ABSTRACT
A moderately halophilic eubacterium, Halomonas elongata, has been used as cell factory to produce fine chemical 1,4,5,6-tetrahydro-2-methyl-4-pyrimidinecarboxylic acid (ectoine), which functions as a major osmolyte protecting the cells from high-salinity stress. To explore the possibility of using H. elongata to biosynthesize other valuable osmolytes, an ectoine-deficient salt-sensitive H. elongata deletion mutant strain KA1 (ΔectABC), which only grows well in minimal medium containing up to 3% NaCl, was subjected to an adaptive mutagenesis screening in search of mutants with restored salt tolerance. Consequently, we obtained a mutant, which tolerates 6% NaCl in minimal medium by overproducing L-glutamic acid (Glu). However, this Glu-overproducing (GOP) strain has a lower tolerance level than the wild-type H. elongata, possibly because the acidity of Glu interferes with the pH homeostasis of the cell and hinders its own cellular accumulation. Enzymatic decarboxylation of Glu to γ-aminobutyric acid (GABA) by a Glu decarboxylase (GAD) could restore cellular pH homeostasis; therefore, we introduced an engineered salt-inducible HopgadBmut gene, which encodes a wide pH-range GAD mutant, into the genome of the H. elongata GOP strain. We found that the resulting H. elongata GOP-Gad strain exhibits higher salt tolerance than the GOP strain by accumulating high concentration of GABA as an osmolyte in the cell (176.94 µmol/g cell dry weight in minimal medium containing 7% NaCl). With H. elongata OUT30018 genetic background, H. elongata GOP-Gad strain can utilize biomass-derived carbon and nitrogen compounds as its sole carbon and nitrogen sources, making it a good candidate for the development of GABA-producing cell factories.
IMPORTANCE
While the wild-type moderately halophilic H. elongata can synthesize ectoine as a high-value osmolyte via the aspartic acid metabolic pathway, a mutant H. elongata GOP strain identified in this work opens doors for the biosynthesis of alternative valuable osmolytes via glutamic acid metabolic pathway. Further metabolic engineering to install a GAD system into the H. elongata GOP strain successfully created a H. elongata GOP-Gad strain, which acquired higher tolerance to salt stress by accumulating GABA as a major osmolyte. With the ability to assimilate biomass-derived carbon and nitrogen sources and thrive in high-salinity environment, the H. elongata GOP-Gad strain can be used in the development of sustainable GABA-producing cell factories.
KEYWORDS: Halomonas elongata, compatible osmolyte, L-glutamic acid, metabolic engineering, γ-aminobutyric acid
INTRODUCTION
Halophilic eubacteria possess varieties of physiological functions to facilitate their adaption to extracellular osmotic changes, which happen dynamically in their habitats. To protect cell and cellular components such as membranes and proteins from hyperosmotic stress under high salinity, certain halophilic bacteria and Archaea maintain their intracellular osmotic homeostasis through biosynthesis, accumulation, and transportation of specific organic compatible solutes, which are small organic osmolytes with high water solubility that can be accumulated to a high concentration in the cells without interfering with cellular activities. Previous studies have shown that many bacteria including not only halophiles but also thermophiles as well as some Archaea including methanogens could efficiently biosynthesize and accumulate various compatible osmolytes including polyols, sugars, amino acids, and amino acid derivatives such as N,N,N-trimethyl glycine (glycine betaine) and 1,4,5,6-tetrahydro-2-methyl-4-primidinecarboxylic acid (ectoine) (1–4).
A moderately halophilic eubacterium, Halomonas elongata, can be found in various high-salinity environments. For example, H. elongata DSM 2581T strain (formerly 1H9 strain) was isolated from a solar salt facility in the Netherlands (5), and H. elongata OUT30018 strain (formerly KS3 strain) was isolated from a high-salinity agricultural field in Khon Kaen, Thailand (6, 7). H. elongata DSM 2581T and OUT30018 strains are known to adapt to dynamic range of salinity around 0.1%–32.5% NaCl and 0.3%–21% NaCl, respectively (6). When exposed to high-salinity environment with salt concentration higher than 3% NaCl, H. elongata cells biosynthesize and accumulate ectoine as a major osmolyte, and when the cells are transferred to a low-salinity medium (3% NaCl; osmotic down shock), the cells secrete ectoine rapidly into the environment to re-establish osmotic equilibrium (8). Formerly, applying this process of synthesis and secretion upon osmotic shock (the so-called bacterial milking process), H. elongata was used in ectoine production (8). Later on, engineered H. elongata strains have been developed to improve ectoine yield, for example, H. elongata DSM 2581T strain that overproduces and excretes ectoine into the culture medium has been successfully used for the production of ectoine at industrial level (9–11). The ability of H. elongata strains to assimilate various carbon and nitrogen sources, including biomass-derived sugars, amino acids, and biogenetic amines, together with its ability to thrive in high-salinity environment (5, 12, 13) makes them valuable as cell factory host for producing useful compounds from biomass waste. Halomonas-based cell factories were recently developed as the next-generation industrial biotechnology for producing various chemicals, demonstrating that Halomonas strains can be used as low-cost chassis for biomanufacturing with high-salinity seawater-based unsterile open fermentation (14).
Different from the H. elongata DSM 2581T, H. elongata OUT30018 can utilize the major putrefactive non-volatile amines, which are histamine and tyramine, as its sole carbon and nitrogen sources (13). Therefore, H. elongata OUT30018 would be one of the most promising cell factories for production of fine chemicals such as ectoine from the putrefactive non-volatile amines in protein-rich bio-waste. Here, we explored the possibility of using H. elongata OUT30018 as cell factory to produce high-value osmolyte, other than ectoine. In this study, an ectoine-deficient salt-sensitive H. elongata mutant strain KA1, which can grow well only in culture medium containing 3% NaCl due to the loss of the ectoine biosynthesis genes (ΔectABC; FERM P-22094) (15), was subjected to an adaptive mutagenesis screening in search of spontaneous suppressor mutants with restored salt tolerance trait. Consequently, a spontaneous phenotypic suppressor mutant, which could grow on a medium containing 6% NaCl was obtained. High-performance liquid chromatography (HPLC) analysis of major osmolytes in the spontaneous suppressor mutant cells revealed that the mutant became more salt tolerant than the KA1 strain by producing and accumulating L-glutamic acid (Glu) at a higher level than the KA1 strain. Therefore, we named this mutant H. elongata Glu-overproducing (GOP) strain. However, the acidic nature of Glu has interfered with its own cellular accumulation and restricted the GOP strain from achieving tolerance to salt stress greater than 7% NaCl in the medium.
The glutamic acid decarboxylase (GAD) system has been known to facilitate intracellular pH homeostasis by consuming protons in a decarboxylation reaction that simultaneously produces an osmolyte γ‐aminobutyric acid (GABA) from Glu (16). To establish the GAD system in the H. elongata GOP strain, a synthetic H. elongata’s codon-usage-optimized (Hop) GadB mutant gene (HopGadBmut), which encodes a mutant GAD with activity under broader pH range than the wild-type (WT) GAD (17), was put under the control of a salt-inducible ectA promoter and introduced into the genome of the H. elongata GOP strain. HPLC analysis confirmed that the resulting H. elongata GOP-Gad strain produced and accumulated GABA in response to salt stress. Moreover, the H. elongata GOP-Gad strain also showed higher salt-stress tolerance than the GOP strain. We concluded that de novo biosynthesis and cellular accumulation of GABA attribute to higher salt tolerance trait of the engineered H. elongata GOP-Gad strain. We present here, the first report demonstrating that Glu and GABA could function as major osmolytes in H. elongata under high-salinity growth condition and that GABA is a better compatible osmolyte than Glu because it can be accumulated to a higher concentration in the cells without interfering with cytosolic pH homeostasis.
RESULTS
Adaptive mutagenesis screening identified a suppressor mutant of an ectoine-deficient salt-sensitive H. elongata KA1 strain
In search of compounds that can be accumulated in H. elongata as osmolytes instead of ectoine, an ectoine-deficient salt-sensitive H. elongata KA1 strain (Table 1), in which the ectoine biosynthesis operon harboring the ectABC genes was removed from the wild-type H. elongata OUT30018 strain (6), was subjected to an adaptive mutagenesis screening to find suppressor mutants with restored salt-tolerant trait. The H. elongata KA1 strain can grow well in liquid M63 medium containing 3% NaCl and grow with slight growth suppression in the M63 medium containing 4% NaCl, but not in the medium containing 6% NaCl. Therefore, in this screening, the H. elongata KA1 strain was precultured for 2 days in liquid M63 medium containing 3% NaCl before the cells were harvested and used as a 5% inoculum for a main culture in liquid M63 medium containing 6% NaCl. After culturing for 7 days, suspension of the cells harvested from the main culture was streaked on a solid M63 medium containing 6% NaCl. As a result, a mutant, which contains a mutation that partially suppressed the salt-sensitive phenotype of H. elongata KA1 strain was identified.
TABLE 1.
Bacterial strains used in this study
| Strains | Phenotypes and descriptions | References |
|---|---|---|
| Halomonas elongata | ||
| OUT30018 | Wild-type strain (Osaka University type culture, formerly designated as KS3). Salt-tolerant phenotype due to the ability to produce and accumulate ectoine as a major osmolyte. | (6) |
| KA1 | Deletion mutant of ectoine biosynthesis genes (ΔectABC) derived from the OUT30018 strain. Deposited as FERM P-22094 strain. Salt-sensitive phenotype due to the inability to produce ectoine. | (15) |
| GOP | Spontaneous suppressor mutant of the salt-sensitive KA1 strain. Improved salt-tolerant phenotype due to the ability to overproduce glutamate. | This study |
| KA1-Gad | Recombinant KA1 strain harboring an artificial bicistronic mCherry-HopGadmut operon, which regulates salt-inducible expression of a red-fluorescent mCherry reporter protein and a mutant glutamate decarboxylase (gadB), which is active at a wide pH range to convert glutamate to GABA. Salt-sensitive phenotype due to the lack of ability to produce GABA to a concentration high enough to function as osmolyte. | This study |
| GOP-Gad | Recombinant GOP strain harboring an artificial bicistronic mCherry-HopGadmut operon, which regulates salt-inducible expression of a red-fluorescent mCherry reporter protein and a mutant glutamate decarboxylase B (gadB), which is active at a wide pH range to convert glutamate to GABA. Improved salt-tolerant phenotype due to the ability to produce and accumulate GABA as a major osmolyte. | This study |
| Escherichia coli | ||
| DH5α | Used as host for pUC57-Kan-based, pBluescript II SK (-)-based, pET-based, and pK18mobsacB-based plasmids, F−, Φ80dlacZ⊿M15, ⊿(lacZYA-argF) U169, hsdR17(rk− mk+), recA1, endA1, relA, deoR, supE44, thi-1, gyrA96, λ- | (18) |
| HB101 | Used as host for pRK2013 plasmid, F−, hsd S20(rB-, mB-), recA13, ara-14, proA2, lacY1, galK2, rpsL20 (str), xyl-5, mtl-1, supE44, leuB6, thi-1 | (19) |
Glycerol is one of the important biomass-derived carbon sources produced as the by-products from oleochemical and biodiesel industries (20, 21). Therefore, glycerol is used instead of glucose as a sole carbon source in the M63 medium used for growth test to determine the level of salt tolerance of the newly isolated mutant. Growth of the mutant was compared to that of the wild-type H. elongata OUT30018 and the salt-sensitive KA1 strains in liquid M63 medium containing 4% glycerol with different concentrations of NaCl. The three bacterial strains were precultured in M63 medium containing 4% glycerol and 3% NaCl before they were used as 5% inoculums for the main cultures in M63 medium containing 4% glycerol and 3%, 6%, 7%, or 8% NaCl. As shown in Fig. 1, the mutant could tolerate growth condition with higher salt stress than the H. elongata KA1 strain, that is, it could grow in M63 medium containing 6% NaCl, while the H. elongata KA1 strain could not. However, the mutant is still less salt tolerant than the wild-type H. elongata OUT30018 and fails to grow in the medium containing more than 7% NaCl.
Fig 1.

Growth of wild-type H. elongata OUT30018 (WT), ectoine-deficient salt-sensitive KA1, and KA1-derived spontaneous mutant strains under different salt-stress conditions. H. elongata strains were precultured in M63 medium supplemented with 4% glycerol and 3% NaCl until optical density at 600 nm (OD600) reached around 0.8 and used as 5% inoculum for main cultures in M63 medium containing 4% glycerol with different NaCl concentrations (3%, 6%, 7%, or 8% NaCl). Photos of the cultures were taken after 48 h of incubation to show differences in cell density.
Identification of major osmolytes accumulated under high-salinity growth conditions in the suppressor mutant of the salt-sensitive H. elongata KA1 strain
To identify compounds, which confer salt tolerance to the isolated mutant, amino acid profile of the mutant grown under no-stress or under salt-stress conditions was determined by HPLC analysis. In extraction step, weight of the wet cell pellet was measured as cell fresh weight (CFW), and cytosolic amino acids accumulated as osmolytes were extracted from the cells by hypo-osmotic shock extraction with pure water mimicking the bacterial milking method (8, 22). As shown in Fig. 2A and B, both the ectoine-producing wild-type H. elongata OUT30018 strain and the ectoine-deficient H. elongata KA1 strain accumulate higher amount of Glu than L-alanine (Ala); however, Glu accumulated in the wild-type H. elongata OUT30018 strain stay essentially the same in the cells grown under salt-stress conditions (6% and 7% NaCl in culture medium) due to the accumulation of ectoine as a major osmolyte (data not shown). As shown in Fig. 2C, the suppressor mutant accumulated higher concentration of Glu and Ala in the cells when grown under high salt-stress condition, i.e. the mutant accumulated 25.58±1.94 μmol/g CFW of Glu and 11.32±2.94 μmol/g CFW of Ala when grown under no stress condition (grown in M63 medium containing 3% NaCl), while it accumulated 32.42±2.27 μmol/g CFW of Glu and 16.99±1.47 μmol/g CFW of Ala when grown in M63 medium containing 7% NaCl. Glu accumulated in the cells of the mutant notably increased with the degree of salt-stress (P ≤ 0.05) and was 1.9-fold higher than that of Ala under salt-stress condition. Moreover, due to the lack of ectoine synthetic pathway in the KA1, GOP and the GOP-Gad strains, Ala concentration in these strains are considerably higher than that accumulated in the wild-type OUT30018 strain probably due to the metabolic shift that increase the amount of pyruvate pool for Ala synthesis (Fig. 3). These results suggested that Glu functions as a major osmolyte that confers higher salt-stress tolerance to the suppressor mutant, therefore, we named this mutant H. elongata Glu overproducing (GOP) strain.
Fig 2.
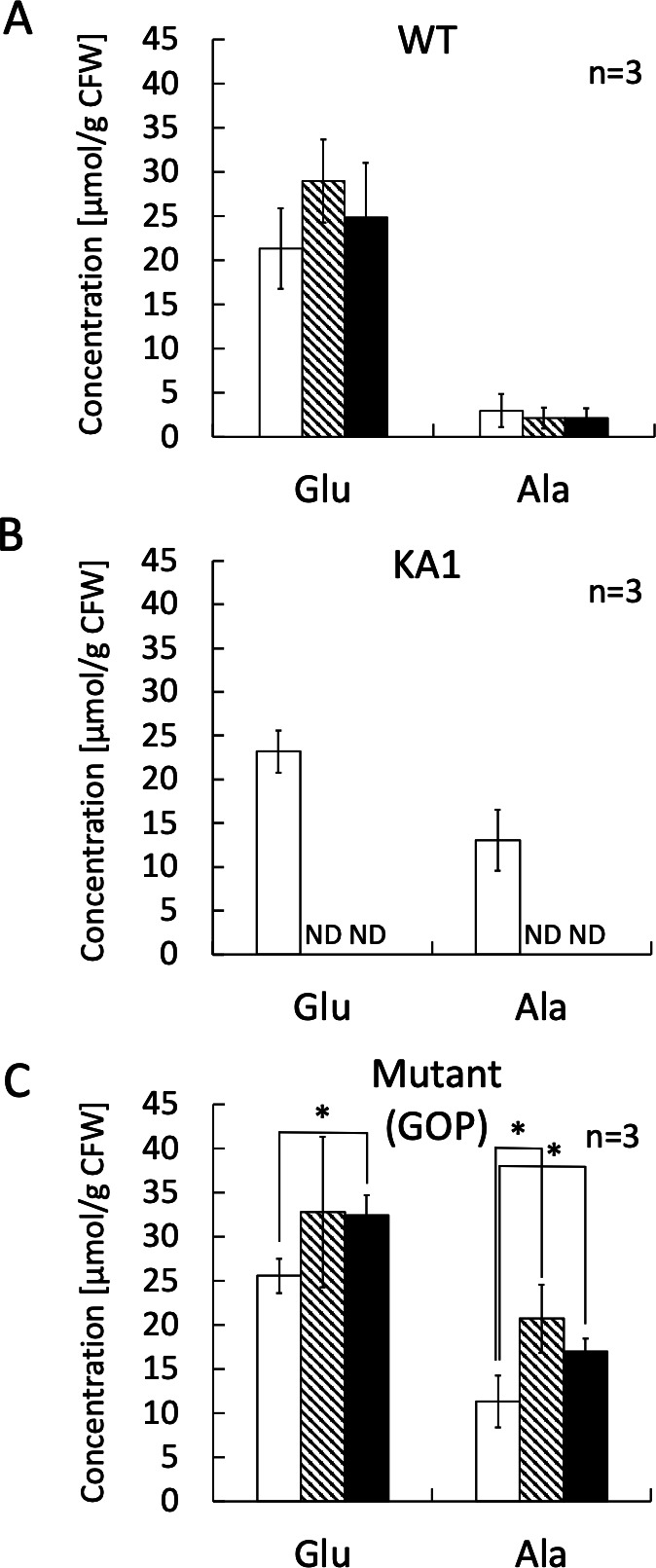
Profiles of L-glutamic acid and L-alanine in the cells of wild-type H. elongata OUT30018, ectoine-deficient salt-sensitive KA1, and KA1-derived spontaneous mutant glutamic acid-overproducing strains culturing in M63 medium containing 4% glycerol with 3% (open column), 6% (hatched column), or 7% (filled column) NaCl. H. elongata strains were precultured in M63 medium containing 4% glycerol with 3%, 6%, or 7% NaCl until OD600 reached more than 0.80 and used as a 5% inoculum for the main cultures in same salinity (3%, 6%, or 7% NaCl) medium. When OD600 of the main cultures reached more than 0.80, free amino acids including Glu and Ala were extracted from the cells by dissolving cell pellets in pure water (20 µL pure water per 1 mg cell fresh weight), and the extracts were analyzed by HPLC. Data were normalized with internal standard norvaline. Values are mean ± standard deviation (n = 3). *P ≤ 0.05. ND, no data; because H. elongata KA1 strain is unable to grow in M63 medium containing 6% or 7% NaCl. (A) Profile of Glu and Ala in WT H. elongata OUT30018 cells. (B) Profile of Glu and Ala in H. elongata KA1 cells. (C) Profile of Glu and Ala in H. elongata GOP cells.
Fig 3.

Schematic of major osmolyte biosynthetic pathways operating in H. elongata OUT30018, KA1, GOP, KA1-Gad, and GOP-Gad strains. (A) H. elongata OUT30018 accumulates ectoine as a result of an expression of the salt-inducible ectABC operon, which contains genes that encode the three enzymes of the ectoine biosynthesis pathway; L-2,4-diaminobutyric acid (DABA) transaminase (DAT) encoded by ectB gene, DABA acetyltransferase (DAA) encoded by ectA gene, and ectoine synthase (ES) encoded by ectC gene. (B) H. elongata KA1 strain does not accumulate ectoine due to the lack of the ectABC gene cluster. This strain can only grow well in the medium containing 3% NaCl. (C) H. elongata GOP strain does not accumulate ectoine due to the lack of the ectABC gene cluster; however, spontaneous mutation in its genome made this strain produces and accumulates higher Glu than the KA1 strain, possibly due to enhanced activity of either glutamate synthetase (GOGAT) or glutamate dehydrogenase (GDH). As a result, this strain has higher salt tolerance than the KA1 strain and can grow in the medium containing 6% and 7% NaCl. (D) H. elongata KA1-Gad strain is engineered to contain a salt-inducible artificial bicistronic mCherry-HopGadBmut operon encoding a red fluorescent reporter protein (mCherry) and a wide pH-range mutant of an L-glutamic acid decarboxylase (GAD), which converts Glu to GABA. This strain does not accumulate ectoine due to the lack of the ectABC gene and does not accumulate Glu to the concentration that is high enough to support GABA accumulation under high-salinity conditions. Therefore, this strain cannot grow in the medium containing more than 4% NaCl. (E) H. elongata GOP-Gad strain is engineered to contain a salt-inducible artificial bicistronic mCherry-HopGadBmut operon encoding an mCherry and a wide pH-range GAD mutant, which converts Glu accumulated in this spontaneous mutant into GABA. This strain grows better than the GOP strain in medium containing 6% and 7% NaCl due to its ability to accumulate GABA as a major osmolyte. Gly, glycerol; DHAP, dihydroxyacetone phosphate; PYR, pyruvate; AcCoA, acetyl coenzyme A; Ac-P, acetyl phosphate; A-AMP, acetyl-AMP; CIT, citrate; ICT, isocitrate; α-KG, α-ketoglutarate; SUC-CoA, succinyl-coenzyme A; SUC, succinate; FUM, fumarate; MAL, malate; OAA, oxaloacetate; Asp, aspartate; ASA, aspartic β-semialdehyde; ADABA, N-γ-acetyl-L-2,4-diaminobutyric acid; GS, glutamine synthetase.
The H. elongata GOP strain was, however, unable to thrive in the M63 medium containing 8% NaCl (Fig. 1), possibly due to the inability of the cell to accumulate the acidic amino acid, Glu, to a concentration high enough to counter the effect of severe salt stress without interfering with intracellular pH homeostasis.
Introduction of custom-engineered salt-stress-responsive glutamic acid decarboxylase system into H. elongata GOP and H. elongata KA1 strains
In acidic environments, the bacterial GAD system facilitates intracellular pH homeostasis through a decarboxylation reaction that raises cellular pH by consuming protons in the reaction that produces γ-aminobutyric acid (GABA) from Glu (16, 23). Although the enzyme GAD is active only in the acidic environment, double mutations in E. coli’s GadB at Glu89 and His465, which involved in a cooperativity system of GAD, could generate a mutant GAD (Glu89Gln/Δ452-466, named GadBmut), which is active even at a neutral pH (active from pH 4 to 8) (17, 24). We saw a potential of using the GadBmut as a tool to mitigate acid stress that is caused by Glu accumulation in the H. elongata GOP strain. In addition to neutralizing the intracellular pH, GadBmut would also generate GABA from Glu in the H. elongata GOP cells. This can be beneficial for the H. elongata GOP cells because GABA was reported to function as a compatible osmolyte in plant cells (25) and in other bacteria (26, 27).
To express GadBmut in H. elongata GOP strain, we designed and constructed an artificial bicistronic mCherry-HopGadBmut operon by putting an mCherry reporter gene, which encodes a red-florescent protein, and a H. elongata’s codon-usage-optimized GadBmut (HopGadBmut) gene under the control of an endogenous salt-stress-inducible ectA promoter which contains two putative binding sites for the vegetative sigma factor σ70 and the hyperosmotic stress responsive sigma factor σ38 (9). Then, as shown in Fig. 4, the bicistronic operon was introduced into the genome of H. elongata GOP and KA1 strains to generate H. elongata GOP-Gad and KA1-Gad strains. Selection of the resulting recombinant H. elongata GOP-Gad and KA1-Gad strains and confirmation of the transgene expression were facilitated by the visualization of the red fluorescence of the mCherry reporter protein. As shown in the upper panel of Fig. 5A, pellets of the recombinant H. elongata GOP-Gad and KA1-Gad cells cultured under optimal salt condition (M63 medium containing 3% NaCl) emitted red fluorescence, which were further intensified in the cells that were grown under salt-stress condition (M63 medium containing 6% NaCl, Fig. 5A, lower panel).
Fig 4.

Schematic of the genomic structure at ectABC locus in H. elongata OUT30018, KA1, GOP, KA1-Gad, and GOP-Gad strains. UectA, 1-kb upstream region of the ectA gene, which contains an ectA promoter with putative binding sites for the osmotically induced sigma factor σ38 and the vegetative sigma factor σ70. This region was used as a target for homologous recombination at the ectABC locus. DectC, 1-kb downstream region of the ectC gene, which contains an ectC terminator. This region was used as a target for homologous recombination at the ectABC locus. ectA, gene, which encodes an L-2,4-diaminobutyric acid (DABA) acetyltransferase; ectB, gene, which encodes a DABA transaminase; ectC, gene, which encodes an ectoine synthase; mCherry, gene, which encodes a red fluorescent reporter protein mCherry. HopGadBmut, synthetic H. elongata’s codon-usage-optimized (Hop) GadB mutant gene (HopGadBmut), which encodes a mutant glutamate decarboxylase (GAD) with activity across broader pH range than the wild-type GAD.
Fig 5.

Salt-inducible production of mCherry reporter protein in recombinant H. elongata KA1-Gad and GOP-Gad strains. H. elongata OUT30018 (WT), GOP-Gad, and KA1-Gad strains cultured in M63 medium containing 4% glycerol with 3% or 6% NaCl until OD600 reached more than 1.00 were used as a 5% inoculum for the main cultures in same salinity (3% or 6% NaCl) M63 medium containing 4% glycerol. When OD600 of the main cultures reached more than 1.00, the cells were pelleted and subjected to tests. (A) Visualization of the salt-inducible production of the mCherry fluorescent reporter protein in the H. elongata GOP-Gad and KA1-Gad strains as shown by mCherry fluorescence of the cell pellets under fluorescent light (Fl) in comparison with bright-field (BF) images. Cell pellet of H. elongata OUT30018 (WT) was used as a negative control. (B) Detection of mCherry protein produced in the H. elongata GOP-Gad and KA1-Gad strains by western blot (WB) analysis. Proteins extracted from the cell pellets shown in (A) were electrophoresed in two identical 5%–20% gradient SDS-polyacrylamide gels. One gel was stained with Coomassie Brilliant Blue (CBB; right panels) for visualization of total protein separated on each lane, while proteins on the other gel were transferred to PVDF membrane and probed with antibody to red fluorescent protein (RFP) in WB analysis (left panels). Protein extracted from H. elongata OUT30018 (WT) was used as a negative control. Rat anti-RFP tag was used as a primary antibody, and goat anti-Rat IgG/IgM (H + L) HRP was used as a secondary antibody. mCherry protein bands were detected at 26 kDa (*).
Figure 5B shows the immunodetection of the mCherry reporter protein in the crude proteins extracted from the recombinant H. elongata GOP-Gad and the KA1-Gad strains grown in liquid M63 media containing 3% or 6% NaCl. Corresponding to the increase in the fluorescent intensity shown in Fig. 5A, the amount of mCherry protein also increased when the cells were grown in medium with high salt content. These results suggest that the expression of the artificial mCherry-HopGadBmut operon is induced by salt stress under the control of the ectA promoter in the recombinant H. elongata GOP-Gad and KA1-Gad strains.
Confirmation of GABA production in H. elongata GOP-Gad and H. elongata KA1-Gad strains
To confirm that the codon-optimized HopGadBmut gene was expressed in the recombinant H. elongata KA1-Gad and GOP-Gad strains and that the HopGadBmut protein could function properly to synthesize GABA from Glu, major osmolytes in cells of the H. elongata KA1-Gad and GOP-Gad strains were profiled in comparison to that of the H. elongata KA1 and GOP strains. All strains were cultured in M63 medium containing 4% glycerol and 3% NaCl, in which the H. elongata KA1 and KA1-Gad strains could grow as well as the H. elongata GOP and GOP-Gad strains, and major osmolytes were extracted and analyzed by HPLC. As shown in Fig. 6, 25.47 ± 4.99 and 24.99 ± 5.12 µmol/g CFW of GABA were detected in the H. elongata KA1-Gad and GOP-Gad strains but not in the H. elongata KA1 and GOP strains. Notably, the concentration of Glu accumulated in the H. elongata KA1-Gad and GOP-Gad strains was 7.17 ± 0.88 and 7.47 ± 2.19 µmol/g CFW, the amount much lower than 28.48 ± 4.07 and 30.21 ± 4.85 µmol/g CFW of Glu accumulated in the H. elongata KA1 and GOP strains.
Fig 6.

Profiles of major osmolytes in H. elongata KA1, KA1-Gad, GOP, and GOP-Gad cells grown in M63 medium containing 3% NaCl. Intracellular concentration of major osmolytes, Glu (open columns), Ala (hatched columns), and GABA (filled columns) in H. elongata KA1, KA1-Gad, GOP, and GOP-Gad cells cultured in M63 medium containing 4% glycerol with 3% NaCl was profiled. Precultures were grown in M63 medium containing 4% glycerol with 3% NaCl to the OD600 of more than 1.00 and used as a 5% inoculum for a main culture in fresh M63 medium containing 4% glycerol with 3% NaCl. When OD600 of the main cultures was 0.5–0.8 during exponential growth phase, osmolytes were extracted from the cells and analyzed by HPLC. Data were normalized with internal standard norvaline. Values are mean ± standard deviation (n = 5). BDL, below detection limit.
This result showed that the engineered HopGadBmut in the recombinant H. elongata KA1-Gad and GOP-Gad strains could successfully convert Glu to GABA even in the absence of coenzyme pyridoxal-5'-phosphate supplementation in the media. This is different from other bacterial strains currently used for developing GABA-producing cell factory (28–30). As growth of the recombinant H. elongata KA1-Gad strain was severely inhibited in M63 medium containing more than 6% NaCl (data not shown), this strain was not used in experiment hereafter.
Salt-inducible bioconversion of Glu to GABA improves salt tolerance of the recombinant H. elongata GOP-Gad strain
To examine the effect of GABA production on the level of salt tolerance of the recombinant H. elongata GOP-Gad strain, growth curves of the H. elongata GOP and GOP-Gad strains, cultured in M63 medium containing 4% glycerol with a series of increasing salt-stress level (3%, 6%, 7%, and 8% NaCl), were plotted and compared. As shown in Fig. 7A and B, although both the GOP and the GOP-Gad strains could proliferate equally well in M63 medium containing 3% NaCl, a significant difference can be observed when they were cultured in M63 medium containing 6% or 7% NaCl. After 7 days of cultivation in M63 medium containing 6% NaCl, OD600 of H. elongata GOP-Gad culture was 0.47 while that of the H. elongata GOP culture was 0.15. When cultured in M63 medium containing 7% NaCl, OD600 at day 7 of H. elongata GOP-Gad culture reached 0.18, while H. elongata GOP strain was barely proliferated (P ≤ 0.05) (Fig. 7C).
Fig 7.

Effect of medium salinity on growths of H. elongata GOP and GOP-Gad strains. H. elongata GOP and GOP-Gad strains were precultured in M63 medium containing 4% glycerol with 3% NaCl until OD600 reached 1.00 before used as an inoculum for the main cultures in M63 medium containing 4% glycerol with 3% (▲), 6% (◆), 7% (●), or 8% (■) NaCl. The starting OD600 of all main cultures was adjusted to 0.01, and OD600 of each cell culture was measured at different time points. Values are mean ± standard deviation (n = 5). (A) Growth curve of H. elongata GOP strain. (B) Growth curve of H. elongata GOP-Gad strain. (C) Growth comparison between GOP (open columns) and GOP-Gad (hatched columns) strains cultured in M63 medium containing 4% glycerol with 3%, 6%, 7%, or 8% NaCl for 7 days. *P ≤ 0.05.
Notably, during routine subculture of the H. elongata GOP-Gad strain, we found substantial variation in growth rate among cultures. To determine the underlying cause of this growth variation, 30 test tubes of H. elongata GOP-Gad cultures were grown in M63 medium containing 4% glycerol and 7% NaCl and intracellular osmolytes of the cells harvested at late-log phase, when OD600 reached 0.80–1.11, were analyzed by HPLC. We found that the GABA concentration of the cultures varied greatly from 3.51 to 34.78 µmol/g CFW (Fig. 8A), which resulted in a large standard deviation of GABA concentration among cultures, as shown in Fig. 8A. After 7 days, evident difference in growth was observed among the cultures (OD600 varied from 0.51 to 1.11), 21 of the 30 cultures had OD600 of more than 0.80 (0.80–1.11) and were categorized as a fast-growing group, while the remaining 9 cultures had OD600 that was less than 0.80 (i.e., 0.51–0.75) and were categorized as a slow-growing group (Fig. 8B). Remarkably, when the amount of Glu and GABA accumulated in the cells of the two groups was compared (Fig. 8C), the average cellular concentration of GABA in the fast-growing group was 26.11 ± 5.56 µmol/g CFW, a much higher concentration than the average 6.19 ± 2.41 µmol/g CFW of the slow-growing group. Moreover, cellular concentration of Glu in the fast-growing group (11.11 ± 3.71 µmol/g CFW) was lower than that found in the slow-growing group (16.16 ± 1.80 µmol/g CFW). These results indicate that the conversion of Glu to GABA was more efficient in the fast-growing group and further confirm that the efficient bioconversion of Glu to GABA positively improves growth vigor of the recombinant GOP-Gad strain grown under salt-stress condition.
Fig 8.

Differences in major osmolytes composition among H. elongata GOP-Gad cultures, which are growing at different rates in high-salinity medium. H. elongata GOP-Gad cells, cultured in M63 medium containing 4% glycerol and 3% NaCl to OD600 of more than 0.80, were used as a 5% inoculum for 30 main cultures in M63 medium containing 4% glycerol with 7% NaCl. When OD600 of the main cultures reached 0.80–1.11, intracellular osmolytes were extracted from the cells and analyzed by HPLC. (A) Profiles of major osmolytes of H. elongata GOP-Gad strain cultured in M63 medium containing 4% glycerol and 7% NaCl. Concentration of Glu (open columns), Ala (hatched columns), and GABA (filled columns) was normalized with internal standard norvaline. Values are mean ± standard deviation (n = 30). (B) Growth profiles of H. elongata GOP-Gad cultures growing at different rates. The cultures were sorted by their OD600 at day 7 of cultivation into two groups. Out of 30 cultures, 21 cultures with OD600 that was equal to or more than 0.80 (OD600 = 0.80–1.11) were categorized as fast-growing cultures (filled columns), and 9 cultures with OD600 that was less than 0.80 (OD600 = 0.51–0.75) were categorized as slow-growing cultures (open columns). Dashed horizontal line indicates the point, where OD600 = 0.80, which separates fast-growing cultures from the slow-growing cultures. (C) Profiles of major osmolytes in the cells of the fast- and the slow-growing GOP-Gad cultures. Because intracellular osmolytes were extracted from the cultured with OD600 between 0.80 and 1.11, intracellular osmolytes of the fast-growing cultures (n = 21 of 30) were extracted after 7 days of cultivation, while those of the slow-growing cultures (n = 9 of 30) were extracted after 8 or 9 days of cultivation.
To determine the ratio of Glu to GABA bioconversion in the H. elongata GOP-Gad strain grown under different salt-stress conditions, the strain was cultured in M63 medium containing 3%, 6%, or 7% NaCl. The fast-growing cells were harvested when OD600 of the cultures of GOP-Gad strain reached around 0.8 at 2, 6, or 7 days in the medium containing 3%, 6%, or 7% NaCl, respectively. Then, major osmolytes were extracted from the cells and analyzed by HPLC. As shown in Fig. 9A, Glu, Ala, and GABA were identified as the top 3 osmolytes in the H. elongata GOP-Gad strain. When grown in M63 medium containing 3% NaCl (no salt stress), Glu (18.30 ± 3.27 µmol/g CFW), Ala (15.45 ± 3.39 µmol/g CFW), and GABA (23.56 ± 3.97 µmol/g CFW) were almost equally accumulated as major osmolytes. When grown in M63 medium containing 6% NaCl (salt stress), Glu (29.13 ± 4.05 µmol/g CFW) was found as a major osmolyte, at the concentration higher than that of Ala (15.89 ± 3.96 µmol/g CFW) and GABA (17.37 ± 2.99 µmol/g CFW). GABA, however, became the major osmolyte of the H. elongata GOP-Gad cells grown in the medium containing 7% NaCl (Fig. 9A). At 34.82 ± 5.33 µmol/g CFW, GABA was accumulated to a much higher concentration than that of Glu (11.97 ± 2.04 µmol/g CFW) and Ala (19.17 ± 2.40 µmol/g CFW). Result in Fig. 9B shows that intracellular GABA/Glu molar ratio of the H. elongata GOP-Gad cells was at the highest when the cells were grown in the medium containing 7% NaCl.
Fig 9.

Effect of medium salinity on GABA accumulation in the H. elongata GOP-Gad strain. (A) Profiles of major osmolytes in the cells of H. elongata GOP-Gad strain cultured in M63 medium containing 4% glycerol with 3%, 6%, or 7% NaCl. The strain was precultured in M63 medium containing 3% NaCl to OD600 of more than 0.8 and used as a 5% inoculum for the main culture in M63 medium containing 4% glycerol with 3%, 6%, or 7% NaCl. After two subcultures, osmolytes of the cells from the third culture were extracted when OD600 reached 0.9–1.2 and were analyzed by HPLC. Concentration of Glu, Ala, and GABA was normalized with internal standard norvaline. Values are mean ± standard deviation (n = 5). Glu, open columns; Ala, hatched columns; GABA, filled columns. (B) Molar ratio of GABA to Glu (GABA/Glu) in the cellular extracts of H. elongata GOP-Gad strain cultured in M63 medium containing 4% glycerol with 3%, 6%, or 7% NaCl. Ratio was calculated from data shown in Fig. 9A. Values are mean ± standard deviation (n = 5). **P ≤ 0.01, ***P ≤ 0.001.
Comparison of major osmolytes extraction methods: wet cells, hypo-osmotic extraction method vs freeze-dried cells, phase-separation extraction method
In all free amino acids profiling experiments in this study, free amino acids were extracted from wet pellets of H. elongata cells by a simple hypo-osmotic shock treatment with pure water, which mimics bacterial milking process (8, 22). To make certain that this simple extraction method could provide reliable estimation of free amino acid and major osmolytes in the cells, a conventional phase-separation extraction (31) was done in parallel with the hypo-osmotic extraction to prepare major osmolyte samples from the same batch of H. elongata GOP-Gad culture. In this comparison experiment, the H. elongata GOP-Gad was cultured in M63 liquid medium containing 3% NaCl until it reached mid-log phase (OD600 = 0.7–0.9). This mid-log phase culture was used as a 5% inoculum for the main culture in 120 mL of M63 liquid medium containing 7% NaCl in 300 mL flask, which was incubated at 37°C with agitation until the culture reached a late-log phase (OD600 = 1.0–1.2). Cell pellets from 50 mL of this late-log phase culture were harvested in duplicate, and the weights of the wet cell pellets were recorded as cell fresh weight (average 75.33 mg CFW/50 mL culture, n = 3). To extract major osmolytes by hypo-osmotic extraction method, pure water was added to one of the pellet samples for hypo-osmotic shock treatment, while the other pellet sample (also average 75.33 mg CFW/50 mL culture, n = 3) was freeze dried for use in phase-separation extraction method. The weight of freeze-dried cell pellet was recorded as cell dry weight (CDW, average 19.0 mg CDW/50 mL culture, n = 3), and major osmolytes in the cell pellets were extracted with methanol/chloroform/water (10:5:3.4, by volume) following the method described by Galinski and Oren (31). The amount of GABA in the extracts was then determined by HPLC, and the results are shown in Fig. 10. The average concentrations of Glu, Ala, and GABA in the extract derived from hypo-osmotic extraction method were 21.40 ± 15.93, 15.94 ± 3.47, and 42.71 ± 21.13 µmol/g CFW while that of the extracts obtained by the phase-separation extraction method were 61.30 ± 26.31, 41.16 ± 17.84, and 176.94 ± 139.06 µmol/g CDW (Fig. 10A and B). After converting the unit of the yield back to be per their origin fresh weights, the average concentrations of Glu, Ala, and GABA in the extracts derived from the phase-separation extraction method became 15.54 ± 6.91, 10.18 ± 3.74, and 42.66 ± 29.60 µmol/g CFW (Fig. 10C). To compare the efficiency of the two extraction methods, ratios of major osmolytes in the extracts obtained by hypo-osmotic method (Fig. 10A) to those obtained by conventional phase-separation method (Fig. 10C) were calculated. As shown in Fig. 10D, the ratios are all equal to or more than 1 (1.38, 1.57, and 1.00 for Glu, Ala, and GABA, respectively) suggest that the hypo-osmotic extraction method could extract more Glu and Ala from the cells than the phase-separation method, while both methods could extract about the same amount of GABA. This result suggests that hypo-osmotic extraction (bacterial milking) method is a reliable extraction method for the quantification of Glu, Ala, and GABA accumulated as major osmolytes in H. elongata cells.
Fig 10.
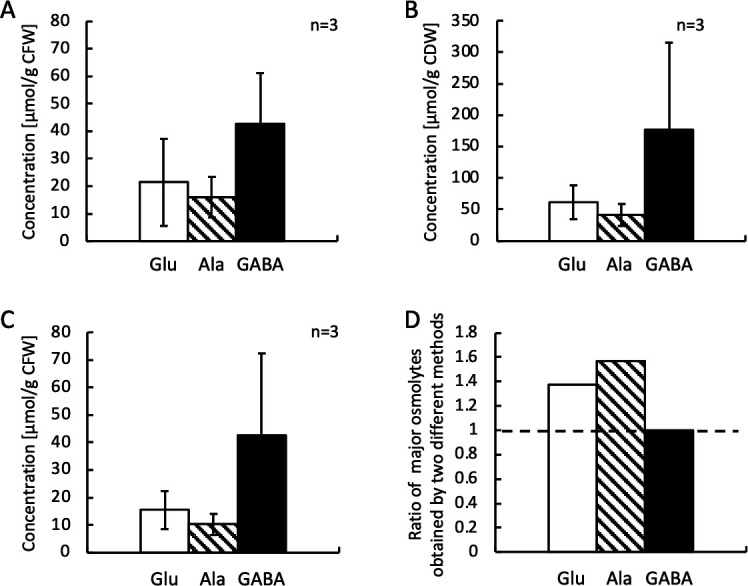
Comparison of major osmolytes extraction methods: wet cells, hypo-osmotic extraction (bacterial milking) method vs freeze-dried cells, conventional phase-separation method. H. elongata GOP-Gad strain was precultured in M63 medium containing 4% glycerol and 3% NaCl until OD600 was more than 0.8 and used as a 5% inoculum for the main culture in 120 mL M63 medium containing 4% glycerol and 7% NaCl (n = 3). When the culture reached late-log phase (OD600 = 1.0–1.2), cells were harvested from 50 mL of the culture in duplicate, and the weights of the wet cell pellets were recorded as cell fresh weight (CFW). Pure water was added to one of the pellet samples to extract the major osmolytes by hypo-osmotic bacterial milking method, while the other pellet sample was freeze dried, and the weight of the dried cell pellet was recorded as cell dry weight (CDW). Then, the major osmolytes were extracted from the dried pellet by adding methanol/chloroform/water (10:5:3.4, by volume) in the conventional phase-separation method. The amount of Glu, Ala, and GABA in the extracts was determined by HPLC. Concentration of Glu (open columns), Ala (hatched columns), and GABA (filled columns) was normalized with internal standard norvaline. (A) Major osmolyte profile of H. elongata GOP-Gad strain growing under high-salinity stress condition derived from sample extracted by wet cells, hypo-osmotic extraction (bacterial milking) method. (B) Major osmolyte profile of H. elongata GOP-Gad strain growing under high-salinity stress condition derived from sample extracted by freeze-dried cells, conventional phase-separation method. (C) A unit conversion of the data shown in B from μmol/g CDW to μmol/g CFW by calculating with original CFW of each sample measured before the freeze-drying process. (D) Ratios of the major osmolytes in the extract derived by wet cells, hypo-osmotic extraction (bacterial milking) method (Fig. 10A) to those derived by freeze-dried cells, conventional phase-separation method (Fig. 10C).
DISCUSSION
The classification of microorganisms based on their preference to salt proposed by Oren (32) divided microorganisms into non-halophilic (grow best in medium containing less than 0.2 M or 1.2% NaCl), slight halophile (grow best in medium containing 0.2–0.5 M or 1.2%–2.9% NaCl), moderate halophile (grow best in medium containing 0.5–2.5 M or 2.9%–14.7% NaCl), borderline extreme halophile (grow best in medium containing 1.5–4.0 M or 8.8%–23.5% NaCl), extreme halophile (grow best in medium containing more than 2.5–5.2 M or 14.7%–30.6% NaCl), halotolerant microorganisms (non-halophile which can tolerate and grow in medium containing less than 2.5 M or 14.7% NaCl), and extremely halotolerant microorganism (non-halophile, which can tolerate and grow in medium containing above 2.5 M or 14.7% NaCl).
To achieve osmotic adaption, these halophilic and halotolerant bacteria accumulate varieties of organic compatible solutes through de novo biosynthesis or by importing the compounds from their environment (33). There was evidence that non-halophilic, slightly halophilic, and halotolerant bacteria accumulate Glu, glutamine (Gln), Ala, proline (Pro), and GABA in their cells in response to increase in NaCl in their environment (1, 4, 26, 32–36). Some bacteria, for example, Halobacillus halophilus, cope with the effect of external salt by accumulating a cocktail of different osmolytes, such as Glu, Gln, Pro, Ala, and ectoine (37).
H. elongata is a moderate halophile, which synthesizes and accumulates ectoine as a major osmolyte when grown in high-salinity environment (3, 6). In this work, to search for novel compounds that could function as osmolytes under the high-salinity condition in H. elongata, an ectoine-deficient and salt-sensitive H. elongata KA1 (15), which only grows well in the medium containing 3% NaCl due to the lack of the three ectoine-synthesis genes (ΔectABC) from the genome of the wild-type H. elongata OUT30018, was subjected to an adaptive mutagenesis screening in search of spontaneous suppressor mutants with restored or improved salt-tolerant trait. As a result of prolonged culturing of the H. elongata KA1 in the medium containing 6% NaCl, a spontaneous phenotypic suppressor mutant, H. elongata Glu-overproducing strain, was obtained. As the name indicated, we found that the H. elongata GOP mutant could grow on a medium containing 6% NaCl by producing and accumulating Glu as a major osmolyte in the cells. Being the metabolic entry point for ammonia, Glu is an important amino acid in the cell. In H. elongata, however, the importance of Glu is even more pronounced. For wild-type H. elongata, both ectoine biosynthesis and Glu synthetase (GOGAT) pathways are activated simultaneously in response to salt stress, and Glu provides amino groups for the production of ectoine (38). In ectoine-deficient H. elongata KA1 and GOP strains, Glu produced through the GOGAT pathway could be accumulated and function as a major osmolyte when grown in medium containing 3% and 6% NaCl (Fig. 2 and 3). Comparably, an ectoine-deficient and salt-sensitive H. elongata SAA4 was found to accumulate Glu and Gln inside the cells when grown in the medium containing 2%–4% NaCl (39). H. elongata SAA4 is a transposon insertion mutant of the ectA gene; therefore, the enzyme L-2,4-diaminobutyric acid (DABA)-transaminase encoded by the ectB gene is still functionally expressed in the cells growing in the medium containing 3%–4% NaCl and produces a small amount of DABA from Glu derived from Gln and α-ketoglutaric acid via the GOGAT pathway (39). As both the H. elongata KA1 and the GOP strains no longer have the ectB gene on their genome, metabolic homeostasis of Glu and Gln in these strains would be different from that of the H. elongata SAA4. Glu also functions as an osmolyte in response to salt stress in other microorganisms. For example, a marine bacterium, Beneckea harveyi, accumulates increasing amount of Glu in the cell when cultured in medium containing 1%–3% NaCl (40), and a Rhizobium sp. strain WR1001, isolated from desert area, accumulates Glu in response to osmotic stress up to 2.9% NaCl (35).
Here, we found that Ala was accumulated together with Glu when the H. elongata GOP strain was cultured in the medium containing 3%–7% NaCl (Fig. 2). Ala was also accumulated in the wild-type H. elongata OUT30018 and the ectoine-deficient H. elongata KA1 strains (Fig. 2B) but not in the closely related H. elongata SAA4 strain, which accumulated Gln together with Glu instead (39). As Gln is a precursor of Glu in most bacteria, the accumulation of Gln in response to osmotic stress may regulate pH homeostasis to avoid increased accumulation of Glu under high-salinity conditions (41). Although a gene encoding a Gln synthetase is present in the genome of H. elongata OUT30018 strain, Gln was not accumulated in the H. elongata OUT30018, KA1, and GOP strains under high-salinity conditions. The basis of this difference will be an interesting subject for further investigation.
We found that the H. elongata GOP strain, which accumulates Glu in response to salt stress, has much lower level of salt tolerance than the wild-type H. elongata OUT30018. Growth of the H. elongata GOP strain was suppressed when cultured in M63 medium containing 7% NaCl (Fig. 1), while the wild-type H. elongata OUT30018 strain could grow well in M63 medium containing more than 15% NaCl. This probably is caused by the inability of the H. elongata GOP strain to accumulate the acidic Glu to a higher concentration without interfering with intracellular pH homeostasis (41). This possibly is also a reason behind the synthesis and accumulation of specific compatible solutes as osmolytes found in halophilic and moderately halophilic bacteria, which are regularly exposed to severe osmotic stress (above 6% NaCl). Among halophilic bacteria, the moderately halophilic bacteria are the most capable to grow across wide salinity range, from 3% to 15% NaCl. To adapt to dynamic changes in the salinity of their environment, these bacteria accumulate not just one but rather two or more compatible osmolytes. For example, H. elongata accumulates Glu and ectoine in response to mild salt stress (4% NaCl), while it accumulates ectoine alone as a major osmolyte under severe salt-stress condition (above 6% NaCl) (3, 6, 41). Similarly, H. halophilus accumulates Glu, Gln, and small amount of Pro in response to mild salt stress (2.3% NaCl) while accumulating Pro as a major osmolyte under severe salt-stress condition (17.5% NaCl) (42). This led us to find solution to mitigate the acidic stress caused by intracellular accumulation of Glu for improving salt-tolerant level of H. elongata GOP strain. In addition, during initial phase of osmotic adaptation to high-salinity environments, potassium ion (K+) may be accumulated as an inorganic compatible solute and as counterions coupled with Glu (1). Therefore, K+ and Glu may function to balance excess positive or negative charges in the bacterial cells prior to the onset of further adaptive responses against high-salinity stress (1). It will be interesting to observe changes in potassium homeostasis in the H. elongata GOP and GOP-Gad cells during their adaptation to salinity stress.
Glutamate decarboxylase system is one of the most effective acid responses and tolerance mechanisms in microorganisms (16). The GAD system facilitates intracellular pH homeostasis by consuming protons during the enzymatic decarboxylation reaction that produces GABA from Glu (16). This system was found in various bacterial species such as Lactobacillus plantarum, L. reuteri, Lactococcus lactis (43), L. brevis (23), Shigella flexneri (44), and E. coli (45, 46). Moreover, several non-halophilic bacteria, including E. coli, Clostridium sporogenes, and Streptococcus faecalis, use the GAD system to produce and accumulate GABA as a compatible osmolyte in response to increasing NaCl concentration (26, 27).
Here, using metabolic engineering approach, we installed the GAD system into the H. elongata GOP strain to mitigate acidic stress caused by Glu accumulation. A salt-stress responsive bicistronic operon harboring an mCherry reporter gene and a H. elongata codon-optimized HopGadBmut gene, which encode a wide pH range mutant GAD enzyme, was engineered and introduced into the genome of the H. elongata GOP strain (Fig. 4). As shown in Fig. 6 and 9, the resulting recombinant H. elongata GOP-Gad strain could convert Glu to GABA, which was accumulated as the major osmolyte in the cells, especially when the cells were grown under salt-stress condition. Most importantly, this accumulation of GABA evidently improved salt tolerance of the H. elongata GOP-Gad strain over that of the H. elongata GOP strain when grown in M63 medium containing 6% and 7% NaCl (Fig. 7).
Intriguingly, we observed variation of growth vigor in some batches of the H. elongata GOP-Gad cells cultured in M63 medium containing 7% NaCl. Further analysis of these cultures revealed that the cells in the fast-growing cultures accumulated significantly more GABA than that of the slow-growing cultures (Fig. 8C). Moreover, when compared with the slow-growing H. elongata GOP-Gad cultures, the cells of the fast-growing cultures emitted stronger red fluorescence of the reporter mCherry protein (data not shown). Because the recombinant HopGadBmut gene was put under the control of the same bicistronic operon that control the expression of the mCherry gene, the higher expression of the HopGadBmut transgene could be responsible for better growth vigor of the fast-growing cultures. Further investigation, such as western blot analysis, to compare the amount of HopGadBmut enzyme produced in these cultures could help clarify this hypothesis.
The difference in expression level of the HopGadBmut transgene as a result of different activation levels of the salt-inducible ectA promoter could also be the reason behind the increase in GABA accumulation and Glu-to-GABA conversion efficiency observed when the H. elongata GOP-Gad cells were grown in the medium with higher salinity (Fig. 9). Intracellular pH of H. elongata GOP-Gad cells cultured under 6% NaCl condition might not be acidic enough for the HopGadBmut enzyme to perform at their optimal activity, while in the cells cultured under higher salt stress (7% NaCl condition) might cause enough drop of the intracellular pH to the level that could increase the activity of the HopGadBmut enzyme.
In this study, to increase the throughput of free amino acids profiling in mutant and recombinant H. elongata cells, free amino acids were extracted from wet cells by hypo-osmotic shock extraction using pure water to mimic the bacterial milking method. To confirm that this simple method is reliable, a conventional method (31), which uses methanol/chloroform/water mixture to extract free amino acids from freeze-dried cells was also done in comparison. As shown in Fig. 10A and C, we found that the ratios of Glu, Ala, and GABA concentrations in the extract derived by hypo-osmotic extraction (bacterial milking) method (Fig. 10A) to those derived by the phase-separation extraction method (Fig. 10C) were 1.38, 1.57, and 1.00, respectively (Fig. 10D). This result shows that the amount of extracted GABA is similar by both methods, while the amount of Glu and Ala extracted by the hypo-osmotic method is slightly higher than those extracted by the phase-separation method. These discrepancies may be caused by different solubilities in methanol of the extracted amino acid osmolytes, which could be neutralized by K+/Na+ counterions by forming potassium/sodium salts to maintain cytosolic charge balance in the cells (47). Due to enhanced solubility in methanol, the neutralized amino acid salts may be trapped in the lower chloroform phase, which contains small amount of methanol. Therefore, our result suggests that hypo-osmotic extraction method is a reliable method for the quantification of cellular content of Glu, Ala, and GABA in H. elongata cells.
Conclusion
In this work, we set out to find a new strain of H. elongata that can produce and accumulate valuable osmolytes other than ectoine during salt stress by mutant screening. As a result, a mutant Glu-overproducing H. elongata GOP strain, which accumulates Glu as its major osmolyte, was obtained. At the start, acidic stress caused by Glu accumulation made the strain seems unsuitable for further use. However, in the end, our metabolic engineering approach to install the GAD system, which converts Glu to GABA, into the H. elongata GOP strain not only successfully alleviated the problem of acidic Glu accumulation in the resulting H. elongata GOP-Gad strain but also increased the salt tolerance of the strain through the accumulation of GABA as a major osmolyte. Therefore, we successfully improved both pH homeostasis and osmotic equilibrium of the H. elongata GOP strain by simply introducing a one-step enzyme reaction of the GAD system.
H. elongata was isolated from brine used in meat fermentation (48); therefore, it is listed as one of the microorganisms with beneficial use (49). With modern fermentation technology, H. elongata has also been used as cell factory to produce ectoine at industry level (8–11). There are evidence that H. elongata OUT30018 strain used in this work could assimilate various biomass-derived carbon sources (glycerol, glucose, xylose, and arabinose) (6, 12) and nitrogen sources (ammonium, amino acids, and their spoilage amines) (13). Other than working as a compatible osmolyte that protects H. elongata GOP-Gad cells from salinity stress, GABA is used in many applications. Among these, GABA is used as food supplements and feed additives for poultry farming (50). GABA has also been used in chemical industry to produce bio-based 2-pyrrolidone for synthesizing a biodegradable polymer, polyamide 4 (51–53). Combination of both the versatility of H. elongata OUT30018 strain and the usefulness of GABA in various industries has made the H. elongata GOP-Gad strain developed in this work as a good candidate for the development of a biomass-based GABA-producing Halomonas cell factory. In future, the GABA-producing Halomonas cell factory could be used for upcycling nitrogen-rich high-salinity waste biomasses to produce GABA-rich H. elongata cells, which can be used as a whole-cell feed additive (named as a single-cell eco-feed). This would contribute to the sustainable development of livestock and aquaculture industries for protection of our planetary health.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions
Bacterial strains and plasmids used in this study are listed in Tables 1 and 2, respectively. Halomonas elongata KA1 strain was derived from the wild-type H. elongata OUT30018 by deletion of the ect operon harboring the ectABC genes, which is responsible for ectoine biosynthesis (15). The ectoine-deficient H. elongata KA1, which has enhanced salt sensitivity than the wild-type H. elongata OUT30018, was used as the initial strain for spontaneous suppressor mutant screening. The H. elongata glutamic acid-overproducing strain was obtained as a suppressor mutant of the salt-sensitive H. elongata KA1 strain.
TABLE 2.
Plasmids used in this study
| Plasmids | Descriptions | References |
|---|---|---|
| pBluescript II SK (-) | Standard cloning vector, for blue-white colony selection; Ampr | (54) |
| pBSK-mCherry | pBluescriptII SK(-) containing an XbaI-BglII-mCherry-SpeI-RBS-BamHI-SmaI insertion fragment. Contains bicistronic operon producing both red fluorescent protein mCherry and lacZ' proteins, designed for red/blue-white colony selection; Ampr. | This study |
| pBSK-mCherry-DectC | pBSK-mCherry containing a BamHI-H. elongata downstream region of ectC (DectC)-HindIII insertion fragment; Ampr. | This study |
| pBSK-UectA-mCherry-DectC | pBSK-mCherry-DectC containing an XbaI-H. elongata upstream region of ectA (UectA)-BglII insertion fragment; Ampr. | This study |
| pBSK-UectA-DectC | pBSK-UectA-mCherry-DectC digested with BglII-BamHI, then self-ligated to remove BglII-mCherry-SpeI-RBS-BamHI fragment; Ampr. | This study |
| pUC57-HopGadBmut | pUC57-Kan containing a XhoI-SpeI-NdeI-HopGadBmut-NheI-BamHI insertion fragment; HopGadBmut gene encodes an H. elongata codon-usage-optimized mutant (Glu89Gln/Δ452–466) of E. coli’s glutamate decarboxylase B (gadB); Kanr. | This study |
| pET-Lipop5-HA | pET15b derivative containing an NcoI-NdeI-HeLipop5-HA-BamHI insertion fragment; used for sub-cloning of the synthetic HopGadBmut gene; Ampr. | (55) |
| pET-HopGadBmut | pET-Lipop5-HA containing an NdeI-HopGadBmut-BamHI insertion fragment; Ampr. | This study |
| pBSK-UectA-mCherry-HopGadBmut-DectC | pBSK-UectA-mCherry-DectC digested with SpeI and BamHI, then ligated with an XbaI-HopGadBmut-BamHI fragment from pET-HopGadBmut; containing a bicistronic operon that encodes a red fluorescent mCherry protein and a mutant gadB protein; Ampr. | This study |
| pK18mobsacB | Suicide vector containing sacB gene, which allows for direct selection of transformants with double-crossover homologous recombination on medium containing 10% sucrose; Kanr, mob, sacB. | (56) |
| pK18mobsacB-UectA-DectC | pK18mobsacB containing XbaI-UectA-DectC-HindIII insertion fragment used in homologous recombination to delete ectABC gene cluster in H. elongata to obtain H. elongata KA1strain; Kanr. | This study |
| pK18mobsacB-UectA-mCherry-HopGadBmut-DectC | pK18mobsacB containing XbaI-UectA-mCherry-HopGadBmut-DectC-HindIII fragment used in homologous recombination to introduce a HopGadBmut gene into the ectABC locus of H. elongata GOP strain and KA1 strain. mCherry was used as a reporter for monitoring salt-induced expression of transgene under the control of an ectA promoter. | This study |
| pRK2013 | Used as a mobilizing helper plasmid in triparental conjugation; Kanr. | (57) |
Codon-optimized HopGadBmut gene (accession number LC649950) encoding E. coli’s glutamic acid decarboxylase mutant (Glu89Gln/Δ452–466) (17) was designed for expression in H. elongata and introduced into H. elongata KA1 and GOP strains to generate γ-aminobutyric acid-producing H. elongata KA1-Gad and GOP-Gad strains, respectively.
For routine bacterial cultures, Luria-Bertani (LB) medium (58) was used. LB medium contains 10 g/L Bacto-tryptone, 5 g/L Bacto-yeast extract, and either 10, 20, or 60 g/L NaCl to generate a series of salt-stress media. LB medium containing 1% NaCl was used for routine culturing of E. coli. LB medium containing 2% NaCl was used for triparental mating to generate H. elongata KA1-Gad and GOP-Gad strains. LB medium containing 6% NaCl was used for routine culturing of H. elongata strains. Solid LB medium was supplemented with 15 g/L agar. The antibiotics, kanamycin (Kan) or ampicillin (Amp), were added to culture media as selection markers and for maintaining the plasmids containing the marker genes in recombinant E. coli (50 mg/L Kan or 100 mg/L Amp) and in H. elongata (100 mg/L Kan) strains. For selection of counter-selectable marker gene (sacB) in H. elongata, 150 g/L sucrose was added to solid LB medium containing 6% NaCl. E. coli and H. elongata strains were cultured in the liquid or on the solid media for 17–24 h at 37°C. Liquid cultures were aerated by shaking in water bath at the speed of 120 rpm.
For mutant screening, growth tests, and osmolyte analyses, H. elongata strains were cultured in M63 minimal medium (59), which consisted of 100 mM KH2PO4, 15 mM (NH4)2SO4, 1 mM MgSO4, and 3.9 µM FeSO4, supplemented with 3% or 6% NaCl and 2% glucose as the sole carbon source in adaptive mutagenesis screening or with 3%, 6%, 7%, or 8% NaCl and 4% glycerol as the sole carbon source for growth analysis and osmolytes profiling. Glycerol was selected as the sole carbon source because it can be found in biomass-derived waste from global biodiesel fuel production including the production established in Nagasaki prefecture, Japan. Initial pH of the media was adjusted to 7.2 with 1 M KOH solution. H. elongata strains were precultured in M63 medium supplemented with 3% NaCl and 4% glycerol before being used as 5% (vol/vol) inoculums for the main cultures.
Adaptive mutagenesis screening for spontaneous mutant of H. elongata KA1 strain with increase in salt tolerance
The ectoine-deficient H. elongata KA1 strain (ΔectABC) precultured for 2 days in M63 medium containing 3% NaCl with 2% glucose as sole carbon source was used as a 5% inoculum for the main culture in M63 medium containing 3% or 6% NaCl with 2% glucose as sole carbon source. After 7 days, an aliquot of 100 µL of the main culture was spread onto solid M63 medium containing 6% NaCl to screen for spontaneous mutant with the enhanced salt tolerance.
Recombinant DNA construction
Primers used in this study are synthesized by Eurofins Genomics K. K., Tokyo, Japan, and sequences of the primers are listed in Table 3. PCR reactions were performed with the primeSTAR HS DNA Polymerase (Takara Bio Inc., Shiga, Japan) or Quick Taq HS DyeMix (Toyobo, Osaka, Japan).
TABLE 3.
Sequences of oligonucleotide primers
| Primers | Sequence (5'−3')a | Descriptions | Restriction enzyme sites |
|---|---|---|---|
| UectA-F | TCTAGATTCGATCTCGATGACTTCCCGCAGC | Forward primer for amplification of an upstream region of H. elongata’s ectA (UectA). | XbaI |
| UectA-R | ACTAGTAGATCTTACGTACATTGTCGTGGTTCGCTGTAGCGAATTTG | Reverse primer for amplification of UectA. | SpeI, BglII, SnaBI |
| DectC-F | ACTAGTGGATCCTAACCCGGCGCAGTATTCTGCCG | Forward primer for amplification of a downstream region of H. elongata’s ectC (DectC). | SpeI, BamHI |
| DectC-R | AAGCTTGGGCATGGTGCATTGTCGAGGGAG | Reverse primer for amplification of DectC. | HindIII |
| mCherry-F | AAATCTAGAAAGATCTGAGGAGGATAACATGGCCATCATCAAGGA | Forward primer for amplification of an mCherry gene. | XbaI, BglII |
| mCherry-R | AAACCCGGGGATCCCATGGTATATCTCCTTCTTAAACTAGTCAGTCCATGCCGCCGGTGGAG | Reverse primer for amplification of an mCherry gene. |
SmaI, BamHI, SpeI |
| HopGadBmut-F | GCGCCAAGAGCATCAGCACCATC | Forward primer for amplification of a partial HopGadBmut fragment. | |
| HopGadBmut-R | GGTAGCTGGCATTCTGCACCTTGG | Reverse primer for amplification of HopGadBmut fragment. |
Restriction endonuclease sites incorporated into primer sequences are underlined.
For construction of pBSK-mCherry to be used as a tool for sub-cloning of DNA fragments, mCherry fragment with ribosome-binding sequence (mCherry-RBS) was PCR amplified with mCherry-F and mCherry-R primer pair using the pmCherry plasmid (Clontech/Takara Bio Inc., Shiga, Japan) as a template. The amplified mCherry-RBS (692 bp) was digested with restriction enzymes, XbaI and SmaI, then inserted into an XbaI-SmaI gap of pBluescript II SK (-) to create pBSK-mCherry (Table 2), which expresses both red fluorescent protein mCherry and lacZ' proteins for red-white and blue-white colony selection.
For construction of a salt-inducible bicistronic mCherry-HopGadBmut operon, the 5′ and 3′ flanking regions of ect operon (ectABC) in the genome of H. elongata, upstream fragment of ectA gene (UectA) and downstream fragment of ectC gene (DectC) were PCR amplified from genomic DNA of H. elongata OUT30018 using UectA-F + UectA-R and DectC-F + DectCR primer pairs. PCR cycles undergo initial denaturation at 98°C for 2 min, followed by 30 cycles of denaturation at 98°C for 10 s, and annealing/extension at 68°C for 1 min 30 s. The amplified DectC fragment (1,227 bp) was digested with restriction enzymes, BamHI and HindIII, then inserted into a BamHI-HindIII gap of pBSK-mCherry to create pBSK-mCherry-DectC, while the amplified UectA fragment (1,203 bp) was digested with restriction enzymes, XbaI and BglII, then inserted into an XbaI-BglII gap of the pBSK-mCherry-DectC to create pBSK-UectA-mCherry-DectC. A synthetic H. elongata’s codon-usage-optimized HopGadBmut gene was designed using the Gene Designer software (60) to encode a mutant version of glutamic acid decarboxylase, which is active under broader pH range than the wild-type GAD (17). The NdeI-HopGadBmut-BamHI fragment was subcloned from pUC57-HopGadBmut (Table 3) using the pET-Lipop5-HA as a sub-cloning vector to generate pET-HopGadBmut plasmid. Then the NdeI-HopGadBmut-BamHI fragment from the pET-HopGadBmut was inserted into an NdeI-BamHI gap of the pBSK-UectA-mCherry-DectC plasmid to generate pBSK-UectA-mCherry-HopGadBmut-DectC. Finally, the XbaI-UectA-mCherry-HopGadBmut-DectC-HindIII fragment from pBSK-UectA-mCherry-HopGadBmut-DectC plasmid was inserted into an XbaI-HindIII gap of the pK18mobsacB to generate the pK18mobsacB-UectA-mCherry-HopGadBmut-DectC. The mCherry and HopGadBmut genes were engineered to express as an artificial bicistronic operon under the control of the ectA promoter in H. elongata. The mCherry gene was used as a reporter to confirm expression of the artificial bicistronic operon in H. elongata.
Generation of the recombinant H. elongata KA1-Gad and GOP-Gad strains
The UectA-mCherry-HopGadBmut-DectC fragment in pK18mobsacB-UectA-mCherry-HopGadBmut-DectC plasmid, which was maintained in E. coli DH5α strain, was introduced into the genome of H. elongata KA1 and GOP strains by a two-step homologous recombination using E. coli HB101/pRK2013-mediated tri-parental conjugation method (61). Three bacterial strains, the donor E. coli DH5α harboring the pK18mobsacB-UectA-mCherry-HopGadBmut-DectC plasmid, the helper E. coli HB101 harboring the pRK2013 conjugative plasmid, and the recipient H. elongata KA1 or GOP strains, were co-cultured for triparental mating on Omnipore membrane (Merck Millipore, Darmstadt, Germany), which was placed on 2% NaCl solid LB medium. After triparental conjugation, cells were cultured on 6% NaCl solid LB medium containing 100 mg/L Kan for the selection of single-crossover strains. Subsequently, Kan-resistant recombinant cells were selected and cultured on 6% NaCl LB solid medium supplemented with 15% sucrose for the selection of the second single-crossover strains with sucrose-tolerant phenotype, in which 50% of the cells would be revertants (reverting back to the original H. elongata KA1 or GOP strains), and another 50% would be recombinants (KA1-Gad or GOP-Gad strains). The H. elongata KA1-Gad or GOP-Gad strains were identified by genomic PCR using a HopGadBmut-specific primer pair (HopGadBmut-F and HopGadBmut-R, Table 3), which amplifies a partial 987-bp fragment of the HopGadBmut gene. PCR cycles undergo initial denaturation at 94°C for 2 min, followed by 35 cycles of denaturation at 94°C for 30 s, and annealing/extension at 68°C for 1 min 20 s.
Crude protein extraction
Production of the mCherry reporter protein was used to indirectly verify the expression of the HopGadBmut gene. The wild-type H. elongata OUT30018 strain (negative control), H. elongata KA1-Gad strain, and H. elongata GOP-Gad strain, cultured in liquid M63 medium containing 3% or 6% NaCl to optical density at 600 nm (OD600) of around 0.8, were used as a 5% inoculum for the main culture in the same salinity medium. When OD600 of the main cultures reached 1, the cells were collected by centrifugation at 13,000 rpm for 3 min, resuspended in 200 µL phosphate-buffered saline, and disrupted by three sets of 30-s sonication to yield cell lysates, which contain crude protein extract. A volume of 15 µL aliquot of lysates from each strain was mixed with 15 µL Ez-Apply dye (Tris-HCl buffer, 1% SDS, 10% sucrose, bromophenol blue (BPB), and 50 mM dithiotreitol (DTT); AE-1430, ATTO Corporation, Tokyo, Japan), homogenized, and heated at 100°C for 3 min to prepare for electrophoresis separation on SDS-polyacrylamide gel.
Western blot analysis
Identical set of crude protein samples was separated by electrophoresis in two 5%–20% gradient SDS-polyacrylamide gels (E-R520L, ATTO Corporation, Tokyo, Japan) in Ez-Run (25 mM Tris, 192 mM glycine, and 0.1% SDS; AE-1410, ATTO Corporation, Tokyo, Japan) buffer. Separated proteins on one of the gels were electrophoretically transferred onto PVDF membranes (WSE-4050, ATTO Corporation, Tokyo, Japan) using western blotting transfer buffer EzBlot (AE-1460, ATTO Corporation, Tokyo, Japan). Non-specific binding sites were blocked using TBS-T solution containing 0.1% EzTween20 (WSE-7235, ATTO Corporation, Tokyo, Japan) in EzTBS buffer (25 mM Tris, 150 mM NaCl; WSE-7230, ATTO Corporation, Tokyo, Japan) with 5% skim milk for 1 h at room temperature. The membrane with transferred protein was washed once with TBS-T and incubated with 5 µL of primary antibody (RFP-tag Rat monoclonal 5F8, 1:1,000, ChromoTek, Planegg-Martinsried, Germany) in 20 mL TBS-T with 5% skim milk for 2 h at room temperature. After three washes in TBS-T with 5% skim milk, the membrane was incubated with 5 µL of second antibody [Goat anti-Rat IgG/IgM (H + L) HRP, 1:1,000, Novus Biologicals USA] in 20 mL TBS-T with 5% skim milk for 1 h at room temperature. After three washes with TBS-T, immunoreactive bands were visualized by incubating the membrane in reaction solution EzWestlumi (AE-1495, ATTO Corporation, Tokyo, Japan). The other SDS-polyacrylamide gel was dyed with EzStain AQUA (Coomassie Brilliant Blue; AE-1340, ATTO Corporation, Tokyo, Japan) at room temperature for 1 h for visualization of total proteins separated in each lane.
Hypo-osmotic extraction (bacterial milking) of free amino acids from H. elongata cells
H. elongata strains were cultured in liquid M63 medium supplemented with 4% glycerol with different levels of salinity until OD600 reached around 0.8 (log phase), then the cells were harvested by centrifugation at 13,000 rpm for 3 min, and the weight of the cell pellet was recorded as cell fresh weight. Mimicking the bacterial milking process (8, 22), the cell pellets were suspended in pure water (20 µL per 1 mg cell fresh weight) for hypo-osmotic extraction of free-amino acids from the cells. After centrifugation at 13,000 rpm for 3 min, the supernatant containing the free amino acids was collected as major osmolytes’ sample.
Phase-separation extraction of free amino acids from H. elongata cells
H. elongata was cultured and harvested, and fresh weight of the cell pellets were determined in the same way as mentioned in the hypo-osmotic extraction (bacterial milking) method. However, this method (31) required the cells to be freeze dried before the extraction; therefore, the cell pellets were frozen overnight at −80°C before they were freeze dried overnight in a Freeze dryer (EYELA FDL-1000, Tokyo Rikakikai Co. Ltd, Tokyo, Japan), and the weight of the freeze-dried pellets was recorded as cell dry weight. The extraction was done by vigorously mixing the freeze-dried cells overnight with the extraction solution, which contains 10:5:3.4 by volume mixture of methanol:chloroform:water (18.4 mL extraction solution/mg CDW). After centrifugation (5,000 × g) to remove cell debris, the supernatant was re-extracted in 1:1 by volume mixture of chloroform:water (10 mL chloroform:distilled water/mg CDW). After vigorous mixing and centrifugation (5,000 × g), the top hydrophilic phase was collected as major osmolytes’ sample.
Amino acids dabsylation
Amino acids dabsylation was performed based on a previous method (62) with a slight modification. A volume of 10 μL aliquot of free amino acids extracted from H. elongata cells or standard amino acids was mixed with 2 µL of 2.5 mM internal standard norvaline and 8 µL of 1 M NaHCO3 pH adjustment solution. Then, the sample was mixed with 40 µL of dabsylation reagents containing 2 mg/L dabsyl chloride dissolved in acetonitrile and incubated at 70°C for 15 min. After the incubation, 440 µL of 250 mM NaHCO3 solution was added, and the samples were centrifuged at 13,000 rpm for 3 min. The supernatant was collected and filtered through a filter vial with 0.2-µm pore size polytetrafluoroethylene (PTFE) membrane (SEPARA Syringeless filter, GVS Japan K.K., Tokyo, Japan) prior to HPLC analysis.
HPLC gradient system for determination of dabsyl amino acids
The determination of dabsyl amino acids (63) derived from H. elongata cells was carried out using high-performance liquid chromatography system (Shimazdu, Kyoto, Japan) equipped with an UV/VIS detector (SPD-10 A VP), an auto sampler (SIL-10 AD VP), two pumps (LC-10 AD VP), degasser (DGU-14A), system controller (SCL-10A Vp), and column oven (CTO-10AC VP). The LabSolutions LC software (Shimadzu, Kyoto, Japan) was used for the system control and data acquisition. Chromatographic separation of dabsyl amino acids was achieved through an analytical C18 column (Poroshell 120 2.7 µm, EC-C18, 4.6 × 75 mm, Agilent Technologies Inc.) with C18 guard column (Poroshell 120 2.7 µm Fast Guard, EC-C18, 4.6 × 5 mm, Agilent Technologies Inc.) using a mobile-phase gradient system consisting of 15% acetonitrile in 20 mM sodium acetate (pH 6.0) (mobile phase A) and 100% acetonitrile (mobile phase B). Dabsyl amino acids were determined by the UV/VIS detector at 468 nm. The injection volume was 10 µL, the flow rate was 0.5 mL/min, and the column temperature was maintained at 25°C. The eluent gradient is listed in Table 4.
TABLE 4.
HPLC gradient condition
| Time (min) | Flow rate (mL/min) | Mobile phase A (%)a | Mobile phase B (%)b | Mode |
|---|---|---|---|---|
| 0.0 | 0.5 | 88.0 | 12.0 | Injection |
| 0.5 | 0.5 | 88.0 | 12.0 | Isocratic |
| 2.0 | 0.5 | 82.0 | 18.0 | Linear gradient |
| 2.5 | 0.5 | 82.0 | 18.0 | Isocratic |
| 3.0 | 0.5 | 80.0 | 20.0 | Linear gradient |
| 3.5 | 0.5 | 80.0 | 20.0 | Isocratic |
| 4.0 | 0.5 | 79.5 | 20.5 | Linear gradient |
| 4.5 | 0.5 | 79.5 | 20.5 | Isocratic |
| 5.0 | 0.5 | 79.0 | 21.0 | Linear gradient |
| 5.5 | 0.5 | 79.0 | 21.0 | Isocratic |
| 6.0 | 0.5 | 78.5 | 21.5 | Linear gradient |
| 6.5 | 0.5 | 78.5 | 21.5 | Isocratic |
| 7.0 | 0.5 | 78.0 | 22.0 | Linear gradient |
| 7.5 | 0.5 | 78.0 | 22.0 | Isocratic |
| 8.0 | 0.5 | 77.5 | 22.5 | Linear gradient |
| 8.5 | 0.5 | 77.5 | 22.5 | Isocratic |
| 9.0 | 0.5 | 77.0 | 23.0 | Linear gradient |
| 9.5 | 0.5 | 77.0 | 23.0 | Isocratic |
| 10.5 | 0.5 | 76.9 | 23.1 | Linear gradient |
| 11.0 | 0.5 | 76.9 | 23.1 | Isocratic |
| 11.5 | 0.5 | 76.8 | 23.2 | Linear gradient |
| 12.0 | 0.5 | 76.8 | 23.2 | Isocratic |
| 12.5 | 0.5 | 76.7 | 23.3 | Linear gradient |
| 13.0 | 0.5 | 76.7 | 23.3 | Isocratic |
| 14.0 | 0.5 | 76.5 | 23.5 | Linear gradient |
| 14.5 | 0.5 | 76.5 | 23.5 | Isocratic |
| 15.0 | 0.5 | 76.4 | 23.6 | Linear gradient |
| 15.5 | 0.5 | 76.4 | 23.6 | Isocratic |
| 16.0 | 0.5 | 76.3 | 23.7 | Linear gradient |
| 16.5 | 0.5 | 76.3 | 23.7 | Isocratic |
| 17.0 | 0.5 | 76.2 | 23.8 | Linear gradient |
| 17.5 | 0.5 | 76.2 | 23.8 | Isocratic |
| 18.0 | 0.5 | 76.1 | 23.9 | Linear gradient |
| 18.5 | 0.5 | 76.1 | 23.9 | Isocratic |
| 19.0 | 0.5 | 76.0 | 24.0 | Linear gradient |
| 19.5 | 0.5 | 76.0 | 24.0 | Isocratic |
| 20.5 | 0.5 | 72.0 | 28.0 | Linear gradient |
| 21.0 | 0.5 | 72.0 | 28.0 | Isocratic |
| 21.5 | 0.5 | 70.0 | 30.0 | Linear gradient |
| 22.0 | 0.5 | 70.0 | 30.0 | Isocratic |
| 22.5 | 0.5 | 20.0 | 80.0 | Linear gradient |
| 23.0 | 0.5 | 20.0 | 80.0 | Isocratic |
| 23.5 | 0.5 | 10.0 | 90.0 | Linear gradient |
| 24.0 | 0.5 | 10.0 | 90.0 | Isocratic |
| 24.5 | 0.5 | 88.0 | 12.0 | Linear gradient |
| 35.0 | 0.5 | 88.0 | 12.0 | Isocratic |
Mobile phase A: 20 mM sodium acetate buffer (pH 6)/acetonitrile (85:15, by volume).
Mobile phase B: acetonitrile.
ACKNOWLEDGMENTS
This work was partially supported by JSPS KAKENHI Grant Numbers 19K12400 and 22K12446, JST Grant Number JPMJPF2117, IFO Grant Number LA-2022-035, the Nagasaki University WISE Program, and the Nagasaki University Priority Research Subject Project. We thank for Drs Takashi Tanaka, Kiyotaka Hara and Fumiyoshi Okazaki for technical advices as well as Drs Kei Nakagawa, Kazuaki Kawamoto, Takashi Watanabe, Hironori Hamasaki, Makoto Kagabu, Mitsuhiko Koyama, and Masaya Nishiyama for helpful discussions.
Z.Z.: Data curation, Formal analysis, Investigation, Validation, Writing–original draft. P.K-N.: Supervision, Writing–original draft, Writing–review and editing. J.O-I.: Investigation, Methodology. H.N.: Conceptualization, Project administration, Funding acquisition, Resources, Supervision, Data curation, Formal analysis, Investigation, Methodology, Validation, Writing–original draft, Writing–review and editing.
Contributor Information
Hideki Nakayama, Email: nakayamah@nagasaki-u.ac.jp.
Pablo Ivan Nikel, Danmarks Tekniske Universitet, The Novo Nordisk Foundation Center for Biosustainability, Kongens Lyngby, Lyngby-Taarbæk, Denmark.
REFERENCES
- 1. Kempf B, Bremer E. 1998. Uptake and synthesis of compatible solutes as microbial stress responses to high-osmolality environments. Arch Microbiol 170:319–330. doi: 10.1007/s002030050649 [DOI] [PubMed] [Google Scholar]
- 2. Roberts MF. 2005. Organic compatible solutes of halotolerant and halophilic microorganisms. Saline Syst 1:5. doi: 10.1186/1746-1448-1-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Galinski EA, Pfeiffer H-P, Trüper HG. 1985. 1,4,5,6-Tetrahydro-2-methyl-4-pyrimidinecarboxylic acid. Eur J Biochem 149:135–139. doi: 10.1111/j.1432-1033.1985.tb08903.x [DOI] [PubMed] [Google Scholar]
- 4. da Costa MS, Santos H, Galinski EA. 1998. An overview of the role and diversity of compatible solutes in Bacteria and Archaea. Adv Biochem Eng Biotechnol 61:117–153. doi: 10.1007/BFb0102291 [DOI] [PubMed] [Google Scholar]
- 5. Vreeland RH, Litchfield CD, Martin EL, Elliot E. 1980. Halomonas elongata, a new genus and species of extremely salt-tolerant bacteria. Int J Syst Evol Microbiol 30:485–495. doi: 10.1099/00207713-30-2-485 [DOI] [Google Scholar]
- 6. Ono H, Okuda M, Tongpim S, Imai K, Shinmyo A, Sakuda S, Kaneko Y, Murooka Y, Takano M. 1998. Accumulation of compatible solutes, ectoine and hydroxyectoine, in a moderate halophile, Halomonas elongata KS3 isolated from dry salty land in Thailand. J Ferment Bioeng 85:362–368. doi: 10.1016/S0922-338X(98)80078-0 [DOI] [Google Scholar]
- 7. Ono H, Sawada K, Khunajakr N, Tao T, Yamamoto M, Hiramoto M, Shinmyo A, Takano M, Murooka Y. 1999. Characterization of biosynthetic enzymes for ectoine as a compatible solute in a moderately halophilic eubacterium, Halomonas elongata. J Bacteriol 181:91–99. doi: 10.1128/JB.181.1.91-99.1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sauer T, Galinski EA. 1998. Bacterial milking: a novel bioprocess for production of compatible solutes. Biotechnol Bioeng 59:128. doi: [DOI] [PubMed] [Google Scholar]
- 9. Schwibbert K, Marin-Sanguino A, Bagyan I, Heidrich G, Lentzen G, Seitz H, Rampp M, Schuster SC, Klenk H-P, Pfeiffer F, Oesterhelt D, Kunte HJ. 2011. A blueprint of ectoine metabolism from the genome of the industrial producer Halomonas elongata DSM 2581T. Environ Microbiol 13:1973–1994. doi: 10.1111/j.1462-2920.2010.02336.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jörg Kunte H, Schwarz T, Galinski E. 2020. 5 the compatible solute ectoine: protection mechanisms, strain development, and industrial production, p 121–152. In Lee N (ed), Biotechnological applications of extremophilic microorganisms. De Gruyter, Berlin, Boston. [Google Scholar]
- 11. Hobmeier K, Oppermann M, Stasinski N, Kremling A, Pflüger-Grau K, Kunte HJ, Marin-Sanguino A. 2022. Metabolic engineering of Halomonas elongata: ectoine secretion is increased by demand and supply driven approaches. Front Microbiol 13:968983. doi: 10.3389/fmicb.2022.968983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tanimura K, Nakayama H, Tanaka T, Kondo A. 2013. Ectoine production from lignocellulosic biomass-derived sugars by engineered Halomonas elongata. Bioresour Technol 142:523–529. doi: 10.1016/j.biortech.2013.05.004 [DOI] [PubMed] [Google Scholar]
- 13. Nakayama H, Kawamoto R, Miyoshi K. 2020. Ectoine production from putrefactive non-volatile amines in the moderate halophile Halomonas elongata. IOP Conf Ser Earth Environ Sci 439:012001. doi: 10.1088/1755-1315/439/1/012001 [DOI] [Google Scholar]
- 14. Ye J-W, Chen G-Q. 2021. Halomonas as a chassis. Essays Biochem 65:393–403. doi: 10.1042/EBC20200159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Konishi K, Nakayama H, Kondo A. 2015. Method for producing compatible solute. In Japan patent 5804353. Japan Patent Office, Tokyo. https://patents.google.com/patent/JP2012029679A/en. [Google Scholar]
- 16. Feehily C, Karatzas KAG. 2013. Role of glutamate metabolism in bacterial responses towards acid and other stresses. J Appl Microbiol 114:11–24. doi: 10.1111/j.1365-2672.2012.05434.x [DOI] [PubMed] [Google Scholar]
- 17. Thu Ho NA, Hou CY, Kim WH, Kang TJ. 2013. Expanding the active pH range of Escherichia coli glutamate decarboxylase by breaking the cooperativeness. J Biosci Bioeng 115:154–158. doi: 10.1016/j.jbiosc.2012.09.002 [DOI] [PubMed] [Google Scholar]
- 18. Inoue H, Nojima H, Okayama H. 1990. High efficiency transformation of Escherichia coli with plasmids. Gene 96:23–28. doi: 10.1016/0378-1119(90)90336-p [DOI] [PubMed] [Google Scholar]
- 19. Boyer HW, Roulland-Dussoix D. 1969. A complementation analysis of the restriction and modification of DNA in Escherichia coli. J Mol Biol 41:459–472. doi: 10.1016/0022-2836(69)90288-5 [DOI] [PubMed] [Google Scholar]
- 20. Anitha M, Kamarudin SK, Kofli NT. 2016. The potential of glycerol as a value-added commodity. Chem Eng J 295:119–130. doi: 10.1016/j.cej.2016.03.012 [DOI] [Google Scholar]
- 21. Gunstone F, Heming M. 2004. FEATURE glycerol — an important product of the oleochemical industry. Lipid Technol 16:177–179. https://www.researchgate.net/publication/267546320_FEATURE_Glycerol_-_an_important_product_of_the_oleochemical_industry. [Google Scholar]
- 22. Cánovas D, Vargas C, Iglesias-Guerra F, Csonka LN, Rhodes D, Ventosa A, Nieto JJ. 1997. Isolation and characterization of salt-sensitive mutants of the moderate halophile Halomonas elongata and cloning of the ectoine synthesis genes. J Biol Chem 272:25794–25801. doi: 10.1074/jbc.272.41.25794 [DOI] [PubMed] [Google Scholar]
- 23. Lyu C, Zhao W, Peng C, Hu S, Fang H, Hua Y, Yao S, Huang J, Mei L. 2018. Exploring the contributions of two glutamate decarboxylase Isozymes in Lactobacillus brevis to acid resistance and γ-aminobutyric acid production. Microb Cell Fact 17:180. doi: 10.1186/s12934-018-1029-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Choi JW, Yim SS, Lee SH, Kang TJ, Park SJ, Jeong KJ. 2015. Enhanced production of γ-aminobutyrate (GABA) in recombinant Corynebacterium glutamicum by expressing glutamate decarboxylase active in expanded pH range. Microb Cell Fact 14:21. doi: 10.1186/s12934-015-0205-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Shelp BJ, Bown AW, McLean MD. 1999. Metabolism and functions of gamma-aminobutyric acid. Trends Plant Sci 4:446–452. doi: 10.1016/s1360-1385(99)01486-7 [DOI] [PubMed] [Google Scholar]
- 26. Measures JC. 1975. Role of amino acids in osmoregulation of non-halophilic bacteria. Nature 257:398–400. doi: 10.1038/257398a0 [DOI] [PubMed] [Google Scholar]
- 27. Yancey PH, Clark ME, Hand SC, Bowlus RD, Somero GN. 1982. Living with water stress: evolution of osmolyte systems. Science 217:1214–1222. doi: 10.1126/science.7112124 [DOI] [PubMed] [Google Scholar]
- 28. Altaib H, Kozakai T, Badr Y, Nakao H, El-Nouby MAM, Yanase E, Nomura I, Suzuki T. 2022. Cell factory for γ-aminobutyric acid (GABA) production using Bifidobacterium adolescentis. Microb Cell Fact 21:33. doi: 10.1186/s12934-021-01729-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Baritugo K-A, Kim HT, David Y, Khang TU, Hyun SM, Kang KH, Yu JH, Choi JH, Song JJ, Joo JC, Park SJ. 2018. Enhanced production of gamma-aminobutyrate (GABA) in recombinant Corynebacterium glutamicum strains from empty fruit bunch biosugar solution. Microb Cell Fact 17:129. doi: 10.1186/s12934-018-0977-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Takahashi C, Shirakawa J, Tsuchidate T, Okai N, Hatada K, Nakayama H, Tateno T, Ogino C, Kondo A. 2012. Robust production of gamma-amino butyric acid using recombinant Corynebacterium glutamicum expressing glutamate decarboxylase from Escherichia coli. Enzyme Microb Technol 51:171–176. doi: 10.1016/j.enzmictec.2012.05.010 [DOI] [PubMed] [Google Scholar]
- 31. Galinski EA, Oren A. 1991. Isolation and structure determination of a novel compatible solute from the moderately halophilic purple sulfur bacterium Ectothiorhodospira marismortui. Eur J Biochem 198:593–598. doi: 10.1111/j.1432-1033.1991.tb16055.x [DOI] [PubMed] [Google Scholar]
- 32. Oren A. 2013. Life at high salt concentrations, p 421–440. In Rosenberg E, DeLong EF, Lory S, Stackebrandt E, Thompson F(ed), The prokaryotes. Springer, Berlin, Heidelberg. [Google Scholar]
- 33. Imhoff JF. 1986. Osmoregulation and compatible solutes in eubacteria. FEMS Microbiol Rev 39:57–66. doi: 10.1111/j.1574-6968.1986.tb01843.x [DOI] [Google Scholar]
- 34. Anderson CB, Witter LD. 1982. Glutamine and proline accumulation by Staphylococcus aureus with reduction in water activity. Appl Environ Microbiol 43:1501–1503. doi: 10.1128/aem.43.6.1501-1503.1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hua S-S, Tsai VY, Lichens GM, Noma AT. 1982. Accumulation of amino acids in Rhizobium sp. strain WR1001 in response to sodium chloride salinity. Appl Environ Microbiol 44:135–140. doi: 10.1128/aem.44.1.135-140.1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Brown AD. 1976. Microbial water stress. Bacteriol Rev 40:803–846. doi: 10.1128/br.40.4.803-846.1976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Saum SH, Müller V. 2008. Regulation of osmoadaptation in the moderate halophile Halobacillus halophilus: chloride, glutamate and switching osmolyte strategies. Saline Syst 4:4. doi: 10.1186/1746-1448-4-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kindzierski V, Raschke S, Knabe N, Siedler F, Scheffer B, Pflüger-Grau K, Pfeiffer F, Oesterhelt D, Marin-Sanguino A, Kunte H-J. 2017. Osmoregulation in the halophilic bacterium Halomonas elongata: a case study for integrative systems biology. PLoS One 12:e0168818. doi: 10.1371/journal.pone.0168818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Göller K, Ofer A, Galinski EA. 1998. Construction and characterization of an NaCl-sensitive mutant of Halomonas elongata impaired in ectoine biosynthesis. FEMS Microbiol Lett 161:293–300. doi: 10.1111/j.1574-6968.1998.tb12960.x [DOI] [PubMed] [Google Scholar]
- 40. Makemson JC, Hastings JW. 1979. Glutamate functions in osmoregulation in a marine bacterium. Appl Environ Microbiol 38:178–180. doi: 10.1128/aem.38.1.178-180.1979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Csonka LN. 1989. Physiological and genetic responses of bacteria to osmotic stress. Microbiol Rev 53:121–147. doi: 10.1128/mr.53.1.121-147.1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Saum SH, Müller V. 2007. Salinity-dependent switching of osmolyte strategies in a moderately halophilic bacterium: glutamate induces proline biosynthesis in Halobacillus halophilus. J Bacteriol 189:6968–6975. doi: 10.1128/JB.00775-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Su MS, Schlicht S, Gänzle MG. 2011. Contribution of glutamate decarboxylase in Lactobacillus reuteri to acid resistance and persistence in sourdough fermentation. Microb Cell Fact 10 Suppl 1:S8. doi: 10.1186/1475-2859-10-S1-S8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Waterman SR, Small PLC. 2003. Identification of the promoter regions and σS-dependent regulation of the gadA and gadBC genes associated with glutamate-dependent acid resistance in Shigella flexneri. FEMS Microbiol Lett 225:155–160. doi: 10.1016/S0378-1097(03)00508-1 [DOI] [PubMed] [Google Scholar]
- 45. Smith DK, Kassam T, Singh B, Elliott JF. 1992. Escherichia coli has two homologous glutamate decarboxylase genes that map to distinct loci. J Bacteriol 174:5820–5826. doi: 10.1128/jb.174.18.5820-5826.1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Castanie-Cornet MP, Penfound TA, Smith D, Elliott JF, Foster JW. 1999. Control of acid resistance in Escherichia coli. J Bacteriol 181:3525–3535. doi: 10.1128/JB.181.11.3525-3535.1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hayashi K, Matsuda T, Takeyama T, Hino T. 1966. Solubilities studies of basic amino acids. Agric Biol Chem 30:378–384. doi: 10.1080/00021369.1966.10858601 [DOI] [Google Scholar]
- 48. Hinrichsen LL, Montel MC, Talon R. 1994. Proteolytic and lipolytic activities of Micrococcus roseus (65), Halomonas elongata (16) and Vibrio sp. (168) isolated from Danish bacon curing brines. Int J Food Microbiol 22:115–126. doi: 10.1016/0168-1605(94)90136-8 [DOI] [PubMed] [Google Scholar]
- 49. Bourdichon F, Casaregola S, Farrokh C, Frisvad JC, Gerds ML, Hammes WP, Harnett J, Huys G, Laulund S, Ouwehand A, Powell IB, Prajapati JB, Seto Y, Ter Schure E, Van Boven A, Vankerckhoven V, Zgoda A, Tuijtelaars S, Hansen EB. 2012. Food fermentations: microorganisms with technological beneficial use. Int J Food Microbiol 154:87–97. doi: 10.1016/j.ijfoodmicro.2011.12.030 [DOI] [PubMed] [Google Scholar]
- 50. Zhang M, Zou X-T, Li H, Dong X-Y, Zhao W. 2012. Effect of dietary gamma-aminobutyric acid on laying performance, egg quality, immune activity and endocrine hormone in heat-stressed Roman hens. Anim Sci J 83:141–147. doi: 10.1111/j.1740-0929.2011.00939.x [DOI] [PubMed] [Google Scholar]
- 51. Kawasaki N, Nakayama A, Yamano N, Takeda S, Kawata Y, Yamamoto N, Aiba S. 2005. Synthesis, thermal and mechanical properties and biodegradation of branched polyamide 4. Polymer 46:9987–9993. doi: 10.1016/j.polymer.2005.06.092 [DOI] [Google Scholar]
- 52. Yamano N, Kawasaki N, Ida S, Nakayama A. 2019. Biodegradation of polyamide 4 in seawater. Polym Degrad Stab 166:230–236. doi: 10.1016/j.polymdegradstab.2019.05.032 [DOI] [Google Scholar]
- 53. Yamano N, Kawasaki N, Takeda S, Nakayama A. 2013. Production of 2-pyrrolidone from biobased glutamate by using Escherichia coli. J Polym Environ 21:528–533. doi: 10.1007/s10924-012-0466-x [DOI] [Google Scholar]
- 54. Alting-Mees MA, Short JM. 1989. pBluescript II: gene mapping vectors. Nucleic Acids Res 17:9494. doi: 10.1093/nar/17.22.9494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Nakayama H. 2015. Cell-surface engineering of Halomonas elongata as an element recycling biotechnology in high salinity environments, p 1642–1652. In Asia Pacific Confederation of Chemical Engineering Congress 2015: APCChE 2015, incorporating CHEMECA 2015. Engineers Australia, Melbourne. https://search.informit.org/doi/10.3316/informit.735447505511224. [Google Scholar]
- 56. Schäfer A, Tauch A, Jäger W, Kalinowski J, Thierbach G, Pühler A. 1994. Small mobilizable multi-purpose cloning vectors derived from the Escherichia coli plasmids pK18 and pK19: selection of defined deletions in the chromosome of Corynebacterium glutamicum. Gene 145:69–73. doi: 10.1016/0378-1119(94)90324-7 [DOI] [PubMed] [Google Scholar]
- 57. Figurski DH, Helinski DR. 1979. Replication of an origin-containing derivative of plasmid RK2 dependent on a plasmid function provided in trans. Proc Natl Acad Sci U S A 76:1648–1652. doi: 10.1073/pnas.76.4.1648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Sambrook J, Russel DW. 2001. Molecular cloning: a laboratory manual. 3rd ed. Cold Spring Harbor Laboratory Press, NewYork. https://www.google.com/books/edition/Molecular_Cloning/Cp6EzQEACAAJ?hl=EN. [Google Scholar]
- 59. Perroud B, Le Rudulier D. 1985. Glycine betaine transport in Escherichia coli: osmotic modulation. J Bacteriol 161:393–401. doi: 10.1128/jb.161.1.393-401.1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Villalobos A, Ness JE, Gustafsson C, Minshull J, Govindarajan S. 2006. Gene designer: a synthetic biology tool for constructing artificial DNA segments. BMC Bioinformatics 7:285. doi: 10.1186/1471-2105-7-285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Lam ST, Lam BS, Strobel G. 1985. A vehicle for the introduction of transposons into plant-associated pseudomonads. Plasmid 13:200–204. doi: 10.1016/0147-619x(85)90043-5 [DOI] [PubMed] [Google Scholar]
- 62. Sato Y, Bounoshita M, Iwaya K, Miyaji T, Saito M. 2010. High-speed separation of dabsyl-amino acids by ultra high-performance liquid chromatography. Bunseki Kagaku 59:823–828. doi: 10.2116/bunsekikagaku.59.823 [DOI] [Google Scholar]
- 63. Syu K-Y, Lin C-L, Huang H-C, Lin J-K. 2008. Determination of theanine, GABA, and other amino acids in green, oolong, black, and Pu-erh teas with dabsylation and high-performance liquid chromatography. J Agric Food Chem 56:7637–7643. doi: 10.1021/jf801795m [DOI] [PubMed] [Google Scholar]


