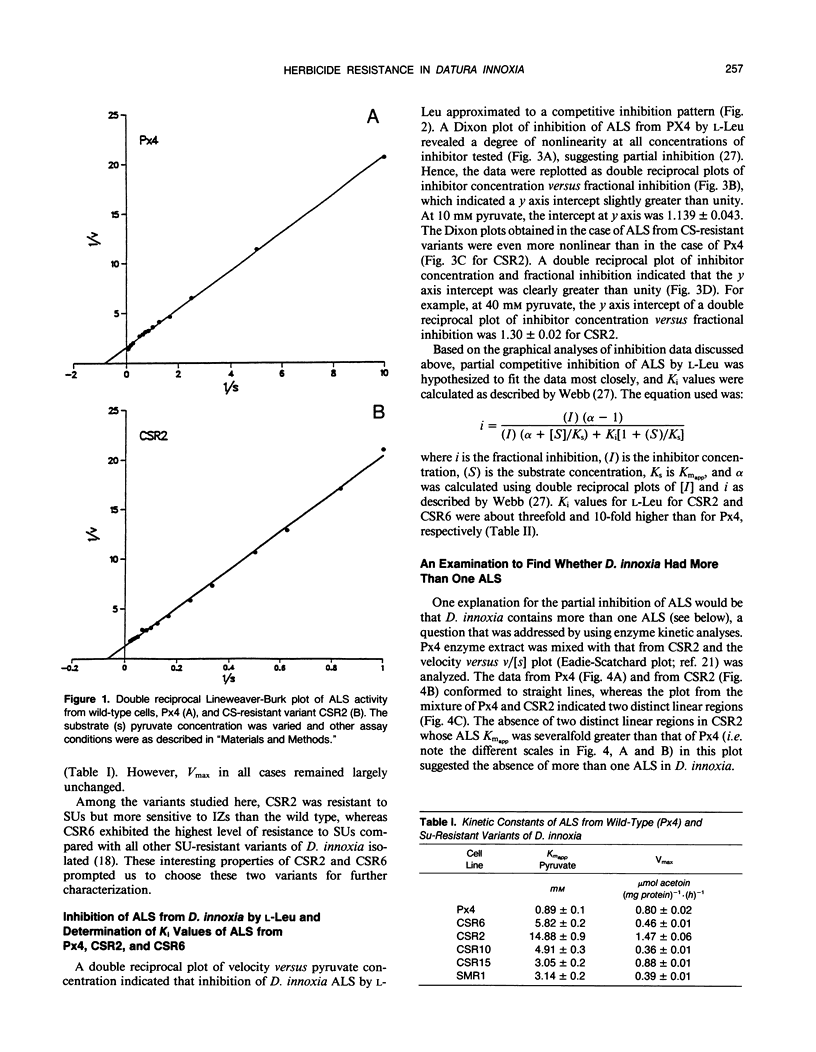
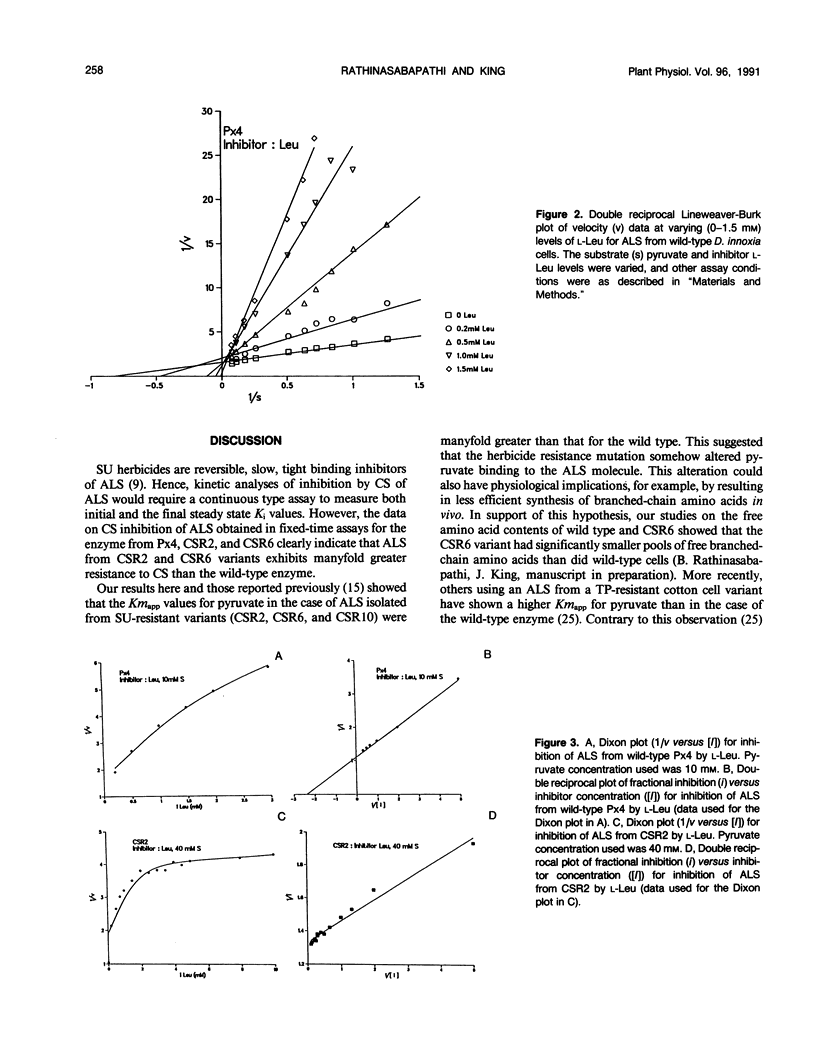
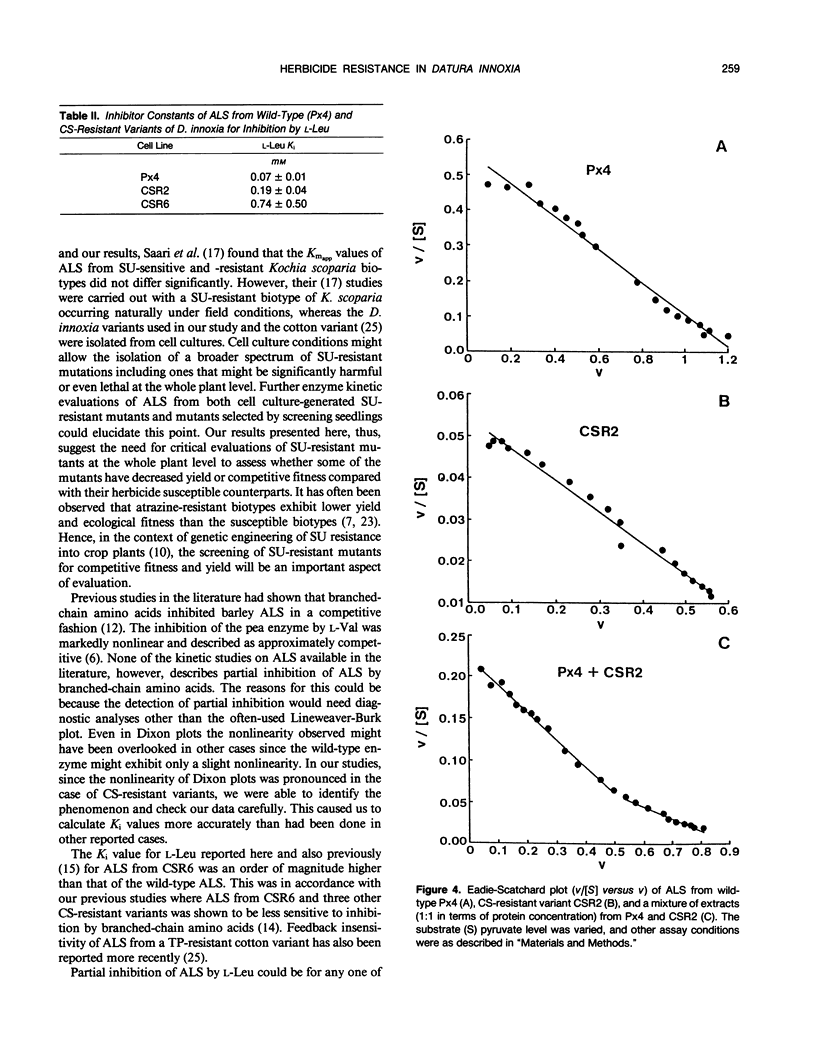
Abstract
Acetolactate synthase (ALS, EC 4. 1.3. 18), the first enzyme in the biosynthesis of branched-chain amino acids, was isolated from wild-type and sulfonylurea-resistant Datura innoxia cell variants and characterized. Apparent Km values of the ALS for pyruvate from three sulfonylurea-resistant variants (CSR2, CSR6, and CSR10) were manyfold greater than that of the wild type. The inhibition of wild-type and herbicide-resistant ALS activity by chlorsulfuron (CS), a sulfonylurea herbicide, and l-leucine (l-Leu), one of the feedback inhibitors of the enzyme, was examined. ALS from two CS-resistant variants exhibited severalfold greater resistance to CS than did the wild-type enzyme. Inhibition of ALS by l-Leu fitted a partially competitive pattern most closely. It is proposed that the herbicide resistance mutation accentuated the partial inhibition characteristics of ALS by l-Leu. ALS from one of the two CS-resistant variants (CSR6) had a Ki for l-Leu an order of magnitude greater than that of the wild-type enzyme. The alterations in kinetic properties observed in the ALS from sulfonylurea-resistant variants are discussed in relation to the possible evolutionary significance of the herbicide binding site of this enzyme, the physiological effects of such biochemical alterations, and their practical utility in genetic studies.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Chaleff R. S., Mauvais C. J. Acetolactate synthase is the site of action of two sulfonylurea herbicides in higher plants. Science. 1984 Jun 29;224(4656):1443–1445. doi: 10.1126/science.224.4656.1443. [DOI] [PubMed] [Google Scholar]
- DIXON M. The determination of enzyme inhibitor constants. Biochem J. 1953 Aug;55(1):170–171. doi: 10.1042/bj0550170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gollop N., Chipman D. M., Barak Z. Inhibition of acetohydroxy acid synthase by leucine. Biochim Biophys Acta. 1983 Oct 17;748(1):34–39. doi: 10.1016/0167-4838(83)90024-9. [DOI] [PubMed] [Google Scholar]
- Holt J. S., Stemler A. J., Radosevich S. R. Differential Light Responses of Photosynthesis by Triazine-resistant and Triazine-susceptible Senecio vulgaris Biotypes. Plant Physiol. 1981 Apr;67(4):744–748. doi: 10.1104/pp.67.4.744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishore G. M., Shah D. M. Amino acid biosynthesis inhibitors as herbicides. Annu Rev Biochem. 1988;57:627–663. doi: 10.1146/annurev.bi.57.070188.003211. [DOI] [PubMed] [Google Scholar]
- LaRossa R. A., Schloss J. V. The sulfonylurea herbicide sulfometuron methyl is an extremely potent and selective inhibitor of acetolactate synthase in Salmonella typhimurium. J Biol Chem. 1984 Jul 25;259(14):8753–8757. [PubMed] [Google Scholar]
- Mazur B. J., Chui C. F., Smith J. K. Isolation and characterization of plant genes coding for acetolactate synthase, the target enzyme for two classes of herbicides. Plant Physiol. 1987 Dec;85(4):1110–1117. doi: 10.1104/pp.85.4.1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhitch M. J. Acetolactate Synthase Activity in Developing Maize (Zea mays L.) Kernels. Plant Physiol. 1988 Jan;86(1):23–27. doi: 10.1104/pp.86.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray T. B. Site of action of chlorsulfuron: inhibition of valine and isoleucine biosynthesis in plants. Plant Physiol. 1984 Jul;75(3):827–831. doi: 10.1104/pp.75.3.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saari L. L., Cotterman J. C., Primiani M. M. Mechanism of Sulfonylurea Herbicide Resistance in the Broadleaf Weed, Kochia scoparia. Plant Physiol. 1990 May;93(1):55–61. doi: 10.1104/pp.93.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxena P. K., King J. Herbicide Resistance in Datura innoxia: Cross-Resistance of Sulfonylurea-Resistant Cell Lines to Imidazolinones. Plant Physiol. 1988 Mar;86(3):863–867. doi: 10.1104/pp.86.3.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxena P. K., King J. Lack of Cross-Resistance of Imidazolinone-Resistant Cell Lines of Datura innoxia P. Mill. to Chlorsulfuron : Evidence for Separable Sites of Action on the Target Enzyme. Plant Physiol. 1990 Nov;94(3):1111–1115. doi: 10.1104/pp.94.3.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaner D. L., Anderson P. C., Stidham M. A. Imidazolinones: potent inhibitors of acetohydroxyacid synthase. Plant Physiol. 1984 Oct;76(2):545–546. doi: 10.1104/pp.76.2.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stowe A. E., Holt J. S. Comparison of Triazine-Resistant and -Susceptible Biotypes of Senecio vulgaris and Their F(1) Hybrids. Plant Physiol. 1988 May;87(1):183–189. doi: 10.1104/pp.87.1.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian M. V., Hung H. Y., Dias J. M., Miner V. W., Butler J. H., Jachetta J. J. Properties of mutant acetolactate synthases resistant to triazolopyrimidine sulfonanilide. Plant Physiol. 1990 Sep;94(1):239–244. doi: 10.1104/pp.94.1.239. [DOI] [PMC free article] [PubMed] [Google Scholar]


