Abstract
MPB70 and MPB80 (MPB70/80) and MPB83 are closely related antigens which are highly expressed in Mycobacterium bovis. MPB70/80 are soluble secreted antigens, while MPB83 is an exported lipoprotein associated with the bacterial surface. In the present study, these antigens had different mobilities in sodium dodecyl sulfate-polyacrylamide gel electrophoresis under reducing and nonreducing conditions. These differences may be explained by the fact that MPB70 and MPB83 both have two internal cysteine residues which would create ring structures by disulfide bonding. We analyzed the structures of MPB70/80 and MPB83 by using monoclonal antibodies (MAbs) raised against bovine purified protein derivative or whole M. bovis cells. MAb 1-5C reacted specifically with MPB70 and MPB80, and MAb MBS43 reacted specifically with MPB83, while the other antibodies, including several previously described MAbs, bound all three antigens. MAbs and polyclonal antibodies reacted strongly with reduced protein and less well with nonreduced protein, indicating involvement of linear epitopes. Epitopes of MAbs Bov-1, 2-6B, 1-5C, and 1-1D were mapped by using synthetic peptides of MPB70. Sequence comparison showed the peptide with the 1-5C-reactive epitope to have three residues different from those in the homologous region of MPB83. Exchanges of A for S in position 112 or Q for E in position 116 abolished the reactivity of MAb 1-5C. Polyclonal rabbit antibodies to native purified MPB70 reacted strongly with peptides 6, 7, and 8 of the N-terminal half of mature MPB70. Cattle sera of experimentally M. bovis-infected animals recognized a broader spectrum of peptides. These findings indicate that there is diagnostic potential for these proteins and that there is also a possible role for antibodies in elucidation of the host-mycobacterium relationship involving a surface-bound and exposed lipoprotein, MPB83, and its highly homologous soluble secreted MPB70/80 counterparts.
MPB70 and MPB80 (MPB70/80) and MPB83 are homologous secreted mycobacterial proteins with limited species distribution (6, 8, 21, 22). These proteins have generated much attention because they are highly expressed in Mycobacterium bovis and minimally expressed in Mycobacterium tuberculosis in vitro (6, 15, 32) and probably in vivo (3, 11).
The MPB70/80 and MPB83 proteins have not been demonstrated in mycobacteria outside the M. tuberculosis complex, and they constitute a group of antigens with considerable potential for improved diagnostic tests for tuberculosis (TB) (9). MPB70 is an important target antigen of humoral and cellular immune responses during infection with bovine and human tubercle bacilli (1, 11, 24, 26) and has been exploited in humoral tests for diagnosis of bovine TB (3, 11). In experimentally infected cattle, the purified protein derivative (PPD) skin test response appears early, and as the disease progresses, it declines as the antibody response to MPB70 appears (11). Cellular in vitro immune responses to purified MPB70 in human TB are also prominent and are comparable to those elicited by MPB64 (26). MPB70 thus also has potential for diagnosis of human TB by means of a simple skin test or by in vitro stimulation of lymphocytes.
There are no differences between M. bovis and M. tuberculosis in the sequences of the expressed proteins encoded by the mpb70/mbt70 and mpb83/mbt83 genes (15, 19, 20, 25, 27). It is not known whether there is a separate gene for MPB80. Comparison of MPB70 and MPB80 has never revealed any serological differences, but they are clearly distinguished by having different pIs (8).
The deduced sequences of MPB70 and MPB83 contain typical hydrophobic signal peptides which are cleaved after translocation. The resulting mature peptides have 63% identical residues. Mature MPB83 differs from MPB70 in that it has a typical lipoprotein consensus motif (15, 29) and a unique insert of 35 amino acids at its N terminus with a putative glycosylation site (15). The 26-kDa lipoprotein form has been confirmed by Triton X-114 extraction (10) and by site-directed mutagenesis with deletion of the cysteine in the consensus motif (29). Flow cytometry of whole mycobacterial cells stained with MBS43 shows that MPB83 is found in association with the bacillary surface (12). The presence of a secretion signal peptide encoded by the mpt70 gene and the predominantly extracellular occurrence of the MPB70 protein (30) indicate that it is a completely soluble secreted antigen not associated with the bacterial surface. The purified native 23-kDa MPB83 molecule (8) is also a soluble secreted (nonlipoprotein) variant of the 26-kDa MPB83 lipoprotein (12). The MPB70/80 and MPB83 antigens are thus an example of highly homologous proteins with different localization in relation to the mycobacterial cell.
Several monoclonal antibodies (MAbs) to MPB70 or MPB83 have been described by independent groups. MAb Bov-1 was originally described as an anti-MPB70 antibody (5). In the SB series (33), the epitopes of SB9 and SB10 were mapped to the N-terminal part of MPB70 by using an extensive panel of synthetic peptides (25). 12/6/1 reacted with both MPB70 and MPB83 (32). MBS43 (4) was recently shown to react only with MPB83 and not with MPB70 (32).
The objective of this study was to provide novel information about the structures of MPB70/80 and MPB83 and to characterize several anti-M. bovis MAbs raised by Lyashchenko et al. (18).
MATERIALS AND METHODS
Bacterial strains and culture fluid preparation.
M. bovis BCG Tokyo substrain 172 was obtained from the National Institute of Health, Tokyo, Japan. The bacilli were grown as surface pellicles on the wholly synthetic Sauton medium for 2 to 3 weeks, and the culture fluid was treated by sterile filtration and ammonium sulfate precipitation as described in detail previously (6).
Purified proteins.
The native proteins MPB70, MPB80, and MPB83 were purified from BCG Tokyo culture fluid and tested for homogeneity as described previously (7, 8, 22). Two different batches of MPB83 were used. MPB70 was reduced with 0.01 M dithiothreitol (DTT) for 30 min at 37°C and alkylated with 0.024 M iodoacetamide for 60 min at room temperature while kept in the dark. The preparation was finally dialyzed against phosphate-buffered saline (PBS).
Peptide synthesis.
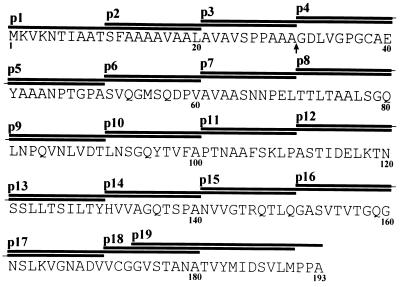
Overlapping synthetic peptides spanning the entire sequence of MPB70 (25) were synthesized by using 9-fluorenylmethoxycarbonyl chemistry and purified by C18 Sep-Pak methodology as described previously (24). A total of 19 20-mer peptides with 10-residue overlaps covered the signal sequence and mature MPB70 protein (see Fig. 5). Ten additional peptides were made to further characterize the MAb 1-5C-reactive epitope (see Fig. 7).
FIG. 5.
Nineteen synthetic overlapping 20-mer peptides covering the entire amino acid sequence of MPB70. The MPB70 sequence is given in the one-letter code for amino acids. The region covered by p1 to p19 is indicated by horizontal bars. The numbers below the sequence are amino acid numbers. Residues 1 to 30 cover the hydrophobic signal sequence, and residues 31 to 193 cover the mature soluble secreted MPB70. The arrow shows the cleavage site between the signal sequence and mature protein.
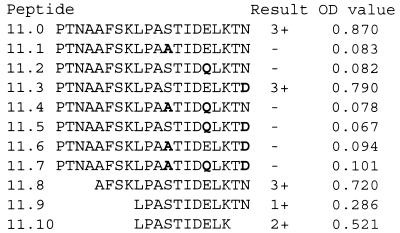
FIG. 7.
Further characterization of the specificity of 1-5C performed with variants of MPB70 peptide 11. Substitutions S→A, E→Q, and N→D singly and in combinations of two and three were made on basis of the MPB83 sequence. Truncated peptides were constructed for precise mapping of the epitope. The OD values in a representative ELISA experiment are given.
Polyclonal antisera.
Rabbit polyclonal antibodies (PAbs) to MPB70 (K33, K34, K35, K216, and K219) (6, 7, 32) and to MPB83 (K483) (8, 32) were produced by immunization with purified proteins in incomplete Freund’s adjuvant by standard procedures (6). The anti-MPB70 antiserum K34 reacts with MPB70 and reacts poorly with MPB83 in Western blotting (32). Polyvalent rabbit anti-BCG immunoglobulin (Ig), obtained by immunization with culture fluid and sonicate of BCG Copenhagen, Danish strain 1331, was a gift from DAKO Immunoglobulins, Copenhagen, Denmark.
MAbs.
Table 1 gives a list of antibodies used in the present study and their reactivities in Western blotting. MAbs 1-5C, 1-1D, and 2-6B were generated by immunizing BALB/c mice with aggregated PPD from M. bovis (Kursk Biofactory, Kursk, Russia) (18). Briefly, 100 μl of 1% CrCl3 (pH 5.0) was added slowly to 2 ml of PPD solution (5 mg/ml in 0.1 M acetate buffer, pH 7.5) with gentle shaking until visible turbidity appeared. The precipitated material was collected by centrifugation and washed twice in PBS. Mice were primed with 0.1 mg of the aggregated PPD subcutaneously or intraperitoneally and reimmunized several times intraperitoneally with the same dose at 3- to 4-week intervals. Antibody responses to PPD were monitored by enzyme-linked immunosorbent assay (ELISA). Mice were boosted intravenously with the same dose, and spleens were harvested 3 days later for cell fusion. Immune splenocytes were fused with X63-Ag8.6.5.3 myeloma cells at a ratio of 1:2 in the presence of 50% polyethylene glycol 3000 at pH 8.0. Culture supernatants were screened by ELISA against PPD from M. bovis or M. avium. Only hybridomas reacting preferentially with bovine PPD were selected for further study. Antibody 7-4D was developed by the same procedure. It was found to be biclonal and to have a contaminating isotype reacting with a low-molecular-mass band of M. tuberculosis and M. bovis not related to the reactivity against MPB70 and MPB83. The specificities of these MAbs at the molecular level have not been described previously. MAb Bov-1 was kindly supplied by S. Haga, Tokyo, Japan (5). MAb SB10 (33) was purchased from Agen Biomedical (Queensland, Australia). MAbs 12/6/1 (32) and MBS43 (4) were kindly supplied by R. G. Hewinson (Central Veterinary Laboratory, Weybridge, United Kingdom).
TABLE 1.
Effect of reducing conditions on the reactivities of antibodies to MPB70/80 and MPB83 in Western blotting
| Antibody | Reactivitya with:
|
||||||
|---|---|---|---|---|---|---|---|
| MPB70, 22 kDa, reducedb | MPB83, 23 kDa, reducedb | MPB70/83, 22/23 kDa, reducedc | MPB70, 15 kDa, unreducedc | MPB83c
|
|||
| 22 kDa, unreduced | 26 kDa, reduced | 25–26 kDa, unreduced | |||||
| MAbs | |||||||
| 1-5C | 2+ | − | ND | − | − | − | − |
| 1-1D | 3+ | 2+ | ND | ± | ± | 2+ | − |
| 7-4D | 1+ | ± | ND | − | − | ± | − |
| 2-6B | 2+ | 1+ | ND | − | − | 2+ | − |
| Bov-1 | 3+ | 1+ | ND | 1+ | 2+ | 1+ | − |
| SB10 | 2+ | 3+ | ND | ± | ± | 2+ | 1+ |
| 12/6/1 | 1+ | 2+ | ND | − | − | 2+ | 2+ |
| MBS43 | − | 2+ | ND | − | − | 2+ | 3+ |
| PAbs | |||||||
| K483 | 2+ | ± | 1+ | 3+ | 2+ | ||
| K33 | 2+ | 1+ | ± | 1+ | ± | ||
| K34 | 2+ | 1+ | − | 1+ | 1+ | ||
| K35 | 2+ | 1+ | 1+ | 1+ | 1+ | ||
The band strength was evaluated visually (see examples of Western blots in Fig. 2 and 3). 1+, weak band; 2+, medium-strong band; 3+, strong band; ±, slightly visible band; −, no visible band; ND, not determined.
Purified protein separated in SDS-PAGE.
BCG Tokyo culture fluid protein separated in SDS-PAGE.
Bovine TB sera.
Anti-MPB70 antibodies in sera from experimentally infected cattle have been described previously (11). For this study, five sera were selected from group 4 animals with strong anti-MPB70 activity in the protein G ELISA (11). Two normal bovine sera obtained from the Veterinary Institute in Oslo, Norway, were used as negative controls.
SDS-PAGE with immunoblotting.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was performed with the Pharmacia system for horizontal electrophoresis in a Multifor II electrophoresis unit 2117 (Pharmacia LKB Biotechnology AB, Uppsala, Sweden) with precast polyacrylamide gels (ExcelGel SDS 8 to 18% gradient or 15% homogeneous). Ten microliters containing 0.5 to 10 μg of total protein was applied per lane. Semidry Western blotting (immunoblotting) was performed with Novablot electrophoretic transfer kit 2117-250 (Pharmacia) onto 0.2-μm-pore-size nitrocellulose membranes (Schleicher & Schuell, Dassel, Germany). Rainbow molecular mass markers (Amersham International plc, Amersham, United Kingdom) were used as standards.
ELISA.
Immunoplate Maxisorp (Nunc, Copenhagen, Denmark) 96-well plates were coated with highly purified native MPB70, MPB80, or MPB83 at 0.5 μg/well. Synthetic peptides were diluted in PBS and applied at 1 μg/well. The plates were then blocked with PBS (pH 7.4) containing 5 mg of bovine serum albumin per ml. In the second layer various antibodies, as specified, were added diluted in PBS with 0.2% Tween 20. Horseradish peroxidase (HRP)-conjugated donkey anti-rabbit Ig (Amersham), HRP-labelled sheep anti-mouse Ig (Amersham), or HRP-labelled rabbit anti-bovine Ig (Cappel, West Chester, Pa.) was used as the secondary antibody. The substrate used was 2,2′-azino-di(ethylbenzthiazoline-sulfate) (ABTS), and the plates were washed four times between each step with PBS containing 0.1% Tween 20. All reaction mixtures were set up in duplicate, with the mean value being used for recording and calculations. Results were read on an MR 7000 ELISA reader (Dynatech Laboratories Inc., Chantilly, Va.).
Capture ELISA.
In double-antibody ELISA, MAbs were diluted in PBS. The blocking step was found to be unnecessary. In the second layer, dilutions of purified native MPB70 or partially reduced and alkylated MPB70 were applied. In the third layer, rabbit PAb K34 raised against purified MPB70 was diluted 1:500, and in the final step, HRP-labelled donkey anti-rabbit Ig was diluted 1:1,000.
RESULTS
Mobilities of purified proteins in SDS-PAGE.
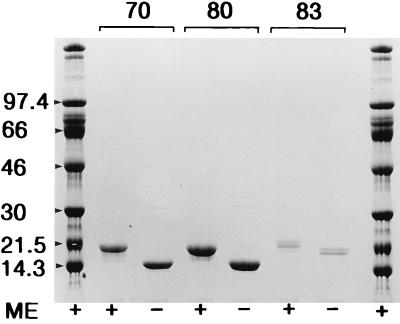
Purified MPB70, MPB80, and MPB83 were subjected to SDS-PAGE with and without mercaptoethanol (ME) in the application buffer (Fig. 1). MPB70 and MPB80 migrated with apparent molecular masses of 22 kDa with ME and 15 kDa in the absence of ME. MPB83 appeared as a double band which stained with MBS43 (data not shown). The doublet appeared under reducing and nonreducing conditions, with equal mobility shifts for both components of the doublet. The mobility of the upper band corresponded to 23 kDa with ME and 22 kDa without ME.
FIG. 1.
MPB70, MPB80, and MPB83 were separated by SDS-PAGE in an 8 to 18% gradient gel and stained with Coomassie brilliant blue. Each lane contained 4 μg of purified protein. +, reducing conditions with ME in the application buffer; −, no ME. Numbers at the left indicate molecular masses in kilodaltons.
Reactivity of MAbs to purified MPB70 and MPB83.
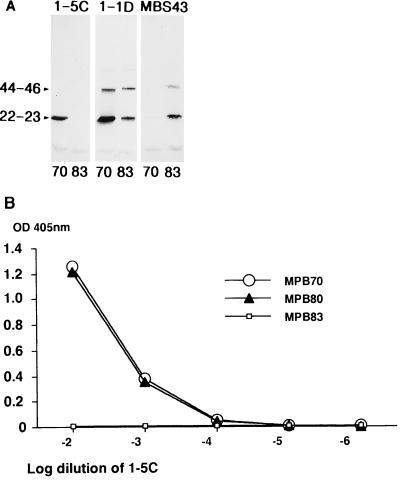
Western blotting after SDS-PAGE of purified native MPB70 and MPB83 is shown in Fig. 2A. Purified MPB70 and MPB83 displayed multiple bands, as previously reported (14, 32), with the bands at apparent molecular masses of 44 to 46 kDa probably representing dimers. MAb 1-5C stained strongly for MPB70 and had no visible band for MPB83. It did not stain the dimer of MPB70. MBS43 reacted strongly with monomeric MPB83 but did not bind significantly to MPB70. The other MAbs (1-1D, 7-4D, 2-6B, and Bov-1) stained both monomer and dimer forms of MPB70 and MPB83, with a more pronounced reactivity toward MPB70. 12/6/1 and SB10 appeared to react more strongly with MPB83 in Western blotting. (These data are summarized in Table 1.) The Western blotting results for 1-5C, 1-1D, and MBS43 were confirmed by ELISA with plates coated with purified MPB70, MPB80, and MPB83 proteins. 1-5C reacted strongly with MPB70 and MPB80 and was negative with MPB83 (Fig. 2B). MBS43 showed the opposite pattern, with positive signals against MPB83 and negative signals against MPB70 (and MPB80), as described previously (32). The titration curves for 1-1D and 7-4D (data not shown) were very similar for the three proteins. All the comparisons of MPB70 and MPB80 showed nearly identical titration curves, confirming previous data showing that these two proteins are very similar serologically (7).
FIG. 2.
Reactivities of MAbs 1-5C, 1-1D, and MBS43 in Western blotting or ELISA. (A) Purified MPB70 and MPB83 were separated under reducing conditions by SDS-PAGE in an 8 to 18% gradient gel and blotted onto nitrocellulose. Each lane contained 0.5 μg of purified protein. Numbers at the left indicate molecular masses in kilodaltons. The staining antibodies are indicated above the lanes. (B) ELISA plates were coated with 0.5 μg of purified MPB70, MPB80, or MPB83 per well, and MAb 1-5C was diluted 10-fold from 1:100 to 1:1,000,000 and added in the second step.
Reactivity of antibodies to MPB70/80 and MPB83 in crude BCG culture fluid.
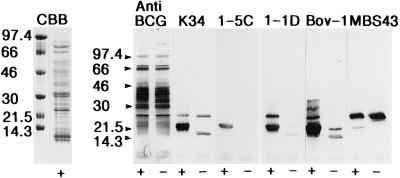
MPB70 is one of the major secreted components in BCG Tokyo culture fluid (30). Crude BCG Tokyo culture fluid was separated by SDS-PAGE, and Western blotting was performed to determine the reactivities of various antibodies to MPB70/80 and MPB83 under reducing and nonreducing conditions. Results for selected antibodies to MPB70/80 and MPB83 are shown in Fig. 3, and the data are summarized in Table 1. Two major bands were detected under reducing conditions with the various antibodies. The broad band at 22 to 23 kDa contains MPB70, MPB80, and the nonlipoprotein form of MPB83, but the individual components cannot be resolved properly by SDS-PAGE. The 26-kDa band represents the lipoprotein version of MPB83. In the nonreduced sample, three bands could be observed, one at 15 kDa representing MPB70/80, a weak band at 22 kDa probably representing the nonlipoprotein form of MPB83, and a band at 25 to 26 to kDa which was slightly ahead of the reduced 26-kDa band, suggesting that the lipoprotein version of MPB83 also migrates faster when nonreduced.
FIG. 3.
BCG Tokyo culture fluid was separated by SDS-PAGE in an 8 to 18% gradient gel. Each lane contained 10 μg of total protein. +, reducing conditions with ME in the application buffer; −, no ME. Numbers at the left indicate molecular masses in kilodaltons. Staining is indicated above the lanes. CBB, BCG Tokyo culture fluid stained with Coomassie brilliant blue. PAbs, polyvalent anti-BCG antibodies (included as a control) and anti-MPB70 (K34); MAbs, 1-5C, 1-1D, Bov-1, and MBS43.
In general, there was strong staining of the bands in the reduced sample and less intense staining of the nonreduced proteins. All of the tested antibodies except 1-5C stained both 22- to 23-kDa and 26-kDa bands in reduced samples; 1-5C stained only one band, MPB70/80 at 22 kDa. This finding suggests that MPB70 and MPB80 are expressed in single forms and that there are no 26-kDa counterparts as seen with MPB83.
1-5C and 2-6B reacted strongly with reduced samples but were completely negative without reduction. Of the MAbs, only Bov-1 stained the nonreduced 15-kDa band properly, while weak 15-kDa bands were seen with 1-1D and SB10. The polyclonal anti-MPB70 also stained the 15-kDa band but with lower intensity than the corresponding reduced 22- to 23-kDa band.
Only three MAbs (SB10, 12/6/1, and MBS43) stained the nonreduced MPB83 lipoprotein at 25- to 26 kDa, while three of the four tested rabbit PAbs stained this band properly, the last one being weakly positive. In particular, the polyclonal anti-MPB83 antibody, K483, stained this band strongly.
Specificities of MAbs by capture ELISA.
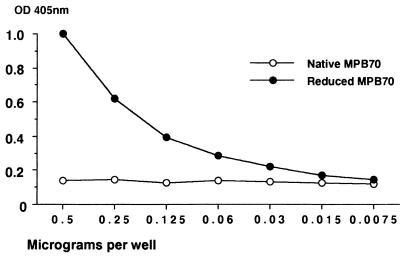
The nonreduced MPB70 was not recognized by immobilized 1-5C, 1-1D, and 7-4D in capture ELISA, while strong reactions were obtained with the reduced sample. The results of a representative experiment using 1-5C are shown in Fig. 4. MAb 12/6/1 was essentially negative in capture ELISA with both reduced and nonreduced MPB70 (data not shown). The capture ELISAs thus confirmed the data obtained by Western blotting showing that various MAbs react preferentially with reduced MPB70. However, when highly purified MPB70 was applied to polystyrene, 1-5C, 1-1D, and 7-4D reacted strongly with both reduced and nonreduced protein (data not shown). These data indicate that considerable conformational changes occur upon binding of nonreduced MPB70 to the ELISA well. With 12/6/1 the reactivity was less with reduced than with native MPB70.
FIG. 4.
Capture ELISA with MAb 1-5C applied at a fixed concentration. Twofold serial dilutions of reduced and nonreduced MPB70 were applied in the next step, and bound antigen was detected with rabbit anti-MPB70 (K34) and HRP-labelled anti-rabbit Ig.
Mapping of MAb epitopes with synthetic peptides.
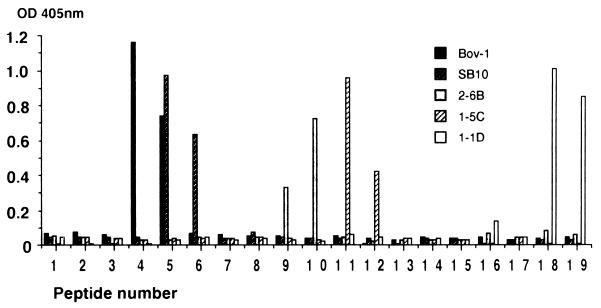
Since the MAbs and PAbs to MPB70/83 reacted strongly with reduced protein and less well with the nonreduced protein, linear epitopes were expected to be involved. Linear antibody epitopes within MPB70 were mapped by using 20-mer peptides with 10-residue overlaps (Fig. 5). Figure 6 shows the results obtained with five MAbs reacting with five different epitopes along the molecule. Bov-1 reacted with peptides 4 and 5. SB10 reacted with peptides 5 and 6, which is in agreement with its original characterization (25). MAb 2-6B reacted with peptides 9 and 10. MAb 1-5C reacted with peptides 11 and 12, while MAb 1-1D reacted with the C-terminal peptides 18 and 19. MAbs 7-4D and 12/6/1 were completely negative (optical density [OD] readings of below 0.2), suggesting that these antibodies react with conformational epitopes of MPB70. HYT27, which is a MAb to the antigen 85 complex (31), was negative as well, being included as a control (data not shown).
FIG. 6.
Characterization of linear epitopes by using the 19 overlapping peptides shown in Fig. 5, each applied at 1 μg/well in duplicate ELISA wells. MAbs Bov-1, SB10, 2-6B, 1-5C, and 1-1D were applied in the second step, and HRP-labelled anti-mouse Ig was applied in the final step.
Fine specificity of MAb 1-5C.
The fine specificity of MAb 1-5C was further investigated because this is the first MAb shown to react only with MPB70/80 and not with MPB83. Comparison of the peptide 11 sequence with the corresponding sequence in MPB83 (15, 20) showed three different amino acid residues in the MPB83 sequence. These three residues were systematically replaced in synthetic peptides. Figure 7 shows their reactivities with MAb 1-5C in ELISA. Substitutions of A for S in position 112 (peptide 11.1) or of Q for E in position 116 (peptide 11.2) abolished the reactivity with 1-5C. Substitution of D for N in position 120 (peptide 11.3) did not affect the reactivity of 1-5C significantly. By using truncated versions of peptide 11 the epitope was further limited to the decapeptide 11.10 (LPASTIDELK).
Mapping of PAb epitopes with synthetic peptides.
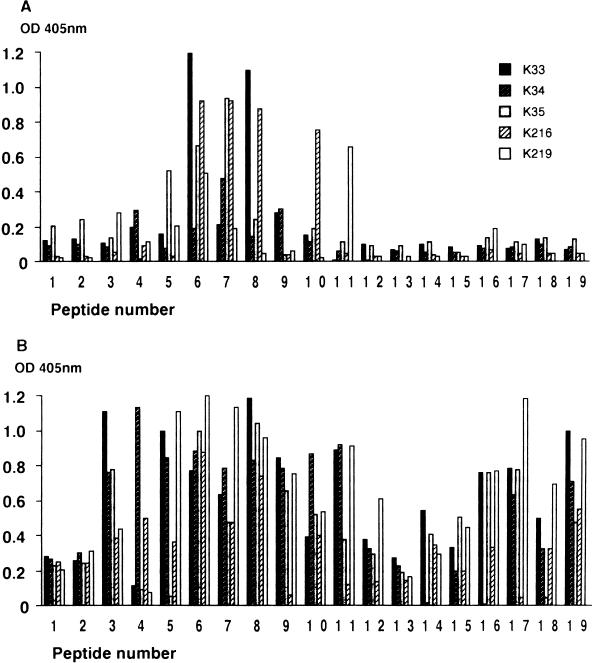
To identify the most immunogenic linear epitopes in MPB70, we analyzed five rabbit PAbs raised by using highly purified native MPB70 as an immunogen. Figure 8A shows their reactivities with the individual peptides in ELISA. Four of the five rabbits showed strong binding to peptide 6, three showed strong binding to peptide 7, two showed strong binding to peptide 8, and one showed strong binding to peptides 5, 10, and 11. The peptides from the C-terminal half of the mature protein, peptides 12 to 19, were negative. The antibody response to linear epitopes of MPB70 upon immunization with native MPB70 thus appeared to be clustered around peptides 5 to 11, with the region spanning peptides 6 to 8 being most immunogenic. Three normal rabbit sera were all negative (OD readings of below 0.2) throughout the peptide series (data not shown).
FIG. 8.
Characterization of linear epitopes by using the 19 overlapping peptides shown in Fig. 5, each applied at 1 μg/well in duplicate ELISA wells. Rabbit PAbs or cattle sera were applied in the second step, and appropriate HRP-labelled secondary antibodies were applied in the final step. (A) Results with rabbit anti-MPB70 PAbs K33, K34, K35, K216, and K219. Peptides 1 and 2 were from the signal sequence, which is cleaved off during translocation and thus is not present in the mature MPB70 used for immunization. Antibody binding to peptides 1 and 2 may be considered background activity. (B) Results with five different cattle sera from animals experimentally infected with M. bovis. Samples were taken 25 weeks after infection.
Mapping of antibody epitopes in infected cattle.
To identify the most immunogenic linear epitopes in MPB70 during experimental infection of cattle with M. bovis, five individual sera taken from cattle 25 weeks after infection were analyzed. Figure 8B shows that the animals had positive antibody responses against a much broader range of peptides than the rabbit sera, but peptides 1 and 2 from the signal sequence were still negative. All five animals were positive with peptides 3, 6, 7, 8, 10, and 19. The strongest responses were measured against peptides 6 and 8. Thus, this region of MPB70 contains the most immunogenic linear epitopes of the molecule upon immunization with purified protein and during infection with bovine tubercle bacilli. In contrast to rabbit sera, bovine sera had antibodies to the C-terminal peptides 16 to 19. This confirms the epitope defined by MAb 1-1D in peptide 19. This antibody epitope was not predicted by the Jameson-Wolf algorithm (16). For a negative control, two normal bovine sera of Norwegian origin were tested (data not shown). The background activities in this system were slightly higher than those with the rabbit sera (OD readings of below 0.3). One of these control sera showed an unexplained positive response to peptide 17 (OD of 0.6).
DISCUSSION
Analysis of MAbs directed against MPB70/80 and MPB83 showed that most MAbs identify shared epitopes on these molecules. There are two exceptions: 1-5C and MBS43 react specifically with MPB70/80 and MPB83, respectively. The reactivities of the MAbs were also found to be highly dependent on the conformational structures of these proteins. Their preferential reactivity with ME- or DTT-treated proteins and mapping of MAb and PAb epitope specificities with synthetic peptides show that linear epitopes of MPB70 are major antibody targets both upon immunization with protein preparations and during infection with M. bovis.
Structural considerations.
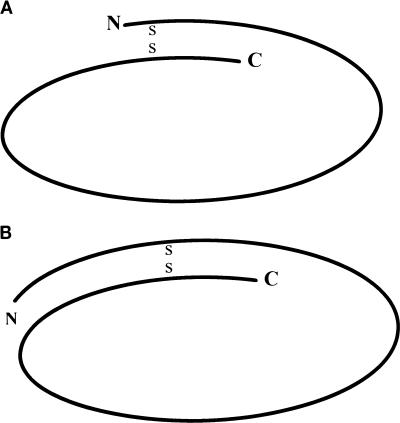
The MPB70 and MPB80 proteins showed a significantly retarded mobility after reduction with ME or DTT. Most monomeric proteins do not exhibit different mobilities with and without reduction. In MPB70, there are two widely spaced cysteines in the polypeptide chain that may alter the three-dimensional structure of the molecules by mediating internal disulfide bonding. Proteins with internal disulfide bridges are not properly unfolded by SDS treatment and therefore migrate faster than extended polypeptides in SDS-PAGE. The mobility difference between reduced and nonreduced MPB70 corresponded to a difference in molecular mass of 7 kDa and represents the difference observed when the 133-amino-acid sequence (13.4 kDa) placed between the two cysteine residues is unfolded. Both the 26-kDa lipoprotein version and the 23-kDa MPB83 protein migrated with a molecular mass difference of about 1 kDa in reduced and nonreduced samples. This difference is quite small compared to that observed with MPB70, although 133 amino acids are enclosed within the disulfide loop in both sequences. The mobility difference between reduced and nonreduced MPB83 is less than expected from the size of its loop (13.6 kDa). This finding suggests that the tertiary structure of MPB83 is more stable than that of MPB70. There are two major structural features that may explain the stabilization of the MPB83 structure: (i) The extra N-terminal piece of 31 amino acids and (ii) carbohydrate and lipid conjugation. The 26-kDa lipoprotein version of MPB83 showed even less of a difference in mobility (reduced versus nonreduced), supporting the idea that carbohydrate and lipid conjugation may play a role in stabilization of its tertiary structure. The mobility difference between reduced and nonreduced MPB80 in SDS-PAGE was comparable to that of MPB70, which indicates that MPB80 has a similar ring structure. Figure 9 shows schematically the proposed ring structures of the mature MPB70 and MPB83 molecules. Internal disulfide bonding was recently demonstrated for antigens 85A and 85B (13), but in this case there is no significant effect on the apparent molecular size in SDS-PAGE upon reduction, because the two cysteines are closely positioned.
FIG. 9.
Schematic structures of MPB70 (A) and MPB83 (B). (A) The oval represents the mature amino acid sequence of MPB70, residues 31 to 193 in Fig. 5. S-S indicates the proposed internal disulfide bond between the cysteine residues at positions 38 and 172 of MPB70. This leaves free N- and C-terminal pieces of 7 and 21 residues, respectively. (B) The oval represents the mature amino acid sequence of MPB83, residues 25 to 220 (15). S-S indicates the proposed internal disulfide bond between the cysteine residues at positions 64 and 198 of MPB83, which leaves free N- and C-terminal pieces of 39 and 22 residues, respectively. N, amino-terminal end; C, carboxy-terminal end.
An additional feature is also of interest. The apparent molecular mass of reduced MPB70 (22 kDa) is much higher than the deduced mass of mature MPB70 (16.3 kDa), which is more consistent with the 15.1-kDa value determined by sedimentation equilibrium centrifugation (22). One possible explanation for its low mobility in SDS-PAGE may be less binding of SDS to the polypeptide chain than is the case with most other proteins. Concerning MPB83, the difference between deduced (19.8 kDa) and apparent (23 kDa) molecular mass is half that for MPB70. This reduction is probably related to glycosylation of MPB83.
Linear epitopes of MPB70.
The presented data show that there is a series of different linear B-cell epitopes on MPB70. The two epitopes previously characterized with MAbs SB9 and SB10 and the presently characterized Bov-1 epitope were within the same N-terminal region as the linear epitopes defined by the rabbit PAbs to MPB70. The sera of experimentally infected cattle also responded strongly within the same region but generally showed a broad range of specificities on the mature MPB70 molecule. The different recognition of epitopes of PAbs from rabbits and cattle may be explained by the fact that the former were obtained by immunization with purified protein, whereas the latter came from M. bovis-infected animals. However, experimental infection of rabbits with M. bovis is necessary to verify this finding. Interestingly, three of the four new anti-bovine PPD MAbs were directed towards linear epitopes different from those characterized by using SB9 and SB10 (25). Five separate linear epitopes distributed along the mature MPB70 are thus defined by MAbs. The data obtained with the rabbit PAbs and those obtained with the sera of experimentally infected cattle show that there are B-cell epitopes along most of the MPB70 sequence except for the 30 residues of the signal peptide.
The 2-6B and 1-1D epitopes may overlap the OSF-2 homology regions I and II, respectively (28). More detailed analysis with truncated peptides is needed to establish the exact positions of these epitopes and whether these antibodies may be of value to study the interaction between OSF-2 and MPB70/80 and MPB83. The 1-1D epitope also overlapped with a previously characterized T-cell epitope (24). Two other previously mapped T-cell epitopes did not coincide with any of the MAb epitopes (1, 24).
Surface-exposed versus hidden epitopes.
The linear epitopes of MPB70 defined by MAbs were apparently not exposed on the surface of the soluble native MPB70 but became available upon reduction of the S-S bridge, as shown by capture ELISA. Reduction of the S-S bond was not required for exposure of these epitopes, because the MAbs bound to nonreduced MPB70 immobilized on polystyrene. The data from Western blotting experiments also generally support that the MAbs and PAbs are primarily directed towards epitopes that become exposed upon reduction. Conformational epitopes on MPB70 probably do exist. There is indirect evidence by the lack of binding of MAbs 12/6/1 and 7-4D to any of the synthetic peptides. These conformational epitopes are apparently not exposed at the surface of MPB70, because the capture ELISA was negative.
It has previously been shown that hyperimmune rabbit sera precipitate soluble MPB70 (6), but the current observations suggest that the majority of B-cell epitopes, conformational or linear, are hidden within the MPB70 molecule. The apparent tendency for formation of antibodies to hidden epitopes may be related to the molecular mimicry between MPB70/MPB83 and OSF-2 (28), which may explain why surface structures of MPB70/MPB83 are less immunogenic.
It is well known that considerable conformational changes may occur when proteins are applied to plastic supports (2). In this instance, it appears to be a great advantage to use purified MPB70 immobilized on polystyrene for detection of anti-MPB70 antibodies towards epitopes hidden within the MPB70 molecule. The bovine TB sera were shown to react extensively to linear epitopes along the MPB70 molecule, and our data suggest that the use of polystyrene-fixed MPB70 is the method of choice for detection of these antibodies.
Immune responses to MPB70 versus MPB83 in bovine TB infection.
The knowledge gained in this study is expected to help improve serological applications as well as our understanding of the immunopathology of mycobacterial infections by providing tools to distinguish between antibodies to specific and cross-reactive epitopes on MPB70 and MPB83. Early antibody responses in bovine infection were shown to be directed towards a broad 26-kDa band in a Western blotting study (23). MPB83 is probably the main lipoprotein constituent of this band and the main target for these early antibody responses. The anti-MPB70 responses appear to develop at a later stage of the disease process (11). In view of the extensive homology between the MPB70 and MPB83 molecules, one would expect that these antibody responses would develop in parallel unless specific epitopes were involved. The new MAbs to specific (1-5C in particular) and cross-reactive epitopes of MPB70 and MPB83 will be of great importance for further differentiation of antibody responses towards these proteins. In view of the fact that MPB83 is a surface-exposed lipoprotein (12, 15, 17, 29) and that MPB70 is efficiently secreted as a soluble protein (30), the study of the specificities and kinetics of development of antibodies to these proteins may give new insight into the immunopathology of mycobacterial infection.
ACKNOWLEDGMENTS
This work was supported by grants from the Anders Jahre Fund for the Promotion of Science and by grants from the European Community (project no. IC18-CT96-0060). Work in Maria L. Gennaro’s laboratory was funded by the National Institutes of Health (grant no. R01 AI36989).
A. Murat Aksoy’s work in Oslo was supported by a government scholarship within the framework of the bilateral cultural agreement between Norway and Turkey, through the Research Council of Norway. Purified proteins were kindly provided by Sadamu Nagai (Osaka, Japan). Shinji Haga (Tokyo, Japan) is greatly acknowledged for supplying the Bov-1 MAb. We thank Gunni Ulvund and Ingunn Ghile for excellent technical assistance and Suzanne Garman Vik for work on the manuscript.
REFERENCES
- 1.Billman-Jacobe H, Radford A J, Rothel J S, Wood P R. Mapping of the T and B cell epitopes of the Mycobacterium bovis protein, MPB70. Immunol Cell Biol. 1990;68:359–365. doi: 10.1038/icb.1990.49. [DOI] [PubMed] [Google Scholar]
- 2.Chuang H Y, Andrade J D. Immunochemical detection by specific antibody to thrombin of prothrombin conformational changes upon adsorption to artificial surfaces. J Biomed Mater Res. 1985;19:813–825. doi: 10.1002/jbm.820190707. [DOI] [PubMed] [Google Scholar]
- 3.Fifis T, Costopoulos C, Corner L A, Wood P R. Serological reactivity to Mycobacterium bovis protein antigens in cattle. Vet Microbiol. 1992;30:343–354. doi: 10.1016/0378-1135(92)90021-k. [DOI] [PubMed] [Google Scholar]
- 4.Goodger J, Nolan A, Russell W P, Dalley D J, Thorns C J, Stuart F A, Croston P, Newell D G. Serodiagnosis of Mycobacterium bovis infection in badgers: development of an indirect ELISA using a 25 kDa antigen. Vet Rec. 1994;135:82–85. doi: 10.1136/vr.135.4.82. [DOI] [PubMed] [Google Scholar]
- 5.Haga S, Nakagawa M, Nagai S, Miura K, Honda M. Purification of MPB70 and production of specific monoclonal antibodies. Hybridoma. 1992;11:483–492. doi: 10.1089/hyb.1992.11.483. [DOI] [PubMed] [Google Scholar]
- 6.Harboe M, Nagai S. MPB70, a unique antigen of Mycobacterium bovis BCG. Am Rev Respir Dis. 1984;129:444–452. doi: 10.1164/arrd.1984.129.3.444. [DOI] [PubMed] [Google Scholar]
- 7.Harboe M, Nagai S, Patarroyo M E, Torres M L, Ramirez C, Cruz N. Properties of proteins MPB64, MPB70, and MPB80 of Mycobacterium bovis BCG. Infect Immun. 1986;52:293–302. doi: 10.1128/iai.52.1.293-302.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harboe M, Nagai S, Wiker H G, Sletten K, Haga S. Homology between the MPB70 and MPB83 proteins of Mycobacterium bovis BCG. Scand J Immunol. 1995;42:46–51. doi: 10.1111/j.1365-3083.1995.tb03624.x. [DOI] [PubMed] [Google Scholar]
- 9.Harboe M, Oettinger T, Wiker H G, Rosenkrands I, Andersen P. Evidence for occurrence of the ESAT-6 protein in Mycobacterium tuberculosis and virulent Mycobacterium bovis and for its absence in Mycobacterium bovis BCG. Infect Immun. 1996;64:16–22. doi: 10.1128/iai.64.1.16-22.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harboe M, Wiker H G. Different localization of MPB70 and MPB83 proteins of Mycobacterium bovis. Med Principles Practice. 1997;6:84–90. [Google Scholar]
- 11.Harboe M, Wiker H G, Duncan J R, Garcia M M, Dukes T W, Brooks B W, Turcotte C, Nagai S. Protein G-based enzyme-linked immunosorbent assay for anti-MPB70 antibodies in bovine tuberculosis. J Clin Microbiol. 1990;28:913–921. doi: 10.1128/jcm.28.5.913-921.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harboe M, Wiker H G, Ulvund G, Lund-Pedersen B, Andersen A B, Hewinson R G, Nagai S. MPB70 and MPB83 as indicators of protein localization in mycobacterial cells. Infect Immun. 1998;66:289–296. doi: 10.1128/iai.66.1.289-296.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harth G, Lee B Y, Wang J, Clemens D L, Horwitz M A. Novel insights into the genetics, biochemistry, and immunocytochemistry of the 30-kilodalton major extracellular protein of Mycobacterium tuberculosis. Infect Immun. 1996;64:3038–3047. doi: 10.1128/iai.64.8.3038-3047.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hasl¢v K, Andersen A B, Bentzon M W. Biological activity in sensitized guinea pigs of MPB70, a protein specific for some strains of Mycobacterium bovis BCG. Scand J Immunol. 1987;26:445–454. doi: 10.1111/j.1365-3083.1987.tb02277.x. [DOI] [PubMed] [Google Scholar]
- 15.Hewinson R G, Michell S L, Russell W P, McAdam R A, Jacobs W R., Jr Molecular characterization of MPT83: a seroreactive antigen of Mycobacterium tuberculosis with homology to MPT70. Scand J Immunol. 1996;43:490–499. doi: 10.1046/j.1365-3083.1996.d01-78.x. [DOI] [PubMed] [Google Scholar]
- 16.Jameson B A, Wolf H. The antigenic index: a novel algorithm for predicting antigenic determinants. Comput Appl Biosci. 1988;4:181–186. doi: 10.1093/bioinformatics/4.1.181. [DOI] [PubMed] [Google Scholar]
- 17.Lounatmaa K, Brander E. Proceedings of the XIIth International Congress for Electron Microscopy. San Francisco, Calif: San Francisco Press, Inc.; 1990. Immunoelectron microscopic localization of 22 kDa protein antigen in the surface layer of Mycobacterium bovis BCG strains; pp. 894–895. [Google Scholar]
- 18.Lyashchenko K P, Bobrovnik S O, Gorbenko E V, Kolesnikova I N, Bukhanevich A M, Lyashko E D, Komissarenko S V. Immunologic characterization of monoclonal antibodies to Mycobacterium bovis and of four affinity-purified mycobacterial antigens. Probl Tuberk. 1993;5:41–44. . (In Russian). [PubMed] [Google Scholar]
- 19.Matsumoto S, Matsuo T, Ohara N, Hotokezaka H, Naito M, Minami J, Yamada T. Cloning and sequencing of a unique antigen MPT70 from Mycobacterium tuberculosis H37Rv and expression in BCG using E. coli-mycobacteria shuttle vector. Scand J Immunol. 1995;41:281–287. doi: 10.1111/j.1365-3083.1995.tb03565.x. [DOI] [PubMed] [Google Scholar]
- 20.Matsuo T, Matsuo H, Ohara N, Matsumoto S, Kitaura H, Mizuno A, Yamada T. Cloning and sequencing of an MPB70 homologue corresponding to MPB83 from Mycobacterium bovis BCG. Scand J Immunol. 1996;43:483–489. doi: 10.1046/j.1365-3083.1996.d01-68.x. [DOI] [PubMed] [Google Scholar]
- 21.Miura K, Nagai S, Kinomoto M, Haga S, Tokunaga T. Comparative studies with various substrains of Mycobacterium bovis BCG on the production of an antigenic protein, MPB70. Infect Immun. 1983;39:540–545. doi: 10.1128/iai.39.2.540-545.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nagai S, Matsumoto J, Nagasuga T. Specific skin-reactive protein from culture filtrate of Mycobacterium bovis BCG. Infect Immun. 1981;31:1152–1160. doi: 10.1128/iai.31.3.1152-1160.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.O’Loan C J, Pollock J M, Hanna J, Neill S D. Immunoblot analysis of humoral immune responses to Mycobacterium bovis in experimentally infected cattle: early recognition of a 26-kilodalton antigen. Clin Diagn Lab Immunol. 1994;1:608–611. doi: 10.1128/cdli.1.5.608-611.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pollock J M, Douglas A J, Mackie D P, Neill S D. Identification of bovine T-cell epitopes for three Mycobacterium bovis antigens: MPB70, 19,000 MW and MPB57. Immunology. 1994;82:9–15. [PMC free article] [PubMed] [Google Scholar]
- 25.Radford A J, Wood P R, Billman-Jacobe H, Geysen H M, Mason T J, Tribbick G. Epitope mapping of the Mycobacterium bovis secretory protein MPB70 using overlapping peptide analysis. J Gen Microbiol. 1990;136:265–272. doi: 10.1099/00221287-136-2-265. [DOI] [PubMed] [Google Scholar]
- 26.Roche P W, Triccas J A, Avery D T, Fifis T, Billman-Jacobe H, Britton W J. Differential T cell responses to mycobacteria-secreted proteins distinguish vaccination with bacille Calmette-Guerin from infection with Mycobacterium tuberculosis. J Infect Dis. 1994;170:1326–1330. doi: 10.1093/infdis/170.5.1326. [DOI] [PubMed] [Google Scholar]
- 27.Terasaka K, Yamaguchi R, Matsuo K, Yamazaki A, Nagai S, Yamada T. Complete nucleotide sequence of immunogenic protein MPB70 from Mycobacterium bovis BCG. FEMS Microbiol Lett. 1989;49:273–276. doi: 10.1016/0378-1097(89)90052-9. [DOI] [PubMed] [Google Scholar]
- 28.Ulstrup J C, Jeansson S, Wiker H G, Harboe M. Relationship of secretion pattern and MPB70 homology with osteoblast-specific factor 2 to osteitis following Mycobacterium bovis BCG vaccination. Infect Immun. 1995;63:672–675. doi: 10.1128/iai.63.2.672-675.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vosloo W, Tippoo P, Hughes J E, Harriman N, Emms M, Beatty D W, Zappe H, Steyn L M. Characterization of a lipoprotein in Mycobacterium bovis (BCG) with sequence similarity to the secreted protein MPB70. Gene. 1997;88:123–128. doi: 10.1016/s0378-1119(96)00806-2. [DOI] [PubMed] [Google Scholar]
- 30.Wiker H G, Harboe M, Nagai S. A localization index for distinction between extracellular and intracellular antigens of Mycobacterium tuberculosis. J Gen Microbiol. 1991;137:875–884. doi: 10.1099/00221287-137-4-875. [DOI] [PubMed] [Google Scholar]
- 31.Wiker H G, Harboe M, Nagai S, Bennedsen J. Quantitative and qualitative studies on the major extracellular antigen of Mycobacterium tuberculosis H37Rv and Mycobacterium bovis BCG. Am Rev Respir Dis. 1990;141:830–838. doi: 10.1164/ajrccm/141.4_Pt_1.830. [DOI] [PubMed] [Google Scholar]
- 32.Wiker H G, Nagai S, Hewinson R G, Russell W P, Harboe M. Heterogenous expression of the related MPB70 and MPB83 proteins distinguish various substrains of Mycobacterium bovis BCG and Mycobacterium tuberculosis H37Rv. Scand J Immunol. 1996;43:374–380. doi: 10.1046/j.1365-3083.1996.d01-61.x. [DOI] [PubMed] [Google Scholar]
- 33.Wood P R, Ripper J, Radford A J, Bundesen P G, Rylatt D B, Cottis L E, John M, Plackett P. Production and characterization of monoclonal antibodies specific for Mycobacterium bovis. J Gen Microbiol. 1988;134:2599–2604. doi: 10.1099/00221287-134-9-2599. [DOI] [PubMed] [Google Scholar]