Abstract
Pseudomonas aeruginosa clinical isolates exhibit invasive or cytotoxic phenotypes. Cytotoxic strains acquire some of the characteristics of invasive strains when a regulatory gene, exsA, that controls the expression of several extracellular proteins, is inactivated. exsA mutants are not cytotoxic and can be detected within epithelial cells by gentamicin survival assays. The purpose of this study was to determine whether epithelial cell invasion precedes and/or is essential for cytotoxicity. This was tested by measuring invasion (gentamicin survival) and cytotoxicity (trypan blue staining) of PA103 mutants deficient in specific exsA-regulated proteins and by testing the effect of drugs that inhibit invasion for their effect on cytotoxicity. A transposon mutant in the exsA-regulated extracellular factor exoU was neither cytotoxic nor invasive. Furthermore, several of the drugs that inhibited invasion did not prevent cytotoxicity. These results show that invasion and cytotoxicity are mutually exclusive events, inversely regulated by an exsA-encoded invasion inhibitor(s). Both involve host cell protein tyrosine kinase (PTK) activity, but they differ in that invasion requires Src family tyrosine kinases and calcium-calmodulin activity. PTK inhibitor drugs such as genistein may have therapeutic potential through their ability to block both invasive and cytotoxicity pathways via an action on the host cell.
In humans, Pseudomonas aeruginosa causes opportunistic infections of the respiratory tract, the cornea, burned skin, and other sites (30). Recent work has shown two categories of P. aeruginosa clinical isolates; one type invades epithelial cells (8, 9), and the other causes epithelial cell cytotoxicity after approximately 3 h of incubation with cells (1, 11). Both types are virulent in animal models of respiratory and corneal infections (8, 13, 21, 26, 31). Invasive and cytotoxic strains differ in the genes that are under the regulatory control of a transcriptional activator called ExsA, encoded by exsA. Several genes are coordinately regulated in this pathway. These include the gene encoding the 49-kDa form of exoenzyme S (exoS) only in invasive strains (13) and the gene for an approximately 70-kDa protein, ExoU (exoU), that is present only in cytotoxic strains (6). The gene encoding the 53-kDa form of exoenzyme S (exoT) is found in both invasive and cytotoxic strains (13). ExoU was recently found to be necessary for cytotoxic activity toward MDCK cells (6).
Cytotoxic strains of P. aeruginosa are inherently capable of invasion. Low levels of invasion are detectable by gentamicin survival assays early in the interaction with corneal or MDCK epithelial cells before cytotoxicity is initiated. If cytotoxicity is disabled by mutation of exsA, invasion can be detected at later time points with both these cell types (10, 13). These observations suggest that invasion and cytotoxicity may be sequential events. In this model, cytotoxic bacteria would enter cells, but subsequent cell killing by the invaded bacteria would allow penetration of antibiotic into the cells, rendering gentamicin survival assays incapable of detecting the presence of intracellular bacteria. This would explain why there is an inverse correlation among clinical isolates between their ability to invade cells as measured by gentamicin assays and their cytotoxic capacity (11).
Mammalian cell invasion and cytotoxicity by bacterial pathogens can involve the activation or inhibition of different signal transduction pathways in the host mammalian cells (2, 4). Studies have shown that inhibitors of mammalian cell signal transduction can prevent cell invasion by some bacterial pathogens (17, 27, 32). Inhibitors of bacterial invasion such as cytochalasin D have been used to show that, for Bordetella pertussis and Shigella flexneri, cytotoxicity can be prevented by inhibiting invasion (20, 23, 34). Cytochalasin D and the protein tyrosine kinase inhibitor genistein block P. aeruginosa invasion of corneal epithelia (9); the effect of these inhibitors on P. aeruginosa-induced cytotoxicity has not been explored.
The aim of this study was to determine if invasion occurs as part of the mechanism by which cytotoxic P. aeruginosa strains kill epithelial cells. If invasion and cytotoxicity are indeed sequential events, then therapeutic approaches aimed at preventing invasion should also block cytotoxicity. Otherwise, different therapeutic strategies might be necessary to manage P. aeruginosa infection according to whether the infecting strain is invasive or cytotoxic.
Two approaches were used to determine the role of invasion in cytotoxicity: (i) a genetic approach, studying transposon mutants of cytotoxic strain PA103, and (ii) a biochemical approach, using mammalian cell signaling inhibitors.
MATERIALS AND METHODS
Bacterial strains and mutants.
Three nonmucoid isolates of P. aeruginosa were tested (serogroup O11, strains 6206 and PA103, and serogroup O6, strain 6294). Strains 6206 and PA103 are cytotoxic for epithelial cells; strain 6294 invades cells without cytotoxicity (13). In this study, various transposon mutants of PA103 were tested to study the relationship between cytotoxicity and invasion. These included (i) an exoT mutant, PA103 exoT::Tc (6); (ii) an exoU mutant, PA103 exoU::Tn5Tc (6); (iii) a double mutant, PA103 exoU::Tn5Tc exsA::Ω (described below); (iv) PA103 exoU::Tn5Tc complemented with exoU in trans (pUCP exoU); or (v) PA103 exoU::Tn5Tc pUCP18, a vector control. Bacterial inocula were prepared from overnight cultures grown on Trypticase soy plates at 37°C (7). Bacterial colonies were suspended in tissue culture medium at various concentrations determined by spectrophotometry (optical density at 650 nm) and confirmed by viable count.
Construction of an exoU exsA double mutation in the chromosome of strain PA103.
To confirm that mutation of exoU in PA103 exoU::Tn5Tc had not affected invasion genes outside the loci regulated by ExsA, we constructed a strain in which exoU and exsA were sequentially inactivated. The starting strain was PA103 exoU::Tn5Tc. A suicide plasmid containing an exsA allele inactivated by the insertion of the Ω fragment (encoding streptomycin resistance) was transferred to PA103 exoU::Tn5Tc by conjugation as previously described (14). The suicide plasmid also contains the counterselectable markers, sacBR, which allows resolution of plasmid and the wild-type exsA allele by selection for growth on medium containing 5% sucrose (14). Sucrose- and streptomycin-resistant isolates were grown for exoenzyme S production, and the supernatants were screened for an exsA::Ω phenotype (absence of ExoT, PopB, PopD, and PcrV) by evaluating the patterns of Coomassie blue-stained protein bands after sodium dodecyl sulfate-polyacrylamide gel electrophoresis (10% separating gel) of concentrated extracellular material (14, 33). From 28 sucrose- and streptomycin-resistant isolates, 13 demonstrated an exsA::Ω phenotype. Southern blot analysis was performed on chromosomal preparations from four isolates with an exsA::Ω phenotype (data not shown). All four strains possessed an Ω insertion in exsA identical to that of the original strain isolated in previous studies using the same gene replacement strategy (14). In addition, all four strains retained the tetracycline resistance marker of Tn5Tc in exoU. Two of these mutants were studied: PA103 exoU::Tn5Tc exsA::Ω(1) and PA103 exoU::Tn5Tc exsA::Ω(2).
Preparation of cell cultures.
Immortalized rabbit corneal epithelial cells (24) were grown in 24- or 96-well tissue culture plates (Corning, New York, N.Y.) as previously described (11). Cells were fed with modified SHEM (18) containing bovine pituitary extract (5 μg/ml) in place of cholera toxin. Cells used in these experiments were grown 3 to 7 days after passaging. Results presented were obtained from cells grown between passages 4 and 16.
Inhibitors.
The following inhibitors of mammalian cell signaling or cytoskeleton function were used in this study (stock concentrations are given in parentheses): genistein (20 mM or 100 mM), tyrphostin A47 (100 mM), cytochalasin D (1 mM), herbimycin A (0.5 mM), wortmannin (5 mM), staurosporine (1 mM), BAPTA-AM [1-2-bis-(1-aminophenoxy)ethane N,N,N′,N′-tetra-acetoxymethyl ester] (5 or 25 mM), W-7 (2.5 mM), U-73122 (10 mM), U-73343 (10 mM), and indomethacin (5 mM). All stock solutions were prepared by dissolving the drug in dimethyl sulfoxide (DMSO; Fisher Scientific, Pittsburgh, Pa.), except for W-7, which was dissolved in distilled water. Stock solutions were stored at −20°C or 4°C as recommended by the manufacturer. All inhibitors were purchased from Calbiochem (La Jolla, Calif.) except genistein, cytochalasin D, and indomethacin, which were obtained from Sigma (St. Louis, Mo.). Working concentrations of each inhibitor were as follows: genistein, 200 μM; tyrphostin A47, 50 μM; cytochalasin D, 10 μM; herbimycin A, 5 μM; wortmannin, 50 nM; staurosporine, 100 nM; BAPTA-AM, 50 μM; W-7, 50 μM; U-73122, 5 μM; U-73343, 5 μM; and indomethacin, 50 μM.
Before exposure to bacteria, corneal cells were treated with inhibitor for 1 h (3.5 h for herbimycin A). Since most drug stock solutions contained DMSO, matching concentrations of DMSO were added to control samples without treatment drugs. In most experiments, the cells were incubated with bacteria in the continued presence of the drug for treated samples. In some experiments, as noted in the text, the drug was not added during the incubation of bacteria with the cells; the cells alone were pretreated with the indicated drug. In other experiments, only the bacteria were pretreated with drugs. Bacterial and cell viability in the presence of each drug was monitored in control samples that were included in every experiment. None of the drugs tested affected bacterial viability or growth (as assessed by standard counts of viable bacteria), nor did they affect corneal cell viability (as assessed by trypan blue staining).
Cytotoxicity assays.
Trypan blue exclusion assays were used to measure the cytotoxic effects of P. aeruginosa strains on a rabbit corneal epithelial cell line (11). Experiments were performed in MEM (minimal essential Eagle medium with Earle’s salts and l-glutamine; Cellgro; Mediatech, Fisher Scientific) buffered with 1 M HEPES–NaOH (pH 7.6), 0.35 g of NaHCO3, and 6 g of bovine serum albumin (Sigma) per liter or in MEM without buffer for inhibitor experiments (pH 7.4, maintained by incubation in 5% CO2). Briefly, bacteria were resuspended in prewarmed medium (37°C) to a concentration of 2 × 106 CFU/ml (strain 6206) or 1 × 107 CFU/ml (strain PA103 and mutants). For inhibitor experiments, corneal epithelial cells were washed once with medium (100 μl), exposed to inhibitor or control solutions in medium (100 μl), and then incubated with 100 μl of bacterial suspension (2 × 105 CFU) with or without inhibitors for 3 h (37°C, 5% CO2, pH 7.4). In experiments with PA103 and its mutants, cells were washed once with medium and exposed directly to bacterial suspensions (106 CFU) for 3 h. Bacterial suspensions were then removed from all samples, and cells were treated with 200 μl of gentamicin solution (200 μg/ml; Biowhittaker, Walkersville, Md.) for 1.5 h to kill extracellular bacteria. This was done to match the methods used for the invasion assays described below and to prevent progression of cytotoxicity beyond the 3-h incubation period. After one washing with MEM (200 μl) to remove the gentamicin, 100 μl of trypan blue solution (0.04% [wt/vol]) (Sigma) was added for 15 min to visualize dead or dying cells. Photographs were taken of the center of each well of cells with a 35-mm camera attached to an Olympus IX70 inverted microscope (10× objective, 10× ocular). At least three wells of cells were used for each strain in each experiment, and all experiments were repeated at least three times.
Trypan blue exclusion assays can be used either as a qualitative method of assessing cytotoxicity or as a semiquantitative method by scoring cytotoxicity with a grading scale ranging from 1 (no cytotoxicity) to 4 (massive cytotoxicity). In previous studies, the results obtained with the semiquantitative method have correlated closely with those from a chromium release quantitative method (11). In this study, we made a quantitative determination of P. aeruginosa cytotoxicity using photographs of trypan blue-stained corneal epithelia. The photographs were divided into equal quadrants, and the numbers of dead cells per quadrant were counted. Quantification of cytotoxicity was performed when cytotoxicity was inhibited or when there was no change in cytotoxic activity. Some inhibitors increased the cytotoxic activity by bacteria. For these inhibitors quantification of dead cells proved to be difficult since there were often areas in which cells were completely destroyed; the data for these samples are shown as photographs.
Invasion assays.
Gentamicin survival assays were used to quantify the extent of bacterial invasion of corneal epithelial cells. These assays were performed as previously described for assessing P. aeruginosa invasion of epithelial cells (9, 11, 13), with minor modifications as described below.
(i) Invasion assay used for comparison of invasion by wild-type and mutant PA103.
An inoculum of 2 × 106 CFU in 200 μl of MEM was used for each well of cells in 24-well tissue culture plates. Each well of cells was calculated to contain 106 epithelial cells; thus the multiplicity of infection was 2. Following a 3-h infection at 37°C, survivors of a 2-h treatment with 200 μg of gentamicin/ml (Sigma) were enumerated by viable bacterial cell counts after the cells were washed with phosphate-buffered saline to remove the antibiotic and cell lysis with a 15-min treatment with 0.25% Triton X-100 (Sigma).
(ii) Invasion assay used to examine the effect of pharmacological inhibitors.
An inoculum of 5 × 104 CFU of strain 6294 suspended in 100 μl of MEM was added to each well of cells grown in 96-well tissue culture plates. Each well was calculated to contain 2.5 × 105 corneal epithelial cells (multiplicity of infection = 0.2). The remainder of the assay was performed as described above for PA103. The use of inhibitors in these assays is as described above. At least six wells were used for each group tested and all experiments were repeated at least three times.
Statistics.
The t test and analysis of variance (ANOVA) were used to analyze the data. P values of <0.05 were considered significant.
RESULTS
PA103 exoU::Tn5Tc is not cytotoxic to, and does not invade, corneal epithelia.
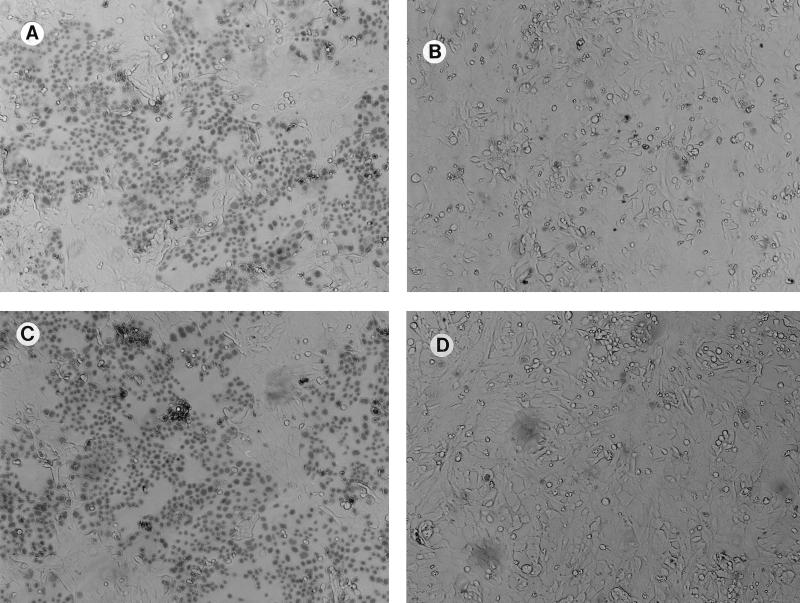
PA103 exoU::Tn5Tc was not cytotoxic to corneal epithelia (Fig. 1). When ExoU expression was restored by complementation, yielding PA103 exoU::Tn5Tc pUCPexoU, cytotoxicity to corneal cells was restored. The exoT mutant, PA103 exoT::Tc showed normal cytotoxicity that was approximately equivalent to that of the parental strain, PA103. These results showed that, as with MDCK cell cytotoxicity, corneal epithelial cell cytotoxicity by PA103 requires ExoU.
FIG. 1.
Cytotoxicity of various isogenic mutants of strain PA103 for corneal epithelial cells measured by trypan blue staining. (A) PA103 exoT::Tc (exoT); (B) PA103 exoU::Tn5Tc (exoU); (C) complementation of exoU by pUCPexoU (PA103 exoU::Tn5Tc pUCPexoU); (D) vector control strain, PA103 exoU::Tn5Tc pUCP18.
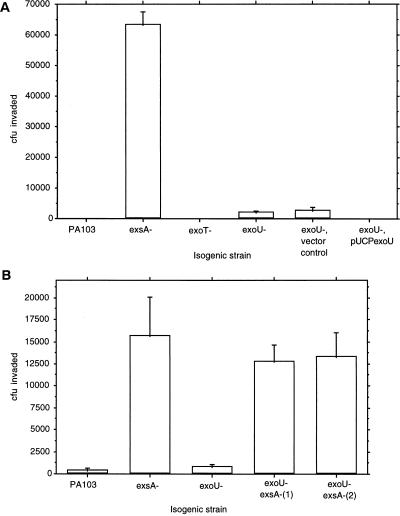
Corneal cell invasion by the various mutants was examined (Fig. 2A). The exoU mutant did not invade the epithelial cells. This was in direct contrast to the exsA mutant, which was capable of corneal epithelial cell invasion. The exoT mutant, which remained cytotoxic, remained noninvasive.
FIG. 2.
The effect of mutations in the ExsA-regulated cytotoxicity pathway on the invasion of strain PA103 into corneal epithelial cells. (A) Comparison between the parent strain PA103 and PA103 exsA::Ω (exsA), PA103 exoT::Tc (exoT), PA103 exoU::Tn5Tc (exoU), PA103 exoU::Tn5Tc pUCP18 (vector control), and PA103 exoU::Tn5Tc complemented with pUCPexoU (exoU+). (B) Different experiment comparing PA103, PA103 exsA::Ω, and PA103 exoU::Tn5Tc with two mutants of PA103 exoU::Tn5Tc in which exsA was inactivated (exoU exsA double mutants).
PA103 exoU::Tn5Tc exsA::Ω(1) and PA103 exoU::Tn5Tc exsA::Ω(2) invade corneal epithelia.
Since PA103 exsA::Ω invades corneal cells (13), PA103 should have the genes necessary for epithelial cell invasion elsewhere on the chromosome. These experiments also suggest that genes regulated by ExsA are not required for invasion. Since ExoU is regulated by ExsA (6, 14), it follows that an exoU mutation should have no effect on invasion. To determine if the mutation of exoU had somehow affected invasion genes outside of the ExsA-regulated pathway, double mutants were constructed in which exsA was inactivated in an exoU mutant, PA103 exoU::Tn5Tc. Both double mutants tested (exoU exsA) acquired the ability to invade cells as effectively as PA103 exsA::Ω (Fig. 2B). In the same experiments, the exoU mutant (PA103 exoU::Tn5Tc) did not invade cells.
These results suggested that the exoU mutation in PA103 exoU::Tn5Tc had not affected the function of genes involved in invasion outside the ExsA-regulated pathway. The outcome of this experiment can be interpreted in two ways: ExsA is a positive regulator of genes that inhibit invasion; alternatively, ExsA may act as a negative regulator of invasion. Since exsA mutants of invasive strains do not show an enhanced ability to invade (13), we favor the former hypothesis, that ExsA is a positive regulator for gene products that inhibit invasion.
Identification of drugs that inhibit P. aeruginosa corneal cell invasion.
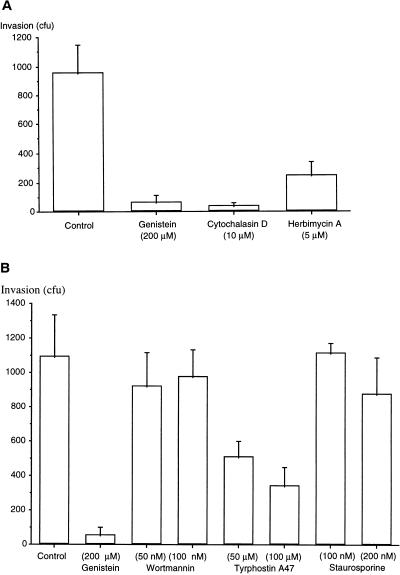
If invasion and cytotoxicity were sequential events, then drugs that block invasion would also reduce cytotoxicity. A noncytotoxic strain, 6294, was used to screen for drugs that blocked invasion through an action on the host corneal epithelial cell. Cytochalasin D (an inhibitor of actin cytoskeleton function) and genistein (a protein tyrosine kinase [PTK] inhibitor) have been found to prevent P. aeruginosa 6294 invasion of primary cultures of rabbit corneal epithelial cells (9). In the present study, the ability of cytochalasin D, genistein, and other inhibitors of mammalian cell signal transduction were tested using the immortalized corneal epithelial cell line. Genistein, cytochalasin D, and herbimycin A (an inhibitor of Src family PTK) all significantly reduced the amount of corneal cell invasion (P < 0.05, ANOVA) (Fig. 3A). Tyrphostin A47, another PTK inhibitor, also inhibited invasion (P < 0.05, ANOVA) (Fig. 3B). In contrast, neither staurosporine (an inhibitor of protein kinase A and C activity) nor wortmannin (an inhibitor of phosphatidylinositol-3 [PI-3] kinase) had any significant effect on P. aeruginosa invasion (P > 0.05, ANOVA) (Fig. 3B).
FIG. 3.
Effect of signal transduction inhibitors on invasion of P. aeruginosa 6294 into corneal epithelial cells. (A) Comparison of the effects of genistein, cytochalasin D, and herbimycin A. (B) Comparison of the effects of wortmannin, tyrphostin A47, and staurosporine with genistein.
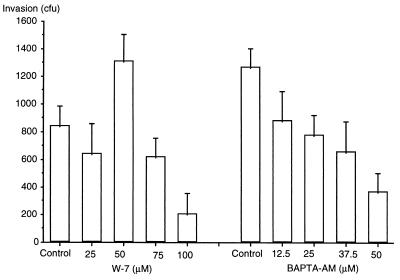
Invasion was significantly reduced by both BAPTA-AM, which blocks increases in intracellular calcium, and higher concentrations of W-7, a calmodulin antagonist (P < 0.05, ANOVA) (Fig. 4). BAPTA-AM reduced invasion in a dose-dependent manner, while W-7 actually increased invasion at lower doses (50 μM). The phospholipase C inhibitor (U-73122) and the negative control (U-73343) had no effect on invasion; negative results were also obtained with indomethacin, an inhibitor of cyclo-oxygenase and phospholipase A2 (data not shown).
FIG. 4.
Effect of various concentrations of W-7 (calmodulin antagonist) and BAPTA-AM (intracellular calcium chelator) on invasion of P. aeruginosa 6294 into corneal epithelial cells.
Inhibitors block cell entry by invasive and cytotoxic strains.
Cytotoxic strains demonstrate low levels of background epithelial cell invasion (residual invasion) (11). Once inhibitors of invasion were screened by using a noncytotoxic strain, some of them were also tested for their effect on residual invasion by cytotoxic strains. Using an inoculum 10-fold larger than that for experiments with the invasive strain, residual invasion by the cytotoxic strain 6206 was reduced from 287 ± 82 (no. of CFU recovered; mean ± standard error) to 3 ± 3 by cytochalasin D (99% inhibition), to 13 ± 7 by genistein (95% inhibition), and to 110 ± 35 by herbimycin A (62% inhibition) (P < 0.05, ANOVA). All of these inhibitors also blocked cell entry by PA103 exsA::Ω (data not shown). These data indicated that the corneal cell entry mechanisms of invasive and cytotoxic P. aeruginosa strains are similar.
Effect of invasion inhibitors on P. aeruginosa cytotoxicity.
Only some of the drugs that inhibited invasion blocked cytotoxicity. The PTK inhibitors genistein and tyrphostin A47 were found to reduce cytotoxicity (Table 1). The Src-PTK inhibitor herbimycin A and the PI-3 kinase inhibitor wortmannin had no detectable effect on corneal cell susceptibility to bacterial killing (Table 1). Although cytochalasin D blocked invasion, this drug made cells even more susceptible to cytotoxicity (Fig. 5). After treatment with this drug, susceptibility was increased to the point that the cell monolayer was destroyed, making it difficult to quantify the number of dead cells in some samples. However, Fig. 5 clearly illustrates that cytochalasin D caused a marked increase in the number of cells that were affected by bacterial cytotoxicity. Both BAPTA-AM and W-7 also blocked invasion, but they enhanced cytotoxicity (data not shown). Staurosporine and the phospholipase C inhibitor U-73122 had no effect on invasion or cytotoxicity.
TABLE 1.
Effect of signaling inhibitors on cytotoxicity induced by P. aeruginosa 6206
| Treatment | No. of affected cells (trypan blue stained)
|
|
|---|---|---|
| Controla | Treated (P) | |
| Genistein (200 μM)b | 468 ± 44 | 79 ± 2 (0.0001) |
| Genistein (200 μM)c (cell pretreatment only) | 241 ± 27 | 174 ± 15 (0.04) |
| Tyrphostin 47 (50 μM) | 359 ± 122 | 72 ± 23 (0.004) |
| Herbimycin A (5 μM)d | 339 ± 66 | 314 ± 50 (0.38) |
| Wortmannin (50 nM) | 187 ± 70 | 179 ± 55 (0.86) |
Cells were not treated with drug.
Cells were pretreated with genistein for 1 h, and then the drug was also added during incubation with bacteria.
Cells were only pretreated with genistein (for 1 h).
Cells were pretreated with herbimycin A for 3.5 h, and then the drug was also added during incubation with bacteria.
FIG. 5.
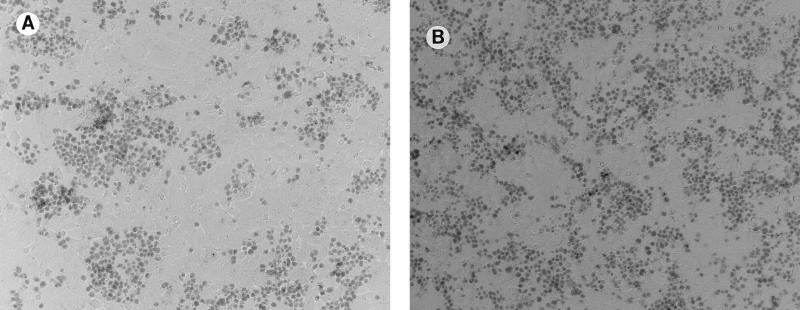
The effect of cytochalasin D on susceptibility of corneal epithelia to P. aeruginosa cytotoxicity. (A) Bacterium-induced cytotoxicity without drug treatment; (B) effect of cytochalasin D (10 μM) treatment of corneal cells before and during exposure to strain 6206 (cytotoxic).
The mechanism by which genistein inhibited cytotoxicity involved an effect on the host cell rather than on the bacteria, since cytotoxicity was inhibited when cells were pretreated with genistein for 1 h prior to exposure to bacteria (Table 1). Pretreating bacteria with genistein had no effect. Similar methods also showed that the enhancement of cytotoxicity by cytochalasin D was via an effect on the host cell (data not shown).
DISCUSSION
The results of earlier studies suggested that P. aeruginosa cytotoxicity may be invasion dependent (11, 13). The new findings presented here suggest that invasion and cytotoxicity are not sequential events. This conclusion is based on two observations: (i) that the mutation of a single gene in the ExsA-regulated pathway (exoU) which caused a loss of cytotoxic capacity did not confer an invasive phenotype, and (ii) that some of the drugs that interfere with host cell signaling events involved in P. aeruginosa invasion did not block susceptibility to P. aeruginosa-mediated cytotoxicity. Indeed, some inhibitors of P. aeruginosa invasion, including cytochalasin D, enhanced susceptibility to P. aeruginosa cytotoxicity.
Mutation of exoU results in a noncytotoxic phenotype for MDCK cells, the absence of overt lung injury in an acute infection model, and, in this study, the loss of cytotoxicity for corneal epithelial cells. If invasion precedes and is required for cytotoxicity, exoU mutants might theoretically exhibit an invasive phenotype unless exoU is responsible for invasion. It is unlikely that ExoU is required for invasion since this protein is not expressed in isolates that are invasive because of an exsA mutation (6, 13). Furthermore, we have shown that the mutation in exoU does not impede invasion since exoU exsA double mutants are as invasive as exsA mutants. One model that fits these data proposes that ExsA regulates the expression of an invasion inhibitor(s). Coordinate regulation of cytotoxicity genes and invasion inhibitors would allow the observed inverse relationship between cytotoxic and invasive phenotypes (11). As the ability to invade does not increase when an invasive strain possesses an exsA mutation, we do not believe that ExsA represses gene products that promote cellular entry.
Treatment of cells with drugs that inhibit invasion confirm that P. aeruginosa cytotoxicity does not depend upon invasion of the host cell. Cytochalasin D, BAPTA-AM, W-7, and herbimycin A all reduced the amount of invasion by both cytotoxic and invasive strains, but they did not reduce cell killing. This observation contrasts with the results of invasion-inhibiting drugs on the cytotoxic activity of other bacterial pathogens, such as S. flexneri and B. pertussis. Apoptosis of macrophages induced by these pathogens can be reduced or prevented by cytochalasin D (20, 34). Noninvasive mutants of S. typhimurium do not kill macrophages (3, 23), suggesting that Salmonella-induced cytotoxicity is invasion dependent.
P. aeruginosa cytotoxicity, rather than being a consequence of invasion, may be mediated from outside the host cell after inhibition of invasion. This observation is consistent with the recent finding that ExsA regulates a type III secretion system. Type III secretion requires contact between host cells and the bacterial pathogen and involves translocation of bacterial proteins across host plasma membranes (29). The identity of a potential invasion inhibitor(s) in P. aeruginosa is not clear; however, ExsA regulates the expression of a family of extracellular proteins apart from ExoS, ExoT, and ExoU (6, 14, 33). Since the genes for the type III secretion pathways encoded by P. aeruginosa and Yersinia spp. show a high level of amino acid homology, it is possible that P. aeruginosa encodes proteins with functions similar to those of Yersinia YopH and YopE. These virulence determinants inhibit epithelial cell entry through alterations in eukaryotic signal transduction pathways (tyrosine phosphatase activity) and disruption of actin microfilaments (15, 28). Either genes for the invasion inhibitor(s) are not present in invasive strains or their production must be down-regulated, since the inactivation of exsA in invasive strains has little effect on invasion (13).
The results of this study suggest that P. aeruginosa invasion and cytotoxicity both involve PTK activity. There is a distinction, however, in the host cell signaling pathways activated by invasive and cytotoxic strains. Our results suggest that invasion, but not cytotoxicity, requires Src family PTK and calcium-calmodulin activity. Src family PTKs are nonreceptor PTKs that are involved in cell signaling. One role of these proteins is recognition of tyrosine-phosphorylated domains on cell surface receptors (e.g., growth factor receptors), with subsequent tyrosine phosphorylation of downstream signaling proteins. Src family PTKs, e.g., pp60Src, have also been shown to reversibly associate with the cell cytoskeleton. The interaction of Src family PTKs with the cytoskeleton is regulated in part by intracellular calcium since BAPTA-AM prevented the reversibility of this association (5). The ability of Src-PTK and calcium-calmodulin inhibitors to reduce P. aeruginosa cell entry may be due to the effects of these drugs on Src-PTK–cytoskeleton interactions. PI-3 kinase (17) and phospholipase C-γ (19) also function as host cell signaling molecules involved in the invasion or interaction of bacterial pathogens with host cells. In this study, however, inhibitors of these signaling proteins had no effect on P. aeruginosa invasion or cytotoxicity.
The mechanism by which the invasion inhibitors cytochalasin D, BAPTA-AM, and W-7 enhanced P. aeruginosa cytotoxicity is not clear; however, three potential mechanisms could be involved. These drugs may enhance cytotoxicity because they block invasion, provided that cytotoxicity requires contact with the outer surface of the host cell membrane. A second possibility is that these inhibitors affect cell function in an additive or synergistic manner with bacterial cytotoxic proteins. It has been shown that the Yersinia cytotoxin YopE disrupts the actin cytoskeleton as part of the mechanism by which it kills cells (28). A third possible mechanism for the enhanced cytotoxicity with these agents may involve their effects on epithelial cell polarity. We previously showed that the basolateral cell surfaces of epithelial cells are more susceptible than the apical surface to P. aeruginosa cytotoxicity (12), and all of the invasion inhibitors that enhanced cytotoxicity in this study can alter the polarity of epithelial cells (22, 25).
In conclusion, this paper provides evidence that P. aeruginosa invasion and cytotoxicity are mutually exclusive events. Cytotoxic strains can be either invasive or cytotoxic depending upon the activity of ExsA, which appears to regulate an invasion inhibitor(s) in addition to genes involved in cytotoxicity. Invasive strains appear to lack the invasion inhibitor expression or function and not only genes involved in cytotoxicity. There is evidence for a role of the host cell in both invasion and cytotoxicity, since inhibitors of host cell signaling can alter these events. Both invasion and cytotoxicity involve PTK activity, but along distinct pathways.
Current forms of therapy for bacterial infections are aimed at inhibiting bacterial growth or survival, placing selective pressure on bacteria to become resistant. This has long been a particular problem for treatment of infections involving P. aeruginosa, which are often life or sight threatening. An alternative approach would be to protect the host from bacterial virulence factors such as epithelial cell invasion and cytotoxicity. For P. aeruginosa, therapeutic intervention would be best aimed at common pathways, such as those that are blocked by PTK inhibitors. Genistein, which is nontoxic, is found in soy foods, and has reversible activity (and which is currently being tested as a cancer chemotherapy drug) (16), might be an ideal drug for such consideration.
ACKNOWLEDGMENTS
We thank Cunrong Li-Yun for technical assistance.
This work was supported by NIH grant RO1 EY11221 and a UC Berkeley Faculty Research Grant to S.M.J.F., grants from Cystic Fibrosis Research, Inc., and the American Lung Association of California to D.J.E., and NIH grants AI31665 and AI01289 to D.W.F.
REFERENCES
- 1.Apodaca G, Bomsel M, Lindstedt R, Engel J, Frank D, Mostov K E, Wiener-Kronish J. Characterization of Pseudomonas aeruginosa-induced MDCK cell injury: glycosylation-defective host cells are resistant to bacterial killing. Infect Immun. 1995;63:1541–1551. doi: 10.1128/iai.63.4.1541-1551.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bliska J B, Galán J E, Falkow S. Signal transduction in the mammalian cell during bacterial attachment and entry. Cell. 1993;73:903–920. doi: 10.1016/0092-8674(93)90270-z. [DOI] [PubMed] [Google Scholar]
- 3.Chen L M, Kaniga K, Galán J E. Salmonella spp. are cytotoxic for cultured macrophages. Mol Microbiol. 1996;21:1101–1115. doi: 10.1046/j.1365-2958.1996.471410.x. [DOI] [PubMed] [Google Scholar]
- 4.Chen Y, Zychlinsky A. Apoptosis induced by bacterial pathogens. Microb Pathog. 1994;17:203–212. doi: 10.1006/mpat.1994.1066. [DOI] [PubMed] [Google Scholar]
- 5.Dash D, Aepfelbacher M, Siess W. The association of pp125FAK, pp60Src, CDC42Hs, and Rap1B with the cytoskeleton of aggregated platelets is a reversible process regulated by calcium. FEBS Lett. 1995;363:231–234. doi: 10.1016/0014-5793(95)00320-9. [DOI] [PubMed] [Google Scholar]
- 6.Finck-Barbançon V, Goranson J, Zhu L, Sawa T, Wiener-Kronish J P, Fleiszig S M J, Wu C, Mende-Mueller L, Frank D W. ExoU expression by Pseudomonas aeruginosa correlates with acute cytotoxicity and epithelial injury. Mol Microbiol. 1997;25:547–557. doi: 10.1046/j.1365-2958.1997.4891851.x. [DOI] [PubMed] [Google Scholar]
- 7.Fleiszig S M J, Efron N, Pier G B. Extended wear enhances Pseudomonas aeruginosa adherence to human corneal epithelium. Investig Ophthalmol Vis Sci. 1992;33:2908–2916. [PubMed] [Google Scholar]
- 8.Fleiszig S M J, Zaidi T S, Fletcher E L, Preston M J, Pier G B. Pseudomonas aeruginosa invades corneal epithelial cells during experimental infection. Infect Immun. 1994;62:3485–3492. doi: 10.1128/iai.62.8.3485-3493.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fleiszig S M J, Zaidi T S, Pier G B. Pseudomonas aeruginosa survival and multiplication within corneal epithelial cells in vitro. Infect Immun. 1995;63:4072–4077. doi: 10.1128/iai.63.10.4072-4077.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fleiszig S M J, Wiener-Kronish J P, Vallas V, Mostov K E, Frank D W. Evidence that all Pseudomonas aeruginosa strains may be inherently capable of invading corneal epithelial cells and that cytotoxicity is regulated by ExsA. ARVO Abstr Investig Ophthalmol Vis Sci. 1996;37:S210. [Google Scholar]
- 11.Fleiszig S M J, Zaidi T S, Preston M J, Grout M, Evans D J, Pier G B. The relationship between cytotoxicity and epithelial cell invasion by corneal isolates of Pseudomonas aeruginosa. Infect Immun. 1996;64:2288–2294. doi: 10.1128/iai.64.6.2288-2294.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fleiszig S M J, Evans D J, Do N, Shin S, Mostov K E. Epithelial cell polarity affects susceptibility to Pseudomonas aeruginosa invasion and cytotoxicity. Infect Immun. 1997;65:2861–2867. doi: 10.1128/iai.65.7.2861-2867.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fleiszig S M J, Wiener-Kronish J P, Miyazaki H, Vallas V, Mostov K E, Kanda D, Sawa T, Yen T S B, Frank D W. Pseudomonas aeruginosa-mediated cytotoxicity and invasion correlate to distinct genotypes at the loci encoding exoenzyme S. Infect Immun. 1997;65:579–586. doi: 10.1128/iai.65.2.579-586.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frank D W, Nair G, Schweizer H P. Construction and characterization of chromosomal insertional mutations of the Pseudomonas aeruginosa exoenzyme S trans-regulatory locus. Infect Immun. 1994;62:554–563. doi: 10.1128/iai.62.2.554-563.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guan K, Dixon J E. Protein tyrosine phosphatase activity of an essential virulence determinant in Yersinia. Science. 1990;249:553–556. doi: 10.1126/science.2166336. [DOI] [PubMed] [Google Scholar]
- 16.Hirano T, Gotoh M, Oka K. Natural flavonoids and lignans are potent cytostatic agents against human leukemic HL-60 cells. Life Sci. 1994;55:1061–1069. doi: 10.1016/0024-3205(94)00641-5. [DOI] [PubMed] [Google Scholar]
- 17.Ireton K, Payrastre B, Chap H, Ogawa W, Sakaue H, Kasuga M, Cossart P. A role for phosphoinositide 3-kinase in bacterial invasion. Science. 1996;274:780–782. doi: 10.1126/science.274.5288.780. [DOI] [PubMed] [Google Scholar]
- 18.Jumblatt M M, Neufeld A H. β-Adrenergic and serotonergic responsiveness of rabbit corneal epithelial cells in culture. Investig Ophthalmol Vis Sci. 1983;24:1139–1143. [PubMed] [Google Scholar]
- 19.Kenny B, Finlay B B. Intimin-dependent binding of enteropathogenic Escherichia coli to host cells triggers novel signaling events including tyrosine phosphorylation of phospholipase C-γ1. Infect Immun. 1997;65:2528–2536. doi: 10.1128/iai.65.7.2528-2536.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khelef N, Zychlinsky A, Guiso N. Bordetella pertussis induces apoptosis in macrophages: role of adenylate cyclase-hemolysin. Infect Immun. 1993;61:4064–4071. doi: 10.1128/iai.61.10.4064-4071.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kudoh I, Wiener-Kronish J P, Hashimoto S, Pittet J, Frank D. Exoproduct secretions of Pseudomonas aeruginosa strains influence severity of alveolar epithelial injury. Am J Physiol. 1994;267:L551–L556. doi: 10.1152/ajplung.1994.267.5.L551. [DOI] [PubMed] [Google Scholar]
- 22.Llorente A, Garred O, Holm P K, Eker P, Jacobsen J, van Deurs B, Sandvig K. Effect of calmodulin antagonists on endocytosis and intracellular transport of ricin in polarized MDCK cells. Exp Cell Res. 1996;227:298–308. doi: 10.1006/excr.1996.0279. [DOI] [PubMed] [Google Scholar]
- 23.Monack D M, Raupach B, Hromockyj A E, Falkow S. Salmonella typhimurium invasion induces apoptosis in infected macrophages. Proc Natl Acad Sci USA. 1996;93:9833–9838. doi: 10.1073/pnas.93.18.9833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Okamoto S, Oji M, Hassell J R, Thoft R A, Pipas J M. Establishment of an immortalized rabbit corneal epithelial cell line using SV40 large T antigen. ARVO Abstr Investig Ophthalmol Vis Sci. 1993;34:S1010. [Google Scholar]
- 25.Paller M S. Lateral mobility of Na+,K+-ATPase and membrane lipids in renal cells. J Membr Biol. 1994;142:127–135. doi: 10.1007/BF00233390. [DOI] [PubMed] [Google Scholar]
- 26.Preston M J, Fleiszig S M J, Zaidi T S, Goldberg J B, Shortridge V D, Vasil M L, Pier G B. Rapid and sensitive method for evaluating Pseudomonas aeruginosa virulence factors during corneal infections in mice. Infect Immun. 1995;63:3497–3501. doi: 10.1128/iai.63.9.3497-3501.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rosenshine I, Duronio V, Finlay B B. Tyrosine protein kinase inhibitors block invasin-promoted bacterial uptake by epithelial cells. Infect Immun. 1992;60:2211–2217. doi: 10.1128/iai.60.6.2211-2217.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rosqvist R, Forsberg A, Wolf-Watz H. Intracellular targeting of the Yersinia YopE cytotoxin in mammalian cells induces actin microfilament disruption. Infect Immun. 1991;59:4562–4569. doi: 10.1128/iai.59.12.4562-4569.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rosqvist R, Magnusson K-E, Wolf-Watz H. Target cell contact triggers expression and polarized transfer of Yersinia YopE cytotoxin into mammalian cells. EMBO J. 1994;13:964–972. doi: 10.1002/j.1460-2075.1994.tb06341.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Salyers A A, Whitt D D. Bacterial pathogenesis: a molecular approach. Washington, D.C: ASM Press; 1994. pp. 260–268. [Google Scholar]
- 31.Tang H, Kays M, Prince A. Role of Pseudomonas aeruginosa pili in acute pulmonary infection. Infect Immun. 1995;63:1278–1285. doi: 10.1128/iai.63.4.1278-1285.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wooldridge K G, Williams P H, Ketley J M. Host signal transduction and endocytosis of Campylobacter jejuni. Microb Pathog. 1996;21:299–305. doi: 10.1006/mpat.1996.0063. [DOI] [PubMed] [Google Scholar]
- 33.Yahr T L, Mende-Mueller L M, Friese M B, Frank D W. Identification of type III secreted products of the Pseudomonas aeruginosa exoenzyme S regulon. J Bacteriol. 1997;179:7165–7168. doi: 10.1128/jb.179.22.7165-7168.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zychlinsky A, Prevost M C, Sansonetti P J. Shigella flexneri induces apoptosis in infected macrophages. Nature. 1992;358:167–169. doi: 10.1038/358167a0. [DOI] [PubMed] [Google Scholar]