over long-term follow-up can help inform treatment selection.
Relative rates of adverse functional outcomes associated with each treatment option for localized PC remain unclear. Evidence is lacking regarding the relative associations of each treatment with functional outcomes, according to whether the patient has a favorable or unfavorable prognosis.3,4,5,6,7,8,9
Therefore, the population-based Comparative Effectiveness Analysis of Surgery and Radiation (CEASAR) study enrolled men diagnosed with localized PC in 2011-2012 and followed them up for approximately 10 years to compare patient-reported outcomes according to treatment received at baseline.
Methods
Study Cohort
This observational cohort study used a rapid case ascertainment system to identify men 80 years or younger diagnosed with clinically localized PC (cT1-T2c, cN0, cM0, prostate-specific antigen [PSA] level <50 ng/mL) among 5 population-based Surveillance, Epidemiology, and End Results (SEER) registries in 2011-2012. A total of 3434 men from SEER registries were enrolled in CEASAR within 6 months of diagnosis. Study abstractors obtained patient-reported questionnaires administered at baseline and at 6 months, 12 months, 3 years, 5 years, and 10 years after treatment. Details have been published.10 In this analysis, we excluded men who did not answer questionnaires at baseline and at least once thereafter or who had missing clinical information (Figure 1).
Figure 1. Definition of the Cohort in the CEASAR Study.
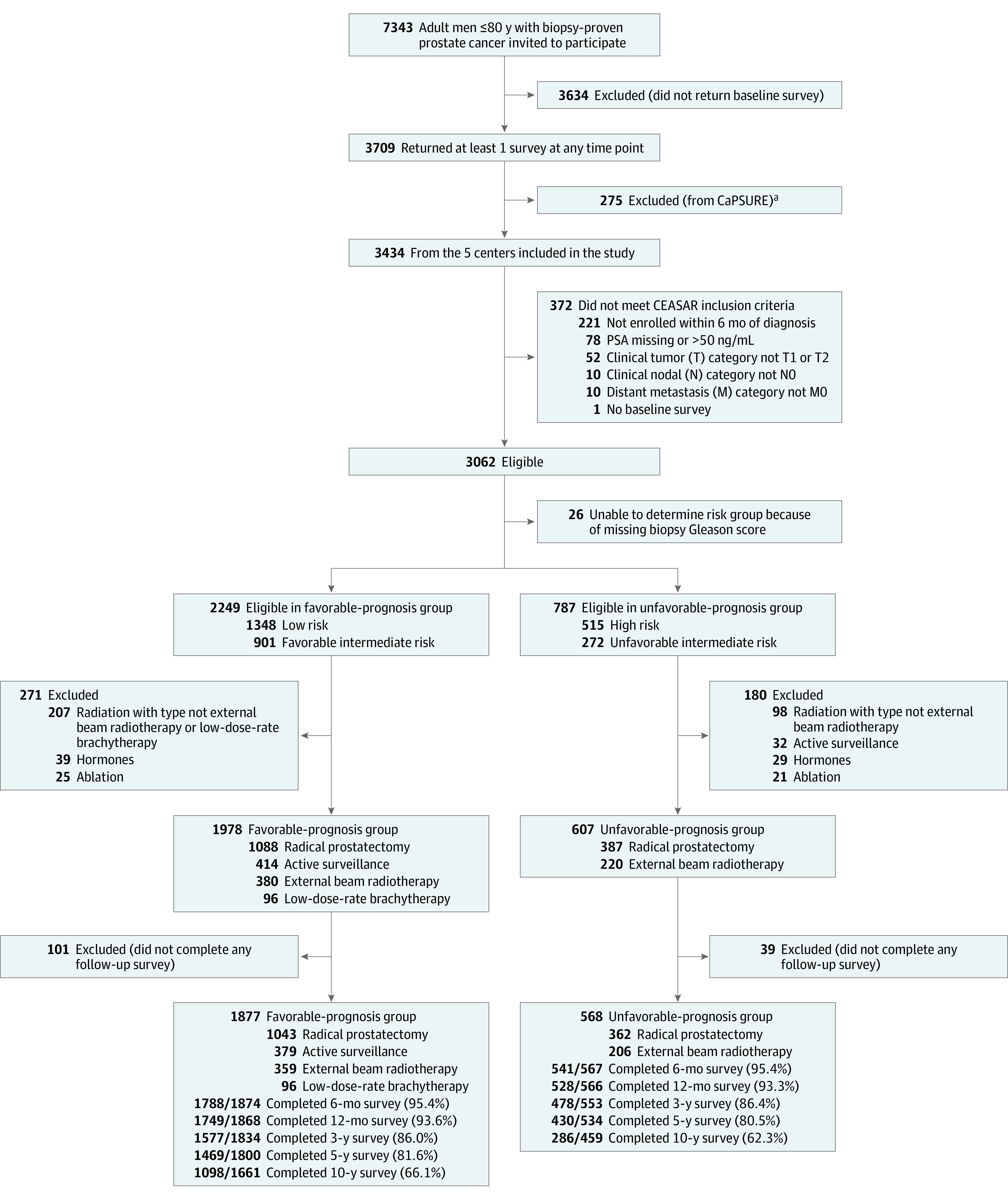
Flow of participants in the Comparative Effectiveness Analyses of Surgery and Radiation (CEASAR) Study of the association between contemporary treatments for localized prostate cancer through 10 years. PSA indicates prostate-specific antigen.
aPatients from CaPSURE were excluded because they did not receive the 10-year survey.
Participants were categorized into 2 groups: (1) favorable-prognosis PC (cT1or T2a/bN0M0, PSA ≤20 ng/mL, and grade group 1-2) corresponding to low- and favorable-intermediate risk PC and (2) unfavorable-prognosis PC (cT2cN0M0, PSA level of 20-50 ng/mL, or grade group 3-5) corresponding to unfavorable intermediate-risk and high-risk PC.1 Institutional review board approvals were obtained from Vanderbilt University Medical Center and enrollment sites. Patients provided written informed consent. Follow-up data were obtained through February 1, 2022.
Exposures (Treatment Modalities)
In the favorable-prognosis PC group, we compared radical prostatectomy, external beam radiotherapy (EBRT) without ADT, low-dose-rate brachytherapy, and AS, defined as no definitive treatment within 1 year of diagnosis or medical record documentation of AS.11 In the unfavorable-prognosis PC group, we compared radical prostatectomy vs EBRT with ADT. Treatment comparisons were selected to be consistent with those recommended by the American Urological Association and National Comprehensive Care Network.1,12
Outcomes
Prespecified primary outcomes were 10-year patient-reported sexual, urinary incontinence, urinary irritation, bowel, and hormone function domain scores, measured with the 26-item Expanded Prostate Cancer Index Composite (EPIC-26).13 Higher EPIC-26 domain scores (range, 0-100) indicate better function. Minimum clinically important differences (MCIDs) were as follows: sexual function, 10 to 12; urinary incontinence, 6 to 9; urinary irritation, 5 to 7; bowel function, 4 to 6; and hormone function, 4 to 6.14,15
Prespecified secondary outcomes were individual items on the EPIC-26 questionnaires, measuring functional quality-of-life concerns, including overall sexual, urinary, and bowel function, in addition to urinary leakage, burning on urination, frequent urination, and bowel urgency. These items used 5 ordinal response options that were dichotomized as moderate/big problem vs no/very small/small, and compared across treatments.3,6,8 Additionally, we included the quality of erections item and dichotomized response options as firm for intercourse vs any of the 3 lower categories. Other prespecified secondary outcomes were health-related quality-of-life domains for physical and mental health, measured using the Medical Outcomes Study 12-Item Short Form at 10 years,16 and overall and PC-specific survival. Higher Medical Outcomes Study 12-Item Short Form scores (range, 0-100) indicate better physical and mental health functioning (no MCID available). Overall and PC-specific survival data were obtained from each SEER registry.
In post hoc analyses, we examined additional clinically relevant individual items from the EPIC-26 (eMethods in Supplement 1).
Covariates
Patient-reported covariates collected at baseline with questionnaires included age, marital status, education, employment, and race and ethnicity, collected as fixed categories to assess for cohort diversity.10 Patient-reported comorbid diseases,17 social support,18 depression,19 and decision-making style20 were assessed at baseline using previously validated questionnaires. Disease characteristics (PSA, stage, grade) and treatment were obtained via medical record abstraction.
Statistical Analysis
Analyses were performed separately for patients with favorable prognosis and for patients with unfavorable prognosis. Baseline characteristics were compared across treatments using Wilcoxon rank sum, Kruskal-Wallis, and χ2 tests where appropriate. Men with favorable-prognosis PC who received AS were analyzed based on the initial AS assignment. We performed 2 post hoc sensitivity analyses. First, analyses were repeated, limiting the AS group to those untreated throughout follow-up. Second, analyses were repeated comparing men treated with EBRT alone vs those treated with EBRT plus ADT in the favorable-prognosis group; only unadjusted analyses were performed because of small sample size in the group receiving ADT.
Associations between initial treatment and EPIC-26 outcomes were assessed using multivariable longitudinal linear regression for each score outcome and logistic regression models for individual EPIC-26 items. These models adjusted for the corresponding baseline EPIC-26 domain score, comorbidity,17 PC risk group (PSA, stage, and grade),12 time since treatment, use of ADT within 1 year from treatment, SEER site, and baseline physical functioning,21 social support,18 depression,19 and participatory decision-making (eMethods in Supplement 1).20 Interaction terms for treatment modality and ADT were included. For the favorable-prognosis PC group, differences in EPIC-26 domain scores were compared across treatment groups among those not receiving ADT; for the unfavorable-prognosis PC group, comparisons were made between radical prostatectomy without ADT and EBRT with ADT. The median interval from enrollment to return of the intended 10-year survey was 9.5 years (IQR, 9.1 to 9.8), and modeling was used to estimate 10-year outcomes, per the original protocol. The longitudinal models adjusted for time since treatment as a continuous variable, allowing for assessment of outcomes at preceding time points to provide context for 10-year outcomes.
Overall and PC-specific survival were estimated using the Kaplan-Meier method by primary treatment.
Missing values for covariates were imputed using the multiple imputation chained equations procedure, as previously described.22,23 No outcome values were imputed. A 2-sided type I error rate of less than .05 was the threshold for statistical significance. Due to the potential inflation of type I error rate from multiple comparisons, secondary outcomes were interpreted as exploratory. Primary outcomes were considered significant only if they met both MCID and statistical significance. Analyses were conducted using R version 4.1 (R Project for Statistical Computing).
Results
Among 3062 potentially eligible men who completed baseline questionnaires, 26 were excluded for missing biopsy grade data, 451 due to the use of nonstandard treatments, and 140 for not completing any follow-up questionnaires. A total of 2445 patients were included, consisting of 77 Asian men (3%), 184 Hispanic men (8%), 350 non-Hispanic Black men (14%), 1797 non-Hispanic White men (74%), and 33 men (1%) of “other” race and ethnicity (including men who selected American Indian/Alaska Native and men who selected other but did not otherwise specify) (Figure 1). Of those included in analyses, survival data were available for 98% (eTable 1 in Supplement 1).
Questionnaire response rates varied from 95% at 6 months to 66% by year 10 (Figure 1). All included covariates had 5% or less missing data (eTable 2 in Supplement 1). Compared with men who responded to the 10-year questionnaires, those who did not respond were older (median age, 66 vs 63 years; P < .001), and included a higher proportion of people who were unmarried (24% vs 17%, P < .001), and had lower education attainment (42% vs 24% completed high school or less, P < .001; eTable 3 in Supplement 1).
At 10 years, 221 of 1834 men (12%) with favorable-prognosis PC had died, 8 (0.4%) from PC. Of men with unfavorable-prognosis PC, 112 of 558 men (20%) died, including 27 (5%) from PC (Figure 2; eTable 1 in Supplement 1). The 10-year estimated PC-specific survival rates, stratified by treatment, in favorable-prognosis PC were 99.7% for AS, 99.5% for radical prostatectomy, 99.3% for EBRT, and 100% for brachytherapy. Among men with unfavorable-prognosis PC, 10-year estimated PC-specific survival rates were 96.4% for radical prostatectomy and 91.7% for EBRT.
Figure 2. Overall and Prostate Cancer–Specific Survival in Patients With Localized Prostate Cancer.
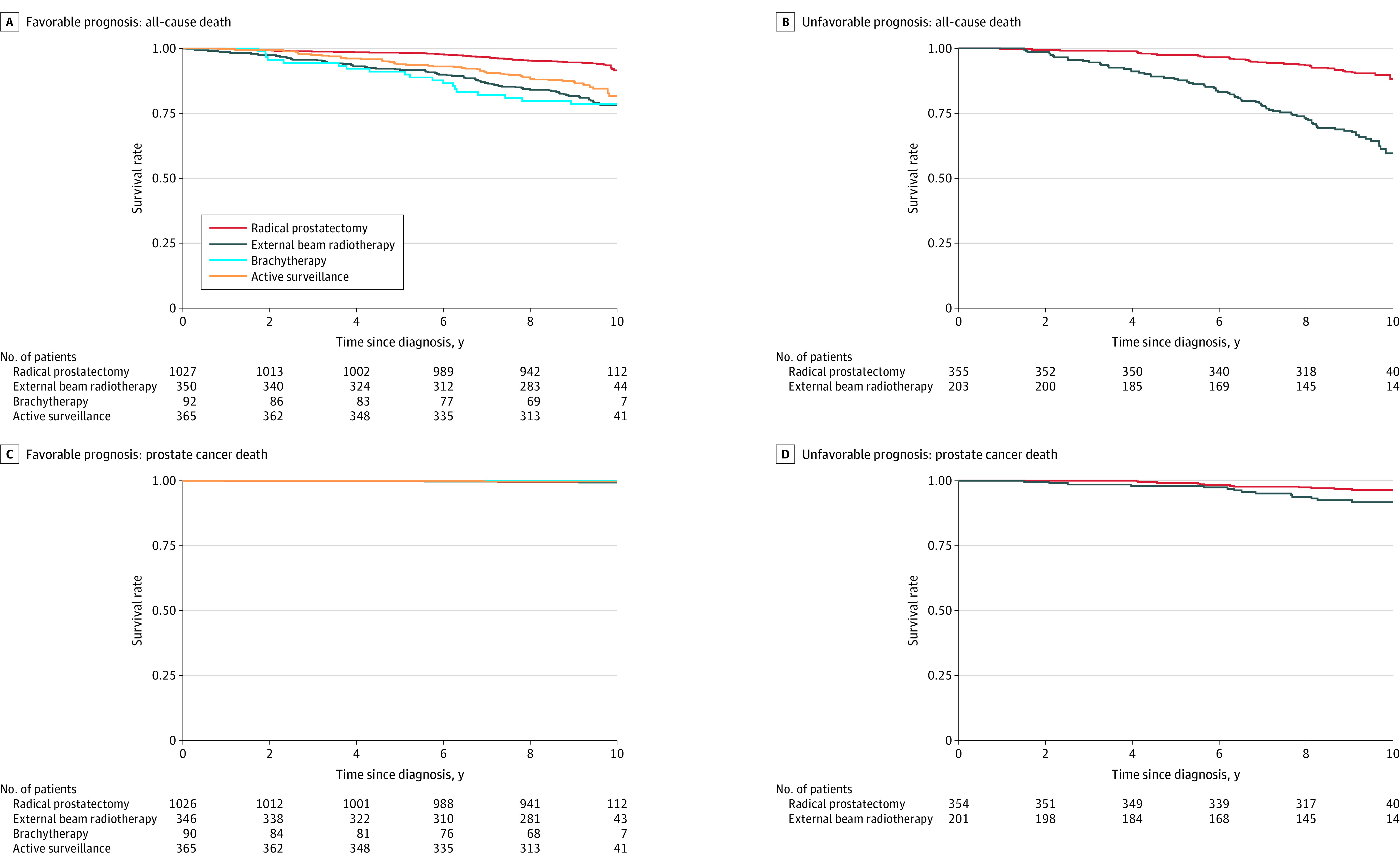
Overall and prostate cancer–specific survival probabilities were calculated using the Kaplan-Meier method for men with favorable-prognosis and unfavorable-prognosis prostate cancer through 10 years. Participants were censored at the date of the last registry follow-up. The median time of observation for the favorable-prognosis group was 9.5 years (IQR, 9.2-9.8), and for the unfavorable-prognosis group was 9.4 years (IQR, 9.0-9.8).
Favorable-Prognosis PC
Of 1877 men with favorable-prognosis PC, 379 (20%) had AS, 1043 (56%) underwent radical prostatectomy (672 [79%] robot-assisted), 359 (19%) had EBRT (278 [79%] intensity-modulated radiotherapy and 273 [83%] image-guided), and 96 (5%) had brachytherapy (Table; eTable 4 in Supplement 1).
Table. Baseline Characteristics of Men Enrolled in the CEASAR Study.
| No. (%) | ||||||
|---|---|---|---|---|---|---|
| Favorable-prognosis prostate cancer | Unfavorable-prognosis prostate cancer | |||||
| Active surveillance (n = 379) | Radical prostatectomy (n = 1043) | EBRT (n = 359) | Brachytherapy (n = 96) | Radical prostatectomy (n = 362) | EBRT (n = 206) | |
| Age at diagnosis, median (IQR), y | 67 (61-72) | 61 (56-65) | 68 (63-73) | 65 (62-70) | 64 (59-68) | 71 (66-74) |
| Race and ethnicity, No. of patientsa | 378 | 1041 | 359 | 96 | 361 | 206 |
| Asian | 9 (2) | 33 (3) | 12 (3) | 0 | 13 (4) | 10 (5) |
| Hispanic | 23 (6) | 92 (9) | 21 (6) | 2 (2) | 31 (9) | 15 (7) |
| Non-Hispanic Black | 52 (14) | 136 (13) | 62 (17) | 10 (10) | 47 (13) | 43 (21) |
| Non-Hispanic White | 287 (76) | 768 (74) | 260 (72) | 81 (84) | 264 (73) | 137 (67) |
| Other | 7 (2) | 12 (1) | 4 (1) | 3 (3) | 6 (2) | 1 (0) |
| Marital status, No. of patientsb | 358 | 979 | 345 | 93 | 343 | 195 |
| Married | 286 (80) | 812 (83) | 253 (73) | 71 (76) | 283 (83) | 147 (75) |
| Education, No. of patients | 361 | 980 | 346 | 95 | 344 | 195 |
| Less than high school | 29 (8) | 84 (9) | 49 (14) | 6 (6) | 40 (12) | 40 (21) |
| High school graduate | 71 (20) | 204 (21) | 76 (22) | 24 (25) | 75 (22) | 36 (18) |
| Some college | 72 (20) | 223 (23) | 75 (22) | 30 (32) | 69 (20) | 47 (24) |
| College graduate | 85 (24) | 222 (23) | 74 (21) | 15 (16) | 84 (24) | 35 (18) |
| Graduate/professional school | 104 (29) | 247 (25) | 72 (21) | 20 (21) | 76 (22) | 37 (19) |
| Comorbidity score, No. of patientsc | 363 | 983 | 347 | 95 | 344 | 197 |
| 0-2 | 96 (26) | 350 (36) | 66 (19) | 32 (34) | 106 (31) | 30 (15) |
| 3-4 | 142 (39) | 419 (43) | 148 (43) | 29 (31) | 147 (43) | 71 (36) |
| ≥5 | 125 (34) | 214 (22) | 133 (38) | 34 (36) | 91 (26) | 96 (49) |
| Employment, No. of patients | 377 | 1035 | 356 | 94 | 359 | 203 |
| Full time | 122 (32) | 606 (59) | 93 (26) | 30 (32) | 152 (42) | 33 (16) |
| Part time | 41 (11) | 71 (7) | 28 (8) | 8 (9) | 33 (9) | 13 (6) |
| Retired | 198 (53) | 307 (30) | 208 (58) | 54 (57) | 154 (43) | 148 (73) |
| Unemployed | 16 (4) | 51 (5) | 27 (8) | 2 (2) | 20 (6) | 9 (4) |
| Risk groups, No. of patientsd | 379 | 1043 | 359 | 96 | 362 | 206 |
| Low risk | 306 (81) | 600 (58) | 169 (47) | 72 (75) | ||
| Favorable intermediate risk | 73 (19) | 443 (42) | 190 (53) | 24 (25) | ||
| Unfavorable intermediate risk | 131 (36) | 66 (32) | ||||
| High risk | 231 (64) | 140 (68) | ||||
| Receipt of androgen deprivation therapy in year 1, No./No. of patients (%) | 2/340 (1) | 11/1036 (1) | 98/355 (28) | 10/95 (11) | 52/355 (15) | 156/204 (76) |
| PSA at diagnosis, median (IQR), ye | 5 (4-7) | 5 (4-7) | 6 (4-8) | 5 (4-7) | 6 (5-9) | 7 (5-14) |
| Clinical tumor stage, No. of patientsf | 373 | 1041 | 359 | 96 | 361 | 205 |
| T1 | 319 (86) | 864 (83) | 294 (82) | 82 (85) | 195 (54) | 118 (58) |
| T2 | 54 (14) | 177 (17) | 65 (18) | 14 (15) | 166 (46) | 87 (42) |
| Biopsy grade group, No. of patientsg | 379 | 1043 | 359 | 96 | 360 | 206 |
| 1 | 337 (89) | 662 (63) | 186 (52) | 77 (80) | 38 (11) | 10 (5) |
| 2 | 42 (11) | 381 (37) | 173 (48) | 19 (20) | 39 (11) | 20 (10) |
| 3 | 151 (42) | 80 (39) | ||||
| 4-5 | 132 (37) | 96 (47) | ||||
| Positive cores at biopsy, median (IQR) [No. of patients] | 1 (1-2) [296] | 3 (1-5) [818] | 2 (1-5) [311] | 2 (1-3) [88] | 4 (2-6) [262] | 4 (2-7) [171] |
| Robot-assisted prostatectomy, No./No. of patients (%) | 672/854 (79) | 212/290 (73) | ||||
| Study site, No. of patients | 379 | 1043 | 359 | 96 | 362 | 206 |
| Utah | 57 (15) | 93 (9) | 6 (2) | 14 (15) | 34 (9) | 8 (4) |
| Atlanta, GA | 49 (13) | 130 (12) | 31 (9) | 22 (23) | 64 (18) | 19 (9) |
| Los Angeles, CA | 128 (34) | 336 (32) | 94 (26) | 18 (19) | 115 (32) | 45 (22) |
| Louisiana | 112 (30) | 287 (28) | 119 (33) | 34 (35) | 103 (28) | 107 (52) |
| New Jersey | 33 (9) | 197 (19) | 109 (30) | 8 (8) | 46 (13) | 27 (13) |
| EPIC-26 baseline scoresh | ||||||
| Sexual function, No. of patients | 352 | 994 | 339 | 94 | 344 | 189 |
| Median (IQR)b | 75 (41-85) | 80 (43-95) | 60 (27-82) | 72 (38-85) | 65 (32-85) | 48 (12-80) |
| Urinary incontinence, No. of patients | 358 | 1005 | 341 | 93 | 347 | 200 |
| Median (IQR)b | 100 (79-100) | 100 (79-100) | 100 (79-100) | 100 (92-100) | 100 (79-100) | 100 (75-100) |
| Urinary irritation, No. of patients | 359 | 1004 | 340 | 93 | 345 | 199 |
| Median (IQR)b | 88 (75-100) | 88 (75-100) | 88 (75-94) | 94 (75-100) | 88 (69-100) | 88 (75-94) |
| Bowel function, No. of patients | 364 | 1021 | 351 | 95 | 354 | 201 |
| Median (IQR)b | 100 (92-100) | 100 (96-100) | 100 (92-100) | 100 (96-100) | 100 (88-100) | 100 (92-100) |
| Hormone function, No. of patients | 363 | 1003 | 341 | 93 | 350 | 192 |
| Median (IQR)b | 95 (85-100) | 95 (85-100) | 95 (80-100) | 100 (81-100) | 90 (80-100) | 90 (80-95) |
| SF-36 baseline scoresi | ||||||
| Physical functioning, No. of patients | 354 | 1006 | 346 | 92 | 354 | 196 |
| Median (IQR)c | 95 (80-100) | 100 (85-100) | 90 (70-100) | 95 (80-100) | 95 (80-100) | 85 (59-100) |
| Emotional well-being, No. of patients | 362 | 1018 | 351 | 94 | 357 | 202 |
| Median (IQR)c | 84 (72-92) | 84 (68-92) | 84 (72-92) | 84 (75-94) | 84 (64-92) | 84 (68-92) |
| Energy and fatigue, No. of patients | 363 | 1018 | 351 | 95 | 358 | 202 |
| Median (IQR)c | 75 (60-85) | 80 (60-87) | 70 (55-85) | 75 (55-88) | 75 (55-85) | 75 (55-85) |
| General health scale, No. of patients | 379 | 1040 | 357 | 96 | 362 | 205 |
| Median (IQR)c | 80 (60-80) | 80 (60-80) | 60 (60-80) | 80 (60-80) | 80 (60-80) | 60 (60-80) |
| Social support scale, No. of patientsj | 377 | 1038 | 358 | 95 | 359 | 201 |
| Median (IQR) | 95 (65-100) | 95 (70-100) | 95 (75-100) | 95 (60-100) | 95 (70-100) | 90 (55-100) |
| Depression scale, No. of patients | 363 | 1014 | 351 | 92 | 359 | 199 |
| Median (IQR)k | 15 (4-26) | 15 (4-30) | 11 (4-30) | 15 (4-33) | 19 (4-33) | 19 (6-35) |
| Participatory decision-making scale, No. of patientsl | 363 | 1032 | 355 | 93 | 357 | 198 |
| Median (IQR) | 83 (68-96) | 86 (71-93) | 79 (64-93) | 86 (75-93) | 82 (68-93) | 75 (57-86) |
Abbreviations: CEASAR, Comparative Effectiveness Analysis of Surgery and Radiation; EBRT, external beam radiotherapy; EPIC-26, 26-item Expanded Prostate Cancer Index Composite; PSA, prostate-specific antigen.
Race and ethnicity were collected in the 12-month survey with the following closed self-reported categories (multiple selection was not allowed): American Indian/Alaska Native; Asian/Oriental/Pacific Islander; Black/African American (not Latino/Hispanic); White/Caucasian (not Latino/Hispanic); Latino/Hispanic/Mexican American; and Other. The “Other” category included 4 men who selected American Indian/Alaska Native and men who selected other but did not otherwise specify.
Not married included separated, divorced, widowed, and never married.
Total Illness Burden Index score measures the severity of comorbidities (score range, 0-23); higher values indicate worse comorbid illnesses.
Risk groups are based on the National Cancer Comprehensive Network prostate cancer guidelines. They are based on tumor stage, PSA level, and biopsy results at time of diagnosis.
Threshold values for elevated PSA levels vary according to age, ranging from ≥2.5 ng/mL for men in their 40s to ≥6.5 ng/mL for men in their 70s. Among men with prostate cancer, PSA level <10 ng/mL is a characteristic of low-risk cancer; ≥10 ng/mL, but <20 ng/mL is a characteristic of intermediate-risk cancer; and ≥20 ng/mL signifies high-risk cancer.24
Tumor staging is based on whether the tumor is felt on a digital rectal examination or seen on imaging. T1 tumors are not felt or seen; T2 tumors are felt on examination and/or seen on imaging.
A positive biopsy core means prostate adenocarcinoma was detected. The tumor is graded according to the highest positive biopsy sampled. Grade group 1 is Gleason score 3 + 3 = 6, group 2 is 3 + 4 = 7, group 3 is 4 + 3 = 7, group 4 is any Gleason score of 8, and group 5 is any Gleason score of 9 or 10.
EPIC measures patient-reported disease-specific function. Scores range from 0-100; higher scores indicate better function.
The Medical Outcomes Short-Form Health Survey 36 (SF-36) has 8 domains. The physical function domain score is a weighted sum of 10 items; emotional well-being, 5 items; energy or fatigue score, 4 items. Each domain score is directly transformed to a scale of 0 to 100, with increasing scores indicating better function or less disability.
The social support scale is a modified domain score created using 5 questions from the Medical Outcomes Study Social Support Scale (score range, 0-100); higher scores indicate greater support.
Epidemiologic Studies Depression Scale was used to derive the depression score (scores were scaled to 100), with higher scores indicating more severe depressive symptoms.
Seven items were scored to determine participatory decision-making (score range, 0 to 100), with higher scores indicating increased patient control, responsibility, and choice.
By 10 years, 110 of 379 men (29%) who initially chose AS transitioned to definitive treatments (51 [46%] with radical prostatectomy, 46 with [42%] EBRT, 8 with [7%] primary ADT, 2 with [2%] ablation, and 3 [3%] with other treatments).
Sexual Function
At 10-year follow-up, there were no significant differences in sexual function domain scores between radical prostatectomy and AS (adjusted mean difference, 7.2 [95% CI, 2.0 to 12.3]; P = .007), EBRT (6.7 [95% CI, 1.2 to 12.1]; P = .02), or brachytherapy (2.2 [95% CI, −7.3 to 11.6]; P = .65). For context, at 3-year follow-up, radical prostatectomy was associated with significantly worse sexual function compared with AS (−17.8 [95% CI, −20.9 to −14.7]), EBRT (−13.9 [95% CI, −17.3 to −10.4]), and brachytherapy (−14.8 [95% CI, −20.1 to −9.5]). At 5-year follow-up, radical prostatectomy was associated with a significant decline in sexual function compared with AS (−10.3 [95% CI, −13.8 to −6.7]; P < .001), but not compared with EBRT (−8.8 [95% CI, −12.5 to −5.0]; P < .001) or brachytherapy (−9.6 [95% CI, −15.6 to −3.6]; P < .002) (Figure 3; eFigure 5 and eTables 5 and 6 in Supplement 1).
Figure 3. Sexual Function, Urinary Incontinence and Irritation, Bowel Function, and Hormone Function in Favorable-Prognosis PC Through 10 Years.
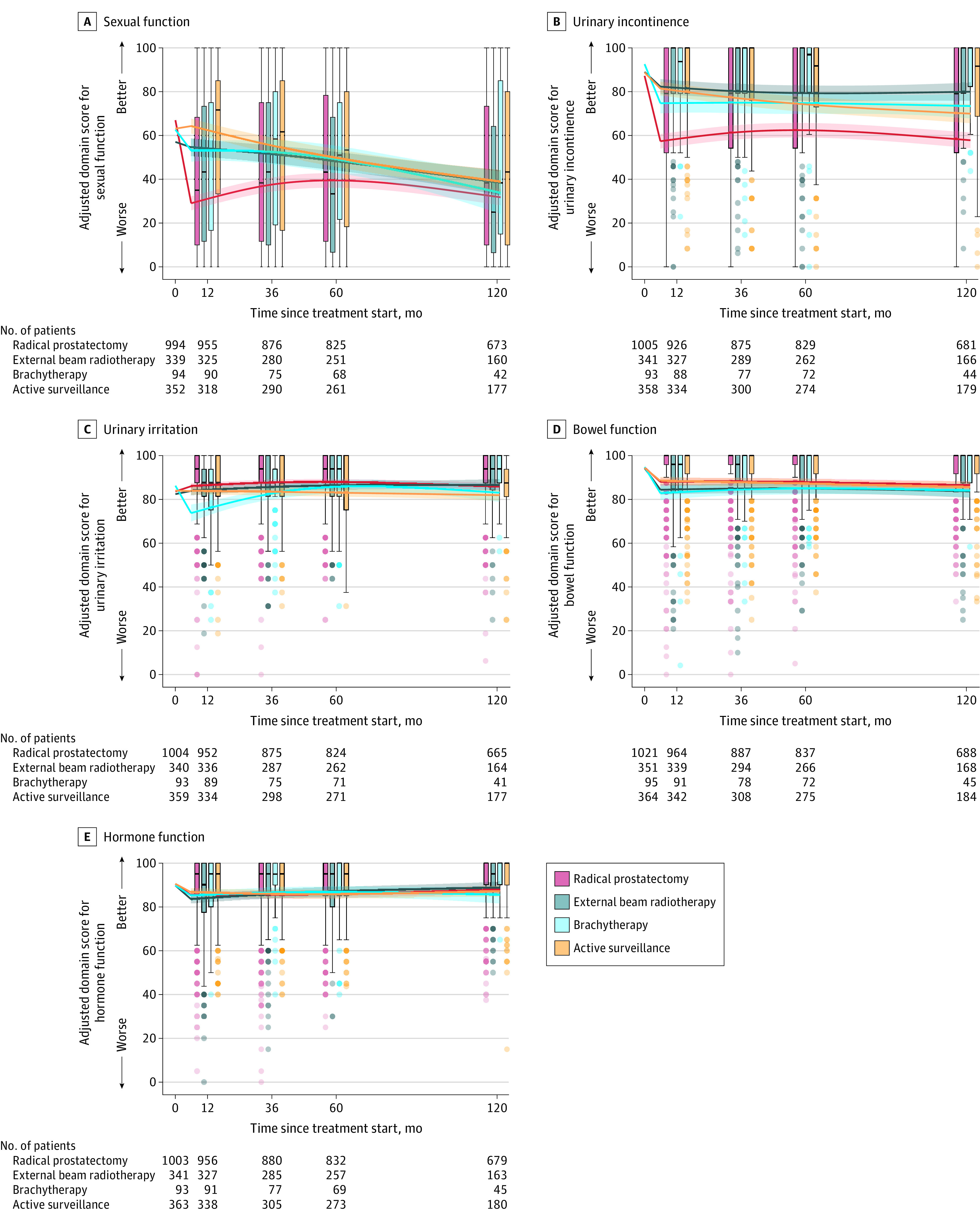
Box plots show unadjusted disease-specific function to 10 years. Crossbars are medians; boxes, IQRs; whiskers extend to the furthest points within 1.5 × IQR; dots, more extreme values with intensity signifying relative number of participants. Curves show adjusted mean EPIC-26 scores starting at unadjusted mean baseline for time zero. Regression models adjusted for baseline domain score, age, race and ethnicity, comorbidities, cancer characteristics, physical function, social support, depression, medical decision-making, and accrual site. Estimated domain scores used mean for continuous variables; mode for categorical variables. Scores and their interpretation are in the Methods. Hormone function assesses symptoms associated with hormone therapy adverse effects. eFigures 1 and 3 in Supplement 1 show more presentations.
Of 678 men (66%) with erections sufficient for intercourse at baseline, 41% (183/449) of the radical prostatectomy, 43% (34/79) of EBRT, 48% (13/27) of brachytherapy, and 46% (57/123) of AS groups had erections sufficient for intercourse at 10 years (eFigure 6 and eTable 7 in Supplement 1).
At 10 years, radical prostatectomy was associated with a higher likelihood of moderate/big problems with sexual function compared with AS (34% for radical prostatectomy vs 24% for AS; adjusted odds ratio [OR], 1.8 [95% CI 1.1-2.8]; P = .02) and with a higher likelihood of erections insufficient for intercourse compared with EBRT (69% for radical prostatectomy vs 74% for EBRT [unadjusted]; adjusted OR, 1.8 [95% CI, 1.1-3.1]; P = .03) (Figure 4; eTables 5 and 6 in Supplement 1).
Figure 4. Perceptions of Erectile Insufficiency, Urinary Leakage, Frequent Urination, and Bowel Function in Men With Favorable-Prognosis Prostate Cancer Through 10 Years.
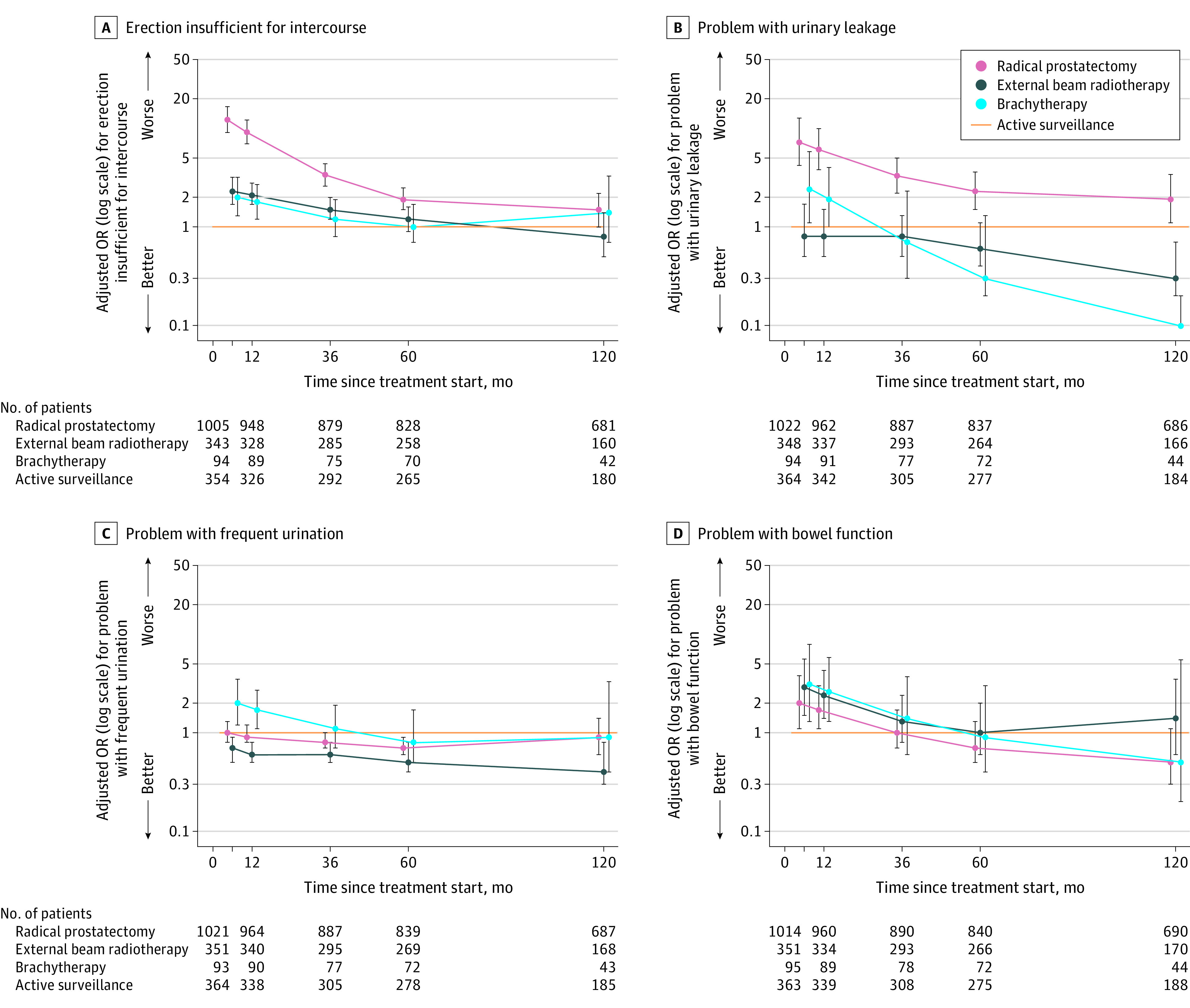
The adjusted odds ratios (ORs) of men reporting a moderate or big problem for the individual items are shown on a logarithmic scale relative to active surveillance through 10 years. The line at y = 1 shows active surveillance (reference). The whiskers indicate 95% CIs. The regression models were adjusted for baseline domain score, age, race and ethnicity, comorbidities, cancer characteristics (stage, grade group, and prostate-specific antigen level), physical function, social support, depression, medical decision-making style, and accrual site. The table at the bottom indicates the total number of men who reported whether the individual functional item was a moderate/big problem vs no/very small/small, or erections insufficient for intercourse. eFigure 10 shows the unadjusted probabilities and eFigure 12 shows additional individual functional items in Supplement 1.
Urinary Incontinence
At 10-year follow-up, radical prostatectomy was associated with significantly worse urinary incontinence scores compared with AS (adjusted mean difference, −12.1 [95% CI, −16.2 to −8.0]), EBRT (−22.0 [95% CI, −25.8 to −18.3]), and brachytherapy (−15.5 [95% CI, −20.8 to −10.2], all P < .001). EBRT was associated with significantly better scores at 10 years compared with AS (9.9 [95% CI, 5.3 to 14.5]; P < .001) (Figure 3; eTables 6 and 8 in Supplement 1).
At 10 years, compared with radical prostatectomy, rates of moderate/big problems with urinary leakage for AS were not significantly different (14% for radical prostatectomy vs 10% for AS; adjusted OR, 1.9 [95% CI, 1.0-3.5]; P = .06), but were significantly higher in the radical prostatectomy group compared with EBRT (14% vs 4%; adjusted OR, 7.2 [95% CI, 2.7-19.0]; P < .001) (Figure 4).
Urinary Irritation
At 10-year follow-up, there were no significant differences in urinary irritation domain scores between AS and radical prostatectomy (adjusted mean difference, 4.0 [95% CI, 1.6 to 6.4]; P < .001), EBRT (4.7 [95% CI, 1.6 to 7.8; P = .003), or brachytherapy (1.2 [95% CI, −3.2 to 5.6]; P = .58). For context, at 1-year follow-up, brachytherapy was associated with significantly worse urinary irritation scores compared with AS (−7.9 [95% CI −11.2 to −4.7]; P < .001) and EBRT (−8.4 [95% CI −11.8 to −5.1]; P < .001), and at 3-year follow-up, brachytherapy was associated with significantly worse urinary irritation compared with radical prostatectomy (−5.0 [95% CI, −7.5 to −2.4]; P < .001) (Figure 3; eTables 6 and 9 in Supplement 1).
At 10 years, EBRT was associated with a lower likelihood of moderate/big problems in urinary function compared with AS (5% for EBRT vs 12% for AS; adjusted OR, 0.3 [95% CI, 0.1-0.7]; P = .007) and radical prostatectomy (5% for EBRT vs 13% for radical prostatectomy; adjusted OR, 0.2 [95% CI, 0.1-0.5]; P < .001) and with a lower likelihood of frequent urination compared with AS (9% for EBRT vs 17% for AS; adjusted OR, 0.4 [95% CI, 0.2-0.9]; P = .03) and radical prostatectomy (9% for EBRT vs 14% for radical prostatectomy; adjusted OR, 0.5 [95% CI, 0.2-1.0]; P = .04) (Figure 4; eTables 6 and 9 in Supplement 1).
Bowel Function
At 10-year follow-up, there were no significant differences in bowel function scores between AS and radical prostatectomy (adjusted mean difference, 0.8 [95% CI, −1.3 to 2.8]; P = .46), EBRT (−2.0 [95% CI, −5.1 to 1.2]; P = .22), or brachytherapy (−1.6 [95% CI, −4.8 to 1.6]; P = .33). For context, at 1-year follow-up, brachytherapy was associated with a significant decline in bowel function compared with AS (−5.1 [95% CI, −7.6 to −2.5]; P < .001) and radical prostatectomy (−4.8 [95% CI, −7.3 to −2.3]; P < .001), but not compared with EBRT (−1.3 [95% CI, −4.1 to 1.4]; P = .83) (Figure 3; eTables 6 and 10 in Supplement 1).
At 10 years, EBRT was associated with a higher likelihood of moderate/big problems with bowel function compared with radical prostatectomy (8% for EBRT vs 3% for radical prostatectomy; adjusted OR, 2.5 [95% CI, 1.1-5.0]; P = .03). At 10-year follow-up, compared with other treatment groups, more men in the EBRT group reported bloody stools (2% for EBRT vs 0% for others; P < .05) and fecal incontinence (5% for EBRT vs 0%-3% for others; P < .05), but the number of events was too small to permit multivariable modeling (Figure 4; eTable 6 in Supplement 1).
Hormone Function
At 10-year follow-up, there were no significant differences in hormone function between AS and radical prostatectomy (adjusted mean difference, 0.7 [95% CI, −1.4 to 2.8]; P = .52), EBRT (1.6 [95% CI, −1.3 to 4.5]; P = .27), or brachytherapy (−1.5 [95% CI, −5.5 to 2.4]; P = .45) (Figure 3; eFigure 7 and eTable 11 in Supplement 1).
General Health-Related Quality of Life
At 10-year follow-up, there were no significant differences in physical health domain scores between AS and radical prostatectomy (adjusted mean difference, 0.5 [95% CI, −0.9 to 1.9]; P = .51), EBRT (−0.7 [95% CI, −2.6 to 1.2]; P = .49), or brachytherapy (0.1 [95% CI, −2.5 to 2.7]; P = .94), or in mental health domain scores between AS and radical prostatectomy (−1.0 [95% CI, −2.5 to 0.6]; P = .22), EBRT (0.5 [95% CI, −1.5 to 2.6]; P = .61), or brachytherapy (0.0 [95% CI, −3.0 to 2.9]; P = .99) (eTables 12 and 13 in Supplement 1).
Sensitivity Analyses
At 10-year follow-up, radical prostatectomy was associated with significantly worse sexual function compared with untreated AS (adjusted mean difference, −12.3 [95% CI, −18.8 to −5.8]; P < .001). Radical prostatectomy and EBRT were associated with significantly better urinary irritation function compared with untreated AS (5.7 [95% CI, 2.6 to 8.8]; P < .001 and 6.5 [95% CI, 2.9 to 10.2]; P < .001, respectively) (eTables 14 and 15 in Supplement 1). The unadjusted domain scores for men with favorable-prognosis PC who received EBRT with and without ADT are shown in eFigure 8 in Supplement 1.
Unfavorable-Prognosis PC
Among 568 men with unfavorable-prognosis PC, 362 (64%) underwent radical prostatectomy (212 [73%] robot-assisted) and 206 (36%) had EBRT (177 [89%] intensity-modulated radiotherapy and 170 [89%] image-guided) (Table; eTable 4 in Supplement 1).
Sexual Function
At 10-year follow-up, there were no significant differences between EBRT with ADT and radical prostatectomy (adjusted mean difference, −1.4 [95% CI, −11.1 to 8.3]; P = .78). For context, there were no significant differences between EBRT with ADT and radical prostatectomy at 1-year follow-up (−5.3 [95% CI, −10.3 to −0.4]; P = .03), at 3-year follow-up (−6.1 [95% CI, −11.7 to −0.4]; P = .04), or at 5-year follow-up (−5.6 [95% CI, −11.8 to 0.6]; P = .07) (Figure 5; eFigure 9 and eTable 16 in Supplement 1).
Figure 5. Sexual Function, Urinary Incontinence, Urinary Irritation, Bowel Function, and Hormone Function in Men With Unfavorable-Prognosis Prostate Cancer Through 10 Years.
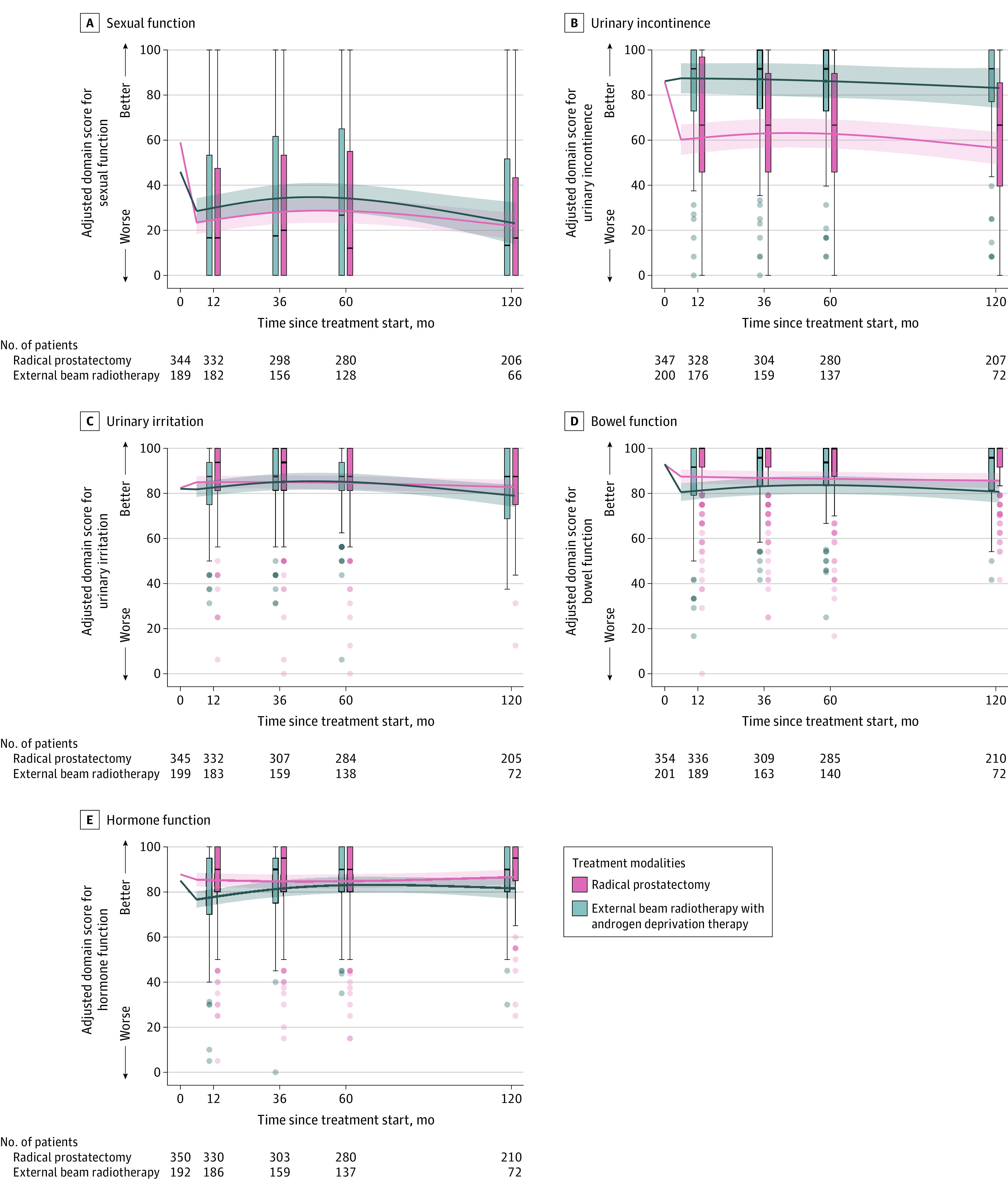
Box plots demonstrate the distribution of unadjusted disease-specific function through 10 years. Crossbars represent medians; boxes, IQRs; whiskers extend to the furthest points within 1.5 × IQR; and more extreme values are shown as dots with intensity signifying the relative number of participants with that value. Curves demonstrate the adjusted-mean Expanded Prostate Cancer Index Composite functional domain scores starting at the unadjusted mean baseline score for time zero. Regression models were adjusted for baseline domain score, age, race and ethnicity, comorbidities, cancer characteristics, physical function, social support, depression, medical decision-making style, and accrual site. Estimated domain scores were calculated using mean for continuous variable and mode for categorical variables. Scores and their interpretation are in the Methods. Shading represents 95% CIs. Hormone function assesses symptoms associated with hormone therapy adverse effects. See eFigure 2 for presentation as radar plots and eFigure 4 for differences between treatments in Supplement 1.
Of 147 men (55%) with erections sufficient for intercourse at baseline, 24% (9/38) and 22% (24/109) in the EBRT and radical prostatectomy groups, respectively, had erections sufficient for intercourse at 10 years (eFigure 6 and eTable 7 in Supplement 1).
At 10 years, there were no significant differences between EBRT with ADT and radical prostatectomy in reporting moderate/big problems with sexual function (32% vs 32%; adjusted OR, 1.0 [95% CI, 0.5-2.3]; P = .91) and erections insufficient for intercourse (81% for EBRT with ADT vs 86% for radical prostatectomy; adjusted OR, 0.6 [95% CI, 0.2-1.5]; P = .25) (Figure 6; eTable 16 in Supplement 1).
Figure 6. Perceptions of Erectile Insufficiency, Urinary Leakage, Frequent Urination, and Bowel Function in Men With Unfavorable-Prognosis Prostate Cancer Through 10 Years.
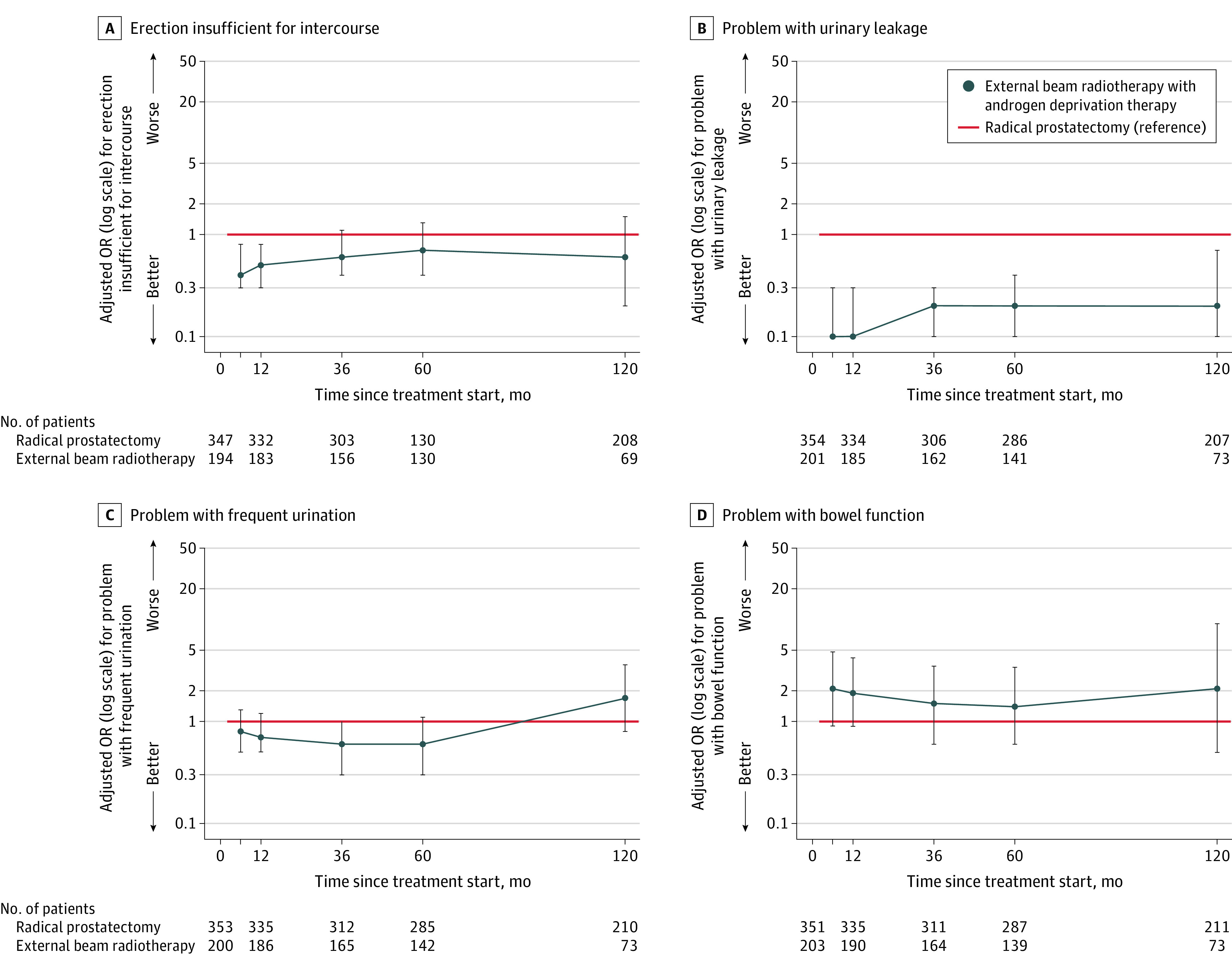
The adjusted odds ratios (ORs) of men reporting a moderate or big problem for the individual items are shown on a logarithmic scale relative to radical prostatectomy through 10 years. The line at y = 1 shows radical prostatectomy (reference). The whiskers indicate 95% CIs. The regression models were adjusted for baseline domain score, age, race and ethnicity, comorbidities, cancer characteristics (stage, grade group, and prostate-specific antigen level), physical function, social support, depression, medical decision-making style, and accrual site. The table at the bottom indicates the total number of men who reported whether the individual functional item was a moderate/big problem vs no/very small/small, or erections insufficient for intercourse. eFigure 11 shows unadjusted probabilities and eFigure 13 shows additional individual functional items in Supplement 1.
Urinary Incontinence
At 10-year follow-up, radical prostatectomy was associated with significantly worse urinary incontinence function compared with EBRT with ADT (adjusted mean difference, −26.6 [95% CI, −35.0 to −18.2]; P < .001) (Figure 5; eTable 17 in Supplement 1). At 10 years, rates of moderate/big problems with urinary leakage were significantly lower in the EBRT with ADT group compared with the radical prostatectomy group (11% for EBRT with ADT vs 25% for radical prostatectomy; adjusted OR, 0.2 [95% CI, 0.1-0.7]; P = .01; Figure 6).
Urinary Irritation
At 10-year follow-up, there were no significant differences in urinary irritation function between EBRT with ADT and radical prostatectomy (adjusted mean difference, 3.7 [95% CI, −1.0 to 8.5]; P = .13) (Figure 5; eTable 18 in Supplement 1). At 10 years, EBRT with ADT was associated with a higher likelihood of reporting a moderate/big problem with burning with urination compared with radical prostatectomy (5% for EBRT with ADT vs 1% for radical prostatectomy; adjusted OR, 9.1 [95% CI, 1.2-71.9]; P = .04) (Figure 6; eTable 18 in Supplement 1).
Bowel Function
At 10-year follow-up, EBRT with ADT was associated with significantly worse bowel function compared with radical prostatectomy (adjusted mean difference, −4.9 [95% CI, −9.2 to −0.7]; P = .02). For context, EBRT with ADT was associated with significantly worse bowel function compared with radical prostatectomy at 6-month follow-up (−6.9 [95% CI, −9.6 to −4.2]; P < .001) and at 1-year follow-up (−6.1 [95% CI, −8.6 to −3.7]; P < .001) (Figure 5; eTable 19 in Supplement 1).
At 10 years, there were no significant differences between EBRT with ADT and radical prostatectomy in the proportions of men reporting moderate/big problems with bowel function (7% for EBRT with ADT vs 3% for radical prostatectomy), bowel urgency (11% for EBRT with ADT vs 5% for radical prostatectomy), increased frequency of bowel movements (7% for EBRT with ADT vs 2% for radical prostatectomy), fecal incontinence (3% for EBRT with ADT vs 1% for radical prostatectomy), or bloody stools (0% for EBRT with ADT vs 0% for radical prostatectomy; all P > .05) and few patients reported these symptoms overall (Figure 6; eTable 19 in Supplement 1).
Hormone Function
At 10-year follow-up, EBRT with ADT was associated with significantly worse hormone function compared with radical prostatectomy (adjusted mean difference, −4.9 [95% CI, −9.5 to −0.3]; P = .04). For context, EBRT with ADT was associated with significantly worse hormone function compared with radical prostatectomy at 6-month follow-up (−8.8 [95% CI, −11.7 to −5.9]; P < .001) and at 1-year follow-up (−7.5 [95% CI, −10.2 to −4.9]; P < .001) (Figure 5; eFigure 7 and eTable 20 in Supplement 1). At 10 years, a higher proportion of men in the EBRT group reported a moderate/big problem with lack of energy compared with radical prostatectomy (18% for EBRT with ADT vs 9% for radical prostatectomy; P = .04) (eTable 20 in Supplement 1).
General Health-Related Quality of Life
At 10-year follow-up, there were no significant differences in physical health domain scores between EBRT with ADT and radical prostatectomy (adjusted mean difference, 0.0 [95% CI, −3.4 to 3.3]; P = .99) or in mental health domain scores (−1.2 [95% CI, −4.6 to 2.3]; P = .51) (eTables 12 and 13 in Supplement 1).
Discussion
In this observational study of patients with localized PC followed up for approximately 10 years, functional outcomes varied by treatment and prognosis at the time of diagnosis. There were no significant differences in sexual function among treatment groups at 10-year follow-up, irrespective of prognostic risk. However, patients with favorable-prognosis PC who underwent radical prostatectomy had significantly worse sexual function than EBRT, brachytherapy, and AS during the first 3 to 5 years, thus experiencing more time with sexual impairment than men undergoing other treatments. In contrast, for men with unfavorable-prognosis PC, no significant differences in sexual function throughout 10-year follow-up were observed between radical prostatectomy and EBRT with ADT. At 10-year follow-up, there were no significant differences in bowel function domain scores among treatments for favorable-prognosis PC, whereas for patients with unfavorable-prognosis PC, EBRT with ADT was associated with significantly worse bowel function domain scores compared with radical prostatectomy. Worse urinary incontinence persisted through 10 years after radical prostatectomy for both favorable and unfavorable prognostic risk compared with other treatment groups. Among men with unfavorable-prognosis PC, those who underwent EBRT with ADT had lower 10-year hormone function scores compared with radical prostatectomy.
The ProtecT randomized trial compared open radical prostatectomy, EBRT with ADT, and active monitoring for localized PC.9 Despite reporting similar patterns of functional outcomes compared with findings in this study, some adverse outcome rates were higher in ProtecT. Specifically, 18% of patients with low-risk PC in ProtecT after both radical prostatectomy and radiation therapy had erections sufficient for intercourse at 10 years compared with 31% and 26% with favorable-prognosis PC in findings in this study after radical prostatectomy and EBRT, respectively. ProtecT reported fecal leakage at least once per week in 12% of EBRT patients, whereas 4.5% of patients reported a moderate/big problem with losing control of bowels in this study. It is possible that use of contemporary techniques, including robotic surgery and intensity-modulated radiotherapy, explains the lower rates of adverse functional outcomes in this study.
Among men with unfavorable-prognosis PC, which requires more intensive treatment than favorable-prognosis PC, there were no clinically meaningful differences in sexual function between EBRT with ADT and radical prostatectomy over the follow-up period. In contrast, urinary incontinence was significantly worse after radical prostatectomy than after EBRT with ADT. Bowel function at 10 years was worse after EBRT and ADT than after radical prostatectomy, although there were no statistically significant differences in reporting of bowel problems, bloody stools, or urgency. The Prostate Cancer Outcomes Study, evaluating older treatment techniques, also suggested development of bowel function problems after radiotherapy compared with radical prostatectomy at 15 years.6,7 Moreover, while hormone function scores among men treated with EBRT and ADT improved initially after completing ADT, they had lower hormone function scores compared with men treated with radical prostatectomy at 10-year follow-up, which may be attributable to residual confounding, despite controlling for age and comorbidities.
Limitations
The study has several limitations. First, the study was observational, and results may have been influenced by confounding, including confounding by indication. Second, MCIDs are subjective and may be influenced by patients’ expectations and preferences. Third, despite the 66% response rate through 10 years of follow-up, the results may have been affected by response bias and missing data. Fourth, some comparisons lacked statistical power, and due to the large number of primary outcomes, some may have been statistically significant by chance. Additionally, in some subgroups, such as those with unfavorable prognosis with erections sufficient for intercourse at baseline who received EBRT or radical prostatectomy treatment, sample sizes were small and may have lacked statistical power to detect important differences. Fifth, guideline-recommended PC diagnostic paradigms and treatments have changed since study inception, and methods of treatment administration have evolved.24,25,26,27,28,29 Sixth, the results may not be generalizable to men with locally advanced disease (cT3+) and men older than 80 years, who were excluded from the study.5,30
Conclusions
Among patients treated for localized PC, radical prostatectomy was associated with worse urinary incontinence but not worse sexual function at 10-year follow-up compared with radiotherapy or AS among people with more favorable prognosis and compared with radiotherapy for those with unfavorable prognosis. Among men with unfavorable-prognosis disease, EBRT with ADT was associated with worse bowel and hormone function at 10-year follow-up compared with radical prostatectomy.
eMethods
eFigure 1. Radar Plots of Adjusted Expanded Prostate Cancer Index Composite Functional Domain Scores for Men With Favorable-Prognosis Prostate Cancer
eFigure 2. Radar Plots of Adjusted Expanded Prostate Cancer Index Composite Functional Domain Scores for Men With Unfavorable-Prognosis Prostate Cancer
eFigure 3. Adjusted-Mean Differences in Functional Outcomes of Men With Favorable-Prognosis Prostate Cancer Through 10 Years
eFigure 4. Adjusted-Mean Differences in Functional Outcomes of Men With Unfavorable-Prognosis Prostate Cancer Through 10 Years
eFigure 5. Unadjusted Mean Functional Outcomes of Men With Favorable- Prognosis Prostate Cancer on the Expanded Prostate Cancer Index Composite (EPIC) Domain Scores
eFigure 6. Unadjusted Sexual Function Outcomes of Men With Favorable and Unfavorable-Prognosis Prostate Cancer Through 10 Years Stratified by Baseline Function
eFigure 7. Unadjusted Hormonal Function Outcomes of Men With Favorable-Prognosis Prostate Cancer Through 10 Years Stratified by Baseline Function
eFigure 8. Unadjusted Functional Outcomes of Men With Favorable-Prognosis Prostate Cancer Treated With External Beam Radiotherapy Through 10 Years Stratified by Receipt of Androgen Deprivation Therapy
eFigure 9. Unadjusted Functional Outcomes of Men With Unfavorable-Prognosis Prostate Cancer Through 10 Years
eFigure 10. Unadjusted Probabilites of Select Individual Items of Men With Favorable-Prognosis Prostate Cancer Through 10 Years
eFigure 11. Unadjusted Probabilites of Select Individual Items of Men With Unfavorable-Prognosis Prostate Cancer Through 10 Years
eFigure 12. Additional Selected Individual Functional Items in Men With Favorable-Prognosis Prostate Cancer Through 10 Years
eFigure 13. Additional Selected Individual Functional Items in Men With Unfavorable-Prognosis Prostate Cancer Through 10 Years
eTable 1. Overall and Prostate Cancer-Specific Survival by Treatment
eTable 2. Number of Missing Data in Study Covariates
eTable 3. Baseline Characteristics of Men in the CEASAR Study by Response to the 10-Year Survey
eTable 4. Treatment Techniques Used in Men With Favorable and Unfavorable Prognosis Prostate Cancer
eTable 5. Unadjusted and Adjusted Sexual Function Outcomes of Men With Favorable-Prognosis Prostate Cancer on the Expanded Prostate Cancer Index Composite (EPIC) Domain Scores and Selected Individual Item Responses by Treatment and Time Point
eTable 6. Pair-wise Comparisons of Adjusted Functional Outcomes of Men With Favorable-Prognosis Prostate Cancer by Treatment
eTable 7. Summary of Sexual Function at 10 Years According to Baseline Function
eTable 8. Unadjusted and Adjusted Urinary Incontinence Function Outcomes of Men With Favorable-Prognosis Prostate Cancer on the Expanded Prostate Cancer Index Composite (EPIC) Domain Scores and Selected Individual Item Responses by Treatment and Time Point
eTable 9. Unadjusted and Adjusted Urinary Irritation Function Outcomes of Men With Favorable-Prognosis Prostate Cancer on the Expanded Prostate Cancer Index Composite (EPIC) Domain Scores and Selected Individual Item Responses by Treatment and Time Point
eTable 10. Unadjusted and Adjusted Bowel Function Outcomes of Men With Favorable-Prognosis Prostate Cancer on the Expanded Prostate Cancer Index Composite (EPIC) Domain Scores and Selected Individual Item Responses by Treatment and Time Point
eTable 11. Unadjusted and Adjusted Hormonal Function Outcomes of Men With Favorable-Prognosis Prostate Cancer on the Expanded Prostate Cancer Index Composite (EPIC) Domain Scores and Selected Individual Item Responses by Treatment and Time Point
eTable 12. Unadjusted General Health-Related Quality of Life Outcomes for Men With Favorable and Unfavorable-Prognosis Prostate Cancer
eTable 13. Adjusted General Health-Related Quality of Life Outcomes for Men With Favorable and Unfavorable Prognosis Prostate Cancer at Year 10
eTable 14. Baseline Characteristics of Men With Favorable-Prognosis Prostate Cancer Enrolled in the CEASAR Study by Treatment Received Including Men Who Were Untreated on Active Surveillance According
eTable 15. Unadjusted and Adjusted Functional Outcomes of Favorable-Prognosis Patients Compared to Untreated Men on Active Surveillance on the Expanded Prostate Cancer Index Composite (EPIC) Domain Scores and Selected Individual Item Responses by Treatment and Time Point
eTable 16. Unadjusted and Adjusted Sexual Function Outcomes of Men With Unfavorable-Prognosis Prostate
eTable 17. Unadjusted and Adjusted Urinary Incontinence Function Outcomes of Men With Unfavorable-Prognosis Prostate Cancer on the Expanded Prostate Cancer Index Composite (EPIC) Domain Scores and Selected Individual Item Responses by Treatment and Time Point
eTable 18. Unadjusted and Adjusted Urinary Irritation Function Outcomes of Men With Unfavorable-Prognosis Prostate Cancer on the Expanded Prostate Cancer Index Composite (EPIC) Domain Scores and Selected Individual Item Responses by Treatment and Time Point
eTable 19. Unadjusted and Adjusted Bowel Function Outcomes of Men With Unfavorable-Prognosis Prostate Cancer on the Expanded Prostate Cancer Index Composite (EPIC) Domain Scores and Selected Individual Item Responses by Treatment and Time Point
eTable 20. Unadjusted and Adjusted Hormonal Function Outcomes of Men With Unfavorable-Prognosis Prostate Cancer on the Expanded Prostate Cancer Index Composite (EPIC) Domain Scores and Selected Individual Item Responses by Treatment and Time Point
eReferences
Data Sharing Statement
References
- 1.Schaeffer EM, Srinivas S, Adra N, et al. NCCN Guidelines Insights: Prostate Cancer, Version 1.2023. J Natl Compr Canc Netw. 2022;20(12):1288-1298. doi: 10.6004/jnccn.2022.0063 [DOI] [PubMed] [Google Scholar]
- 2.Hamdy FC, Donovan JL, Lane JA, et al. ; ProtecT Study Group . Fifteen-year outcomes after monitoring, surgery, or radiotherapy for prostate cancer. N Engl J Med. 2023;388(17):1547-1558. doi: 10.1056/NEJMoa2214122 [DOI] [PubMed] [Google Scholar]
- 3.Barocas DA, Alvarez J, Resnick MJ, et al. Association between radiation therapy, surgery, or observation for localized prostate cancer and patient-reported outcomes after 3 years. JAMA. 2017;317(11):1126-1140. doi: 10.1001/jama.2017.1704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hoffman KE, Penson DF, Zhao Z, et al. Patient-reported outcomes through 5 years for active surveillance, surgery, brachytherapy, or external beam radiation with or without androgen deprivation therapy for localized prostate cancer. JAMA. 2020;323(2):149-163. doi: 10.1001/jama.2019.20675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen RC, Basak R, Meyer AM, et al. Association between choice of radical prostatectomy, external beam radiotherapy, brachytherapy, or active surveillance and patient-reported quality of life among men with localized prostate cancer. JAMA. 2017;317(11):1141-1150. doi: 10.1001/jama.2017.1652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Resnick MJ, Koyama T, Fan KH, et al. Long-term functional outcomes after treatment for localized prostate cancer. N Engl J Med. 2013;368(5):436-445. doi: 10.1056/NEJMoa1209978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mazariego CG, Egger S, King MT, et al. Fifteen year quality of life outcomes in men with localised prostate cancer: population based Australian prospective study. BMJ. 2020;371:m3503. doi: 10.1136/bmj.m3503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sanda MG, Dunn RL, Michalski J, et al. Quality of life and satisfaction with outcome among prostate-cancer survivors. N Engl J Med. 2008;358(12):1250-1261. doi: 10.1056/NEJMoa074311 [DOI] [PubMed] [Google Scholar]
- 9.Donovan J, Hamdy F, Lane J, et al. Patient-reported outcomes 12 years after localized prostate cancer treatment. NEJM Evid. 2023;2(4). doi: 10.1056/EVIDoa2300018 [DOI] [PubMed] [Google Scholar]
- 10.Barocas DA, Chen V, Cooperberg M, et al. Using a population-based observational cohort study to address difficult comparative effectiveness research questions: the CEASAR study. J Comp Eff Res. 2013;2(4):445-460. doi: 10.2217/cer.13.34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wallis CJD, Morton G, Herschorn S, et al. The effect of selection and referral biases for the treatment of localised prostate cancer with surgery or radiation. Br J Cancer. 2018;118(10):1399-1405. doi: 10.1038/s41416-018-0071-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eastham JA, Auffenberg GB, Barocas DA, et al. Clinically localized prostate cancer: AUA/ASTRO guideline, part I: introduction, risk assessment, staging, and risk-based management. J Urol. 2022;208(1):10-18. doi: 10.1097/JU.0000000000002757 [DOI] [PubMed] [Google Scholar]
- 13.Szymanski KM, Wei JT, Dunn RL, Sanda MG. Development and validation of an abbreviated version of the expanded prostate cancer index composite instrument for measuring health-related quality of life among prostate cancer survivors. Urology. 2010;76(5):1245-1250. doi: 10.1016/j.urology.2010.01.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jayadevappa R, Malkowicz SB, Wittink M, Wein AJ, Chhatre S. Comparison of distribution- and anchor-based approaches to infer changes in health-related quality of life of prostate cancer survivors. Health Serv Res. 2012;47(5):1902-1925. doi: 10.1111/j.1475-6773.2012.01395.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Skolarus TA, Dunn RL, Sanda MG, et al. ; PROSTQA Consortium . Minimally important difference for the Expanded Prostate Cancer Index Composite Short Form. Urology. 2015;85(1):101-105. doi: 10.1016/j.urology.2014.08.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ware J Jr, Kosinski M, Keller SDA. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34(3):220-233. doi: 10.1097/00005650-199603000-00003 [DOI] [PubMed] [Google Scholar]
- 17.Litwin MS, Greenfield S, Elkin EP, Lubeck DP, Broering JM, Kaplan SH. Assessment of prognosis with the total illness burden index for prostate cancer: aiding clinicians in treatment choice. Cancer. 2007;109(9):1777-1783. doi: 10.1002/cncr.22615 [DOI] [PubMed] [Google Scholar]
- 18.Sherbourne CD, Stewart AL. The MOS social support survey. Soc Sci Med. 1991;32(6):705-714. doi: 10.1016/0277-9536(91)90150-B [DOI] [PubMed] [Google Scholar]
- 19.Andresen EM, Malmgren JA, Carter WB, Patrick DL. Screening for depression in well older adults: evaluation of a short form of the CES-D (Center for Epidemiologic Studies Depression Scale). Am J Prev Med. 1994;10(2):77-84. doi: 10.1016/S0749-3797(18)30622-6 [DOI] [PubMed] [Google Scholar]
- 20.Kaplan SH, Greenfield S, Gandek B, Rogers WH, Ware JE Jr. Characteristics of physicians with participatory decision-making styles. Ann Intern Med. 1996;124(5):497-504. doi: 10.7326/0003-4819-124-5-199603010-00007 [DOI] [PubMed] [Google Scholar]
- 21.McHorney CA, Ware JE Jr, Raczek AE. The MOS 36-Item Short-Form Health Survey (SF-36): II, psychometric and clinical tests of validity in measuring physical and mental health constructs. Med Care. 1993;31(3):247-263. doi: 10.1097/00005650-199303000-00006 [DOI] [PubMed] [Google Scholar]
- 22.Schafer JL. Analysis of Incomplete Multivariate Data. Chapman and Hall/CRC; 1997. doi: 10.1201/9781439821862 [DOI] [Google Scholar]
- 23.White IR, Royston P, Wood AM. Multiple imputation using chained equations: issues and guidance for practice. Stat Med. 2011;30(4):377-399. doi: 10.1002/sim.4067 [DOI] [PubMed] [Google Scholar]
- 24.Wei JT, Barocas D, Carlsson S, et al. Early detection of prostate cancer: AUA/SUO guideline part I: prostate cancer screening. J Urol. 2023;210(1):46-53. doi: 10.1097/JU.0000000000003491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Borregales LD, DeMeo G, Gu X, et al. Grade migration of prostate cancer in the United States during the last decade. J Natl Cancer Inst. 2022;114(7):1012-1019. doi: 10.1093/jnci/djac066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kasivisvanathan V, Rannikko AS, Borghi M, et al. ; PRECISION Study Group Collaborators . MRI-targeted or standard biopsy for prostate-cancer diagnosis. N Engl J Med. 2018;378(19):1767-1777. doi: 10.1056/NEJMoa1801993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Al Hussein Al Awamlh B, Barocas DA, Zhu A, et al. Use of active surveillance vs definitive treatment among men with low- and favorable intermediate-risk prostate cancer in the US between 2010 and 2018. JAMA Intern Med. 2023;183(6):608-611. doi: 10.1001/jamainternmed.2022.7100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dalela D, Jeong W, Prasad MA, et al. A pragmatic randomized controlled trial examining the impact of the retzius-sparing approach on early urinary continence recovery after robot-assisted radical prostatectomy. Eur Urol. 2017;72(5):677-685. doi: 10.1016/j.eururo.2017.04.029 [DOI] [PubMed] [Google Scholar]
- 29.Miller LE, Efstathiou JA, Bhattacharyya SK, Payne HA, Woodward E, Pinkawa M. Association of the placement of a perirectal hydrogel spacer with the clinical outcomes of men receiving radiotherapy for prostate cancer: a systematic review and meta-analysis. JAMA Netw Open. 2020;3(6):e208221. doi: 10.1001/jamanetworkopen.2020.8221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Donovan JL, Hamdy FC, Lane JA, et al. ; ProtecT Study Group . Patient-reported outcomes after monitoring, surgery, or radiotherapy for prostate cancer. N Engl J Med. 2016;375(15):1425-1437. doi: 10.1056/NEJMoa1606221 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods
eFigure 1. Radar Plots of Adjusted Expanded Prostate Cancer Index Composite Functional Domain Scores for Men With Favorable-Prognosis Prostate Cancer
eFigure 2. Radar Plots of Adjusted Expanded Prostate Cancer Index Composite Functional Domain Scores for Men With Unfavorable-Prognosis Prostate Cancer
eFigure 3. Adjusted-Mean Differences in Functional Outcomes of Men With Favorable-Prognosis Prostate Cancer Through 10 Years
eFigure 4. Adjusted-Mean Differences in Functional Outcomes of Men With Unfavorable-Prognosis Prostate Cancer Through 10 Years
eFigure 5. Unadjusted Mean Functional Outcomes of Men With Favorable- Prognosis Prostate Cancer on the Expanded Prostate Cancer Index Composite (EPIC) Domain Scores
eFigure 6. Unadjusted Sexual Function Outcomes of Men With Favorable and Unfavorable-Prognosis Prostate Cancer Through 10 Years Stratified by Baseline Function
eFigure 7. Unadjusted Hormonal Function Outcomes of Men With Favorable-Prognosis Prostate Cancer Through 10 Years Stratified by Baseline Function
eFigure 8. Unadjusted Functional Outcomes of Men With Favorable-Prognosis Prostate Cancer Treated With External Beam Radiotherapy Through 10 Years Stratified by Receipt of Androgen Deprivation Therapy
eFigure 9. Unadjusted Functional Outcomes of Men With Unfavorable-Prognosis Prostate Cancer Through 10 Years
eFigure 10. Unadjusted Probabilites of Select Individual Items of Men With Favorable-Prognosis Prostate Cancer Through 10 Years
eFigure 11. Unadjusted Probabilites of Select Individual Items of Men With Unfavorable-Prognosis Prostate Cancer Through 10 Years
eFigure 12. Additional Selected Individual Functional Items in Men With Favorable-Prognosis Prostate Cancer Through 10 Years
eFigure 13. Additional Selected Individual Functional Items in Men With Unfavorable-Prognosis Prostate Cancer Through 10 Years
eTable 1. Overall and Prostate Cancer-Specific Survival by Treatment
eTable 2. Number of Missing Data in Study Covariates
eTable 3. Baseline Characteristics of Men in the CEASAR Study by Response to the 10-Year Survey
eTable 4. Treatment Techniques Used in Men With Favorable and Unfavorable Prognosis Prostate Cancer
eTable 5. Unadjusted and Adjusted Sexual Function Outcomes of Men With Favorable-Prognosis Prostate Cancer on the Expanded Prostate Cancer Index Composite (EPIC) Domain Scores and Selected Individual Item Responses by Treatment and Time Point
eTable 6. Pair-wise Comparisons of Adjusted Functional Outcomes of Men With Favorable-Prognosis Prostate Cancer by Treatment
eTable 7. Summary of Sexual Function at 10 Years According to Baseline Function
eTable 8. Unadjusted and Adjusted Urinary Incontinence Function Outcomes of Men With Favorable-Prognosis Prostate Cancer on the Expanded Prostate Cancer Index Composite (EPIC) Domain Scores and Selected Individual Item Responses by Treatment and Time Point
eTable 9. Unadjusted and Adjusted Urinary Irritation Function Outcomes of Men With Favorable-Prognosis Prostate Cancer on the Expanded Prostate Cancer Index Composite (EPIC) Domain Scores and Selected Individual Item Responses by Treatment and Time Point
eTable 10. Unadjusted and Adjusted Bowel Function Outcomes of Men With Favorable-Prognosis Prostate Cancer on the Expanded Prostate Cancer Index Composite (EPIC) Domain Scores and Selected Individual Item Responses by Treatment and Time Point
eTable 11. Unadjusted and Adjusted Hormonal Function Outcomes of Men With Favorable-Prognosis Prostate Cancer on the Expanded Prostate Cancer Index Composite (EPIC) Domain Scores and Selected Individual Item Responses by Treatment and Time Point
eTable 12. Unadjusted General Health-Related Quality of Life Outcomes for Men With Favorable and Unfavorable-Prognosis Prostate Cancer
eTable 13. Adjusted General Health-Related Quality of Life Outcomes for Men With Favorable and Unfavorable Prognosis Prostate Cancer at Year 10
eTable 14. Baseline Characteristics of Men With Favorable-Prognosis Prostate Cancer Enrolled in the CEASAR Study by Treatment Received Including Men Who Were Untreated on Active Surveillance According
eTable 15. Unadjusted and Adjusted Functional Outcomes of Favorable-Prognosis Patients Compared to Untreated Men on Active Surveillance on the Expanded Prostate Cancer Index Composite (EPIC) Domain Scores and Selected Individual Item Responses by Treatment and Time Point
eTable 16. Unadjusted and Adjusted Sexual Function Outcomes of Men With Unfavorable-Prognosis Prostate
eTable 17. Unadjusted and Adjusted Urinary Incontinence Function Outcomes of Men With Unfavorable-Prognosis Prostate Cancer on the Expanded Prostate Cancer Index Composite (EPIC) Domain Scores and Selected Individual Item Responses by Treatment and Time Point
eTable 18. Unadjusted and Adjusted Urinary Irritation Function Outcomes of Men With Unfavorable-Prognosis Prostate Cancer on the Expanded Prostate Cancer Index Composite (EPIC) Domain Scores and Selected Individual Item Responses by Treatment and Time Point
eTable 19. Unadjusted and Adjusted Bowel Function Outcomes of Men With Unfavorable-Prognosis Prostate Cancer on the Expanded Prostate Cancer Index Composite (EPIC) Domain Scores and Selected Individual Item Responses by Treatment and Time Point
eTable 20. Unadjusted and Adjusted Hormonal Function Outcomes of Men With Unfavorable-Prognosis Prostate Cancer on the Expanded Prostate Cancer Index Composite (EPIC) Domain Scores and Selected Individual Item Responses by Treatment and Time Point
eReferences
Data Sharing Statement


