ABSTRACT
In bacteria, the second messenger cyclic di-GMP (c-di-GMP) is synthesized and degraded by multiple diguanylate cyclases (DGCs) and phosphodiesterases. A high level of c-di-GMP induces biofilm formation and represses motility. WspR, a hybrid response regulator DGC, produces c-di-GMP when it is phosphorylated. FlhF, a signal recognition particle-type GTPase, is initially localized to the cell poles and is indispensable for polar flagellar localization in Pseudomonas aeruginosa. In this study, we report that deletion of flhF affected biofilm formation and the c-di-GMP level in P. aeruginosa. Phenotypic analysis of a flhF knockout mutant revealed increased biofilm formation, wrinkled colonies on Congo red agar, and an elevated c-di-GMP level compared to the wild-type strain, PAO1. Yeast and bacterial two-hybrid systems showed that FlhF binds to the response regulator HsbR, and HsbR binds to WspR. Deletion of hsbR or wspR in the ΔflhF background abolished the phenotype of ΔflhF. In addition, confocal microscopy demonstrated that WspR-GFP was distributed throughout the cytoplasm and formed a visible cluster at one cell pole in PAO1 and ΔhsbR, but it was mainly distributed as visible clusters at the lateral side of the periplasm and with visible clusters at both cell poles in ΔflhF. These findings suggest that FlhF influences the subcellular cluster and localization of WspR and negatively modulates WspR DGC activity in a manner dependent on HsbR. Together, our findings demonstrate a novel mechanism for FlhF modulating the lifestyle transition between motility and biofilm via HsbR to regulate the DGC activity of WspR.
IMPORTANCE
Cyclic di-GMP (c-di-GMP) is a second messenger that controls flagellum biosynthesis, adhesion, virulence, motility, exopolysaccharide production, and biofilm formation in bacteria. Recent research has shown that distinct diguanylate cyclases (DGCs) or phosphodiesterases (PDEs) produce highly specific outputs. Some DGCs and PDEs contribute to the total global c-di-GMP concentration, but others only affect local c-di-GMP in a microenvironment. However, the underlying mechanisms are unclear. Here, we report that FlhF affects the localization and DGC activity of WspR via HsbR and is implicated in local c-di-GMP signaling in Pseudomonas aeruginosa. This study establishes the link between the c-di-GMP signaling system and the flagellar localization and provides insight for understanding the complex regulatory network of c-di-GMP signaling.
KEYWORDS: c-di-GMP, subcellular clustering, biofilm formation, FlhF, WspR
INTRODUCTION
Pseudomonas aeruginosa is a Gram-negative opportunistic pathogen that causes acute and chronic infections. Its ability to form biofilm favors persistent infection, particularly in a nosocomial setting (1–3). The bacterial flagellum contributes to biofilm formation, particularly surface attachment and adhesion during the initial stages (4, 5). Cyclic di-GMP (c-di-GMP) is a second messenger that controls, for example, flagellum biosynthesis, adhesion, virulence, motility, exopolysaccharide production, and biofilm formation in bacteria (6–8). A high intracellular c-di-GMP level induces biofilm formation, whereas a low level inhibits biofilm formation and stimulates bacterial motility (9). However, the underlying mechanisms are unclear.
In P. aeruginosa and other bacteria, c-di-GMP is synthesized from two molecules of GTP by diguanylate cyclase (DGC), which has a GGDEF domain and is degraded into 5′-phosphoguanylyl-(3,′5′)-guanosine (pGpG), and/or GMP by phosphodiesterase (PDE), which has an EAL or HD-GYP domain (10, 11). Forty-three genes in P. aeruginosa encode GGDEF, EAL, or HD-GYP proteins (12). The abundance of DGCs and PDEs in a bacterial genome may be correlated with the presence of distinct regulatory systems. Bacteria use local c-di-GMP signaling to produce high-specificity regulatory outputs to avoid undesired crosstalk; in this process, several DGCs and PDEs function simultaneously (13). In P. aeruginosa, the DGC HsbD mediates local c-di-GMP signaling via the HptB/HsbR/HsbA/HsbD pathway (14). In this pathway, when HptB is inactive or absent, HsbR acts as a kinase and phosphorylates HsbA. Phosphorylated HsbA (HsbA-P) interacts with the diguanylate cyclase HsbD, leading to polar localization of HsbD and an increase in the cellular c-di-GMP level, resulting in hyper-biofilm formation and hyper-twitching phenotypes. In contrast, when HptB is phosphorylated, it transfers a phosphoryl group to HsbR, thereby repressing the kinase activity of the latter.
The DGC WspR is the output response regulator of the Wsp signal transduction system and produces c-di-GMP when phosphorylated and is inhibited when c-di-GMP binds to its GGEEF domain (15). The Wsp system uses WspA to detect surface-associated signals, leading to the phosphorylation of WspR and triggering the DGC activity of WspR, resulting in the synthesis of c-di-GMP and biofilm formation (16). In P. aeruginosa, phosphorylated WspR (WspR-P) tends to form tetramers and visible subcellular clusters; this cluster formation potentiates the DGC activity of WspR (17).
FlhF is a signal recognition particle-type GTPase that determines the locations of flagella (18). It is initially localized to the cell pole and is indispensable for polar flagellar localization in P. aeruginosa, Vibrio cholerae, and Pseudomonas putida (18–20). In P. aeruginosa, the absence of flhF results in lateral flagella and diminished motility (18, 21), although the underlying mechanism is unknown. Here, we report that an flhF deletion mutant of P. aeruginosa PAO1 had a hyper-biofilm-formation phenotype and produced wrinkled colonies on Congo red agar. Our data suggest that FlhF binds to HsbR, and HsbR binds to WspR, thus modulating the subcellular localization of WspR. Therefore, FlhF influences local c-di-GMP pools in P. aeruginosa.
RESULTS
The flhF mutant has enhanced biofilm formation, wrinkled colonies, and an elevated level of c-di-GMP
FlhF regulates flagellum biosynthesis and is required for normal swimming motility in bacteria with polar flagella (18). We constructed an unmarked deletion of flhF in P. aeruginosa PAO1. The ΔflhF mutant strain was non-motile (Fig. 1A). Interestingly, it had greater biofilm formation than PAO1 in borosilicate tubes stained with crystal violet. Quantification of biofilm formation after crystal violet staining showed that the mutant was about 1.3-fold greater than PAO1 (Fig. 1B). In addition, it formed red, multi-wrinkled, and slightly dry colonies on Congo red agar at 25°C, whereas PAO1 and the ΔflhF-complemented strain formed smooth colonies (Fig. 1C).
Fig 1.
The ΔflhF mutant displays high c-di-GMP phenotypes. (A) The ΔflhF mutant is defective for swarming and swimming motility. (B) Deletion of flhF increased the biofilm formation in P. aeruginosa. Biofilm formation by the indicated strains was displayed with crystal violet staining (top) and quantified by optical density measurement (bottom). (C) Congo red binding phenotype was visualized after 2 days of incubation. The wrinkly phenotype was observed in the indicated P. aeruginosa strains. (D) The relative intracellular level of c-di-GMP was measured with the transcriptional pKD-cdrA reporter. (E) Western blotting analysis of CdrA-Flag showed the relative level of c-di-GMP in bacterial strains. PAO1 and ΔflhF mutant harbored a control plasmid (pAK1900). The plasmid p-2133 was used to express the phosphodiesterase PA2133. The protein level of CdrA-Flag from the indicated strains was examined by western blotting. The tagged proteins were detected using a Flag antibody. RNA polymerase α (α-RNAP) antibody was used as a loading control. EV represents the empty vector in this and subsequent experiments. Data are shown as mean ± SEM from three experiments at least. The error bars indicate standard deviations (*P < 0.05, **P < 0.01, and ***P < 0.001, Student’s t-test).
The second messenger c-di-GMP positively regulates the production of biofilm-matrix components. Wrinkled colonies on Congo red agar can be used as an indicator of the c-di-GMP level in P. aeruginosa (22). In P. aeruginosa, the adhesin CdrA is a structural component of the biofilm matrix and is highly upregulated by c-di-GMP (23–25). Therefore, the expression level of cdrA is indicative of the c-di-GMP level (26, 27). We compared the c-di-GMP levels in PAO1 and ΔflhF using a transcriptional pKD-cdrA reporter system. As shown in Fig. 1D, the ΔflhF mutant showed significantly higher cdrA expression than PAO1 and the flhF-complemented strain. Western blotting showed that the CdrA level in the mutant was elevated compared to PAO1 (Fig. 1E). To verify this finding, a plasmid expressing the PDE PA2133 (p-2133) was introduced into the mutant. Overexpression of PA2133 significantly reduced biofilm formation and restored colony morphology on Congo red agar and cdrA expression to those of PAO1. Therefore, the greater biofilm formation and wrinkled colonies on Congo red agar of the ΔflhF mutant were attributed to the increased intracellular concentration of c-di-GMP.
HsbR interacts with FlhF and is essential for the phenotype of ΔflhF
To investigate the FlhF functional network, a yeast two-hybrid random genome fragment library of PAO1 was used to screen for proteins that interact with FlhF. Using pGBKT7-flhF as a bait expression vector, we identified two potential FlhF-interacting factors (HsbR and FleN) in the pGADT7-Library (data not shown). In the genomes of some bacteria with polar flagella, fleN is located next to flhF. Mutation of fleN leads to multiple polar flagella and non-motility (21). HsbR was identified as a response regulator of the HptB/HsbR/HsbA/HsbD pathway that controls flagellar gene expression and biofilm formation (14). On this basis, we constructed a pGADT7-hsbR plasmid; a yeast two-hybrid assay indicated that FlhF physically interacted with HsbR (Fig. 2A).
Fig 2.
FlhF regulates biofilm formation via interaction with HsbR. (A) A yeast two-hybrid assay reveals an interaction between FlhF and HsbR. The yeast two-hybrid reporter strain AH109 containing the indicated plasmids was incubated on SD/-Trp/-Leu medium and SD/-Trp/-Leu/-His/-Ade medium at 30°C for 2 days. pGADT7-largeT and pGBKT7-p53 were used as positive controls; pGADT7 and pGBKT7 were used as negative controls. The plasmids harbored by the AH109 strains are indicated on the right. (B) Colony morphologies were demonstrated by growth on Congo red agar plates after 2 days of incubation. The wrinkly phenotype was observed in the indicated P. aeruginosa strains. (C) Biofilm formation by the indicated strains was displayed with crystal violet staining (top) and quantified by optical density measurement (bottom). Each experiment was repeated three times at least. The error bars indicate standard deviations (**P < 0.01 and ****P < 0.0001, Student’s t-test).
To investigate the role of hsbR in ΔflhF biofilm formation, we generated an hsbR deletion in PAO1 and ΔflhF mutant background. Introduction of the hsbR mutation in the ΔflhF mutant abrogated the wrinkled colonies on Congo red agar and the greater biofilm formation of ΔflhF. Unlike ΔflhF, ΔflhFΔhsbR and ΔhsbR formed smooth colonies similar to PAO1 (Fig. 2B). Moreover, there were no differences in biofilm formation among ΔflhFΔhsbR, ΔhsbR, and PAO1 (Fig. 2C). Overexpression of hsbR in ΔflhFΔhsbR induced wrinkled colonies on Congo red agar and enhanced biofilm formation, like that of ΔflhF. Therefore, hsbR is necessary for enhanced biofilm formation and red, wrinkled colonies of ΔflhF. HsbR is a response regulator and contains the receiver (REC), phosphatase (PP2C), and histidine kinase/ATPase (HATP) domains (28, 29). A yeast two-hybrid assay demonstrated a direct interaction between FlhF and the REC or PP2C domain (Fig. 3). Therefore, HsbR is implicated in the FlhF signaling pathway.
Fig 3.
Analyses of interactions between FlhF and HsbR subdomains. (A) Schematic representation of the HsbR subdomains. REC domain (amino acids 13–125), PP2C domain (amino acids 186–386), and HATP domain (amino acids 442–571). (B) Yeast two-hybrid analyses of interactions between FlhF and HsbR subdomains. The reporter strain AH109 containing the indicated plasmids was incubated on SD/-Trp/-Leu medium and SD/-Trp/-Leu/-His/-Ade medium at 30°C for 2 days. pGADT7-largeT and pGBKT7-p53 were used as positive controls; pGADT7 and pGBKT7 were used as negative controls. The plasmids harbored by the AH109 strains are indicated on the right. Each experiment was repeated three times at least.
HsbR interacts with WspR and inhibits the DGC activity of WspR
The HptB/HsbR/HsbA/HsbD pathway controls P. aeruginosa biofilm formation and motility (14). In this pathway, the HsbA phosphorylation state is the key. When HptB is absent or not phosphorylated, HsbR acts as a kinase and phosphorylates HsbA (HsbA-P), which interacts with the diguanylate cyclase HsbD, leading to an increase in the c-di-GMP level and a hyper-biofilm-formation phenotype. Therefore, to investigate whether ΔflhF affects biofilm formation and the level of c-di-GMP via HsbR, HsbA, and HsbD, we constructed ΔflhFΔhsbD and ΔflhFΔhsbA double mutants. Deletion of hsbD or hsbA in the ΔflhF background did not abolish the ΔflhF mutant phenotype according to biofilm formation and Congo red assays. The ΔflhFΔhsbD and ΔflhFΔhsbA double mutants displayed wrinkled colonies and a similar level of biofilm formation of ΔflhF (Fig. 4), suggesting that hsbA and hsbD are components of the HptB, but not the FlhF signaling pathway.
Fig 4.
HsbA and HsbD are not involved in the phenotypes of ΔflhF. Biofilm formation by the indicated P. aeruginosa strains was displayed with crystal violet staining (top), and the wrinkly phenotype of indicated strains was observed by growth on Congo red agar plates after 2 days of incubation (bottom). Each experiment was repeated three times at least.
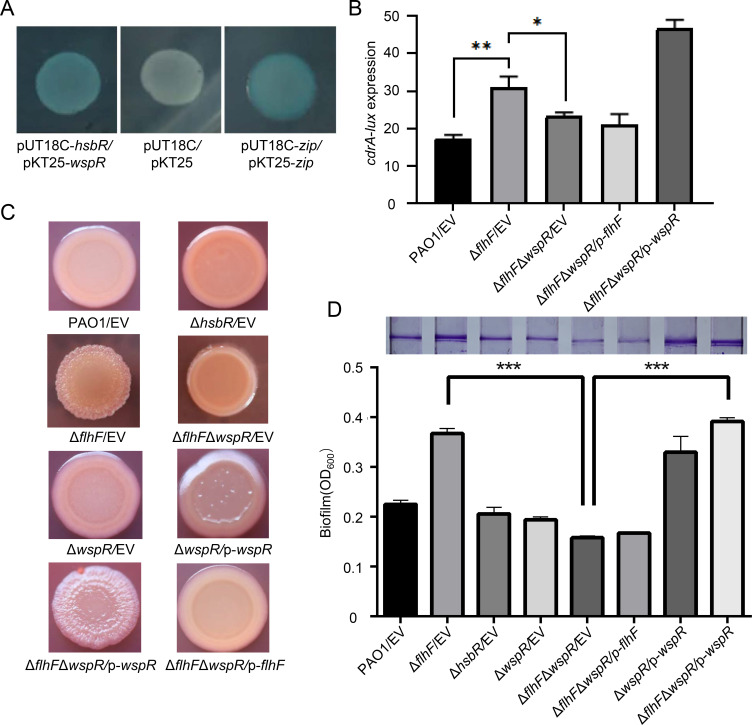
Because ΔflhF had an elevated c-di-GMP level, we investigated whether HsbR interacts with other DGCs or PDEs responsible for the metabolism of c-di-GMP. We cloned 43 genes encoding DGCs or PDEs from P. aeruginosa into the pKT25 plasmid. The protein-protein interactions between HsbR and these 43 proteins were determined using a bacterial two-hybrid assay. As shown in Fig. 5A, HsbR strongly interacted with WspR. In addition, PA0338, ProE, PA0290, PA4781, PA2572, PA4396, FimX and PA1181 interacted weakly with HsbR (data not shown).
Fig 5.
WspR impacts the phenotypes of ΔflhF. (A) The bacterial two-hybrid assay reveals an interaction between HsbR and WspR. The bacterial two-hybrid reporter Escherichia coli BTH101 recombinant strains harboring the indicated plasmids were separately streaked on LB/X-Gal/IPTG (isopropyl β-D-thiogalactoside) plates. The pUT18C-zip and pKT25-zip plasmids were used as positive controls. The pUT18C and pKT25 plasmids were used as negative controls. (B) The relative intracellular level of c-di-GMP was measured with the transcriptional pKD-cdrA reporter. (C) Colony morphologies were demonstrated by growth on Congo red agar plates after 2 days of incubation. The wrinkly phenotype was observed in the indicated P. aeruginosa strains. (D) Biofilm formation by the indicated strains was displayed with crystal violet staining (top) and quantified by optical density measurement (bottom). Each experiment was repeated three times at least. The error bars indicate standard deviations (*P < 0.05, **P < 0.01, and ***P < 0.001, Student’s t-test).
WspR is a diguanylate cyclase that produces c-di-GMP and stimulates biofilm formation (17). To determine whether wspR is required for the ΔflhF phenotype, we engineered ΔwspR and ΔflhFΔwspR mutant strains. The cdrA expression was decreased in ΔflhFΔwspR compared to ΔflhF (Fig. 5B). Congo red assays demonstrated that deletion of wspR in the ΔflhF background abolished the wrinkled colonies of the ΔflhF mutant (Fig. 5C). Concomitantly, a decreased biofilm formation was observed in the ΔflhFΔwspR mutant (Fig. 5D). Overproduction of wspR in ΔflhFΔwspR restored the biofilm phenotype to that of ΔflhF. Therefore, WspR is responsible for the wrinkled colonies and elevated biofilm formation of the ΔflhF mutant, suggesting that FlhF negatively regulates the DGC activity of WspR.
FlhF affects the subcellular cluster of WspR
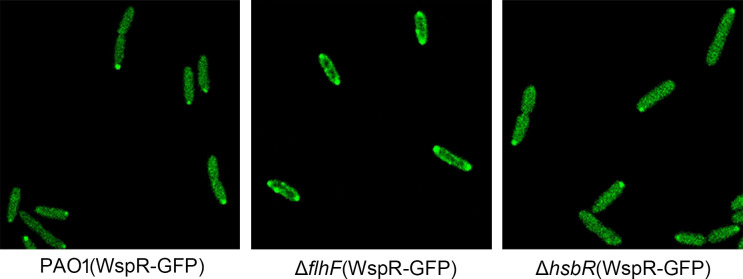
FlhF is localized at the cell pole in P. aeruginosa (19). Because FlhF interacts with HsbR, and HsbR interacts with WspR, we speculated that the localization of WspR might be affected by FlhF in P. aeruginosa. To investigate this, GFP was fused to the C-terminus of WspR. WspR-GFP was expressed in PAO1, ΔflhF, and ΔhsbR, respectively. We observed that WspR-GFP diffused throughout the cell and formed one small visible cluster at one pole in PAO1 cell according to fluorescence microscopy (Fig. 6). However, WspR-GFP in the ΔflhF background formed subcellular clusters at the cell periphery, with greater visible aggregation at both cell poles compared to PAO1. The fluorescent aggregates in ΔflhF were more visible and brighter than in PAO1. Interestingly, WspR-GFP in ΔhsbR also diffused throughout the cytoplasm with one cluster aggregated at one pole, as in PAO1.
Fig 6.
Fluorescence micrographs of strain PAO1 derivatives expressing the WspR-GFP. Overnight cultures of wild-type PAO1, ΔflhF mutant, and the ΔhsbR expressing WspR-GFP were subcultured in fresh Luria–Bertani (LB) with 0.5 mM IPTG for 3 h. Bacterial cells were fixed and examined by fluorescence microscopy.
Previous study has shown that the phosphorylated WspR tends to form visible clusters in P. aeruginosa cells, whereas unphosphorylated WspR is diffused in cells and not visible (15, 17). In general, the DGC activity of WspR is enhanced by such clustering (17). Based on this, it can be predicted that WspR is phosphorylated and highly active because it formed more visible bright clusters in the ΔflhF background. Consistent with this, phenotypes of ΔflhF with enhanced biofilm formation and wrinkled colonies on Congo red agar support this prediction. In contrast to ΔflhF, WspR-GFP did not form more clusters in PAO1 and ΔhsbR backgrounds, suggesting that WspR is not phosphorylated and has low activity in the presence of FlhF or in the absence of HsbR.
DISCUSSION
c-di-GMP is a key bacterial intracellular signaling molecule that controls the transition between the motile and biofilm states. The P. aeruginosa genome has 43 genes encoding DGCs or PDEs responsible for the metabolism of intracellular c-di-GMP and regulates different cellular processes in a highly specific manner (12). However, how bacteria gather information to coordinate the c-di-GMP level and trigger dependent behaviors is unclear.
In P. aeruginosa, FlhF accumulates at both cell poles and directs the assembly of a single flagellum at one pole, whereas ΔflhF strains have nonpolar flagella and reduced motility (18). However, the mechanism underlying how FlhF coordinates the position of the flagella and motility remains unknown. In this study, the ΔflhF mutant showed increased biofilm formation and wrinkled colonies on Congo red agar compared to PAO1 (Fig. 1B and C). In general, this higher biofilm formation and red colony phenotypes are thought to be correlated with increased c-di-GMP levels (17). Indeed, overexpression of PDE PA2133 in ΔflhF abolished the high biofilm formation and wrinkled colonies. These characteristics of the ΔflhF mutant suggest that FlhF is associated with the metabolism of c-di-GMP.
The HptB/HsbR/HsbA/HsbD pathway is involved in motility and biofilm formation (14). In this pathway, HsbR acts as a kinase and phosphorylates HsbA, which in turn binds HsbD and stimulates its DGC activity, leading to an increase of c-di-GMP. In this study, we found that FlhF interacted with HsbR using a yeast two-hybrid cDNA library of PAO1 (Fig. 2). Deletion of hsbR in the ΔflhF mutant background abolished the wrinkled colonies and hyper-biofilm formation of the ΔflhF mutant, whereas ΔflhFΔhsbD and ΔflhFΔhsbA had phenotypes like that of the ΔflhF mutant. Thus, we are able to conclude that the regulation of flhF requires hsbR but not hsbA or hsbD (Fig. 4).
Furthermore, we present evidence that HsbR interacts with WspR. Deletion of wspR in the ΔflhF background resulted in a phenotype like PAO1. (Fig. 5). Interestingly, WspR-GFP formed visible subcellular clusters with strong fluorescence and was mainly localized at the periphery of ΔflhF cells; however, it diffused in the cytoplasm in ΔhsbR and PAO1 cells (Fig. 5). The expression of WspR was not regulated by FlhF and HsbR (data not shown). Previous studies showed that the subcellular cluster formation appears to be an intrinsic property of phosphorylated WspR. The increased WspR cluster is associated with increased DGC activity (15, 17, 30). This suggests that WspR DGC activity was activated by the phosphorylation in the ΔflhF mutant, resulting in a high level of c-di-GMP. Consistent with this, the ΔflhF mutant showed higher biofilm formation, wrinkled colonies on Congo red agar and marked visible clusters of WspR-GFP.
Based on the results presented above, we suggest a model in which FlhF inhibits the DGC activity of WspR by inhibiting the HsbR-mediated phosphorylation, maintaining a low c-di-GMP concentration in PAO1 cell (Fig. 7A), and promoting cell motility (Fig. 7B). On the contrary, in the ΔflhF mutant, WspR is phosphorylated by HsbR, and WspR-P forms subcellular clusters that further stimulate its DGC activity, leading to the synthesis of c-di-GMP and promoting biofilm formation (Fig. 7C). In this model, the DGC activity of WspR is repressed in wild-type PAO1 cell.
Fig 7.
Model for FlhF affecting the sublocation of WspR through HsbR and yielding a local c-di-GMP. Green gradient in the cell illustrates the distribution of c-di-GMP levels. (A) Model for the FlhF-modulated c-di-GMP production through HsbR and WspR. FlhF inhibits the DGC activity of WspR by inhibiting the HsbR-mediated phosphorylation of WspR. WspR-P tends to form visible subcellular clusters with high DGC activity. (B) WspR-GFP was diffused in the cytoplasm and formed a visible cluster at one pole in PAO1, yielding low concentration c-di-GMP and inducing motility. WspR could produce a local high c-di-GMP concentration at the pole where it formed clusters. (C) WspR formed oligomers and localized in the periphery of the ΔflhF cell, yielding an increased global concentration of c-di-GMP, resulting in biofilm formation.
Interestingly, WspR-GFP forms a visible cluster at one pole in PAO1 and ΔhsbR cells but not at both poles like in the ΔflhF mutant (Fig. 7B and C). It appears that the concentration of c-di-GMP in both cell poles is different in PAO1 cells, and the microenvironment of the pole with the WspR-P cluster should have a high c-di-GMP level. Our findings also showed that HsbR binds several DGC/PDE proteins in addition to WspR. Thus, this raises the question of whether polar flagella and motility are related to the locations of DGCs or PDEs and/or local c-di-GMP signaling. This is an intriguing question that is worth following up.
Overall, FlhF affects the subcellular localization of WspR via HsbR and is implicated in local c-di-GMP signaling in P. aeruginosa. These findings provide further insight into how distinct DGCs and PDEs modulate bacterial behavior.
MATERIALS AND METHODS
Bacterial strains, media, and plasmids
The bacterial strains, yeast strains, plasmids, and primers used in this study are listed in Tables 1 and 2. For solid media, Bacto Agar was added to a final concentration of 1.5%. Antibiotics were used when required at the following concentrations: for Escherichia coli, 25 µg/mL gentamicin (Gm), 50 µg/mL kanamycin (Kan), 100 µg/mL carbenicillin (Cb), and 10 µg/mL tetracycline (Tc) in LB medium. For P. aeruginosa, 100 µg/mL Gm in LB medium or 150 µg/mL in Pseudomonas isolation agar, and 600 µg/mL trimethoprim (Tmp) and 300 µg/mL Cb in LB medium.
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Relevant characteristics | Source |
|---|---|---|
| E. coli | ||
| DH5α | deoR, recA, endA, hsdR, supE, thi, gyrA, relA | Stratagene |
| BTH101 | F-, cya-99, araD139, galE15, galK16, rpsL1, (Strr), hsdR2 mcrAl, mcrB1 | Stratagene |
| P. aeruginosa | ||
| PAO1 | Wildtype | Lab stock |
| ΔflhF | flhF deletion mutant of PAO1 | This study |
| ΔhsbR | hsbR deletion mutant of PAO1 | This study |
| ΔhsbA | hsbA deletion mutant of PAO1 | This study |
| ΔhsbD | hsbD deletion mutant of PAO1 | This study |
| ΔwspR | wspR deletion mutant of PAO1 | This study |
| ΔflhFΔhsbR | flhF and hsbR deletion mutant of PAO1 | This study |
| ΔflhFΔhsbD | flhF and hsbD deletion mutant of PAO1 | This study |
| ΔflhFΔhsbA | flhF and hsbA deletion mutant of PAO1 | This study |
| ΔflhFΔwspR | flhF and wspR deletion mutant of PAO1 | This study |
| Saccharomyces cerevisiae | ||
| AH109 | MATa, trp1-901, leu2-3, 112 ura3-52 his3-200 gal4Δ gal80Δ, LYS2::GAL1UAS-GAL1TATA-HIS3, GAL2UAS-GALTATA-ADE2, URA3: :MEL1UAS -MEL1TATA-lacZ | Stratagene |
| Plasmids | ||
| mini-CTX-lacZ | Integration plasmid; Tcr | (31) |
| mini-CTX-cdrA-Flag | Expression plasmid, mini-CTX-lacZ containing the entire cdrA gene and the 3× flag sequence; Tcr | This study |
| pMS402 | Expression reporter plasmid carrying the promoterless luxCDABE; Kanr, Tmpr | Lab collection |
| pKD-cdrA | pMS402 containing cdrA promoter region; Kanr, Tmpr | (32) |
| pEX18Amp |
oriT+
sacB+ gene replacement vector with multiple cloning sites from pUC18; Cbr |
(33) |
| pEX18Amp-flhF | flhF deletion plasmid; Cbr | This study |
| pEX18Amp-hsbR | hsbR deletion plasmid; Cbr | This study |
| pEX18Amp-wspR | wspR deletion plasmid; Cbr | This study |
| pEX18Amp-hsbA | hsbA deletion plasmid; Cbr | This study |
| pEX18Amp-hsbD | hsbD deletion plasmid; Cbr | This study |
| pAK1900 | E. coli-P. aeruginosa shuttle cloning vector carrying plac upstream of MCS; Cbr | (34) |
| p-2133 | pAK1900 with the coding sequence of PA2133; Cbr | (32) |
| p-flhF | pAK1900 with the coding sequence of flhF; Cbr | This study |
| p-hsbR | pAK1900 with the coding sequence of hsbR; Cbr | This study |
| p-wspR | pAK1900 with the coding sequence of wspR; Cbr | This study |
| pGBKT7 | pGBKT7 shuttle vector between E. coli and AH109; Kanr | Clontech |
| pGBKT7-p53 | pGBKT7 with the coding sequence of p53, yeast two-hybrid positive control; Kanr | Clontech |
| pGBKT7-flhF | pGBKT7 with the coding sequence of flhF; Kanr | This study |
| pGADT7 | pGADT7 shuttle vector between E. coli and AH109; Cbr | Clontech |
| pGADT7-largeT | pGADT7 with the coding sequence of largeT, yeast two-hybrid positive control; Cbr | Clontech |
| pGADT7-hsbR | pGADT7 with the coding sequence of hsbR; Cbr | This study |
| pGADT7-REC | pGADT7 with the coding sequence of hsbR (REC); Cbr | This study |
| pGADT7-PP2C | pGADT7 with the coding sequence of hsbR (PP2C); Cbr | This study |
| pGADT7-HATP | pGADT7 with the coding sequence of hsbR (HATP); Cbr | This study |
| pKT25 | Bacterial two-hybrid plasmid, lac promoter and the T25 fragment for C-terminal heterologous protein fusion; Kanr | (35) |
| pKT25-zip | Leucine zipper of GCN1 cloned into pKT25 for bacterial two-hybrid positive control; Kanr | (35) |
| pKT25-wspR | pKT25 with the coding sequence of wspR; Kanr | This study |
| pUT18C | Bacterial two-hybrid vector, designed to create C-terminal heterologous protein fusion; Cbr | (35) |
| pUT18C-zip | Leucine zipper of GCN1 cloned into pUT18C for bacterial two-hybrid positive control; Cbr | (35) |
| pUT18C-hsbR | pUT18C with the coding sequence of hsbR; Cbr | This study |
| pME6032 | Shuttle vector between P. aeruginosa and E. coli for gene expression; Tcr | (36) |
| pME-wspR-GFP | pME6032 containing the entire WspR-GFP sequence; Tcr | This study |
TABLE 2.
Primers used in this study
| Primer | Sequence (5′→3′) | Application |
|---|---|---|
| pEX-flhF-up-S | CCGgaattcCTTCCTGATGCCCTCGGTG | Constructing flhF deletion mutant |
| pEX-flhF-up-A | CTAGtctagaGTCGCGGACCAGTTTCATG | |
| pEX-flhF-down-S | CTAGtctagaGACGCCATGGCCGAGATG | |
| pEX-flhF-down-A | CCCaagcttGCACTCCAGAGGCCGCTGT | |
| pEX-hsbR-up-S | AAAggatccCACCTGCGGGAACAGCTC | Constructing hsbR deletion mutant |
| pEX-hsbR-up-A | GAAtctagaGCCGACGGAAAGGTGGTGTCC | |
| pEX-hsbR-down-S | GGCtctagaCTCGGCGATGAGAATCGTCAG | |
| pEX-hsbR-down-A | AATaagcttGCTCACCGCCGCCAAGGC | |
| pEX-hsbA-up-S | ATAggtaccGTGCTTGTCCAGCAATGC | Constructing hsbA deletion mutant |
| pEX-hsbA-up-A | ATAggatccCGAATGTCGAAAAGACTT | |
| pEX-hsbA-down-S | GTAggatccAACTAAGTGCGTTCGAAA | |
| pEX-hsbA-down-A | AATgtcgacCGCCAGACTTGACGTAAG | |
| pEX-hsbD-up-S | ACTgaattcAAGCGCAGCTCCACCTGG | Constructing hsbD deletion mutant |
| pEX-hsbD-up-A | TTAtctagaGCGCGCTTTACGAAGCCA | |
| pEX-hsbD-down-S | TGCtctagaTAGAGATTGGAACGGGAGTCG | |
| pEX-hsbD-down-A | AAAaagcttGCGCCAGGCGCTGTCGGC | |
| pEX-wspR-up-S | AAAggatccTGGCCCGCAGCATCTTCA | Constructing wspR deletion mutant |
| pEX-wspR-up-A | AAAtctagaCCGTCCAGAGGGGCGCCC | |
| pEX-wspR-down-S | ATAtctagaACCAGGTGGGCCTGATGGAACAGC | |
| pEX-wspR-down-A | TTTaagcttTGACCCAGTGCGCCGCCG | |
| pAK-flhF-S | CTCtctagaTGCATGTGCTGGCCTACCAG | Constructing flhF-complemented plasmid |
| pAK-flhF-A | ATTaagcttGTATTGGCGGGAACGATGCG | |
| pAK-hsbR-P-S | AAAtctagaCGCAAGGAAGCGGACCCGCGCCAGGTT | Constructing hsbR-complemented plasmid |
| pAK-hsbR-P-A | GGAAGTCTCCTGGTAGGCGTGGCGG | |
| pAK-hsbR-S | GCCTACCAGGAGACTTCCATGAATCCCCCGGCGGCG | |
| pAK-hsbR-A | TTAggtaccTTATGCCTGAGGCGACCA | |
| pAK-wspR-S | AAAtctagaCCGGGTTCGACTCCGGCC | Constructing wspR-complemented plasmid |
| pAK-wspR-A | AAAggtaccTCAGCCCGCCGGGGCCGG | |
| mini-CTX-cdrA-Flag-S | TATctcgagGGAAGGTTCCTTGGCGGC | For CdrA western blotting |
| mini-CTX-cdrA-Flag-A | ATTaagcttGCCGCCCAGGTTGCTGGT | |
| pGBKT7-flhF-S | CCCagatctATGCAAGTCAAACGCTTC | For yeast two-hybrid |
| pGBKT7-flhF-A | TATgtcgacACCTTGTGTTGTCGTCTT | |
| pGADT7-hsbR-S | AAAcatatgATGAATCCCCCGGCGG | |
| pGADT7-hsbR-A | CTTggatccTTATGCCTGAGGCGACC | |
| pGADT7-REC-S | AAAcatatgATGATTCTCATCGCCGAGGAC | |
| pGADT7-REC-A | AAAggatccGTTGATCTTGGCTTCGAG | |
| pGADT7-PP2C-S | AAAggatccGTTGATCTTGGCTTCGAG | |
| pGADT7-PP2C-A | AGCggatccCTGCAGCCGCACTTCGAG | |
| pGADT7-HATP-S | AAAcatatgATGGGCGCGCTGTATACGGTA | |
| pGADT7-HATP-A | AGCggatccAAACTCCACGGACACCAC | |
| pGADT7-5′AD | CTATTCGATGATGAAGATACCCCACCAAAC | |
| pGADT7-3′AD | TCTACCACGTGCTACGTGTC | |
| wspR-GFP-1-S | TTAgaattcATGCACAACCCTCATGAG | For WspR-GFP expression |
| wspR-GFP-1-A | GCCCGCCGGGGCCGGCGG | |
| wspR-GFP-2-S | CCGCCGGCCCCGGCGGGCTCTAAAGGTGAAGAACTG | |
| wspR-GFP-2-A | TTAggtaccTTATTTGTAGAGCTCATCCATG | |
| pKT25-wspR -S | CACtctagagATGAAGCAGATGGGTAGCATG | For bacterial two-hybrid |
| pKT25-wspR -A | AAAggtaccTACGGCCGAACCTGTCGC | |
| pUT18C-hsbR-S | AAAtctagaTATGAATCCCCCGGCGGCG | |
| pUT18C-hsbR-A | TTAggtaccTTATGCCTGAGGCGACCA |
Saccharomyces cerevisiae AH109 was cultured in yeast peptone dextrose adenine medium at 30°C with shaking at 220 rpm or on synthetic dropout (SD) minimal medium agar omitting both L-leucine and L-tryptophan or L-leucine, L-tryptophan, histidine, and adenine.
Plasmid construction
To construct mini-CTX-cdrA-Flag, a unique domain of the cdrA fragment (320 bp upstream and 1,500 bp downstream of the ATG start codon) was amplified by PCR from PAO1 using the primers mini-CTX-cdrA-Flag-S/mini-CTX-cdrA-Flag-A. This DNA fragment was cloned into the corresponding restriction sites of mini-CTX-Flag, which originated from the plasmid mini-CTX-lacZ (31, 37), yielding mini-CTX-cdrA-Flag.
GFP translational fusion at the C-terminus of wspR was performed as follows. Briefly, the wspR coding sequence without the stop codon was amplified using the primers wspR-GFP-1-S/wspR-GFP-1-A, and the GFP coding sequence was amplified from the pKEN-GFPmut3* plasmid using the primers wspR-GFP-2-S/wspR-GFP-2-A (38). Next, the wspR and GFP fragments were ligated by overlap PCR using the primers wspR-GFP-1-S/wspR-GFP-2-A. The resulting wspR-GFP fusion gene was digested and subcloned into the shuttle plasmid pME6032 to produce pME-wspR-GFP.
Construction of mutant strains
The deletion mutants were constructed by homologous recombination as described previously (33). The flhF gene was deleted by PCR-amplifying a 1.5-kb fragment upstream of flhF using the primers pEX-flhF-up-S/pEX-flhF-up-A and a 1.5-kb fragment downstream of flhF using the primers pEX-flhF-down-S/pEX-flhF-down-A. The two PCR fragments were ligated into the pEX18Amp plasmid, resulting in pEX18Amp-flhF, in which the entire flhF open reading frame had been deleted. The resultant plasmid was electroporated into PAO1 for allelic exchange. The deletion mutants were selected on LB agar with Cb for the first homologous recombinants, and LB agar with 10% sucrose for the second. The flhF deletion mutant was complemented with the pAK1900 plasmid carrying flhF. The other mutants were generated using similar strategies and were verified by PCR and DNA sequencing.
Congo red colony morphology assay
To observe colony morphology on Congo red agar, 1.8 µL overnight culture was spotted on LB agar containing 40 µg/mL Congo red and 1 µg/mL Coomassie brilliant blue (39). The plate was incubated at 30°C overnight followed by room temperature for 1 day. Colony morphology was recorded using a digital camera.
Swimming and swarming motility assays
Swimming and swarming motility assays were performed as described elsewhere (40). The swimming medium consisted of 0.3% agar with 1% tryptone and 0.5% NaCl. The swarming medium consisted of 0.5% agar with 0.8% nutrient broth and 0.5% glucose. Bacterial cultures were diluted to an OD600 of 1, and 2 µL aliquots were center-spotted onto the agar surface. Swimming-motility plates were incubated at 30°C for 14–16 h, and swarming-motility plates were incubated at 37°C for 14–16 h. Bacterial motility was imaged using a camera (LAS-3000, Tanon, China).
Biofilm assay
Biofilm formation in LB was assayed as described previously with minor modifications (41). Briefly, overnight cultures were diluted 1:1,000 into a borosilicate tube containing 3 mL of fresh LB medium. After 19-h incubation at 25°C without shaking, planktonic bacteria were gently removed. To quantify biofilm formation, the tubes were washed three times with sterile water and stained with 0.1% crystal violet for 20 min at room temperature. Next, the plates and tubes were washed with water to remove unbound dye and air-dried. Dried biofilm was solubilized using 95% absolute ethanol, and the OD600 was measured using a microplate reader (Synergy2X, BioTek, USA). Experiments were performed in triplicate, and results are shown as means ± SD.
Gene-expression assays
The plasmid pMS402 carrying a promoter-less luxCDABE reporter gene cluster was used to construct the reporter plasmid (42). The cdrA promoter region was amplified by PCR and cloned into pMS402, yielding pKD-cdrA. The gene expression level was monitored by measuring luminescence (counts per second) production as described previously with minor modifications (43). P. aeruginosa cultures were incubated overnight in 50 mL tubes, and 100 µL aliquots were transferred to a black 96-well plate with a transparent bottom to measure luminescence and bacterial growth (OD595) in a Synergy 2 Microplate Reader (BioTek) every 2 h for 24 h. A P. aeruginosa strain carrying the promoter-less pMS402 was used as the vector control.
Yeast two-hybrid screening
A yeast two-hybrid random cDNA library of wild-type PAO1, the pGADT7-Library, was donated by Rob Lavigne (44). FlhF was used as a bait protein to screen this library. Full-length flhF was amplified from PAO1 genomic DNA by PCR using primers containing BglI and SalI sites and ligated into the same sites in the pGBKT7 plasmid, yielding the bait-plasmid pGBKT7-flhF, which was transformed into competent AH109 cells according to the manufacturer’s instructions. The bait-containing AH109 cells were transformed with the prey cDNA library pGADT7-Library plated on SD/-Trp/-Leu and SD/-Trp/-Leu/-His/-Ade agars and incubated at 30°C for 2–3 days. Positive colonies were transferred to SD/-Trp/-Leu liquid medium and incubated for 2 days for plasmid isolation. The plasmids were identified by PCR using the primers pGADT7-5′AD and pGADT7-3′AD and sequenced and analyzed by BLAST searching against the Pseudomonas genome database (https://blast.ncbi.nlm.nih.gov). A strain containing pGBKT7-53 (BD fusion of p53) and pGADT7-largeT (AD fusion of large T antigen) was used as the positive control, and a strain containing pGBKT7 and pGADT7 was the negative control.
Full-length hsbR and hsbR DNA regions encoding the REC, PP2C, and HATP domains were amplified from PAO1 genomic DNA by PCR using primers containing NdeI and BamHI sites and ligated into the same sites in the pGADT7 plasmid. This yielded the prey-plasmids pGADT7-REC, pGADT7-PP2C, and pGADT7-HATP, respectively.
Bacterial two-hybrid assay
The bacterial two-hybrid assay was used to analyze protein-protein interactions. The coding regions of hsbR and wspR were amplified from PAO1 genomic DNA by PCR using the primer pairs pUT18C-hsbR-S/A and pKT25-wspR-S/A, respectively. The hsbR PCR products were cloned into the pUT18C plasmid, yielding pUT18C-hsbR, and the wspR PCR product was cloned into the pKT25 plasmid, yielding pKT25-wspR. The 43 genes encoding DGCs and PDEs were amplified from PAO1 genomic DNA by PCR and cloned into the pKT25 plasmid. Next, derivatives of pUT18C and pKT25 were co-transformed into the reporter strain E. coli BTH101. After incubation for 90 min on Super Optimal Broth with Catabolic Repressor (SOC) medium at 37°C, bacteria were selected on LB plates with 100 µg/mL Cb, 50 µg/mL Kan, and 0.5 mM IPTG. Individual colonies were picked and cultured overnight at 37°C in LB broth supplemented with both Cb and Kan. This cell suspension was used for screening on LB/X-Gal/IPTG agar. The blue clones on LB/X-Gal/IPTG agar are considered as positive interaction. The pUT18C-zip and pKT25-zip plasmids were used as the positive controls.
Western blotting analysis of CdrA
The cdrA gene encodes a large protein of 220 KDa, which cannot be detected by western blotting. To overcome this limitation, a unique domain of the cdrA gene (320 bp upstream and 1,500 bp downstream of ATG) from PAO1 was cloned to generate the plasmid mini-CTX-cdrA-Flag. Mini-CTX-cdrA-Flag integrates into the attB site in P. aeruginosa.
Western blotting analysis of CdrA-Flag was performed as described previously (45). P. aeruginosa cells were cultured overnight in LB medium, diluted 1:100, and incubated at 37°C for 2 h. Cells were harvested by centrifugation and resuspended in a loading buffer. Proteins were separated by SDS-PAGE and transferred to PVDF membranes by semi-dry transfer electrophoresis. CdrA-Flag was analyzed using an anti-Flag antibody.
Confocal microscopy
Sample preparation and microscopy were performed as described previously (15). Overnight cultures of strains containing fluorescent fusions were subcultured in fresh LB with 0.5 mM IPTG for 3 h. Next, cells were collected and diluted to an OD600 of 0.1 in PBS (pH 7.4). Resuspended cells were spotted on a 0.8% agarose PBS pad on a microscope slide and covered with a coverslip. Confocal microscopy was performed using an Olympus IX71 microscope with a UPlanSApro 100×/1.40 Oil objective (Olympus, Japan) and a coolSNAP HQ (Photometrics, USA) CCD camera.
ACKNOWLEDGMENTS
The authors would like to thank Dr. Haihua Liang from the Southern University of Science and Technology for his helpful and constructive comments that improved the manuscript substantially.
This study was supported by grants from the Natural Science Basic Research Program of Shaanxi (2021JZ-42 and 2022JQ-184), the National Natural Science Foundation of China (32370192), and the Natural Science Foundation of Shaanxi Academy of Basic Research (22JHQ056).
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Contributor Information
Weina Kong, Email: kongwn@nwu.edu.cn.
Tietao Wang, Email: WangTietao@nwu.edu.cn.
Yani Zhang, Email: yani@nwu.edu.cn.
Isaac Cann, University of Illinois Urbana-Champaign, Urbana, Illinois, USA.
REFERENCES
- 1. Mulcahy LR, Isabella VM, Lewis K. 2014. Pseudomonas aeruginosa biofilms in disease. Microb Ecol 68:1–12. doi: 10.1007/s00248-013-0297-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lyczak JB, Cannon CL, Pier GB. 2002. Lung infections associated with cystic fibrosis. Clin Microbiol Rev 15:194–222. doi: 10.1128/CMR.15.2.194-222.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Høiby N, Bjarnsholt T, Moser C, Bassi GL, Coenye T, Donelli G, Hall-Stoodley L, Holá V, Imbert C, Kirketerp-Møller K, Lebeaux D, Oliver A, Ullmann AJ, Williams C, ESCMID Study Group for Biofilms and Consulting External Expert Werner Zimmerli . 2015. ESCMID guideline for the diagnosis and treatment of biofilm infections 2014. Clin Microbiol Infect 21 Suppl 1:S1–25. doi: 10.1016/j.cmi.2014.10.024 [DOI] [PubMed] [Google Scholar]
- 4. Sauer K, Camper AK, Ehrlich GD, Costerton JW, Davies DG. 2002. Pseudomonas aeruginosa displays multiple phenotypes during development as a biofilm. J Bacteriol 184:1140–1154. doi: 10.1128/jb.184.4.1140-1154.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sauer Karin, Stoodley P, Goeres DM, Hall-Stoodley L, Burmølle M, Stewart PS, Bjarnsholt T. 2022. The biofilm life cycle: expanding the conceptual model of biofilm formation. Nat Rev Microbiol 20:608–620. doi: 10.1038/s41579-022-00767-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jenal U, Reinders A, Lori C. 2017. Cyclic di-GMP: second messenger extraordinaire. Nat Rev Microbiol 15:271–284. doi: 10.1038/nrmicro.2016.190 [DOI] [PubMed] [Google Scholar]
- 7. Petrova OE, Sauer K. 2009. A novel signaling network essential for regulating Pseudomonas aeruginosa biofilm development. PLoS Pathog 5:e1000668. doi: 10.1371/journal.ppat.1000668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hengge R. 2009. Principles of c-di-GMP signalling in bacteria. Nat Rev Microbiol 7:263–273. doi: 10.1038/nrmicro2109 [DOI] [PubMed] [Google Scholar]
- 9. Ha D-G, O’Toole GA. 2015. c-di-GMP and its effects on biofilm formation and dispersion: a Pseudomonas aeruginosa review. Microbiol Spectr 3:MB–0003. doi: 10.1128/microbiolspec.MB-0003-2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Orr MW, Donaldson GP, Severin GB, Wang JX, Sintim HO, Waters CM, Lee VT. 2015. Oligoribonuclease is the primary degradative enzyme for pGpG in Pseudomonas aeruginosa that is required for cyclic-di-GMP turnover. Proc Natl Acad Sci U S A 112:E5048–57. doi: 10.1073/pnas.1507245112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cohen D, Mechold U, Nevenzal H, Yarmiyhu Y, Randall TE, Bay DC, Rich JD, Parsek MR, Kaever V, Harrison JJ, Banin E. 2015. Oligoribonuclease is a central feature of cyclic diguanylate signaling in Pseudomonas aeruginosa. Proc Natl Acad Sci U S A 112:11359–11364. doi: 10.1073/pnas.1421450112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Valentini M, Filloux A. 2016. Biofilms and cyclic di-GMP (c-di-GMP) signaling: lessons from Pseudomonas aeruginosa and other bacteria. J Biol Chem 291:12547–12555. doi: 10.1074/jbc.R115.711507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hengge R. 2021. High-specificity local and global c-di-GMP signaling. Trends Microbiol 29:993–1003. doi: 10.1016/j.tim.2021.02.003 [DOI] [PubMed] [Google Scholar]
- 14. Valentini Martina, Laventie B-J, Moscoso JA, Jenal U, Filloux A. 2016. The diguanylate cyclase HsbD intersects with the HptB regulatory cascade to control Pseudomonas aeruginosa biofilm and motility. PLoS Genet 12:e1006473. doi: 10.1371/journal.pgen.1006473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Güvener ZT, Harwood CS. 2007. Subcellular location characteristics of the Pseudomonas aeruginosa GGDEF protein, WspR, indicate that it produces cyclic-di-GMP in response to growth on surfaces. Mol Microbiol 66:1459–1473. doi: 10.1111/j.1365-2958.2007.06008.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. O’Neal L, Baraquet C, Suo Z, Dreifus JE, Peng Y, Raivio TL, Wozniak DJ, Harwood CS, Parsek MR. 2022. The Wsp system of Pseudomonas aeruginosa links surface sensing and cell envelope stress. Proc Natl Acad Sci U S A 119:e2117633119. doi: 10.1073/pnas.2117633119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Huangyutitham V, Güvener ZT, Harwood CS. 2013. Subcellular clustering of the phosphorylated WspR response regulator protein stimulates its diguanylate cyclase activity. mBio 4:e00242-13. doi: 10.1128/mBio.00242-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Murray TS, Kazmierczak BI. 2006. FlhF is required for swimming and swarming in Pseudomonas aeruginosa. J Bacteriol 188:6995–7004. doi: 10.1128/JB.00790-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pandza S, Baetens M, Park CH, Au T, Keyhan M, Matin A. 2000. The G-protein FlhF has a role in polar flagellar placement and general stress response induction in Pseudomonas putida. Mol Microbiol 36:414–423. doi: 10.1046/j.1365-2958.2000.01859.x [DOI] [PubMed] [Google Scholar]
- 20. Correa NE, Peng F, Klose KE. 2005. Roles of the regulatory proteins FlhF and FlhG in the Vibrio cholerae flagellar transcription hierarchy. J Bacteriol 187:6324–6332. doi: 10.1128/JB.187.18.6324-6332.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Dasgupta N, Arora SK, Ramphal R. 2000. fleN, a gene that regulates flagellar number in Pseudomonas aeruginosa. J Bacteriol 182:357–364. doi: 10.1128/JB.182.2.357-364.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hickman JW, Tifrea DF, Harwood CS. 2005. A Chemosensory system that regulates biofilm formation through modulation of cyclic diguanylate levels. Proc Natl Acad Sci U S A 102:14422–14427. doi: 10.1073/pnas.0507170102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Flemming HC, Wingender J. 2010. The biofilm matrix. Nat Rev Microbiol 8:623–633. doi: 10.1038/nrmicro2415 [DOI] [PubMed] [Google Scholar]
- 24. Rybtke M, Berthelsen J, Yang L, Høiby N, Givskov M, Tolker-Nielsen T. 2015. The LapG protein plays a role in Pseudomonas aeruginosa biofilm formation by controlling the presence of the CdrA adhesin on the cell surface. Microbiologyopen 4:917–930. doi: 10.1002/mbo3.301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Borlee BR, Goldman AD, Murakami K, Samudrala R, Wozniak DJ, Parsek MR. 2010. Pseudomonas aeruginosa uses a cyclic-di-GMP-regulated adhesin to reinforce the biofilm extracellular matrix. Mol Microbiol 75:827–842. doi: 10.1111/j.1365-2958.2009.06991.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lee CK, Schmidt WC, Webster SS, Chen JW, O’Toole GA, Wong GCL. 2022. Broadcasting of amplitude- and frequency-modulated c-di-GMP signals facilitates cooperative surface commitment in bacterial lineages. Proc Natl Acad Sci U S A 119:e2112226119. doi: 10.1073/pnas.2112226119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhang YY, Xia B, Li M, Shi J, Long YQ, Jin YX, Bai F, Cheng ZH, Jin SG, Wu WH. 2018. HigB reciprocally controls biofilm formation and the expression of type III secretion system genes through influencing the intracellular c-di-GMP level in Pseudomonas aeruginosa . Toxins 10:424. doi: 10.3390/toxins10110424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hsu JL, Chen HC, Peng HL, Chang HY. 2008. Characterization of the histidine-containing phosphotransfer protein B-mediated multistep phosphorelay system in Pseudomonas aeruginosa PAO1. J Biol Chem 283:9933–9944. doi: 10.1074/jbc.M708836200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Houot L, Fanni A, de Bentzmann S, Bordi C. 2012. A bacterial two-hybrid genome fragment library for deciphering regulatory networks of the opportunistic pathogen Pseudomonas aeruginosa. Microbiology (Reading) 158:1964–1971. doi: 10.1099/mic.0.057059-0 [DOI] [PubMed] [Google Scholar]
- 30. De N, Pirruccello M, Krasteva PV, Bae N, Raghavan RV, Sondermann H. 2008. Phosphorylation-independent regulation of the diguanylate cyclase WspR. PLoS Biol 6:e67. doi: 10.1371/journal.pbio.0060067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Becher A, Schweizer HP. 2000. Integration-proficient Pseudomonas aeruginosa vectors for isolation of single-copy chromosomal lacZ and lux gene fusions. Biotechniques 29:948–950, doi: 10.2144/00295bm04 [DOI] [PubMed] [Google Scholar]
- 32. Jiao H, Li F, Wang T, Yam JKH, Yang L, Liang H, Whiteley M. 2021. The pyocin regulator PrtR regulates virulence expression of Pseudomonas aeruginosa by modulation of Gac/Rsm system and c-di-GMP signaling pathway. Infect Immun 89. doi: 10.1128/IAI.00602-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hoang TT, Karkhoff-Schweizer RR, Kutchma AJ, Schweizer HP. 1998. A broad-host-range FLP-FRT recombination system for site-specific excision of chromosomally-located DNA sequences: application for isolation of unmarked Pseudomonas aeruginosa Mutants. Gene 212:77–86. doi: 10.1016/s0378-1119(98)00130-9 [DOI] [PubMed] [Google Scholar]
- 34. Poole K, Neshat S, Krebes K, Heinrichs DE. 1993. Cloning and nucleotide sequence analysis of the ferripyoverdine receptor gene fpvA of Pseudomonas aeruginosa . J Bacteriol 175:4597–4604. doi: 10.1128/jb.175.15.4597-4604.1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Karimova G, Pidoux J, Ullmann A, Ladant D. 1998. A bacterial two-hybrid system based on a reconstituted signal transduction pathway. Proc Natl Acad Sci U S A 95:5752–5756. doi: 10.1073/pnas.95.10.5752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Heeb S, Itoh Y, Nishijyo T, Schnider U, Keel C, Wade J, Walsh U, O’Gara F, Haas D. 2000. Small, stable shuttle vectors based on the minimal pVS1 replicon for use in gram-negative, plant-associated bacteria. Mol Plant Microbe Interact 13:232–237. doi: 10.1094/MPMI.2000.13.2.232 [DOI] [PubMed] [Google Scholar]
- 37. Chen G, Gan J, Yang C, Zuo Y, Peng J, Li M, Huo W, Xie Y, Zhang Y, Wang T, Deng X, Liang H. 2020. The SiaA/B/C/D signaling network regulates biofilm formation in Pseudomonas aeruginosa. EMBO J 39:e105997. doi: 10.15252/embj.2020105997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wilks JC, Slonczewski JL. 2007. pH of the cytoplasm and periplasm of Escherichia coli: rapid measurement by green fluorescent protein fluorimetry. J Bacteriol 189:5601–5607. doi: 10.1128/JB.00615-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Almblad H, Harrison JJ, Rybtke M, Groizeleau J, Givskov M, Parsek MR, Tolker-Nielsen T. 2015. The cyclic AMP-vfr signaling pathway in Pseudomonas aeruginosa is inhibited by cyclic di-GMP. J Bacteriol 197:2190–2200. doi: 10.1128/JB.00493-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Rashid MH, Kornberg A. 2000. Inorganic polyphosphate is needed for swimming, swarming, and twitching motilities of Pseudomonas aeruginosa. Proc Natl Acad Sci U S A 97:4885–4890. doi: 10.1073/pnas.060030097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. O’Toole GA, Kolter R. 1998. Initiation of biofilm formation in Pseudomonas fluorescens WCS365 proceeds via multiple, convergent signalling pathways: a genetic analysis. Mol Microbiol 28:449–461. doi: 10.1046/j.1365-2958.1998.00797.x [DOI] [PubMed] [Google Scholar]
- 42. Duan K, Dammel C, Stein J, Rabin H, Surette MG. 2003. Modulation of Pseudomonas aeruginosa gene expression by host microflora through interspecies communication. Mol Microbiol 50:1477–1491. doi: 10.1046/j.1365-2958.2003.03803.x [DOI] [PubMed] [Google Scholar]
- 43. Zhang Y, Qin J, Tan B, Kong W, Chen G, Zhang C, Liang H. 2017. The p-type ATPase PA1429 regulates quorum-sensing systems and bacterial virulence. Front Microbiol 8:2449. doi: 10.3389/fmicb.2017.02449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wagemans J, Delattre A-S, Uytterhoeven B, De Smet J, Cenens W, Aertsen A, Ceyssens P-J, Lavigne R. 2015. Antibacterial phage ORFans of Pseudomonas aeruginosa phage LUZ24 reveal a novel MvaT inhibiting protein. Front Microbiol 6:1242. doi: 10.3389/fmicb.2015.01242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Song Y, Yang C, Chen G, Zhang Y, Seng Z, Cai Z, Zhang C, Yang L, Gan J, Liang H. 2019. Molecular insights into the master regulator CysB-mediated bacterial virulence in Pseudomonas aeruginosa. Mol Microbiol 111:1195–1210. doi: 10.1111/mmi.14200 [DOI] [PubMed] [Google Scholar]