Abstract
Sex-related disparities in force production of humans have been widely observed. Previous literature has attributed differences in peripheral traits, such as muscle size, to explain these disparities. However, less is known about potential sex-related differences in central neuromuscular traits and many comparable studies, not exploring sex-related differences, exhibit a selection-bias in the recruitment of subjects making the generalization of their findings difficult. Utilizing high-density electromyography arrays and motor unit (MU) decomposition, the aim of the current study is to compare MU yield and discharge properties of the tibialis anterior between male and female humans. Twenty-four subjects (10 females) performed two submaximal (20%) isometric dorsiflexion contractions. On average, males yielded nearly twice the amount of MUs as females. Further, females had significantly higher MU discharge rate, lower MU action potential amplitude, and lower MU action potential frequency content than males despite similar levels of torque and MU discharge variability. These findings suggest differences in central neuromuscular control of force production between sexes; however, it is unclear how lower yield counts affect the accuracy of these results.
Keywords: Motor unit decomposition, High density sEMG, Electromyography
1. Introduction
Human force production is regulated by a wide array of peripheral and central factors. Sex-related differences in absolute force production have been largely attributed to peripheral neuromuscular factors. Primary candidates include changes in cross-sectional area, metabolic function, fiber-type composition, and muscle fatigability (Bishop et al., 1987; Staron et al., 2000; Hicks et al., 2001; Kent-Braun et al., 2002). In contrast, few studies have investigated the role of central factors in the disparity of muscle force production between sexes.
There are several lines of evidence to suggest that the neural drive to muscle may be different between sexes. Previous investigations demonstrate differences in the number and size (Breedlove and Arnold, 1983; Grisham et al., 1992; Mierzejewska-Krzyżowska et al., 2014) of motoneurons within sexually dimorphic motor pools, such as the spinal nucleus of the bulbocavernosus, responsible for innervating multiple perineal muscles (Schroder, 1980). These differences appear to be a result of hormone dependent motoneuron death in the prenatal development period since both sexes initially form similar numbers of motoneurons within these spinal motor pools (Cihak et al., 1970; Nordeen et al., 1985). It is unclear if there are sex-related differences in the number and size of spinal motoneurons in other motor pools, where the muscle structure and function may be more similar across sexes. For example, Yuan et al. (2000) observed no difference in the number of motoneuron cell bodies in the anterior horn of the spinal cord (at C5 and L3 locations) of human cadavers between sexes. Complicating the issue, the majority of other human cadaveric studies assessing motoneuron counts do not differentiate between sexes in the reporting of their findings or methods (Christensen, 1959; Santo Neto et al., 1998; Neto et al., 2004; Santo Neto and Marques, 2008; Cheah et al., 2019), so it is difficult to assess the generalizability of these studies across sexes.
If no gross anatomical sex-related differences in spinal motoneurons exist, it remains feasible that the CNS may be driven and coordinated differently to account for differences in anthropometric and peripheral neuromuscular traits. Previous studies investigating the differences in motor unit behavior, between sexes, have relied on proxy measures such as surface electromyography (sEMG) to infer motoneuron activity from the amplitude (Visser and de Rijke, 1974; Krishnan and Williams, 2009; Lenhardt et al., 2009; Bolgla et al., 2014) and median signal frequency of sEMG (Cioni et al., 1994). While these surrogate measures of neuromuscular control provide important data to investigate potential differences between sexes, these assessment techniques have numerous technical and methodological limitations (Farina et al., 2014; Vigotsky et al., 2017).
Given the limitations of traditional sEMG, a number of investigators have utilized other techniques to investigate sex-related differences in the neuromuscular system of humans. Trevino et al. (2019) utilized sEMG motor unit decomposition algorithms, ultrasonography, and muscle biopsies to identify sex-related differences in muscle fiber composition, cross-sectional area, motor unit action potential waveforms (MUAP) between sexes. These findings could indicate peripheral neuromuscular differences, specifically the relative size of muscle fibers associated with higher threshold motor units compared to lower threshold units (Trevino et al., 2019). However, the authors did not report the motor unit discharge rates (MUDR) or other measures of central neuromuscular factors. Other investigators have utilized fine-wire EMG, with heterogeneous results (Christie and Kamen, 2010; Harwood et al., 2014; Peng et al., 2018) to investigate the discharge of single motor units. Fine-wire EMG benefits from its focal selectivity in the detection MUs, making it possible to quantify the discharges of individual motor units. Consequently, because of its selectivity, fine-wire EMG is spatially restricted to small samples of motor unit activity, potentially limiting the generalizability of those findings across the entire motor pool (Duchateau and Enoka, 2011). Utilizing fine-wire EMG, Christie and Kamen (2010) observed that males had significantly higher MUDR than females during maximal, volitional, isometric, contractions (MVIC) of the tibialis anterior. Similarly, Inglis and Gabriel (2020) observed that males have higher MUDR during MVIC contractions of the tibialis anterior. However, females demonstrated higher MUDR during sub-maximal intensities of the same motor pool. Further, when Inglis and Gabriel utilized a sub-set of strength-matched participants, they found females had higher MUDR during both submaximal and maximal volitional contractions. These data suggest there may be differences in the neural drive to muscle between sexes.
High-density electromyography arrays (HDsEMG) and motor unit decomposition techniques have the potential to identify and track dozens of individual motor units (Negro et al., 2016). This wealth of information may help reconcile potential sex-related differences in neural drive that contribute to the differential force output observed in previous studies. To date, no previous studies have investigated the potential sex-related central neuromuscular properties with high spatial resolution techniques within the tibialis anterior motor pool. Here, using HDsEMG, we aim to compare motor unit yield and discharge properties of the tibialis anterior between male and female humans. We hypothesize females will yield fewer MUs from MU decomposition and contain higher MUDR, than males, during 20% MVIC submaximal contractions.
2. Methods
2.1. Participants
Twenty-four human subjects (10 females, mean age 26.5 ± 4.98 years; mean BMI 26.4 ± 3.6) were drawn from the university population. Participants with no known neuromuscular impairments were recruited for this study. All procedures were approved by the Institutional Review Board at Temple University (Protocol # 23971). All subjects provided written informed consent prior to participation.
2.2. Experimental Set-Up
In order to assess MU yield and discharge properties across male and female subjects we utilized HDsEMG during a series of submaximal contractions (Fig. 1). Prior to the experimental session, the skin over the right tibialis anterior (TA) was shaved, abraded with high-grit sandpaper, and cleansed with water. One 64-channel high-density electrode array (8 mm inter-electrode distance, 5 columns × 13 rows, ELSCH064NM2; OT Bioelettronica; Turin, Italy) is coated with conductive adhesive paste (AC Cream; Spes Medica; Genova, Italy) and placed on the skin over the muscle according to SENIAM guidelines and secured with medical tape (3 M Transpore; St. Paul, MN). The medial border of the HDsEMG array was placed parallel with and aligned to the anterior tibial crest, the center of the array was positioned on the largest part of the muscle belly approximately in the upper 2/3 of the lower leg. Participants were seated in an isokinetic dynamometer (Biodex; Shirley, NY) and secured with straps across the torso and upper leg in a comfortable position of 60–90° hip flexion and 15° knee flexion. The ankle was secured to a footplate achieving 15° degrees of plantarflexion and affixed to a six degrees of freedom load cell (JR3; Woodland, CA). The ankle’s axis of rotation was centered to the load cell in this position. Real-time feedback of torque output was provided on a large format monitor positioned directly in front of the participant, approximately 5 ft (1.5 m) from the subject, to assist task performance.
Fig. 1. Raw data.
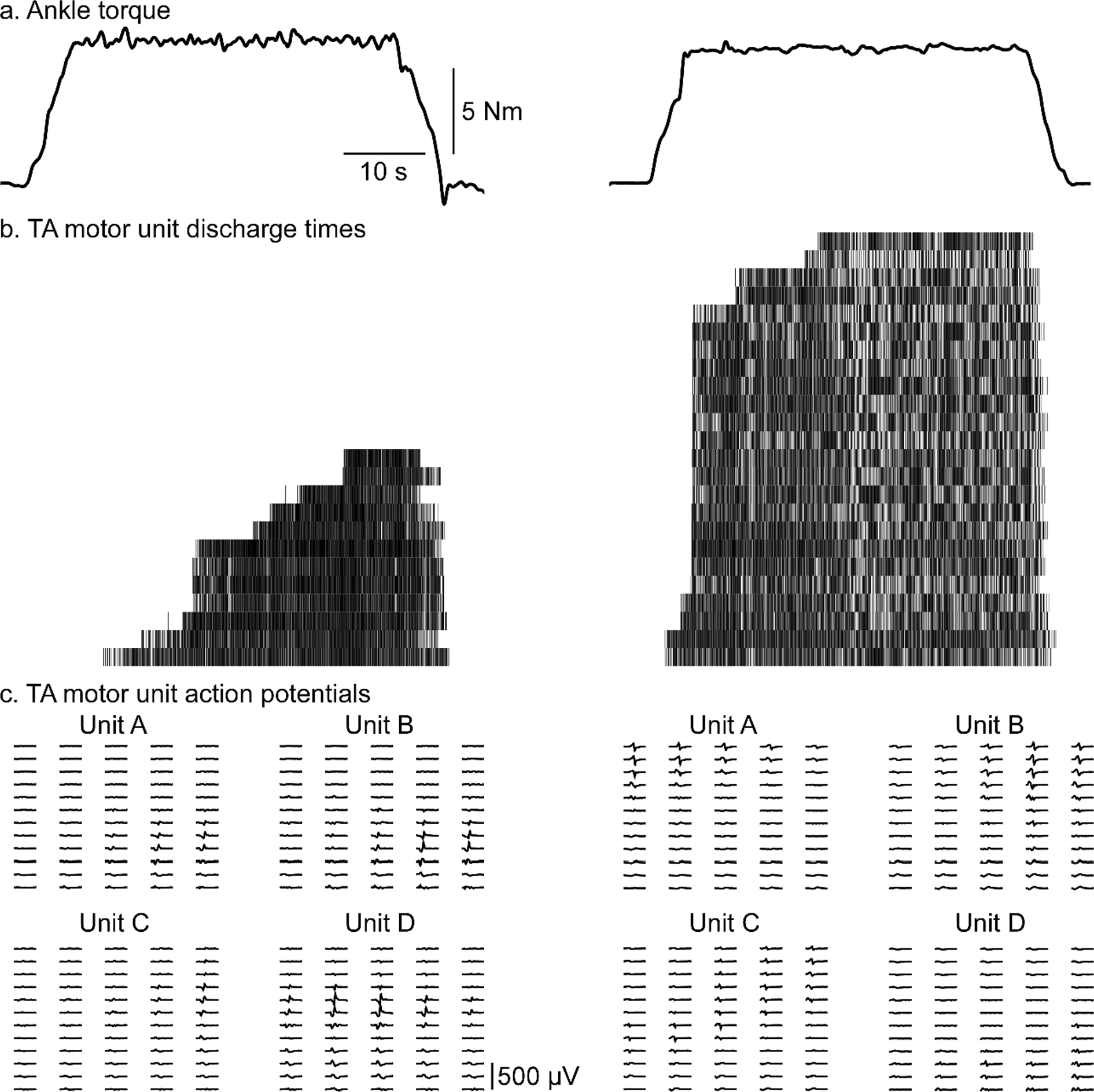
Data of two subjects (left female and right male) during 20% MVIC submaximal hold contractions. Dorsiflexion torque (solid black line) during submaximal hold contraction (A). A raster plot of motor unit discharge, each row represents a unique motor unit with each hashmark representing the discharge of that unit (B). Representative motor unit action potential wave forms are displayed in the bottom section (C).
2.3. Protocol
A series of submaximal isometric contractions were utilized, in which the force output could be controlled, to ensure relatively similar levels of effort were achieved between groups and within-subjects. During this time, MN activity was recorded with HDsEMG. Prior to data collection, participants were provided time to acclimate to the equipment to mitigate any learning effects during the torque generating tasks. At the beginning of each experimental session, participants performed three maximal volitional isometric contractions (MVIC) of the TA. Each MVIC consisted of a ~ 3 s increase to maximum, a ~ 3 s sustained maximal, and a ~ 3 sec decrease to rest. Each MVIC was separated by at least 2 min. For the purpose of EMG decomposition and subsequent motor unit analysis, participants were asked to produce and hold an isolated joint torque at a fixed percentage (20%) of their MVIC. Participants held the 20% of their MVIC for 40 s during these contractions. Participants performed a total of two isometric hold contractions. To mitigate the effects of fatigue and/or potentiation, two minutes of rest between contractions was imposed.
2.4. Data collection
During isometric contractions, differential HDsEMG data was recorded using the OTBioLab software (OT Bioelettronica; Turin, Italy). Torque was baseline corrected and filtered using a 20 Hz low pass filter, and this signal was used for visual feedback of task performance during the TA contractions. EMG was amplified at 150x, filtered at 20–900 Hz, and collected at 2048 Hz using a 16-bit A/D converter (Quattrocento, OT Bioelettronica; Turin, Italy), simultaneously with the torque data.
2.5. EMG conditioning
To quantify EMG amplitude during maximal and submaximal contractions, a single differential channel (channel 27) was selected, in the center of the surface array, from which EMG activity was recorded. This recording was full wave rectified and low pass filtered at 20 Hz. The maximum EMG amplitude value of each MVIC attempt was calculated from this filtered data. The average EMG amplitude of the 20% submaximal contractions was calculated from the filtered EMG data 2 s after the start of and 2 s prior to the completion of the torque plateau from each contraction.
2.6. Motor unit decomposition
HDsEMG array recordings were inspected offline, and up to 64 acceptable monopolar channels were isolated for subsequent processing. The selected HDsEMG array channels were then decomposed into the discharge of individual motor units using a convolutive blind source separation algorithm (Negro et al., 2016). This algorithm has been extensively validated and provides an accurate method for decomposing motor unit spike trains (e.g. Thompson et al., 2018). Multiple different permutations of the decomposition parameters were applied to ensure the highest accuracy and motor unit yield. For this, several cost functions (Holobar et al., 2012; Chen and Zhou, 2016; Negro et al., 2016) and three extension factors (8, 16, and 24) were used, yielding a total of 27 decomposition combinations for each trial, of which the version with the highest average silhouette and yield of suitable motor units was chosen for further analysis.
A semi-automated MU cleaning process was performed by an experienced investigator to ensure the accurate detection of each spike of each motor unit, similar to previous reports (Afsharipour et al., 2020; Hassan et al., 2020; Cudicio et al., 2021). All remaining motor unit spike trains were identified and incorporated into the subsequent data set for further analysis. The total count of these spike trains, for each subject, are referred to as MU Yield. The mean discharge rate and coefficient of variation (CoV) of the inter-spike intervals (ISI) of each MU were calculated from the motor unit data 2 s after the start of and 2 s prior to the completion of the torque plateau from each contraction (ensuring that only the isolated steady-state firing of each motor unit, during stable torque levels, were considered for analysis). Recruitment thresholds for each MU were defined as the torque at which each motor unit’s first identified spike occurred.
2.7. Motor unit action potential (MUAP) analysis
Motor unit action potential (MUAP) waveforms across the array were calculated by spike-triggered averaging the HDsEMG data using the discharge of each motor unit (Fig. 1c). The peak-to-peak amplitude and median frequency content of the waveforms at each electrode were calculated on these reconstructed MUAPs. Spatial distribution of MUAPs across the array was calculated by identifying the number of channels (total of 58 channels on each array), for each MU identified, in which MUAP peak-to-peak amplitude exceeded 50% of each MUAPs maximum peak-to-peak amplitude.
2.8. Statistical analysis
The normality of the data was tested with the Shapiro-Wilk test and revealed normal distributions for MU characteristics, torque, and MU yield. An unpaired t-test was used to compare differences in subject characteristics, maximal torque, and maximal EMG amplitude. The consistency of key variables obtained during the two submaximal hold contractions was assessed using a two-way mixed effect, consistency, single rater intraclass correlation analysis (ICC2,1) (Shrout and Fleiss, 1979). According to guidelines set forth by (Koo and Li, 2016), ICC values less than 0.5, between 0.5 and 0.75, between 0.75 and 0.9, and greater than 0.90 are interpreted as poor, moderate, good, and excellent reliability, respectively. A two-way repeated-measures analysis of variance was used to test the main effect of Sex and Contraction for the key motor unit variables during the submaximal hold contractions. All statistical analysis were performed using MATLAB 2017b (MathWorks, Natick, USA), and significance was accepted at the p ≤ value 0.05. Results are reported as mean and (±standard deviation).
3. Results
3.1. Subject characteristics
Subjects, by sex group, did not statistically differ in age (female (25.97 years (4.46 ±); male (28.36 years (5.84 ±)), weight (female (73.91 kg (9.89 ±); male (84.25 kg (8.99 ±)), nor BMI (female (25.67 BMI (3.96 ±); male (27.36 BMI (3.78 ±)). However, female (159.69 cm (4.72 ±)) subjects were on average shorter (175.68 cm (6.68 ±)) than their male counterparts (p = 0.005).
3.2. Ankle torque
Key results are presented in Table 1. On average, females generated less torque (20.6 Nm (11.0 ±)) during MVIC attempts than males (38.5 Nm(11.1 ±)) (p < 0.005). During the 20% MVIC submaximal hold contractions, when torque was normalized to each subject’s maximum MVIC, no significant difference was observed between groups. However, females displayed higher CoV of torque than males.
Table 1.
Motor Unit and Torque Characteristics During 20% MVIC Contractions.
| Female (n = 10) |
Male (n = 14) |
Significance | |||
|---|---|---|---|---|---|
| Mean | ICC | Mean | ICC | ||
|
| |||||
| MU yield | 13.4 MUs (9.0 ± ) | 0.98 | 27.7 MUs (7.3 ± ) | 0.91 | 0.015* |
| Mean Torque | 4.2 Nm (2.1 ± ) | 0.96 | 7.7 Nm (2.7 ± ) | 0.98 | 0.045* |
| Normalized Torque | 20.1% (1.6 ± ) | 0.98 | 20.0% (1.6 ± ) | 0.97 | 0.24 |
| Torque CoV | 3.4% (1.2 ± ) | 0.91 | 2.3% (1.0 ± ) | 0.89 | 0.039* |
| Mean sEMG Amplitude | 62.3 μV (21.2 ± ) | 0.79 | 94.0 μV (42.9 ± ) | 0.85 | 0.03* |
| Normalized sEMG Amplitude | 8.82% (6.5 ± ) | 0.79 | 13.51% (6.0 ± ) | 0.84 | 0.66 |
| Median Frequency of sEMG | 89.9 Hz (21.1 ± ) | 0.98 | 93.8 Hz (25.4 ± ) | 0.85 | 0.88 |
| MU Discharge Rate | 12.7 pps (0.8 ± ) | 0.72 | 11.4 pps (1.6 ± ) | 0.87 | 0.02* |
| CoV of ISI | 16.2% (2.6 ± ) | 0.80 | 17.1% (5.3 ± ) | 0.87 | 0.25 |
| Recruitment Threshold | 16.6% (3.5 ± ) | 0.94 | 12.3% (3.7 ± ) | 0.81 | 0.005* |
| Max p2p MUAP Amplitude | 204.3 μV (21.2 ± ) | 0.91 | 334.1 μV (165.6 ± ) | 0.94 | 0.007* |
| Mean p2p MUAP Amplitude | 163.74 μV (60.70 ± ) | 0.92 | 260.37 μV (117.43 ± ) | 0.95 | 0.001* |
| Median MUAP Frequency | 97.18 Hz (23.80 ± ) | 0.92 | 121.20 Hz (16.67 ± ) | 0.94 | 0.008* |
| Normalized MUAP distribution | 18.58% (4.6 ± ) | 0.96 | 16.46% (3.8 ± ) | 0.97 | 0.55 |
Abbreviations: Motor unit (MU); Maximum volitional isometric contraction (MVIC); Coefficient of Variation (CoV); Inter-Spike Interval (ISI); peak-to-peak (p2p); Motor unit action potential (MUAP). Statistically significant (< 0.05) p-value (*).
3.3. Tibialis anterior EMG
The average maximum sEMG amplitude was not significantly different between females (275.43 μV (119.07 ±)) and males (225.65 μV (100.85 ±)), during MVIC attempts (p = 0.111). However, on average, females expressed lower mean sEMG amplitude during submaximal hold contractions than males. No difference was observed in the median frequency content of sEMG during the submaximal hold contractions.
3.4. Tibialis anterior motor unit yield
A total of 48 submaximal contractions yielded 522 motor unit spike trains for subsequent analysis. On average, we were able to extract nearly twice as many motor units in males as compared to females (Table 1). This yield was consistent across contractions, with ICC values representing excellent levels of reliability. ICC values are utilized to ensure within-subject reliability is maintained and both contractions are completed as similarly as possible (Martinez-Valdes et al., 2016). As noted more specifically in subsequent sections, all motor unit and force generation characteristics presented with good (0.75 to 0.9) to excellent reliability (greater than 0.9), suggesting participants performed both contractions similarly. Despite these findings, MU yield varied in a non-uniform manner across contractions and sex. A Bland-Altman plot was used in quantifying the agreement between the two contractions by visualizing the mean difference and constructing limits of agreement (Fig. 2). We observed very little variation in MU yield among subjects with very low yield, however, as MU yield increased so did the variation in yield.
Fig. 2. Bland-Altman plot.
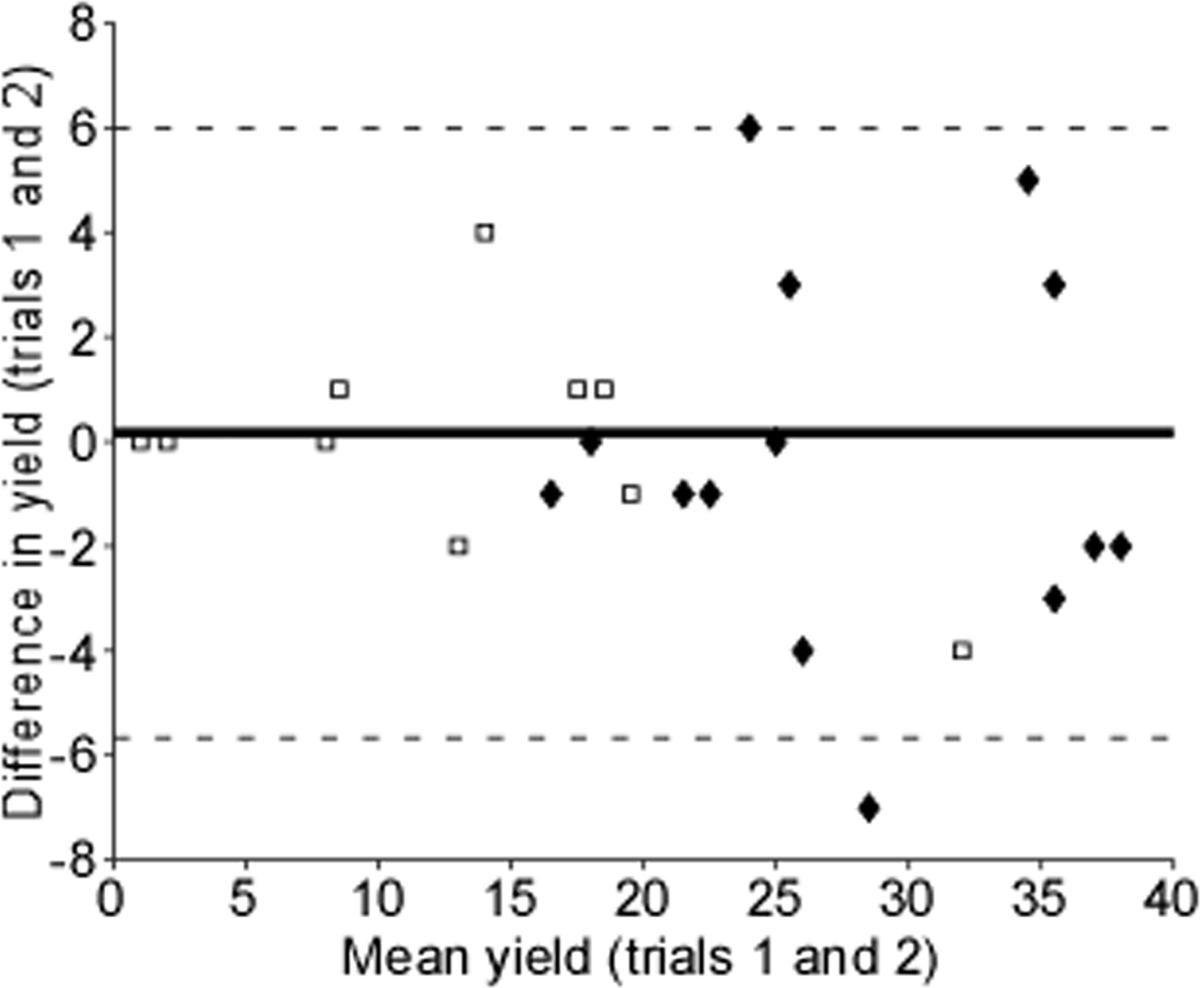
The difference in motor unit yield is plotted against the mean yield of each subject (open squares represent females, solid diamonds represent male subjects). The solid line represents the mean difference, and the dashed lines represent two standard deviations.
3.5. Tibialis anterior motor unit discharge characteristics
On average, females had higher MUDR during the submaximal hold contractions, than males. Additionally, females had higher MU recruitment thresholds during submaximal hold contractions than males. Further, the range of recruitment threshold for females (14.22% (8.63 ±)) was less than males (18.36% (3.07 ±)) (p = 0.02). The CoV of the interspike interval (ISI) did not differ between females and males. Mean motor unit discharge rates and recruitment thresholds during the submaximal hold contractions showed high ICC values representing good-to-excellent levels of reliability.
After observing the disparity in MU recruitment thresholds between sexes, an additional investigation was performed. We discovered that 50.5% (135 spike trains) of female MUs were recruited two or more seconds after the torque had already plateaued; in contrast, males only had this occur for 11.3% (88 spike trains) of their MUs. To observe the effects of these “late” recruited MUs, a sub-analysis of MUDR and other key factors were performed with these units excluded from the data set. With the removal of “late” MUs; the aforementioned differences in MUDR and variability were preserved, and a significant difference in MUDR was found within this sub-set of data (females: 12.48 pps (0.88 ±) vs. males: 11.33 pps (0.91 ±) (p = 0.01).
3.6. Tibialis anterior motor unit action potential morphology
On average, females had a smaller maximum peak-to-peak MUAP amplitude, smaller mean peak-to-peak MUAP amplitude, and lower frequency content of their MUAPs than males. The peak-to-peak maximum MUAP amplitude and frequency content of the MUAP, during the 20% MVIC hold contractions, showed high ICC values representing excellent levels of reliability.
When observing the spatial distribution of MUAPs across the array, normalized to each MUAPs maximum P2P amplitude, females and males did not statistically differ in the percentage of activity across the entire HDsEMG array.
3.7. Factors underlying decomposition yield
Initially, we considered that the magnitude of subcutaneous tissue may influence the detection of the EMG signal in a way that is detrimental to motor unit yield. Specifically, we expected there would be a negative relationship between the amount of subcutaneous tissue and motor unit yield. BMI was used as a crude measure of this subcutaneous tissue. Indeed, when pooled across sex, there was a significant relationship between BMI and motor unit yield, but this relationship was positive (r = 0.38; p = 0.04).
To further explore this relation, we added BMI as a factor in the ANOVA. A significant main effect of BMI (p = 0.031) was observed, and the main effect of sex remained significant (p = 0.015). A significant interaction between BMI and sex was also observed (p = 0.047). This interaction appears to be driven by the poor yield in females with lower BMI (Fig. 3).
Fig. 3. MU Yield as a function of body mass index.
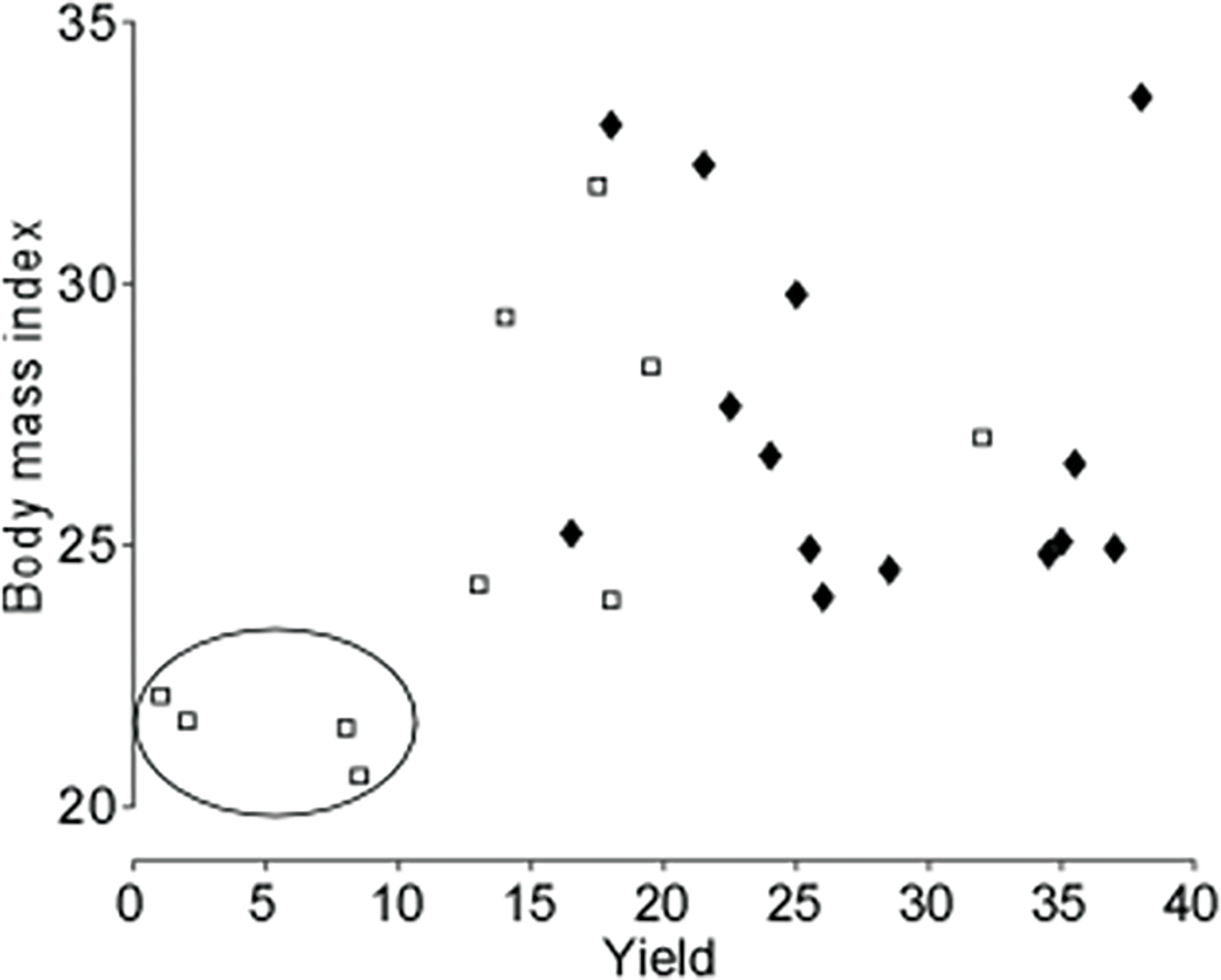
The MU yield is averaged between Trial 1 and Trial 2 for each subject. Open squares represent female subjects and solid diamonds represent male subjects. A circle is drawn around a small sub-set of female subjects with relatively low BMI and low motor unit yield.
4. Discussion
In this study, we assessed the potential sex-related differences in central neuromuscular characteristics and MU yield of the tibialis anterior motor pool using HDsEMG and motor unit decomposition techniques. Consistent with previous work (Hoffman et al., 1979; Bishop et al., 1987), we observed males generated greater torque than females during maximal contractions, this may be due to sex-related differences in peripheral neuromuscular characteristics (muscle fiber size, type, and composition) (English et al. 1999; Drzymala-Celichowska et al. 2012; Mierzejewska-Krzyżowska et al. 2011). However, during intensity-matched (20% MVIC) contractions, females had significantly higher MUDR, higher mean MU recruitment thresholds, lower MUAP peak-to-peak amplitude, and lower MUAP frequency content than males despite similar relative levels of torque and MU discharge variability. Notably, during the 20% MVIC contractions, males yielded nearly twice the amount of MUs as females via HDsEMG decomposition.
4.1. Sex differences in the detection of motor units
In an attempt to maximize MU yield, we utilized 27 different versions of the decomposition algorithms to extract optimal MU yield in both sexes (See Methods). From this pool of algorithms, we chose the best decomposition based on accuracy level, visual inspection, and number of motor units yielded. Additionally, we observed high levels of repeatability of these findings within the same person and across trials. Suggesting the factors associated with the ability (or inability) of the HDsEMG signal to be decomposed may be relatively consistent within and across experimental sessions.
Recently, others have also observed similar findings of low MU yield in female subjects using HDsEMG and MU decomposition (Afsharipour et al., 2020; Del Vecchio et al., 2020; Hug et al., 2021). In general, MUs within female subjects are more difficult for the decomposition algorithms to discriminate among others in the same motor pool. Filtering effects on the HDsEMG signal during collection and decomposition could give a biased perspective of the true MU behavior during these tasks, although it is unknown as to exactly which mechanisms cause this occurrence more readily in females. Superimposition of MUs with lower MUAP amplitude and higher MUDR could be more difficult for the decomposition algorithm to discriminate between, but future work should evaluate these effects on MU yield (Haynes and Kim, 2021).
Previous studies have suggested higher levels of subcutaneous fat as a confounding variable in filtering effects of HDsEMG (Farina et al., 2002a; Kuiken et al., 2003). In the current study, body mass index (BMI), an anthropometric estimate of subcutaneous fat (Hall and Cole, 2006), did not statistically differ between groups. Further, we found that females with the lowest BMI also had the lowest MU yield (see Fig. 3). This finding might emphasize the importance of lean muscle mass (e.g., cross-sectional area) rather than subcutaneous fat in MU yield for these participants.
Motor unit type and distribution throughout the TA motor pool may also direct the observed differences in the MU yield between sexes. The TA was chosen for its reliability in extracting MUs (Del Vecchio et al., 2020), its wide MU recruitment range (Del Vecchio et al., 2018), and for providing a relatively easy-to-access interface for the HDsEMG arrays, which helps mitigate movement artifacts. However, it is not clear if the composition of MUs within the TA motor pool is similar between males and females. Previous research in humans (Miller et al., 1993) and animals (English et al. 1999; Drzymala-Celichowska et al. 2012; Mierzejewska-Krzyżowska et al. 2011), have shown sex-related differences in muscle fiber size and composition. Further, Trevino et al. (2019) have shown males elicit larger MUAPs in higher threshold units compared to females, suggesting a sex-related difference in muscle fiber composition characteristics. These differences are supported by our findings of lower MUAP peak-to-peak amplitude in females. It is possible that by having a higher percentage of low threshold MUs with increased MUDR, the decomposition algorithms are not as effective at differentiating female MUAPS. Further, it is also possible the MU recruitment strategies of males and females differ (Nishikawa et al., 2017) or motor unit behavior may be altered due to overt differences in peripheral neuromuscular traits (Celichowski and Drzymała, 2006). While MUAP shape, amplitude, and frequency content give vital clues of the approximation of the MU, the decomposition algorithms may more readily identify male MUs through this action.
4.2. Sex differences in the neural control of motor output
In the current study, females exhibited higher MUDR under submaximal contraction, and these findings are replicated in similar studies using fine-wire EMG (Peng et al., 2018; Inglis and Gabriel, 2020). Further, the current study found a significant difference in MU recruitment thresholds between sexes. The occurrence of late recruited units and shift in mean recruitment threshold may be a result of differing motor control strategies, such as an increase in reliance of co-contraction of the antagonist muscle to maintain force steadiness during the task (Mengarelli et al., 2017) or may be a result of MU exclusion bias due to filtering effects (e.g., subcutaneous fat) or MU superimposition (Haynes and Kim, 2021). It is important to note, if lower threshold MUs are more vulnerable to exclusion bias in HDsEMG collection (as we suspect it could be the case in the current study), this will imply even greater differences between sexes in MUDR as lower threshold MUs typically have greater MUDR than higher threshold MUs (De Luca and Erim, 1994). Moreover, females exhibited lower MUAP peak-to-peak amplitude, a finding supported by other recent work (Trevino et al., 2019) and lower MUAP frequency content, suggesting there may be sex-related differences in innervation number (Drzymała-Celichowska and Krutki, 2015) or the size of MUs utilized during these 20% MVIC contractions. Given the lower and more compressed female MU recruitment range, higher MUDR, and differing MUAP characteristics in the current study, these findings could suggest alternative force generation strategies or differences in central neuromuscular properties between sexes.
It is noteworthy to mention that global EMG measures, such as median frequency of EMG and 100% MVIC EMG amplitude, did not differ significantly between sexes. These global EMG measures have been used in the previous studies to quantify neural drive to various motor pools, and our findings shed light on the inadequacy of interpreting sEMG findings (Keenan et al., 2006; Del Vecchio et al., 2017; Vigotsky et al., 2017; Martinez-Valdes et al., 2018; Dideriksen and Farina, 2019).
The spatial differences in MUAP waveforms across the 64-channel array were only significantly different when not normalized to maximum peak-to-peak amplitude, suggesting this is a representation in the disparity of MUAP amplitude, not spatial characteristics. When MUAP amplitude was normalized to each MU, there was no spatial difference between sexes, suggesting no gross spatial differences in MU recruitment.
5. Sex differences observed in the HDsEMG literature
To our knowledge, no other study has explicitly assessed sex-related differences in MU yield of humans with HDsEMG. The methods used in the current study allow for the quantification of the concurrent discharge of a large amount of individual human motoneurons. These techniques make it possible to accurately and reliably infer the underlying motoneuron activity from decomposed EMG signals (Thompson et al., 2018). Further, these methods offer numerous methodological and practical benefits over traditional surface EMG and fine-wire EMG methods, particularly with regard to the mitigation of crosstalk, amplitude cancellation, and improved spatial resolution (Farina et al., 2002b; Del Vecchio et al., 2017; Cudicio et al., 2021; Germer et al., 2021). The application of this technique provides a new means to quantify the organization and distribution of human spinal motoneurons between the sexes.
Despite the advantages of this approach, the majority of recent (year 2000 and later) HDsEMG studies, including participants with no known neuromuscular impairments, contained very few female participants (See Appendix), with the majority of studies opting to include males exclusively. The approximately 7 to 1 male/female disparity across these studies severely limits the generalizability of this powerful technology and our understanding of human physiology, especially if the limited data we acquire from female subjects is significantly less data than what is possible to extract from males. Currently, it is unclear from these experiments if sex-related differences exist in central neuromuscular factors that affect force production. If sex-related differences exist in central neuromuscular traits, it is imperative to revisit canonical findings in the literature and replicate them in both sexes.
5.1. Limitations
Some limitations of this study include the lack of varying contraction types, MVIC intensities, and differing motor pools. It is possible these findings may vary under these aforementioned conditions – however previous work (from the tibialis anterior and other muscles) is consistent with the conclusion that the current yield observed with contemporary HDsEMG decomposition approaches is lower in females than males. Further, in the context of female subjects, the phase of menstruation cycle and use of hormonal birth control were not controlled for. It is not clear how differences in these factors may affect motor unit behavior. Additionally, muscle size (e.g., cross-sectional area) was not directly measured in the current study, so it is possible the HDsEMG arrays were not adequately capturing the same percentage of MUs in each subject, however, given the relative size of the TA muscle to the size of the array it seems unlikely this would meaningfully affect the findings of the present study. Lastly, although calculated BMI is correlated with body fat levels (Hall and Cole, 2006), direct measures of subcutaneous fat were not measured in the current study. It is possible differences in local distribution of subcutaneous fat and muscle mass may augment BMI values and the interpretation of these results. Future studies should focus on the estimation of peripheral properties from populations of motor units (Negro and Orizio, 2017) in female subjects to fully understand their influence on discharge rate properties.
5.2. Conclusion
Sex differences in the phenotypic expression of peripheral neuromuscular traits contribute greatly to the disparity in absolute torque generation between sexes. However, emerging examples of divergent central neuromuscular factors, such as those shown in the current study, illuminate the possibility of sex differences in central mechanisms as well. These differences may be a result of augmented neural drive in females to compensate for overt differences in peripheral traits or they may be a result of more elusive central neuromuscular differences.
The discharge of single motor units provides insights into potential sex-related differences in the neural control of the motor output. However, recording from a relatively large number of concurrently active motor units is necessary to accurately understand the neural drive to each motor pool. The decomposition of the HDsEMG signal may be a means to capture such data however, females provide significantly lower motor unit yield counts than males – it remains unclear how lower yields will affect the accuracy of these calculations. Future work should investigate the potential sex-related differences in decomposition algorithms and signal filtering effects on this outcome. Lastly, it is imperative we strive to narrow the selection bias gap in this research niche, as it may have long-term impacts on the development of this technology and our understanding of human motor behavior.
Acknowledgements
We wish to thank Tyler Kmiec for his expertise and assistance with the motor unit cleaning process.
Funding Sources
This work was supported by NS098509 to Charles J. Heckman.
Abbreviations:
- MU
motor unit
- sEMG
surface electromyography
- MUAP
motor unit action potential waveforms
- MUDR
motor unit discharge rate
- MVIC
maximal volitional isometric contraction
- HDsEMG
high-density electromyography
- TA
tibialis anterior
- CoV
coefficient of variation
- ISI
inter-spike intervals
- ICC
intraclass correlation analysis
- BMI
body mass index
Appendix A
A literature search was performed to quantify the representation of males and females in hdEMG decomposition. To identify studies considered, several databases and search engines were used: PubMed (NLM) and IEEE Xplore. Multiple search terms were used including “high density EMG,” “high density electromyography,” “hd EMG,” and “motor unit decomposition.”
Over 1,000 articles were initially rendered, utilizing secondary search functions based on relevance from the abstract and a publication date after the year 2000; this list was reduced to ~300.
Of the remaining articles, 59 were selected to form the basis for the current review due to explicit mention of sex in the methods section and inclusion of only participants with no known neuromotor pathology (Fig. 1a). In total, 799 subjects were extracted from the search findings (97 female and 702 male). Additionally, 40 of the 59 studies (67.7%) recruited solely male subjects. This finding is likely an under representation of the totality of HDsEMG literature as many articles that were excluded from the review did not explicitly mention sex or gender in the reporting of the participant demographics (see Fig. A1).
Fig. A1. Literature Review Results.
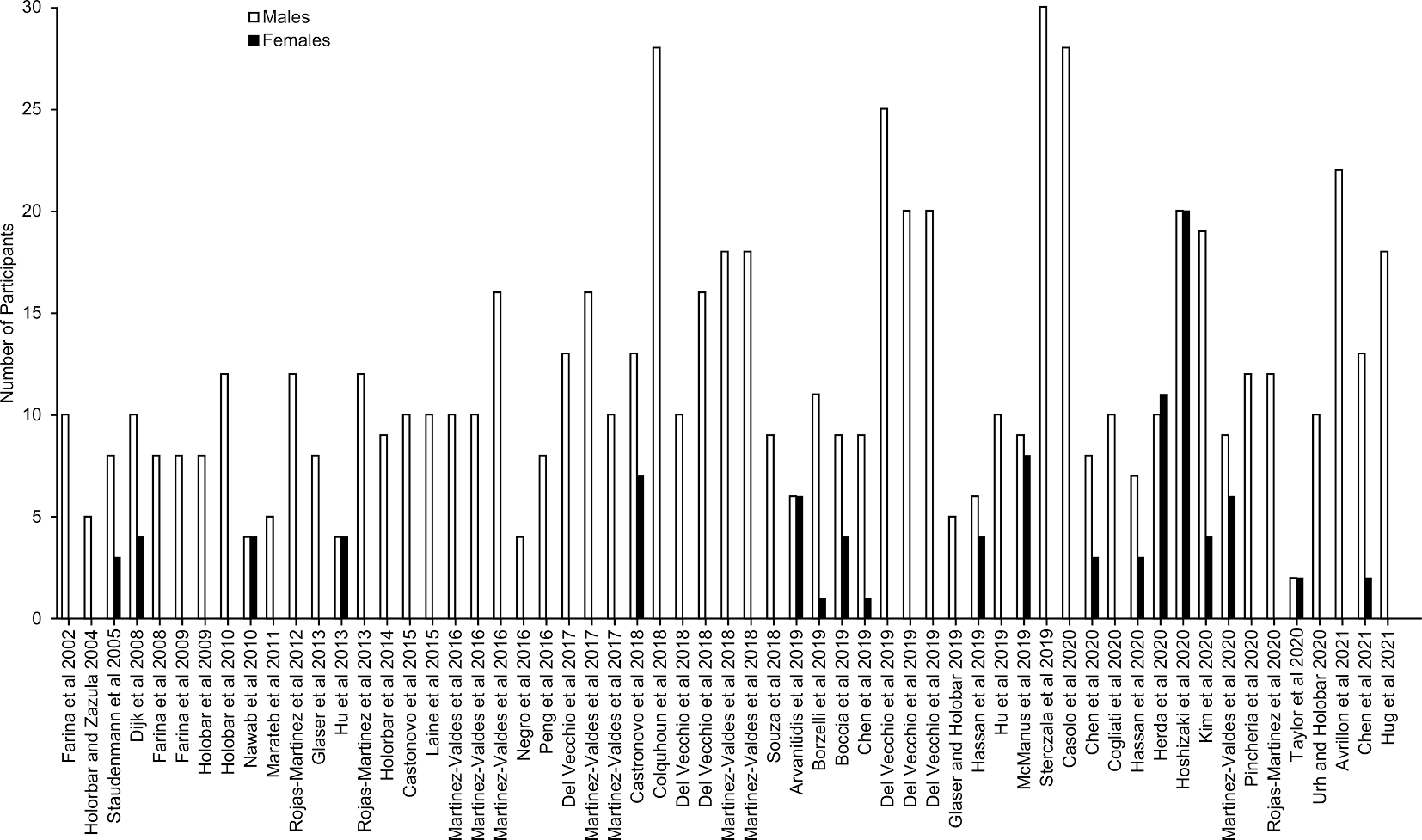
59 peer-reviewed journal articles, listed by publication date, selected from literature search to quantify the representation of males and females in hdEMG decomposition.
Footnotes
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Afsharipour B, Manzur N, Duchcherer J, Fenrich KF, Thompson CK, Negro F, Quinlan KA, Bennett DJ, Gorassini MA, 2020. Estimation of self-sustained activity produced by persistent inward currents using firing rate profiles of multiple motor units in humans. J. Neurophysiol. 124 (1), 63–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop P, Cureton K, Collins M, 1987. Sex difference in muscular strength in equally-trained men and women. Ergonomics 30 (4), 675–687. [DOI] [PubMed] [Google Scholar]
- Bolgla L, Cook N, Hogarth K, Scott J, West C, 2014. Trunk and hip electromyographic activity during single leg squat exercises do sex differences exist? Int. J. Sports Phys. Ther. 9, 756–764. [PMC free article] [PubMed] [Google Scholar]
- Breedlove SM, Arnold AP, 1983. Sex differences in the pattern of steroid accumulation by motoneurons of the rat lumbar spinal cord. J. Comp. Neurol. 215 (2), 211–216. [DOI] [PubMed] [Google Scholar]
- Celichowski J, Drzymała H, 2006. Differences between properties of male and female motor units in the rat medial gastrocnemius muscle. J. Physiol. Pharmacol. 57, 83–93. [PubMed] [Google Scholar]
- Cheah A, Lee EY, Lim AYT, 2019. Upper Extremity Axon Counts and Clinical Implications for Motor Nerve Transfer. Plast Reconstr. Surg. 144 (6). [DOI] [PubMed] [Google Scholar]
- Chen M, Zhou P, 2016. A Novel Framework Based on FastICA for High Density Surface EMG Decomposition. IEEE Trans. Neural Syst. Rehabil. Eng.: A Publ. IEEE Eng. Med. Biol. Soc. 24 (1), 117–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen E, 1959. Topography of terminal motor innervation in striated muscles from stillborn infants. Am. J. Phys. Med. 38, 65–78. [PubMed] [Google Scholar]
- Christie A, Kamen G, 2010. Short-term training adaptations in maximal motor unit firing rates and afterhyperpolarization duration. Muscle Nerve 41 (5), 651–660. [DOI] [PubMed] [Google Scholar]
- Cihak R, Gutmann E, Hanzlikova V, 1970. Involution and hormone-induced persistence of the M. sphincter (levator) ani in female rats. J. Anat. 106, 93–110. [PMC free article] [PubMed] [Google Scholar]
- Cioni R, Giannini F, Paradiso C, Battistini N, Navona C, Starita A, 1994. Sex differences in surface EMG interference pattern power spectrum. J. Appl. Physiol. 1985 (77), 2163–2168. [DOI] [PubMed] [Google Scholar]
- Cudicio A, Martinez-Valdes E, Cogliati M, Orizio C, Negro F, 2021. The force-generation capacity of the tibialis anterior muscle at different muscle-tendon lengths depends on its motor unit contractile properties. Eur. J. Appl. Physiol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deluca C, Erim Z, 1994. Common drive of motor units in regulation of muscle force. Trends Neurosci. 17 (7), 299–305. [DOI] [PubMed] [Google Scholar]
- Del Vecchio A, Holobar A, Falla D, Felici F, Enoka RM, Farina D, 2020. Tutorial: Analysis of motor unit discharge characteristics from high-density surface EMG signals. J. Electromyogr. Kinesiol.: Off. J. Int. Soc. Electrophysiol. Kinesiol. 53, 102426. [DOI] [PubMed] [Google Scholar]
- Del Vecchio A, Negro F, Felici F, Farina D, 2017. Associations between motor unit action potential parameters and surface EMG features. J. Appl. Physiol. 1985 (123), 835–843. [DOI] [PubMed] [Google Scholar]
- Del Vecchio A, Negro F, Felici F, Farina D, 2018. Distribution of muscle fibre conduction velocity for representative samples of motor units in the full recruitment range of the tibialis anterior muscle. Acta Physiol. (Oxf) 222 (2). [DOI] [PubMed] [Google Scholar]
- Dideriksen JL, Farina D, Haith AM, 2019. Amplitude cancellation influences the association between frequency components in the neural drive to muscle and the rectified EMG signal. PLoS Comput. Biol. 15 (5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drzymala-Celichowska H, Karolczak J, Redowicz MJ, Bukowska D, 2012. The content of myosin heavy chains in hindlimb muscles of female and male rats. J. Physiol. Pharmacol. 63, 187–193. [PubMed] [Google Scholar]
- Drzymała-Celichowska H, Krutki P, 2015. Slow motor units in female rat soleus are slower and weaker than their male counterparts. J. Muscle Res. Cell Motil. 36 (3), 287–295. [DOI] [PubMed] [Google Scholar]
- Duchateau J, Enoka RM, 2011. Human motor unit recordings: origins and insight into the integrated motor system. Brain Res. 1409, 42–61. [DOI] [PubMed] [Google Scholar]
- English AW, Eason J, Schwartz G, Shirley A, Carrasco DI, 1999. Sexual dimorphism in the rabbit masseter muscle: Myosin heavy chain composition of neuromuscular compartments. Cells Tissues Organs 164, 179–191. [DOI] [PubMed] [Google Scholar]
- Farina D, Cescon C, Merletti R, 2002a. Influence of anatomical, physical, and detection-system parameters on surface EMG. Biol. Cybern. 86 (6), 445–456. [DOI] [PubMed] [Google Scholar]
- Farina D, Merletti R, Enoka RM, 2014. The extraction of neural strategies from the surface EMG: an update. J. Appl. Physiol. 1985 (117), 1215–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farina D, Merletti R, Indino B, Nazzaro M, Pozzo M, 2002b. Surface EMG crosstalk between knee extensor muscles: experimental and model results. Muscle Nerve 26 (5), 681–695. [DOI] [PubMed] [Google Scholar]
- Germer CM, Farina D, Elias LA, Nuccio S, Hug F, Del Vecchio A, 2021. Surface EMG cross talk quantified at the motor unit population level for muscles of the hand, thigh, and calf. J. Appl. Physiol. 1985 (131), 808–820. [DOI] [PubMed] [Google Scholar]
- Grisham W, Casto JM, Kashon ML, Ward IL, Byron Ward O, 1992. Prenatal flutamide alters sexually dimorphic nuclei in the spinal cord of male rats. Brain Res. 578 (1–2), 69–74. [DOI] [PubMed] [Google Scholar]
- Hall DM, Cole TJ, 2006. What use is the BMI? Arch. Dis. Child 91, 283–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harwood B, Cornett KMD, Edwards DL, Brown RE, Jakobi JM, 2014. The effect of tendon vibration on motor unit activity, intermuscular coherence and force steadiness in the elbow flexors of males and females. Acta Physiol. (Oxf) 211 (4), 597–608. [DOI] [PubMed] [Google Scholar]
- Hassan A, Thompson CK, Negro F, Cummings M, Powers RK, Heckman CJ, Dewald JPA, McPherson LM, 2020. Impact of parameter selection on estimates of motoneuron excitability using paired motor unit analysis. J. Neural Eng. 17 (1), 016063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes EMK, Kim C, 2021. Antagonist surface electromyogram decomposition and the case of the missing motor units. J. Neurophysiol. 126 (6), 1943–1947. [DOI] [PubMed] [Google Scholar]
- Hicks AL, Kent-Braun J, Ditor DS, 2001. Sex differences in human skeletal muscle fatigue. Exerc. Sport Sci. Rev. 29 (3), 109–112. [DOI] [PubMed] [Google Scholar]
- Hoffman T, Stauffer RW, Jackson AS, 1979. Sex difference in strength. Am. J. Sports Med. 7 (4), 265–267. [DOI] [PubMed] [Google Scholar]
- Holobar A, Glaser V, Gallego JA, Dideriksen JL, Farina D, 2012. Non-invasive characterization of motor unit behaviour in pathological tremor. J. Neural Eng. 9 (5), 056011. [DOI] [PubMed] [Google Scholar]
- Hug F, Avrillon S, Del Vecchio A, Casolo A, Ibanez J, Nuccio S, Rossato J, Holobar A, Farina D, 2021. Analysis of motor unit spike trains estimated from high-density surface electromyography is highly reliable across operators. J. Electromyogr. Kinesiol.: Off. J. Int. Soc. Electrophysiol. Kinesiol. 58, 102548. [DOI] [PubMed] [Google Scholar]
- Inglis JG, Gabriel DA, 2020. Sex differences in motor unit discharge rates at maximal and submaximal levels of force output. Appl. Physiol. Nutr. Metab. 45 (11), 1197–1207. [DOI] [PubMed] [Google Scholar]
- Keenan KG, Farina D, Merletti R, Enoka RM, 2006. Amplitude cancellation reduces the size of motor unit potentials averaged from the surface EMG. J. Appl. Physiol. 1985 (100), 1928–1937. [DOI] [PubMed] [Google Scholar]
- Kent-Braun JA, Ng AV, Doyle JW, Towse TF, 2002. Human skeletal muscle responses vary with age and gender during fatigue due to incremental isometric exercise. J. Appl. Physiol. 1985 (93), 1813–1823. [DOI] [PubMed] [Google Scholar]
- Koo TK, Li MY, 2016. A Guideline of Selecting and Reporting Intraclass Correlation Coefficients for Reliability Research. J. Chiropr. Med. 15 (2), 155–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan C, Williams GN, 2009. Sex differences in quadriceps and hamstrings EMG-moment relationships. Med. Sci. Sports Exerc. 41, 1652–1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuiken TA, Lowery MM, Stoykov NS, 2003. The effect of subcutaneous fat on myoelectric signal amplitude and cross-talk. Prosthet Orthot Int. 27, 48–54. [DOI] [PubMed] [Google Scholar]
- Lenhardt SA, McIntosh KC, Gabriel DA, 2009. The surface EMG-force relationship during isometric dorsiflexion in males and females. Electromyogr. Clin. Neurophysiol. 49, 227–234. [PubMed] [Google Scholar]
- Martinez-Valdes E, Laine CM, Falla D, Mayer F, Farina D, 2016. High-density surface electromyography provides reliable estimates of motor unit behavior. Clin. Neurophysiol.: Off. J. Int. Federation Clin. Neurophysiol. 127 (6), 2534–2541. [DOI] [PubMed] [Google Scholar]
- Martinez-Valdes E, Negro F, Falla D, De Nunzio AM, Farina D, 2018. Surface electromyographic amplitude does not identify differences in neural drive to synergistic muscles. J. Appl. Physiol. 1985 (124), 1071–1079. [DOI] [PubMed] [Google Scholar]
- Mengarelli A, Maranesi E, Burattini L, Fioretti S, Di Nardo F, 2017. Co-contraction activity of ankle muscles during walking: A gender comparison. Biomed. Signal Process. Control 33, 1–9. [Google Scholar]
- Mierzejewska-Krzyżowska B, Bukowska D, Taborowska M, Celichowski J, 2014. Sex differences in the number and size of motoneurons innervating rat medial gastrocnemius muscle. Anat Histol. Embryol. 43 (3), 182–189. [DOI] [PubMed] [Google Scholar]
- Mierzejewska-Krzyżowska B, Drzym˙ a a-Celichowska H, Celichowski J, 2011. Gender differences in the morphometric properties of muscle fibres and the innervation ratio of motor units in rat medial gastrocnemius muscle. Anatomia, Histologia, Embryologia 40, 249–255. [DOI] [PubMed] [Google Scholar]
- Miller AEJ, MacDougall JD, Tarnopolsky MA, Sale DG, 1993. Gender differences in strength and muscle fiber characteristics. Eur. J. Appl. Physiol. Occup. Physiol. 66 (3), 254–262. [DOI] [PubMed] [Google Scholar]
- Negro F, Muceli S, Castronovo AM, Holobar A, Farina D, 2016. Multi-channel intramuscular and surface EMG decomposition by convolutive blind source separation. J. Neural. Eng. 13 (2), 026027. [DOI] [PubMed] [Google Scholar]
- Negro F, Orizio C, 2017. Robust estimation of average twitch contraction forces of populations of motor units in humans. J. Electromyogr. Kinesiol. 37, 132–140. [DOI] [PubMed] [Google Scholar]
- Neto HS, Filho JM, Passini R Jr., Marques MJ, 2004. Number and size of motor units in thenar muscles. Clin. Anat. 17, 308–311. [DOI] [PubMed] [Google Scholar]
- Nishikawa Y, Watanabe K, Takahashi T, Hosomi N, Orita N, Mikami Y, Maruyama H, Kimura H, Matsumoto M, 2017. Sex differences in variances of multi-channel surface electromyography distribution of the vastus lateralis muscle during isometric knee extension in young adults. Eur. J. Appl. Physiol. 117 (3), 583–589. [DOI] [PubMed] [Google Scholar]
- Nordeen EJ, Nordeen KW, Sengelaub DR, Arnold AP, 1985. Androgens prevent normally occurring cell death in a sexually dimorphic spinal nucleus. Science 229 (4714), 671–673. [DOI] [PubMed] [Google Scholar]
- Peng YL, Tenan MS, Griffin L, 2018. Hip position and sex differences in motor unit firing patterns of the vastus medialis and vastus medialis oblique in healthy individuals. J. Appl. Physiol. 1985 (124), 1438–1446. [DOI] [PubMed] [Google Scholar]
- Neto HS, de Carvalho VC, Marques MJ, 1998. Estimation of the number and size of human flexor digiti minimi muscle motor units using histological methods. Muscle Nerve 21 (1), 112–114. [DOI] [PubMed] [Google Scholar]
- Neto HS, Marques MJ, 2008. Estimation of the number and size of motor units in intrinsic laryngeal muscles using morphometric methods. Clin. Anat. 21 (4), 301–306. [DOI] [PubMed] [Google Scholar]
- Schroder HD, 1980. Organization of the motoneurons innervating the pelvic muscles of the male rat. J. Comp. Neurol. 192, 567–587. [DOI] [PubMed] [Google Scholar]
- Shrout PE, Fleiss JL, 1979. Intraclass correlations: uses in assessing rater reliability. Psychol. Bull. 86, 420–428. [DOI] [PubMed] [Google Scholar]
- Staron RS, Hagerman FC, Hikida RS, Murray TF, Hostler DP, Crill MT, Ragg KE, Toma K, 2000. Fiber type composition of the vastus lateralis muscle of young men and women. J. Histochem. Cytochem. 48 (5), 623–629. [DOI] [PubMed] [Google Scholar]
- Thompson CK, Negro F, Johnson MD, Holmes MR, McPherson LM, Powers RK, Farina D, Heckman CJ, 2018. Robust and accurate decoding of motoneuron behaviour and prediction of the resulting force output. J. Physiol. 596 (14), 2643–2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trevino MA, Sterczala AJ, Miller JD, Wray ME, Dimmick HL, Ciccone AB, Weir JP, Gallagher PM, Fry AC, Herda TJ, 2019. Sex-related differences in muscle size explained by amplitudes of higher-threshold motor unit action potentials and muscle fibre typing. Acta Physiol. (Oxf) 225 (4). [DOI] [PubMed] [Google Scholar]
- Vigotsky AD, Halperin I, Lehman GJ, Trajano GS, Vieira TM, 2017. Interpreting Signal Amplitudes in Surface Electromyography Studies in Sport and Rehabilitation Sciences. Front. Physiol. 8, 985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser SL, de Rijke W, 1974. Influence of sex and age on EMG contraction pattern. Eur. Neurol. 12, 229–235. [DOI] [PubMed] [Google Scholar]
- Yuan H.u., Goto N, Akita H, Goto J, Jin SR, 2000. Sexual dimorphism of the motoneurons in the human spinal cord. Okajimas Folia Anat. Jpn. 77 (5), 143–148. [DOI] [PubMed] [Google Scholar]


