Abstract
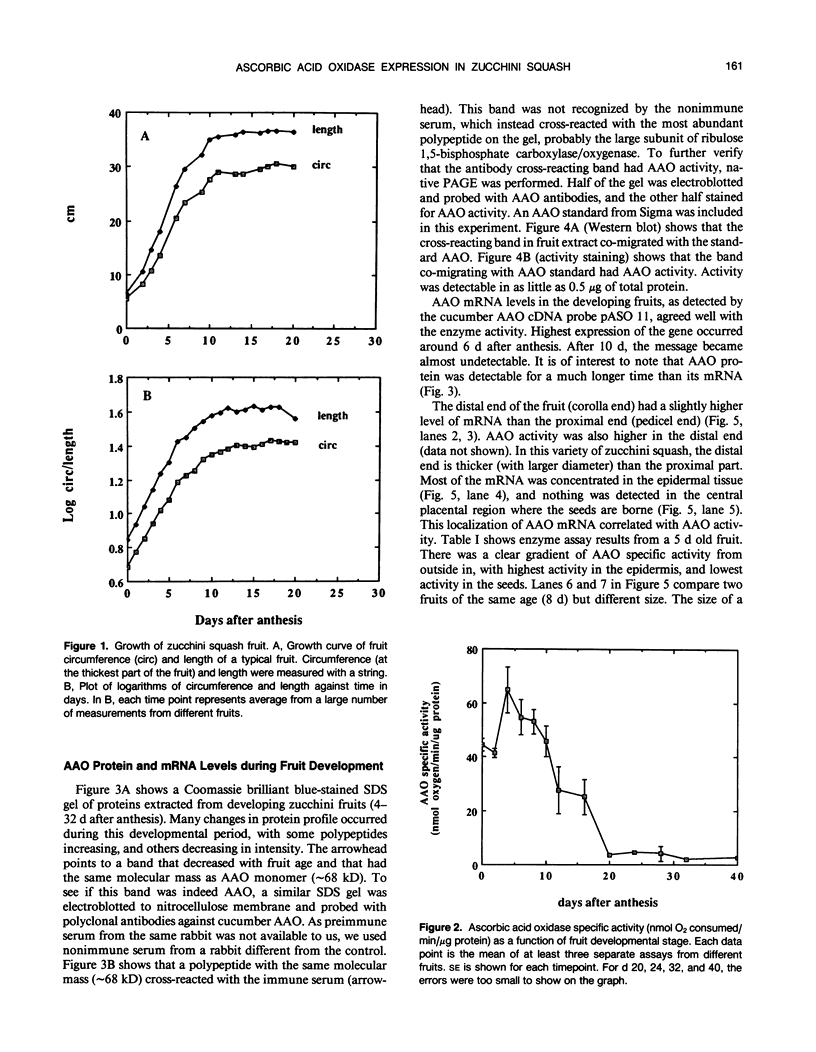
The expression of ascorbic acid oxidase was studied in zucchini squash (Cucurbita pepo L.), one of the most abundant natural sources of the enzyme. In the developing fruit, specific activity of ascorbic acid oxidase was highest between 4 and 6 days after anthesis. Protein and mRNA levels followed the same trend as enzyme activity. Highest growth rate of the fruit occurred before 6 days after anthesis. Within a given fruit, ascorbic acid oxidase activity and mRNA level were highest in the epidermis, and lowest in the central placental region. In leaf tissue, ascorbic acid oxidase activity was higher in young leaves, and very low in old leaves. Within a given leaf, enzyme activity was highest in the fast-growing region (approximately the lower third of the blade), and lowest in the slow-growing region (near leaf apex). High expression of ascorbic acid oxidase at a stage when rapid growth is occurring (in both fruits and leaves), and localization of the enzyme in the fruit epidermis, where cells are under greatest tension during rapid growth in girth, suggest that ascorbic acid oxidase might be involved in reorganization of the cell wall to allow for expansion. Based on the known chemistry of dehydroascorbic acid, the end product of the ascorbic acid oxidase-catalyzed reaction, we have proposed several hypotheses to explain how dehydroascorbic acid might cause cell wall “loosening.”
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Belanger F. C., Brodl M. R., Ho T. H. Heat shock causes destabilization of specific mRNAs and destruction of endoplasmic reticulum in barley aleurone cells. Proc Natl Acad Sci U S A. 1986 Mar;83(5):1354–1358. doi: 10.1073/pnas.83.5.1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake M. S., Johnston K. H., Russell-Jones G. J., Gotschlich E. C. A rapid, sensitive method for detection of alkaline phosphatase-conjugated anti-antibody on Western blots. Anal Biochem. 1984 Jan;136(1):175–179. doi: 10.1016/0003-2697(84)90320-8. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Church G. M., Gilbert W. Genomic sequencing. Proc Natl Acad Sci U S A. 1984 Apr;81(7):1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalton D. A., Hanus F. J., Russell S. A., Evans H. J. Purification, properties, and distribution of ascorbate peroxidase in legume root nodules. Plant Physiol. 1987 Apr;83(4):789–794. doi: 10.1104/pp.83.4.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayan J., Dawson C. R. Substrate specificity of ascorbate oxidase. Biochem Biophys Res Commun. 1976 Nov 22;73(2):451–458. doi: 10.1016/0006-291x(76)90728-2. [DOI] [PubMed] [Google Scholar]
- Gildensoph L. H., Briskin D. P. Modification of an essential arginine residue associated with the plasma membrane ATPase of red beet (Beta vulgaris L.) storage tissue. Arch Biochem Biophys. 1989 May 15;271(1):254–259. doi: 10.1016/0003-9861(89)90276-2. [DOI] [PubMed] [Google Scholar]
- HEWITT E. J., DICKES G. J. Spectrophotometric measurements on ascorbic acid and their use for the estimation of ascorbic acid and dehydroascorbic acid in plant tissues. Biochem J. 1961 Feb;78:384–391. doi: 10.1042/bj0780384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda S. I. Ascorbic Acid Oxidase in Barley Roots. Plant Physiol. 1955 Mar;30(2):174–181. doi: 10.1104/pp.30.2.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JENSEN W. A., KAVALJIAN L. G. The cytochemical localization of ascorbic acid in root tip cells. J Biophys Biochem Cytol. 1956 Jan 25;2(1):87–92. doi: 10.1083/jcb.2.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasher J. S., Allen K. E., Kasamo K., Slayman C. W. Characterization of an essential arginine residue in the plasma membrane H+-ATPase of Neurospora crassa. J Biol Chem. 1986 Aug 15;261(23):10808–10813. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Marchesini A., Capelletti P., Canonica L., Danieli B., Tollari S. Evidence about the catecholoxidase activity of the enzyme ascorbate oxidase extracted from Cucurbita pepo medullosa. Biochim Biophys Acta. 1977 Oct 13;484(2):290–300. doi: 10.1016/0005-2744(77)90085-7. [DOI] [PubMed] [Google Scholar]
- Messerschmidt A., Huber R. The blue oxidases, ascorbate oxidase, laccase and ceruloplasmin. Modelling and structural relationships. Eur J Biochem. 1990 Jan 26;187(2):341–352. doi: 10.1111/j.1432-1033.1990.tb15311.x. [DOI] [PubMed] [Google Scholar]
- Morré D. J., Crane F. L., Sun I. L., Navas P. The role of ascorbate in biomembrane energetics. Ann N Y Acad Sci. 1987;498:153–171. doi: 10.1111/j.1749-6632.1987.tb23759.x. [DOI] [PubMed] [Google Scholar]
- NEWCOMB E. H. Effect of auxin on ascorbic oxidase activity in tobacco pith cells. Proc Soc Exp Biol Med. 1951 Mar;76(3):504–509. doi: 10.3181/00379727-76-18538. [DOI] [PubMed] [Google Scholar]
- Ohkawa J., Okada N., Shinmyo A., Takano M. Primary structure of cucumber (Cucumis sativus) ascorbate oxidase deduced from cDNA sequence: homology with blue copper proteins and tissue-specific expression. Proc Natl Acad Sci U S A. 1989 Feb;86(4):1239–1243. doi: 10.1073/pnas.86.4.1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters J. L., Castillo F. J., Heath R. L. Alteration of Extracellular Enzymes in Pinto Bean Leaves upon Exposure to Air Pollutants, Ozone and Sulfur Dioxide. Plant Physiol. 1989 Jan;89(1):159–164. doi: 10.1104/pp.89.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs M. M., Freeling M., Okimoto R. The anaerobic proteins of maize. Cell. 1980 Jul;20(3):761–767. doi: 10.1016/0092-8674(80)90322-0. [DOI] [PubMed] [Google Scholar]
- Smith E. L. Reversible blocking at arginine by cyclohexanedione. Methods Enzymol. 1977;47:156–161. doi: 10.1016/0076-6879(77)47019-8. [DOI] [PubMed] [Google Scholar]