Abstract
Objective
Bone marrow defects of the jaw (BMDJ) surrounding dental implants, in combination with impaired bone-to-implant contact (BIC), are difficult to detect in X-rays. This study evaluated BMDJ surrounding titanium (Ti-Impl) and ceramic (Cer-Impl) dental implants and incomplete BIC using a new trans-alveolar ultrasonography device (TAU) with numerical scaling for BIC.
Methods
The titanium stimulation test (Ti-Stim) was used to detect immune overactivation in response to titanium. Bone density surrounding implants was measured using TAU. We also validated osteoimmune dysregulation.
Results
TAU values showed reduced BIC and decreased osseointegration for Ti-Impl. Moreover, TAU values in the Cer-Impl group were more than twice those in the Ti-Impl cohort. The multiplex analysis of C-C motif chemokine 5 (CCL5, also known as RANTES) expression revealed a 20-fold increase in BMDJ surrounding Ti-Impl. Higher levels of CCL5 inflammation were present in the positive Ti-Stim group.
Conclusions
Our data indicate that Cer-Impl have an osteoimmune advantage over Ti-Impl. The key determinant for osteoimmune sustainability appears to be the absence of inflammation at the implant site. We therefore recommend the use of TAU to assess the implant site prior to implantation.
Keywords: Osseointegration, osteoimmunology, ceramic implant, titanium implant, trans-alveolar ultrasound sonography, titanium stimulation test
Introduction
Peri-implantitis has been extensively researched, and is characterized by inflammation of the dental implant bed in the late stage of implant placement. Marginal peri-implantitis, triggered by anaerobic microflora, leads to progressive bone loss from the alveolar crest. Although the precise incidence depends on the case definition that is adopted, a recent systematic review estimated that approximately 23% of dental implants are affected by peri-implantitis. 1 Given its prevalence, the management of marginal peri-implantitis is a common problem in general dental practice. 2 The role of titanium as a local inflammatory driver of peri-implantitis, both in vivo and in vitro, has also been discussed in the literature.3–6 Osseointegration, defined as “functional ankylosis” between the implant and the jawbone, is the primary treatment objective of the implantologist. Osseointegration refers to a direct structural and functional connection between living bone and the surface of a load-bearing artificial implant. 6 Successful osseointegration is considered to occur when new bone is deposited directly at the bone–implant interface and the implant exhibits mechanical stability. 7
The question remains: what happens when this so-called “bone-to-implant contact” (BIC) does not take place over the entire surface of the implant? On the one hand, the implant is sufficiently healed—to the satisfaction of both the implantologist and patient—and serves to mechanically improve chewing ability and occlusion. On the other hand, chronic inflammation may occur in areas where osseointegration is impaired, to the immunological detriment of the patient.8,9 The interdisciplinary field of osteoimmunology underlies critical discoveries concerning bone regeneration and the development of new therapeutic strategies for bone diseases. 10 Bone cells interact with immune cells under both physiological and pathological conditions. 11 Furthermore, the bone marrow cavity is critical for the proper development of the immune system, and houses stem cells that are important for immune system maintenance. Both within and outside the medullary cavity, cytokines produced by immune cells have important effects on the regulation of bone homeostasis and the development of systemic immunological diseases. 11
In the present study, we focused on the role of osteoimmunology (i.e., the relationship between the immune system and bone metabolism) and the immunoregulatory properties of bone remodeling in the osseointegration of dental implants. The central questions that we aimed to address were as follows. First, which immunological processes are involved in the transition from impaired local osseointegration to systemic osteoimmunological dysfunction? Second, what—if any—differences exist in the development of BIC between titanium (Ti-Impl) and ceramic (Cer-Impl) implants? And third, which are the most appropriate methods for assessing BIC impairments associated with unsuccessful osseointegration?
Materials and methods
Definition of terms
To distinguish bone resorption leading to implant loss from marginal mucositis and peri-implantitis, we have referred to bone metabolism disorders as “para-implantitis” in the present study. Para-implantitis is defined by an absence of periodontal pocketing and an abacterial, silent, chronic inflammatory process that occurs adjacent to the dental implant. This inflammatory process does not result in marginal bone loss around the implant, but rather leads to disintegration of the region of the implant bed that lacks direct contact with the oral environment.
We have also defined a new unit of measurement for bone density, which was determined using a newly developed trans-alveolar ultrasonography (TAU) device. We have named this unit of measurement the cavitation-TAU unit (CTU).
The reporting of this study conforms to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines. 12
Study cohort
Two groups of patients with dental implants made from different materials were assessed for BIC and the osteoimmune response of the para-implant bone area: one group had Ti-Impl, and the comparison group had Cer-Impl. Patients in the Ti-Impl group were tested for possible hypersensivity to titanium using the titanium stimulation test (Ti-Stim; outlined in the “Investigative methods” section). From this cohort, two smaller groups were selected: one comprising patients with a positive Ti-Stim (tumor necrosis factor [TNF]-α ≥ 40 pg/mL; interleukin [IL]-1 ≥ 30 pg/mL) and another comprising those with a negative Ti-Stim (TNF-α < 40 pg/mL; IL-1 <30 pg/mL). We then compared BIC measurements among the following three groups: the Ti-Impl group with a positive Ti-Stim, the Ti-Impl group with a negative Ti-Stim, and the Cer-Impl group.
Inclusion and exclusion criteria
For inclusion, patients needed to have a firmly osseointegrated and load-bearing Ti-Impl or Cer-Impl of a minimum duration of 4 months. Patients were excluded if they had a loosened implant, were unable to tolerate occlusal loading because of pain, or were using cortisone and bisphosphonates (because of their effects on bone metabolism). The use of medication to treat systemic disease was not considered grounds for exclusion.
Ethical considerations
All clinical samples were provided by patients undergoing surgical treatment at our clinic. Each patient expressed an interest in determining whether chronic inflammation was present in the jawbone and, if so, whether it was associated with a pre-existing chronic immune disorder or systemic disease. The presence of bone marrow defects of the jaw (BMDJ) associated with osteoimmune dysregulation was confirmed preoperatively in each patient using panoramic radiography, cone beam computed tomography (CBCT)/digital volume tomography (DVT), and TAU examinations. Postoperatively, local C-C motif chemokine 5 (CCL5, also known as regulated on activation, normal T-cell expressed and secreted [RANTES]) expression levels were determined using samples excised from each patient’s jawbone. The present study was conducted as a retrospective case–control study, and was classified as such by the Institute for Medical Diagnostics (IMD), Berlin, according to DIN EN 15198/DIN EN 17025; it thus received exemption from the need for ethical approval. All patients provided their written informed consent to participate in this study. The present study was patient-centered; the samples and data were obtained directly in the course of routine clinical practice and the normal medical care of the patients, and were evaluated retrospectively. Institutional approval was not required to publish case details. Patients who were taking medication to alleviate any symptoms were not required to discontinue such medication, other than those affecting bone metabolism. All patient details have been de-identified.
All procedures performed in this study involving human participants were approved by a forensically accredited institute, the IMD-Berlin, according to DIN EN 15198/DIN EN 17025. Furthermore, they were conducted in compliance with the Helsinki Declaration of 1975 as revised in 2013 or comparable ethical standards.
Investigative methods
The death of local para-implant bone marrow cells leads to abnormal osteoimmune function, which then results in a chronic inflammatory microenvironment that impairs bone regeneration and repair. The risk factors of osteonecrosis are generally multifactorial, but the etiology and pathogenesis remain unclear. 13 To describe para-implant osteonecrotic defects and associated bone mass loss and bone structure disintegration, as well as to analyze osteoimmune abnormalities, we conducted the following investigations (which have been infrequently used, to date) both before and after therapeutic surgical implant removal. 1) We preoperatively investigated potential individual systemic immunological reactions to titanium using the Ti-Stim. 14 2) We preoperatively examined para-implant bone density using TAU (because the radiographic imaging of bony structures surrounding implants is currently limited by deflection and scattering phenomena). 3) We postoperatively performed a local study of osteoimmune cytokine profiles using a bead-based multiplex analysis 15 to control for the local dysregulation of para-implant immune messengers with respect to para-implant inflammatory osteoimmune dysregulation. 16
We hope that fellow researchers will reproduce our methodology in future studies.
Ti-Stim
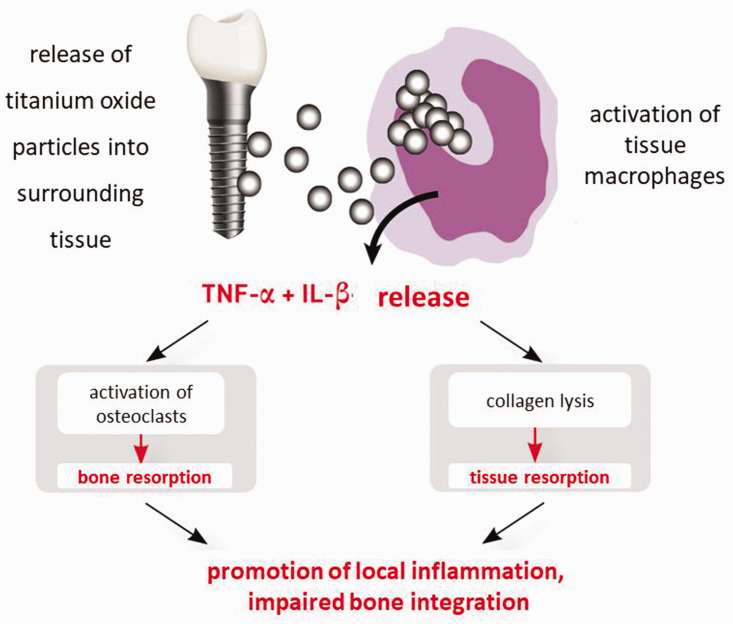
Titanium has a very low allergenic potency compared with many other metals. This is because titanium ions are immediately oxidized after their release from implants as a result of their high oxidation tendency (i.e., oxidized titanium ions have no hapten effects). Individual titanium hypersensitivity is most commonly caused by the excessive pro-inflammatory reactivity of macrophages. After contact with titanium oxide particles, macrophages react by releasing the pro-inflammatory cytokines tumor necrosis factor (TNF)-α and interleukin (IL)-1. The intensity of cytokine release depends on individual genetic variants (polymorphisms). The Ti-Stim (Figure 1) was developed and validated by IMD-Berlin (Berlin, Germany) to address this issue. The Ti-Stim involves a blood test to examine whether a patient’s macrophages produce an increased or decreased inflammatory response (a positive or negative result, respectively) following contact with titanium particles. 14 In patients with positive findings, macrophages in the tissue surrounding the implantation site are overactivated in response to released titanium particles and induce primarily local—but possibly also systemic—inflammation.17–19
Figure 1.
Macrophage-mediated local inflammation is promoted by titanium sensitization and tumor necrosis factor (TNF)-α and interleukin (IL)-1β release (source: IMD-Berlin).
Measurement of bone density using TAU
To overcome the diagnostic uncertainties of conventional radiography in the measurement of bone density surrounding an implant, a TAU device has recently been developed.20,21
Validation of TAU bone density measurements
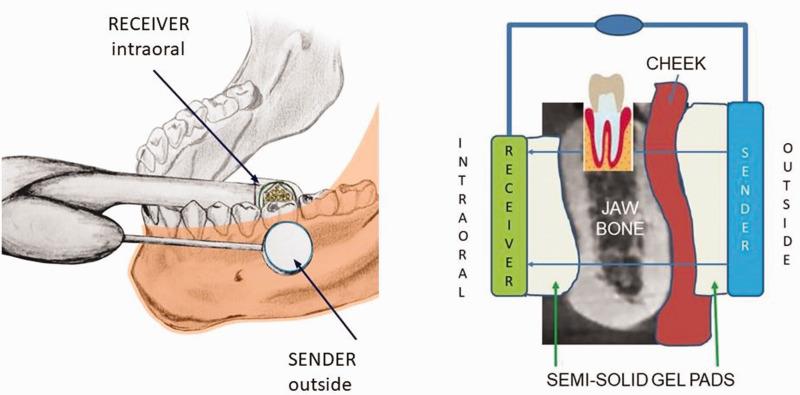
TAU measurements are based on ultrasonic principles in which sound is best conducted through solid material, more weakly conducted through aqueous environments, and slowly conducted through air. That is, solid structures attenuate ultrasound to a lesser degree than fatty or aqueous structures. The TAU device consists of an ultrasonic transmitter that is placed on the skin over the specific tooth and jaw area to be measured. Additionally, a thumbnail-sized receiver is placed intra-orally over the specific dental area to be assessed. Interference-free acoustic coupling is achieved with a gel pad that is placed both intra- and extra-orally (Figure 2). Each dental area is measured individually. The receiver has 91 piezoelectric fields that register the trans-alveolar sound waves, which are then converted into a colored pulse via a computer unit; sound waves of different speeds are represented in different colors (Figure 3). The TAU monitor displays the various structures that are detected based on mineralization density, using two- and three-dimensional graphic representations of bone density.22–24
Figure 2.
Measurement of jawbone density using a trans-alveolar ultrasonography device. Note: individual adjustments of the measuring unit can be made in response to varied jaw conditions using specially designed gel pads.
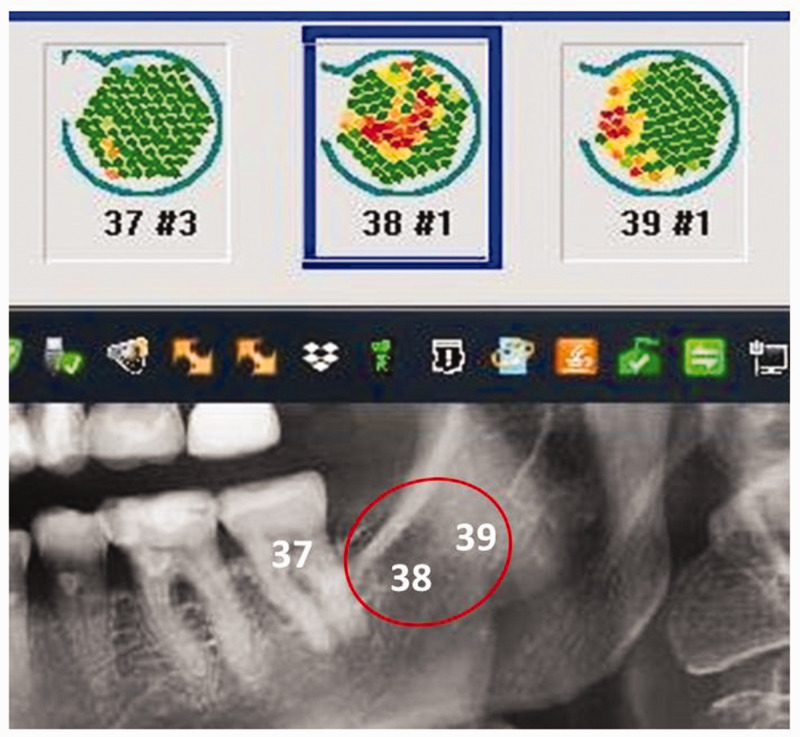
Figure 3.
Comparison of X-ray image (two-dimensional-panoramic radiography) and trans-alveolar ultrasonography measurements of the same jawbone area, showing regions of varying bone density in green (dense, inconspicuous structures) and red (low-density, conspicuous structures). In contrast to the two-dimensional-panoramic radiography and three-dimensional digital volume tomography/cone beam computed tomography images, the trans-alveolar ultrasonography measurements in edentulous retromolar area 38/39 indicate a region that is suspicious in terms of jaw osteolysis. This finding, shown in red, is the result of increased attenuation of the ultrasound beam caused by fatty degeneration of the formerly dense medullary cancellous bone. This finding may be compared with that of the area at healthy tooth 37, which indicates the presence of solid bone structure with normal bone density (shown in green).
Numerical evaluation of local attenuation coefficients using TAU
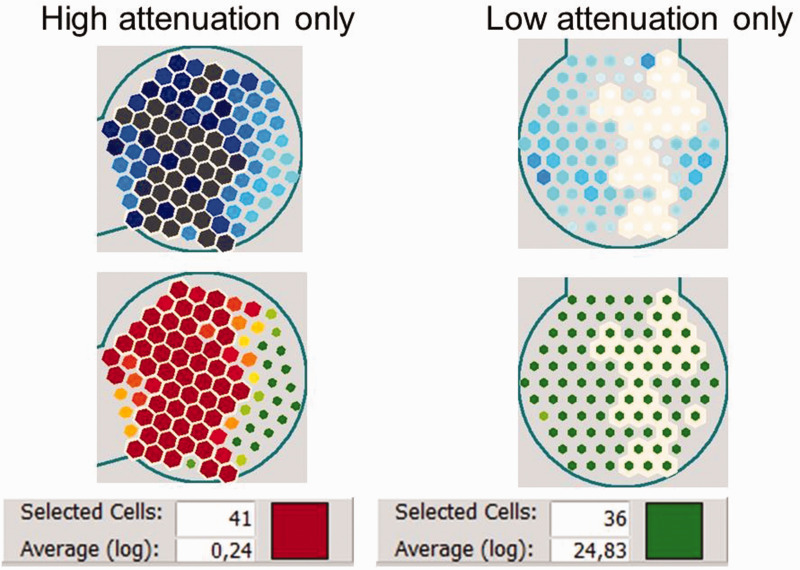
In radiography, an algorithm is used to convert gray levels to density measures (Hounsfield units). By contrast, the TAU device provides an exact numerical measurement of bone density for each jaw region within the scope of the 10-mm receiver (usually per tooth). 25 To do this, the TAU software provides a numerical representation of the attenuation coefficients within the measurement range of the device. When the operator clicks on the darkest of the 91 sensor fields of a given measurement, the software retrieves the data for this specific field and displays the measured value automatically using a logarithmic evaluation. The sensor fields with the highest attenuation values measured by TAU, indicating an area of impaired BIC, are then shown in red or black. Next, the TAU software calculates the logarithmic average of the sum of the values of the sensor fields with the lowest density, displayed in red, as the “Average(log)” (Figure 4, left panel). In the same way, the logarithmic average of the values of the sensor fields with the highest density, shown in green (equivalent to reduced attenuation caused by solid structures), is also calculated (Figure 4, right panel). In the following sections, the numbers for these logarithmic averages (i.e., “Average(log)” as displayed by TAU) are represented in CTU.
Figure 4.
Measurement of the cavitation-trans-alveolar ultrasonography (TAU) unit (CTU) via the logarithmic average of the values of the sensor fields using the TAU device. The numerical representations of TAU attenuation coefficients for diminished bone density (left panel) and dense material (right panel) are shown. Selected sensor cells (left panel: high attenuation; right panel: low attenuation) are indicated by a white outline. The evaluation is presented in the bottom window for a number of selected sensor cells; the result is displayed as a logarithmic mean, which is associated with a corresponding color (left panel: red corresponds to high attenuation; right panel: green corresponds to low attenuation). The TAU software thus allows the mean value to be calculated over a freely selected range of the 91 piezoelectric sensors.
The logarithmic averaging used in TAU means that the color change from red to green occurs at relatively low values, (e.g., 2); however, the value range extends to 100. Green fields are much more important than red fields when linear averaging. For example, when a single green field with a value of 100 and 10 red fields with a value of 1 are selected, the linear average results are (100 + 10 × 1)/11 = 10, which is presented as the color green. However, with logarithmic averaging, the logarithms of the values are averaged and the exponential value is shown; thus, log10(100) = 2, log10(1) = 0 would be the average value of the logarithms, leading to (2 + 10 × 0)/11 = 0.18. The exponential value would then be 100.18 = 1.52, which would be displayed as red to orange. In practice, the sensor fields with the darkest colors in the left black/blue scale are clicked on by the operator.
CTU and jaw bone density
Using the aforementioned software-guided evaluation of bone density, we introduced a new unit for measuring jawbone density using the TAU device: CTU. CTU represents the attenuation coefficient of ultrasound waves within the cortical bone and medullary cavity of the jawbone. Tables 1 and 2 present the correlations between the color codes used by the TAU device and the respective CTU values, tissue consistencies, anatomical structures, and suspected diagnoses.
Table 1.
Green/red color code for trans-alveolar ultrasonography (TAU) and the corresponding cavitation-TAU unit (CTU), consistency, anatomical structure, and suspected diagnosis for each color.
| CTU | Color | Consistency | Anatomy/suspected diagnosis |
|---|---|---|---|
| 0.24 | Gray 
|
Hollow space | Error? |
| 0.23 | Dark red 
|
Dissolved/liquid | Osteolysis |
| 0.62 | Light red 
|
Soft/fatty | Osteonecrosis |
| 1.18 | Orange 
|
Moderately soft | Osteitis |
| 1.68 | Yellow-orange 
|
Slightly soft | Ischemia |
| 1.85 | Light yellow 
|
Nerve structure | Inferior alveolar nerve |
| 1.96 | Light green 
|
Moderately firm | Healthy spongiosa |
| 3.98 | Green 
|
Dense/solid | Cortical bone/linea obliqua |
Table 2.
Black/blue color code for trans-alveolar ultrasonography (TAU) and the corresponding cavitation-TAU unit (CTU), consistency, anatomical structure, and suspected diagnosis for each color.
| CTU | Color | Consistency | Anatomy/Suspected Diagnosis |
|---|---|---|---|
| 0.08 | Gray 
|
Hollow space | Error? |
| 0.24 | Black 
|
Fatty-degenerated | Osteolysis |
| 0.37 | Dark Blue 
|
Soft/fatty | Osteonecrosis |
| 0.58 | Light gray 
|
Moderately soft | Ischemia |
| 4.69 | Light blue, large 
|
Fatty, healthy | Inferior alveolar nerve |
| 7.16 | Light blue, small 
|
Firm, less dense | Healthy spongiosa |
| 64.95 | White 
|
Hard, dense | Cortical bone/linea obliqua |
| 295.1 | Gold 
|
Soft tissue | Oral mucosa/cheek |
Why CTU? Limitations of the radiological assessment of inflammatory para-implant bone environments
When investigating whether local inflammation is present in the jawbone area surrounding individual Ti-Impl, the Ti-Stim fails to provide a diagnostically conclusive answer. However, the assessment of potentially impaired osseointegration is also severely limited by radiological constraints with respect to implantology. The primary concern is the presence of typical beam-hardening artifacts in DVT/CBCT, which are caused by radiation deflecting off the Ti-Impl or Cer-Impl. These artifacts cause substantial image degradation and often misrepresent the region of interest. They should thus be recognized and understood—along with normal CBCT anatomy—to enable the accurate assessment of image volumes and avoid inaccurate diagnoses. 26 Theoretical and experimental results have indicated the occurrence of many beam-hardening artifacts with a typical implant diameter. 27 Artifacts are common in the currently used CBCT, and are induced by discrepancies between mathematical modeling and the actual physical imaging process. Given that artifacts may interfere with the diagnostic process using CBCT datasets, all users should be aware of their presence. 28
Cer-Impl generate many more artifacts than Ti-Impl. In one study, the average intensity of artifacts surrounding Cer-Impl were three times greater than those surrounding Ti-Impl. 29 Because more- or less-pronounced image artifacts can be present, appearing as white or light gray bands or lines, the bone–implant interface cannot be accurately reconstructed. Previous studies have emphasized the difficulties and uncertainties associated with interpreting bone structures around implants in DVT diagnostics. 30 However, no technique has yet been developed to visualize whether bone or soft tissue surrounds Ti-Impl. 30
Postoperative analysis of osteoimmune cytokine profiles in impaired BIC
Cells communicate with each other via the release of chemical messengers or signaling molecules (i.e., ligands) that bind to the surface receptors of neighboring cells. Cytokines, which function as extracellular ligands, are secreted by a broad range of cells including immune cells and various stromal cells. The cytokine milieu in the extracellular space often dictates the type of immune response that is mounted by cells. Cytokines are mediators of paracrine and intracrine cell communication within a highly complex mediator network. 31 Pro-inflammatory chemokines are secreted by injured tissue and recruit macrophages, neutrophils, and other immune cells to remove harmful stimuli and regulate the immune system. 32 In the case of osteonecrosis, the persistence of these harmful factors stimulates local immune cells to continuously secrete inflammatory factors, ultimately resulting in chronic inflammation. 33
Cytokine assessments in postoperative samples of BMDJ
Samples with conspicuous osteoimmunological abnormalities that were excised during surgery were cooled and analyzed in the laboratory to determine their individual cytokine profiles (Figure 5). At IMD-Berlin (inspected by Deutsche Akkreditierungsstelle, the national accreditation body of the Federal Republic of Germany 34 ), the samples were homogenized by mechanical force in 200 μL of cold protease inhibitor buffer (Complete Mini Protease Inhibitor Cocktail; Roche Diagnostics GmbH, Penzberg, Germany). The homogenate was then centrifuged for 15 minutes at 13,400 rpm. Next, the supernatant was collected and centrifuged for a further 25 minutes at 13,400 rpm. In the 15 supernatants of tissue homogenate, we measured CCL5, fibroblast growth factor (FGF)-2, IL-1 receptor antagonist (ra), IL-6, IL-8, monocyte chemotactic protein-1 (MCP1), and TNF-α. These measurements were performed using the Human Cytokine/Chemokine Panel I (MPXHCYTO-60K; Merck KGaA, Darmstadt, Germany) according to the manufacturer’s instructions. The findings were analyzed using the Luminex® 200™ with xPonent® Software (Luminex Co, Austin, TX, USA).
Figure 5.
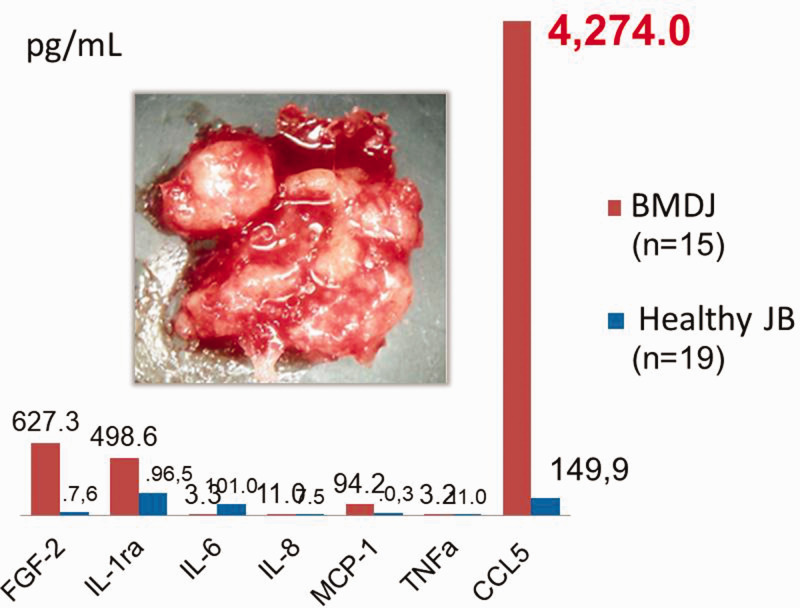
Analysis of seven cytokines in bone marrow defects of the jaw (BMDJ) in a cohort of patients with chronic facial pain/neuralgia (n = 15) compared with healthy jawbones (JB). Blue columns show the median cytokine expression values in healthy JBs; red columns show the median cytokine expression values in the 15 BMDJ samples. The image inside the graph shows a sample of fatty degeneration in the jawbone found in a BMDJ from one patient in the cohort. CCL, C-C motif chemokine; FGF, fibroblast growth factor; IL, interleukin; MCP, monocyte chemotactic protein; TNF, tumor necrosis factor.
CCL5 overexpression in BMDJ
In previous studies, we have highlighted the characteristic overexpression of the proinflammatory chemokine CCL5 in BMDJ with fatty degenerative osteonecrosis (FDOJ).35,36 The overexpression of this single chemokine appears to be unique to the jawbone. In the current study, we also used the chemokine CCL5 as an immune marker for areas of the jaw with osteonecrotic changes. The panel shown in Figure 5 presents the cytokine analysis of 15 BMDJ samples from patients with chronic facial pain/neuralgia. This panel illustrates the cryptic inflammatory process that occurs in cases of BMDJ without any classic signs of inflammation (i.e., the overexpression of a single chemokine, CCL5). The absence of excessive levels of the inflammatory cytokines TNF-α and IL-6 may explain the lack of acute para-implant pain episodes, meaning that the affected individual is unaware of the chronic inflammatory process.
Definitions of osseointegration
Successful osseointegration according to Albrektsson et al
Osseointegration was discovered in 1962 37 and was originally defined as direct contact between an implant surface and bone. Today, osseointegration is used to encompass a foreign body reaction in which bone is formed to protect tissue from the foreign material (i.e., the implant). 30 In recent decades, there has been a paradigm shift in which the notion of implants as inert biomaterials has been replaced by the view that such foreign bodies induce immunomodulatory interactions with the host. 38 Wennerberg and Albrektsson described the pathomechanism of para-implant osseointegration as an immunomodulatory interaction with the implanted foreign material. 7 Osseointegration appears to be an immunologically and inflammatory-driven process with the goal of shielding the foreign material placed in the body; bone cells form and remodel hydroxyl apatite. The biological microenvironment surrounding the implant enables osteogenic cells to bind to its surface and secrete biomolecules that promote early bone formation. 39 Micro-roughness—a term used to describe a specific implant surface topography—has been reported to have a positive effect on metabolic activity 40 and increase para-implant osteogenesis in vivo. 41 The new bone formation processes increase the possibility of contact osteogenesis with a “specific cell differentiation pattern.”42,43 According to other authors,44,45 osteogenesis results from the coupling activity between osteoblasts and osteoclasts. From an immunological perspective, osseointegration is considered a healing mechanism that controls the interactions between an implant and a bone bed. In summary, osseointegration is defined as a time-dependent healing process whereby the clinically asymptomatic rigid fixation of alloplastic materials is achieved and maintained in bone during functional loading.46,47 Its histological appearance resembles functional ankylosis with no intervention of fibrous or connective tissue between the bone and the implant surface. 6 Figure 6 presents the contrast between ideal osseointegration in theory 6 and the clinical reality of partially failed osseointegration.
Figure 6.
Contrast between theoretical ideal osseointegration and the clinical reality of partially failed osseointegration. Left panel: complete ankylosis surrounding a titanium dental implant (by permission of T. Albrektsson). 6 Right panel: osteoimmune pathology adjacent to an implant, resulting in partially failed osseointegration.
Partially failed osseointegration
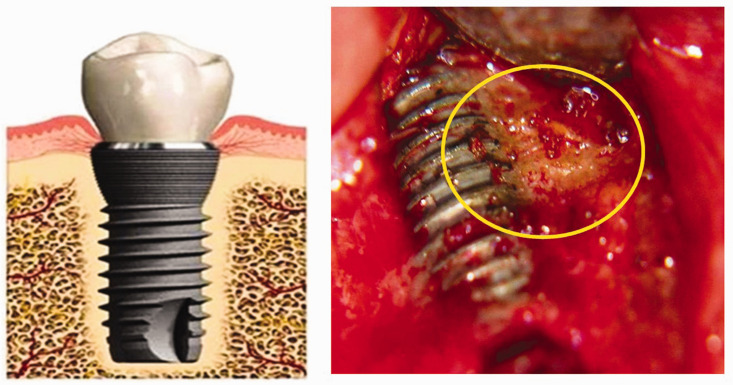
The ideal of a completely osseointegrated and fully loadable implant gives rise to the following question: is para-implant osteogenesis always completely developed? Alternatively, does the physiological foreign body reaction that is characteristic of osseointegration ultimately result in failed osseointegration, at least partially? (See examples in Figures 6, 7, and 8.) Furthermore, is the clinical picture complicated by so-called “silent” inflammation that follows as a secondary event? These questions do not focus on cases of premature implant loss caused by obvious mechanical failure, but on the much less noticeable process of a transition from successful ankylosis to osteoimmune pathology with important local and systemic sequelae. Long-term osteoimmune success depends on a foreign body equilibrium that, if disturbed, may lead to impaired function of the implant. This occurs through a breakdown process, resulting in bone resorption because cells—such as osteoclasts of various origins and possibly even macrophages—degrade more bone than is formed via osteoblastic activity. 48 In summary, the osseointegration of long-term implants is often incomplete, with gaps remaining between the implant surface and surrounding hard tissue, thus resulting in impaired healing and incomplete osseointegration. 49
Figure 7.
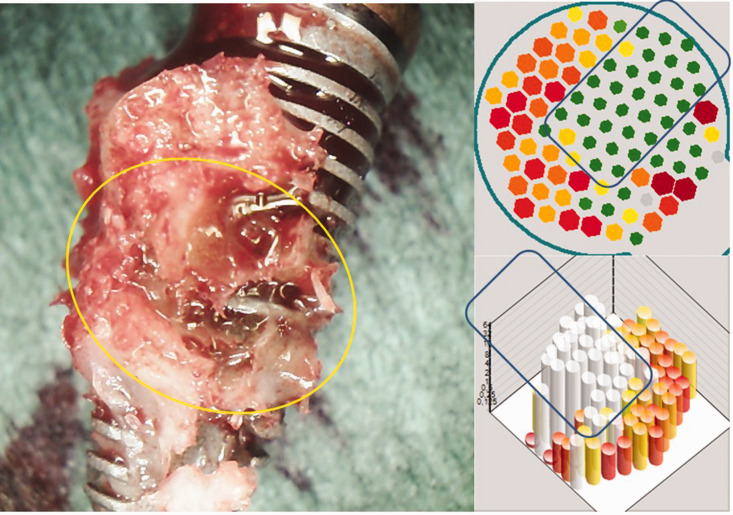
Postoperative and trans-alveolar ultrasonography (TAU) images from of an excised implant. Left image: immediate postoperative image of the excised implant, showing attached tissue that is characteristic of bone marrow defects of the jaw/fatty degeneration in the jawbone. Upper right image: corresponding TAU image showing the solid area of the implant in green in two dimensions (represented by the outlined region), and the adjacent area of osteolysis in the para-implant bed in red. Lower right image: three-dimensional representation of the osteolytic region surrounding the implant (shown in red) and the clearly delineated area of the implant (outlined in white).
Figure 8.
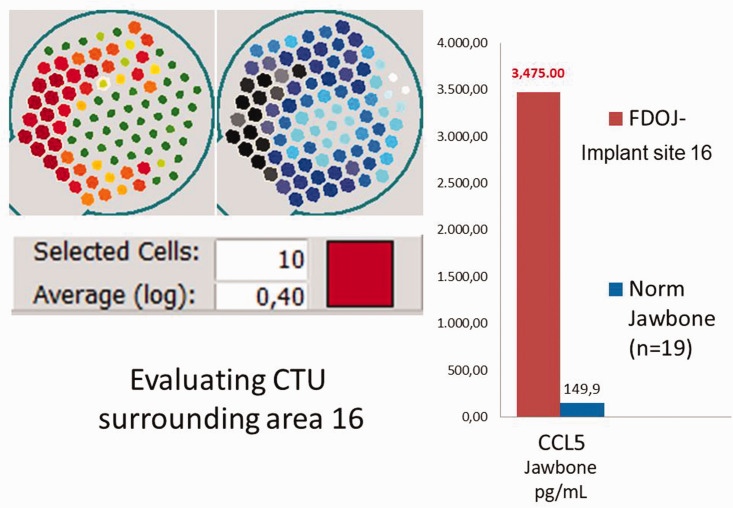
Left panel: numerical evaluation of the trans-alveolar ultrasonography (TAU) measurement of bone density in area 16 (in cavitation-TAU units [CTU]). Right panel: postoperative multiplex analysis of local C-C motif chemokine 5 (CCL5) overexpression in the para-implant alveolar bone, as measured in the TAU image shown on the left. This analysis revealed an approximately 30-fold overexpression of CCL5 (3475 pg/mL versus the normal value of 149.9 pg/mL). FDOJ, fatty degeneration in the jawbone; Norm, normal; Impl, implantation.
Statistical analysis
Quantitative data were analyzed using descriptive statistics, which were calculated using IBM SPSS Statistics for Windows, version 19 (IBM Corp., Armonk, NY, USA). The median and mean values and data distribution were calculated. Differences between cohorts were analyzed using Student’s t-test, Spearman’s rho, or Mann–Whitney U-test. The two-sided unpaired t-test was used to determine differences within groups, and Spearman’s coefficient was used to analyze correlations among the cytokine profile analyses. The significance level was set at P < 0.05.
Clinical examples illustrating the main research questions
The following case reports from our clinic, which include the diagnostic protocols used in each case, further illustrate the main questions addressed in the present study. These examples demonstrate our multi-layered investigative approach into the impaired implant osseointegration and gap formation that are associated with osteoimmune dysregulation.
Para-implantitis in Case 1: Ti-Impl and intraosseous inflammation
A 57-year-old woman with an implant of 7 years of duration presented with migraine on the right side only, as well as atypical facial pain—present since her implant placement—in the right upper jaw only. On the basis of an integrated diagnosis and the patient’s pain symptoms, the implant was surgically removed.
Radiography and measurement of bone density using TAU
Preoperatively, two radiographic studies (orthopantomography [OPG] and CBCT) and an additional TAU measurement of the para-implant alveolar bone were performed. Both radiographs showed inconspicuous structures and allowed for no further assessment of alveolar bone density, particularly because of artifacts present in the CBCT images (Figure 9). Figure 7 shows an immediately postoperative image of the removed implant, with regions of BMDJ/FDOJ attached to the implant that were associated with partially failed osseointegration. The corresponding area of reduced bone density surrounding the implant is shown in the TAU image in red, and indicates the presence of BMDJ/FDOJ.
Figure 9.
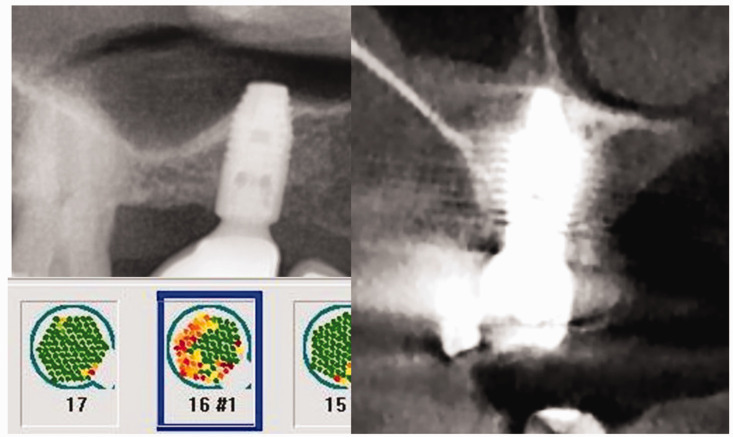
Comparison of different imaging modalities. Upper left image: orthopantomogram showing completely inconspicuous bone surrounding the implant at area 16. Right image: the degree of mineralization of the para-implant bone environment, as shown in this cone beam computed tomography image, is impossible to assess; in particular, flare artifacts (visible as bright streaks) prevent a detailed analysis. Lower left image: the corresponding trans-alveolar ultrasonography measurement of bone density in this area indicates the conspicuous region surrounding the titanium dental implant at area 16 (in red), representing reduced mineralization density.
Preoperative Ti-Stim
The result of the preoperative Ti-Stim performed in Case 1 was clearly positive, with 323 pg/mL of IL-1 (<30.0 pg/mL) and 263 pg/mL of TNF-α (<40.0 pg/mL).
Numerical evaluation of TAU measurement of bone density in CTU
Numerical scaling for TAU is necessary to provide an objective representation of bone density. By selecting the darkest red- or black-/blue-colored sensor fields of the jaw region under investigation (in this case, the Ti-Impl site in area 16), the TAU device creates a logarithmic average (log) of the attenuation intensity, as shown in the left panel of Figure 8. In Case 1, this value was 0.40 CTU, which falls within the range of values in CTU for areas of FDOJ in BMDJ.
Postoperative analysis of the osteoimmune cytokine profile
The osteoimmune cytokine profile was analyzed using multiplex analysis for CCL5 expression in the alveolar jawbone surrounding the Ti-Impl. The postoperative CCL5 analysis from the osteolytic alveolar bone surrounding the Ti-Impl (area 16) revealed approximately 30-fold overexpression of CCL5 (3475 pg/mL) compared with normal values (149.9 pg/mL) (Figure 5).16,35,36
Postoperative histology
In addition to CCL5 overexpression, histological examination confirmed the existence of FDOJ; in particular, the “formation of oil cysts” was noted in the osteolytic medullary region. The histopathological report for the BMDJ sample of Case 1 stated: “Sample excised from area 16: Medullary tissue … with exclusively fatty marrow; this displays necrobiotic changes and mucinous degeneration zones as well as small so-called oil cyst formations. Lastly, also small fibrotic zones, generally consistent with changes in the context of fatty-degenerative osteolysis of the jawbone.”
Case 1 conclusions
Case 1 presented a problem that is widely unknown; although two-and three-dimensional radiography allowed for only a limited assessment of the para-implant alveolar bone, TAU imaging indicated severely reduced bone density (in red). After the implant was removed, osteonecrosis in the corresponding jawbone area was noted as fatty degeneration of the medullary cavity. CCL5 overexpression and histological examination confirmed the presence of silent inflammatory osteonecrosis that was preoperatively indicated using TAU, with a CTU value of only 0.40. The cryptic bone degeneration surrounding the implant was considered a pathological immune process and was therefore clinically removed to improve the patient’s health.
Para-implantitis in Case 2: Cer-Impl and intraosseous inflammation
Case 2 had an implant of 9 months of duration at the site of a prior root canal-treated tooth (#14); the implant was well osseointegrated and prosthetically restored. However, the patient reported chronic pain in the right maxilla for the previous 6 months. Based on an integrated diagnosis and the patient’s pain symptoms, the implant was surgically removed.
Radiography and measurement of bone density using TAU
Preoperatively, two-dimensional OPG, three-dimensional CBCT, and an additional TAU measurement of the para-implant alveolar bone were performed in Case 2. Both the OPG and CBCT showed inconspicuous structures and allowed for no further assessment of alveolar bone density, in particular because of artifacts present in the CBCT images (Figure 10).
Figure 10.
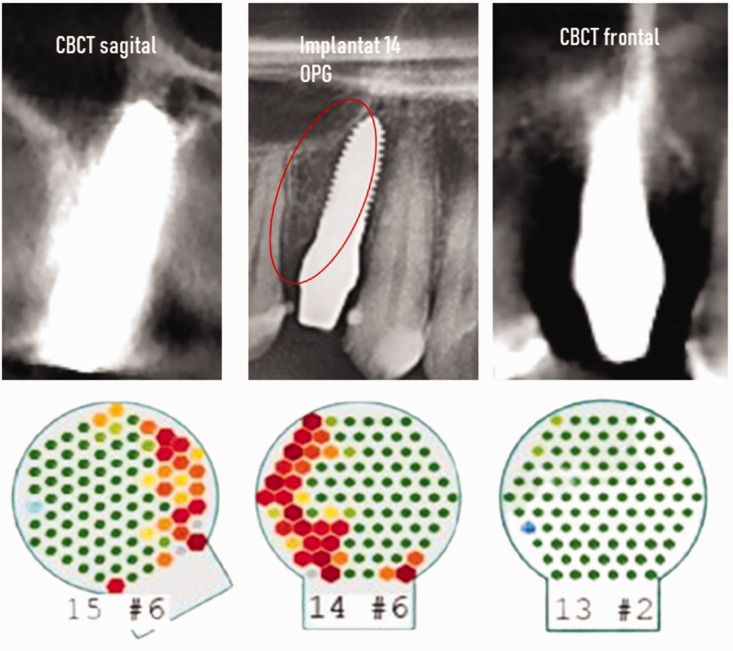
Comparison of two- (middle image) and three-dimensional radiographic images of the ceramic implant in area 14 of Case 2, showing the frontal (left image) and sagittal (right image) views in cone beam computed tomography (CBCT). The orthopantomogram (OPG) was unremarkable and the CBCT images were unable to be interpreted because of the presence of artifacts. Lower image: measurements of bone density with trans-alveolar ultrasonography (TAU) in areas 15, 14, and 13 (left to right, respectively). Although the alveolar bone density surrounding healthy teeth 13 and 15 is shown in green, indicating normal bone metabolism, the interdental space between tooth #15 and the distal side of the adjacent ceramic implant is shown in red, indicating osteolysis. Implantat, implantation site.
Numerical evaluation of TAU measurement of bone density in CTU
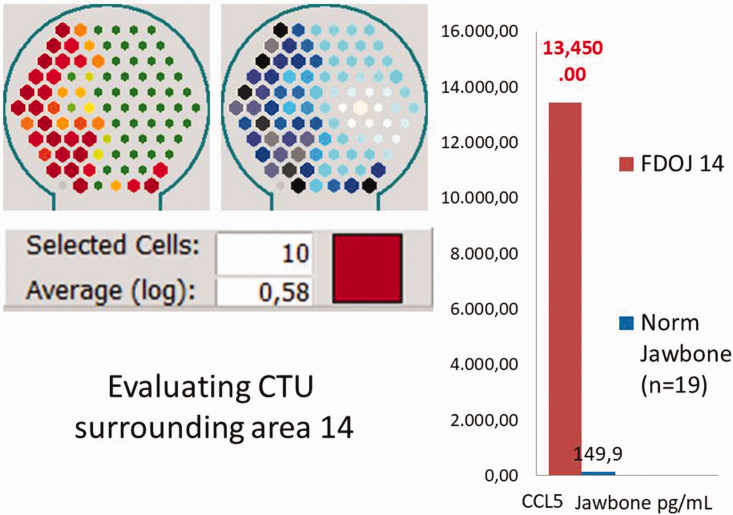
By selecting the darkest red- or black-/blue-colored sensor fields of the Cer-Impl site at area 14, a logarithmic average of the attenuation intensity was generated (Figure 11, left panel). In Case 2, this value was 0.58 CTU, which falls within the normal range of values in CTU for FDOJ in BMDJ (Figure 11, right panel).
Figure 11.
Evaluation of the cavitation-trans-alveolar ultrasonography (TAU) unit (CTU) surrounding area 14. Left panel: numerical evaluation of the TAU measurement of bone density in CTU. Right panel: postoperative C-C motif chemokine 5 (CCL5) analysis of osteolytic alveolar bone at ceramic dental implant (Cer-Impl) 14, revealing an almost 100-fold overexpression of proinflammatory CCL5 (13,350 pg/mL versus the normal value of 149.9 pg/mL). FDOJ, fatty degeneration in the jawbone; Norm, normal.
Postoperative analysis of the osteoimmune cytokine profile
The postoperative osteoimmune cytokine profile was analyzed using multiplex analysis for CCL5 expression in the alveolar jawbone surrounding the Cer-Impl (area 14). There was almost 100-fold overexpression of proinflammatory CCL5 (13,350 pg/mL) compared with normal values (149.9 pg/mL) (Figure 12, right panel).
Figure 12.
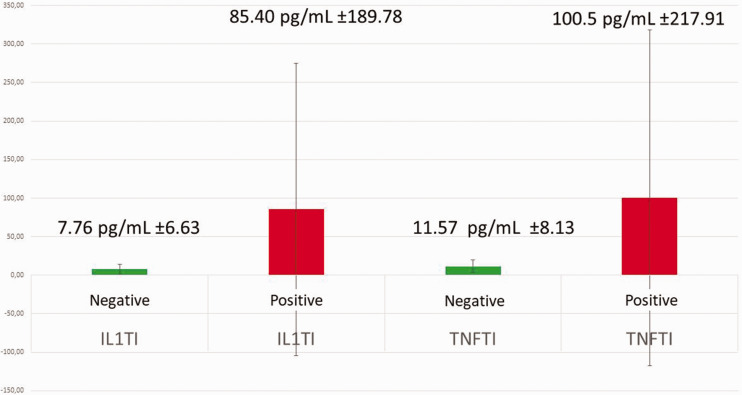
Distribution of titanium stimulation test (Ti-Stim) results for 894 patients, each with a clinically firm, osseointegrated titanium dental implant. Negative Ti-Stim: 710 patients; positive Ti-Stim: 184 patients, including cytokine expression of interleukin (IL)-1 and tumor necrosis factor (TNF)-α in each group.
Postoperative histology
The postoperative histological findings also described the presence of pathology at area 14: “Sample excised from the oral cavity (area 14) with scarring, with band-like chronic and florid inflammation. Small foci of inflammation in the stratified epithelia is also seen. From a morphological point of view, this could conceivably be a radicular cyst.”
Case 2 conclusions
The deflection artifacts present in two- and three-dimensional radiographic imaging caused by the Cer-Impl allowed for only the limited assessment of the para-implant bone. In contrast, TAU imaging revealed severely diminished bone density distal from the Cer-Impl, at area 14 (in red). Following implant removal, we analyzed the cytokines in tissue excised from the para-implant region; our findings were characteristic of fatty degeneration of the bone and there was very high proinflammatory CCL5 expression. A histological assessment confirmed the disrupted bone metabolism that was indicated by TAU (with a CTU value of only 0.58). The cryptic inflammatory process surrounding the implant in area 14 was considered a pathological immune process and was therefore clinically removed to improve the patient’s health.
Results
Ti-Stim in the Ti-Impl group
We performed Ti-Stim in 894 patients (342 male patients/552 female patients) between January 2010 and September 2022. The mean age of the patients in each group was as follows: total Ti-Stim group, 63.24 ± 0.404 years; total positive Ti-Stim group, 61.74 ± 0.353 years; total negative Ti-Stim group, 64.64 ± 0.455 years. These patients sought the clarification and alleviation of issues via dental treatment. The Ti-Stim was used to examine possible systemic exposure to titanium with adverse osteoimmunological effects. In each case, there was no acute inflammatory peri-implantitis and no loosening of (or occlusal discomfort from) the Ti-Impl. The Ti-Stim results for the 894 patients were as follows: negative Ti-Stim, 710 patients (79.26%) and positive Ti-Stim, 184 patients (20.74%). In the positive Ti-Stim group, mean cytokine expression was as follows: TNF-α, 100.5 ± 217.91 pg/mL and IL-1, 85.40 ± 189.78 pg/mL. In the negative Ti-Stim group, mean cytokine expression was as follows: TNF-α, 11.57 ± 8.13 pg/mL and IL-1, 7.76 ±6.63 pg/mL. These results are presented in Figure 12.
CTU values in the Ti-Impl cohort with positive or negative Ti-Stim
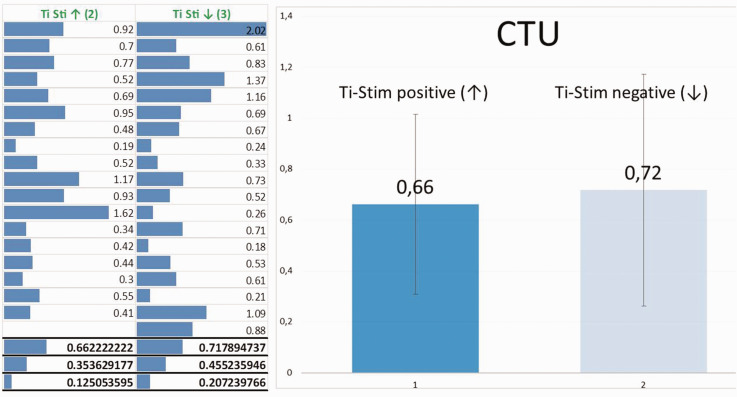
Within the negative Ti-stim group of 708 patients, we randomly chose and examined 19 patients (6 male patients/13 female patients; mean age, 65.05 ± 10.227 years) to obtain the CTU for a single Ti-Impl in each patient, to determine the CTU that indicated reduced osseointegration and possible associated osteoimmune dysregulation. Within the group of 184 subjects with a positive Ti-Stim, we randomly chose and examined 18 patients (6 male patients/12 female patients; mean age, 61.67 ± 11.675 years) to determine the CTU for a single Ti-Impl in each patient. Figure 13 shows a comparison of the CTU values from both groups, with a small advantage for the Ti-Stim negative group.
Figure 13.
Mean, standard deviation, and variance of cavitation-trans-alveolar ultrasonography (TAU) unit (CTU) values in the positive and negative titanium stimulation test (Ti-Stim) groups. As determined by TAU, the CTU mean value was 0.66 CTU for the positive Ti-Stim group and 0.72 CTU for the negative Ti-Stim group; these values were not significantly different. Similarly, although the positive Ti-Stim group appeared to have lower values (median = 0.54) than the negative Ti-Stim group (median = 0.67) for the dependent variable, this difference was not significant (U = 162.5, r = 0.04; Mann–Whitney U-test).
CTU values for bone density in the Cer-Impl and Ti-Impl groups
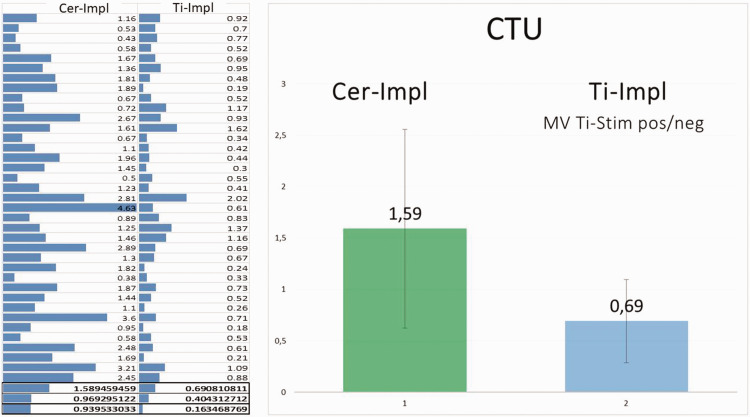
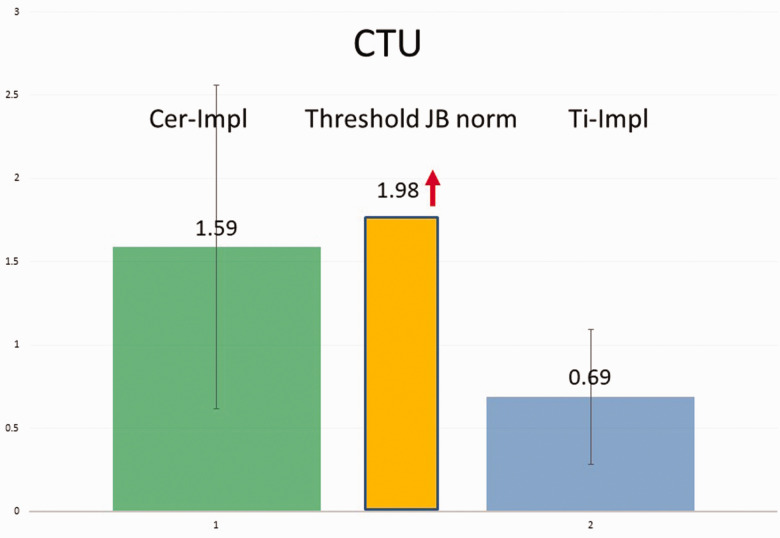
In the Cer-Impl group (mean age: 57.28 ± 0.969 years) bone density (in CTU) was measured using TAU in 37 patients (5 male patients/32 female patients). This measurement was taken at a firm, osseointegrated, and load-bearing Cer-Impl in each case. The results are presented in Figure 14. The median bone density value of the Cer-Impl group was 1.59 CTU, whereas the median value of all 37 Ti-Impl patients was 0.69 CTU.
Figure 14.
Comparisons of cavitation-trans-alveolar ultrasonography (TAU) unit (CTU) findings among the groups. Left panel: table showing the CTU mean, standard deviation, and variance values of the groups with ceramic (Cer-Impl; left column) and titanium (Ti-Impl; right column) dental implants. The mean CTU value (MV), as determined using TAU, was higher in the Cer-Impl group (1.59 CTU) than in the Ti-Impl group (0.69 CTU) with respect to the dependent variable (U = 247.5, P < 0.001, r = 0.55; Mann–Whitney U-test). Right panel: graphical display of the information showing that the Cer-Impl group had higher values for the dependent variable (median = 1.44) than the titanium stimulation test (Ti-Stim) group (median = 0.61).
CTU values for bone density in the Cer-Impl group and Ti-Impl groups with positive and negative Ti-Stim
The Cer-Impl group had significantly higher values than the positive Ti-Stim group for the dependent variable (median 1.44 vs. 0.54 CTU, respectively; Mann–Whitney U-test, U = 107.5, P < 0.001, r = 0.55). The Cer-Impl group also had significantly higher values than the negative Ti-Stim group for the dependent variable (median 1.44 vs. 0.67 CTU, respectively; Mann–Whitney U-test, U = 140, P < 0.001, r = 0.49).
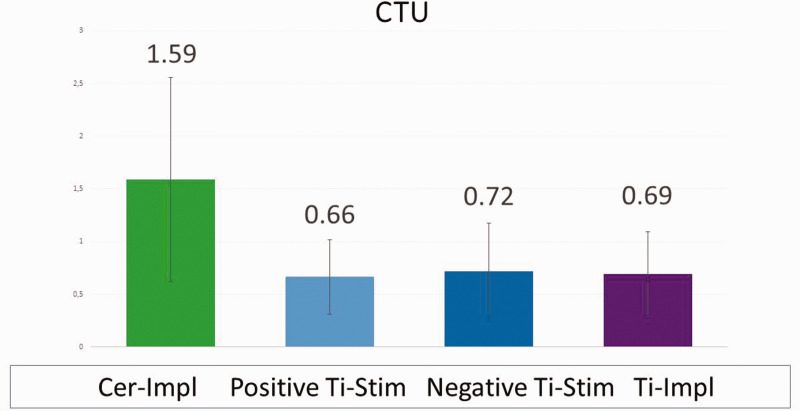
Figure 15 shows the distribution of CTU values in the positive and negative Ti-Stim groups, the combined Ti-Impl cohort, and the Cer-Impl group. The Cer-Impl group had significantly higher BIC than all of the Ti-Impl groups (1.59 vs. 0.69 CTU, respectively). By contrast, there were no significant differences in bone density between the Ti-Impl groups.
Figure 15.
Median cavitation-trans-alveolar ultrasonography (TAU) unit (CTU) values of the four groups. The considerably higher CTU value of the ceramic dental implant (Cer-Impl) group indicates significantly increased para-implant bone density in this group compared with the titanium dental implant (Ti-Impl) groups. There was a significantly decreased CTU value, and a corresponding reduction in bone-to-implant contact and osseointegration, in the total Ti-Impl cohort (right column) compared with the Cer-Impl group (left column). Ti-Stim, titanium stimulation test.
Postoperative evaluation of CCL5 expression in Ti-Impl groups with positive or negative Ti-Stim
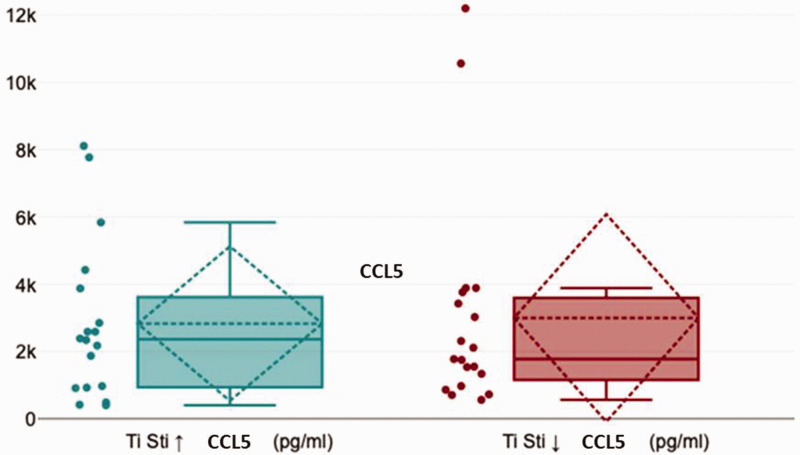
In patients with a positive Ti-Stim, a CTU value < 1.0, and/or chronic pain symptoms documented in the patient’s medical history, we considered that there was a medical indication for Ti-Impl removal. After Ti-Impl removal from 19 patients in the positive Ti-Stim group and 18 patients in the negative Ti-Stim group, we examined CCL5 expression in the para-implant BMDJ with fatty degenerative changes. Figure 16 displays the distribution of CCL5 expression in both the positive and negative Ti-Stim groups, with a slight reduction in the negative Ti-Stim group. We identified no relevant medical indications for Cer-Impl removal except in one patient, who was documented as Case 2 in the present report.
Figure 16.
Distribution of C-C motif chemokine 5 (CCL5) expression in the positive and negative titanium stimulation test (Ti-Stim) groups, showing a slight, but non-significant, reduction in the negative Ti-Stim group (U = 165.5, r = 0.03; Mann–Whitney U-test).
CTU values in the positive Ti-Stim group versus the negative Ti-Stim group in the logarithmic ratio
The linear comparison of bone density in titanium-sensitized (0.66 CTU) versus non-titanium-sensitized (0.72 CTU) patients was calculated as follows: 100.66:100.72 =100.66–0.72 = 10−0.06 = 0.87. The logarithmic reciprocal calculation was as follows: 100.72: 100.6 = 100.06 = 1.48
The logarithmic bone density CTU surrounding the Ti-Impl in titanium-sensitized patients was thus reduced by a factor of 1.48 relative to non-titanium-sensitized patients.
CTU values in the Cer-Impl group versus the total Ti-Impl cohort in the logarithmic ratio
The linear comparison of bone density between Ti-Impl (0.69 CTU) and Cer-Impl (1.59 CTU) patients was as follows: 100.69:101.59 = 10−0.90 = 0.125. The logarithmic reciprocal calculation was as follows: 101.59:100.69 = 100.90 = 7.94.
The calculated logarithmic bone density CTU was thus 7.94 times greater surrounding the Cer-Impl than surrounding the Ti-Impl.
CTU values of all groups compared with the threshold value for a healthy jawbone
In the present study, we defined the color code of TAU attenuation with corresponding CTU values. Given the logarithmic nature of ultrasound attenuation, these values are subject to a wide range of variation. However, from a threshold value of 1.98 CTU and higher, corresponding para-implant bone densities indicate an immunologically healthy BIC. Figure 17 compares the CTU values in the para-implant regions surrounding Ti-Impl and Cer-Impl in our study cohort with the lowest threshold value for healthy bone density (1.98 CTU).
Figure 17.
Graph comparing the cavitation-trans-alveolar ultrasonography (TAU) unit (CTU) values of the ceramic dental implant (Cer-Impl) group (left column) and titanium dental implant (Ti-Impl) cohort (right column) to the lowest threshold value for healthy bone density of 1.98 CTU (middle column). The Cer-Impl group had significantly higher values for the dependent variable (median = 1.44) than the positive Ti Stim group (median = 0.54 CTU; U = 107.5, P < 0.001, r = 0.55; Mann–Whitney U-test).
CTU values of Ti-Impl compared with the threshold value for healthy jawbone in logarithmic ratio
The linear comparison of bone density in the Ti-Impl group (0.69 CTU) versus normal bone density (1.98 CTU) was as follows: 100.69:101.98 = 100.69–1.98 = 10−1,29 =0.051. The logarithmic reciprocal calculation was as follows: 101.98:100.69 =101.29 = 19.49
The calculated logarithmic bone density CTU surrounding the Ti-Impl was thus reduced by a factor of 19.49 compared with the normal medullary jawbone.
CTU values of Cer-Impl compared with the threshold value for healthy jawbone in logarithmic ratio
The linear comparison of bone density in the Cer-Impl group (1.59 CTU) versus normal bone density (1.98 CTU) was as follows: 101.59:101.98 = 10−0.39 = 0.407. The logarithmic reciprocal calculation was as follows: 101.98:101.59 = 100.39 = 2.45
The calculated logarithmic bone density CTU surrounding the Cer-Impl was thus reduced by a factor of 2.45 compared with the normal medullary jawbone.
Discussion
CTU values in the positive Ti-Stim group versus the negative Ti-Stim group
The CTU values of the two Ti-Stim groups varied only slightly; the positive Ti-Stim group, with CTU values approximately 5% lower than those in the negative Ti-Stim group, also appeared to have decreased BIC (although these findings were not significant). That is, there were likely reduced CTU values, and hence also decreased BIC and osseointegration, in the positive Ti-Stim group. This finding is consistent with previous studies that have reported an increased risk of Ti-Impl failure in patients with positive Ti-Stim.50–52 The clinical relevance of these titanium polymorphisms (i.e., possible hypersensivity to titanium, see the Investigative methods section) is supported by reports that patients with high responder polymorphisms are at increased risk for titanium-associated inflammation and are susceptible to periprosthetic bone loss as the degree of inflammation increases.50–52
CTU values in the Cer-Impl group versus the total Ti-Impl cohort
The BIC of the Cer-Impl group (median 1.59 CTU) was more than twice that of the total Ti-Impl cohort (median 0.69 CTU), indicating that bone density was more than 100% greater in the Cer-Impl group. Together, our findings indicate a greater decrease in BIC and osseointegration in the positive Ti-Stim group than in the Cer-Impl group.
CCL5 expression in Ti-Impl groups
Previous research has indicated a tendency of patients with Ti-Impl to develop para-implant BMDJ, which has an impact on the local overexpression of CCL5, 46 with a median value of 2913 pg/mL; this finding is approximately equal to that of both Ti-Stim groups in the present study. Although the negative Ti-Stim group appeared to have less inflammatory reduction in BIC than the positive Ti-Stim group, this difference was not significant. Overall, however, CCL5 expression was approximately 20-fold higher in the Ti-Impl cohort compared with normal expression (149.9 pg/mL). This chronic proinflammatory CCL5 overexpression seemed to occur more frequently with Ti-Impl, independent of the Ti-Stim result.
The importance of CCL5 signaling in chronic immune diseases has been extensively discussed in the literature.53–57 Cases of BMDJ caused by fatty degeneration of the alveolar bone can function as chronic sources of proinflammatory CCL5 signaling, and have been documented in previous studies.58–60
Bone quality before implant insertion: risk assessments
Our comparison of the CTU threshold value for a healthy jawbone versus the CTU values for the regions surrounding Ti-Impl and Cer-Impl suggest that incomplete osseointegration occurs in both implant groups, regardless of the choice of implant material. Moreover, although physiologically unfavorable para-implant BMDJ occurred in both implant groups, the CTU values indicated a significantly lower BMDJ incidence in patients with Cer-Impl than in those with Ti-Impl. Given that impaired BIC (which is associated with osteoimmune dysregulation) also occurs with Cer-Impl, the metabolic and inflammatory status of the implant site prior to implantation likely plays a pivotal role in implant osseointegration. Together, our data indicate that the evaluation of pre-implant bone quality may be a key factor in achieving complete osseointegration. It is thus recommended that the bone quality of the implant site is assessed prior to implant placement using the more informative TAU method, to assess the risk of poor osseointegration and avoid adverse osteoimmunological outcomes. However, because pre-implant measurements of bone quality were unavailable for the implants investigated in the present study, our data does not provide any conclusions in this respect.
Summary
The research presented herein illustrates the transition from the disturbed physiology of a foreign body reaction of osseointegration into the pathology of silent chronic inflammation in dental implantology. The current study thus draws attention to a potential local cryptic process with possible systemic effects, in which incomplete BIC interacts osteoimmunologically via proinflammatory chemokines (CCL5). This cryptic process necessitates a broader perspective that takes into account the following considerations:
The Ti-Stim is an important step toward the detection of possible chronic immune dysregulation caused by Ti-Impl. However, the proportion of individuals that this applies to—even those in a state of severe immune dysregulation—is only approximately 20%. To avoid the mistaken retention of Ti-Impl solely on the basis of a negative Ti-Stim, a more refined diagnosis may be possible via bone density measurement using a TAU device and appropriate CTU staging.
CCL5 overexpression, with possible systemic effects as a result of released titanium particles, 48 is amplified by chronic osteoimmunological stimulation in areas of impaired BIC.
In the present study, TAU was used to visualize this hidden pathology in 37 patients with Ti-Impl to measure the density of the surrounding bone, which showed decreased CTU values. The staging of reduced bone density using objective criteria such as CTU—especially for the BIC area of existing implants—and associated evidence of chronic inflammation (i.e., reduced CTU values) provide a strong indication for the removal of suspicious implants.
The TAU device facilitates the evaluation of bone quality prior to implant placement. Given that the use of Cer-Impl does not necessarily rule out potentially impaired BIC and increased osteoimmune risks, the use of TAU before implantation (to determine alveolar bone metabolism) is important to ensure a successful outcome.
The correlation between laboratory values of CCL5 overexpression and corresponding CTU values precludes a fundamental error with respect to measurements made using the newly introduced TAU device and the staging of reduced bone density by reference to CTU.
Consistent with our findings of relatively reduced BIC surrounding Ti-Impl, researchers have reported obligate bone marrow edema in jaws adjacent to dental implants. 61 In this previous study, the magnetic resonance imaging signal intensity in the bone marrow areas of jaws with dental implants was significantly higher than in those without dental implants. Notably, this study also reported that bone marrow edema was observed in magnetic resonance imaging despite a lack of clinically abnormal findings, and that bone marrow edema was associated with dental implants.
The tension between immunological considerations, which tend to be undervalued, and the emphasis on mechanical success in dental implantology are often unappreciated. It is critical that the apparently successful placement of a firmly fixed implant is evaluated in light of immunologically relevant criteria. From this perspective, the present study presents three practical, scientifically validated, and easy-to-perform methods of investigation: blood testing to determine titanium sensitivity, CCL5 analyses, and the use of a newly developed TAU device to supplement radiographic imaging studies.
Study limitations
Relatively little data were obtained in the present study, and our selection of the measured alveolar implant environments was relatively arbitrary. This meant that we were unable draw strong conclusions regarding the objectives of this study. Therefore, the TAU ultrasound method for evaluating BMDJ and the CTU scaling method presented here remain under scientific investigation.
Conclusions
The aim of the present study was to evaluate BIC using diagnostic criteria and staging systems that are not currently used in clinical practice. In addition, we explored methods that may enhance diagnostic efficiency and the assessment of impaired BIC as well as potential osteoimmune dysregulation with possible systemic sequelae. Firm implant attachment, marginal mucositis, and peri-implantitis are readily amenable to diagnostic evaluation. However, this is not the case for the osteoimmune processes in the concealed area of para-implant osseointegration. To our knowledge, we are the first to demonstrate the following. First, hitherto-neglected reduced BIC and partially failed osseointegration was able to be detected by measuring the para-implant bone density with TAU. Second, measurements using TAU for Cer-Impl (using CTU values) were higher than those for Ti-Impl. Patients with Cer-Impl showed significantly improved long-term osseointegration compared with those with Ti-Impl. Third, the use of Cer-Impl instead of Ti-Impl does not fundamentally exclude the possibility of impaired osseointegration. Fourth, implantation in the osteoimmunologically uncompromised alveolar bone (as assessed using TAU) appears to be a key factor for the successful osseointegration and long-term osteoimmune sustainability of dental implants. Finally, our analyses of areas of incomplete BIC showed local overexpression of a single chemokine, CCL5, which may have systemic sequelae; for example, systemic inflammatory disorder. 16 Together, our findings suggest that the evaluation of bone density and bone metabolism in the selected alveolar region should be performed using TAU prior to dental implant placement, irrespective of the choice of implant material.
To our knowledge, this research adds a new diagnostic approach in view of the immunomodulatory effects of biomaterials that come into direct contact with body tissues. There are likely further complex mechanisms in dental implantology that have not yet been fully appreciated, given that osseointegration involves a continuous and dynamic host defense reaction. 62 The results of our combined diagnostic (Ti-Stim and CTU) and pathogenetic (CCL5) findings support the consistent use of Cer-Impl. Additional clinical and multicenter studies, particularly with respect to the stability of the assessed implants, are necessary to further validate the methods and statements presented in our study.
Acknowledgements
The authors thank all patients who participated in this study, as well as all the staff who contributed directly or indirectly to the study. The manuscript was edited, with additional translation from German to English, by Natasha Gabriel.
Footnotes
The TAU apparatus and associated software was provided without charge for the purposes of this study by CaviTAU® (Munich, Germany). The corresponding author (JL) is the holder of patents used in the TAU apparatus (CaviTAU®).
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
ORCID iD: Johann Lechner https://orcid.org/0000-0001-9800-0276
Author contributions
JL: conceptualized and developed the methodology and wrote the manuscript. VvB: generated the lab data, verified the results, and analyzed the bone samples using multiplex analysis. FN: performed the clinical validation of data and analytical calculations. FS: provided study materials, performed the TAU measurements of implants, and corrected the manuscript. We confirm that the manuscript has been read and approved by all named authors.
References
- 1.Derks J, Tomasi C. Peri-implant health and disease. A systematic review of current epidemiology. J Clin Periodontol 2015; 42: S158–S171. doi: 10.1111/jcpe.12334. [DOI] [PubMed] [Google Scholar]
- 2.Tabanella G, Nowzari H, Slots J. Clinical and microbiological determinants of ailing dental implants. Clin Implant Dent Relat Res 2009; 11: 24–36. doi: 10.1111/j.1708-8208.2008.00088.x. [DOI] [PubMed] [Google Scholar]
- 3.Kotsakis GA, Olmedo DG. Peri-implantitis is not periodontitis: scientific discoveries shed light on microbiome-biomaterial interactions that may determine disease phenotype. Periodontol 2000 2021; 86: 231–240. doi: 10.1111/prd.12372. [DOI] [PubMed] [Google Scholar]
- 4.Safioti LM, Kotsakis GA, Pozhitkov AE, et al. Increased levels of dissolved titanium are associated with peri-implantitis - a cross-sectional study. J Periodontol 2017; 88: 436–442. doi: 10.1902/jop.2016.160524. [DOI] [PubMed] [Google Scholar]
- 5.Nemec M, Behm C, Maierhofer V, et al. Effect of titanium and zirconia nanoparticles on human gingival mesenchymal stromal cells . Int J Mol Sci 2022; 23: 10022. doi: 10.3390/ijms231710022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Albrektsson T, Brånemark PI, Hansson HA, et al. Osseointegrated titanium implants. Requirements for ensuring a long-lasting, direct bone-to-implant anchorage in man. Acta Orthop Scand 1981; 52: 155–170. doi: 10.3109/17453678108991776. [DOI] [PubMed] [Google Scholar]
- 7.Trindade R ,Albrektsson T, Tengvall P, et al. Foreign body reaction to biomaterials: on mechanism s for buildup and breakdown of osseointegration. Clin Implant Dent Relat Res 2014; 18: 192–203. doi: 10.1111/cid.12274. [DOI] [PubMed] [Google Scholar]
- 8.Walsh MC, Kim N, Kadono Y, et al. Osteoimmunology: interplay between the immune system and bone metabolism. Annu Rev Immunol 2006; 24: 33–63. doi:10.1146/annurev.immunol.24.021605.090646. [DOI] [PubMed] [Google Scholar]
- 9.Lorenzo J, Horowitz M, Choi Y. Osteoimmunology: interactions of the bone and immune system. Endocr Rev 2008; 29: 403–440. doi:10.1210/er.2007-0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li Z, Sheng P, Li C, et al. Osteoimmunology in bone regeneration. Biomed Res Int 2020; 2020: 6297356. doi: 10.1155/2020/6297356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Takayanagi H. Osteoimmunology: shared mechanisms and crosstalk between the immune and bone systems. Nat Rev Immunol 2007; 7: 292–304. doi:10.1038/nri2062. [DOI] [PubMed] [Google Scholar]
- 12.Von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med 2007; 147: 573–577. doi: 10.1016/j.jclinepi.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 13.Yang SO, Park JM, Son HJ, et al. Osteonecrosis. In: Yang SO, Oh SW, Choi YY, Ryu JS. (eds) Atlas of Nuclear Medicine in Musculoskeletal System. Singapore: Springer, 2022, pp.83–91. doi: 10.1007/978-981-19-2677-8_8. [Google Scholar]
- 14.Dörner T, Haas J, Loddenkemper C, et al. Implant-related inflammatory arthritis. Nat Clin Pract Rheumatol 2006; 2: 53–56. doi: 10.1038/ncprheum0087. [DOI] [PubMed] [Google Scholar]
- 15.Cox C. Bead-based multiplex immunoassays: procedures, tips, and tricks. Methods Mol Biol 2021; 2261: 263–276. doi: 10.1007/978-1-0716-1186-9_16. [DOI] [PubMed] [Google Scholar]
- 16.Lechner J, Von Baehr V. RANTES and fibroblast growth factor 2 in jawbone cavitations: triggers for systemic disease? Int J Gen Med 2013; 6: 277–290. doi: 10.2147/IJGM.S43852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jansson H, Hamberg K, De Bruyn H, et al. Clinical consequences of IL-1 genotype on early implant failures in patients under periodontal maintenance. Clin Implant Dent Relat Res 2005; 7: 51–59. doi: 10.1111/j.1708-8208.2005.tb00047.x. [DOI] [PubMed] [Google Scholar]
- 18.Laine M, Leonhardt A, Roos-Jansåker AM, et al. IL-1RN gene polymorphism is associated with peri-implantitis. Clin Oral Implants Res 2006; 17: 380–385. doi: 10.1111/j.1600-0501.2006.01249.x. [DOI] [PubMed] [Google Scholar]
- 19.Montes CC, Alvim-Pereira F, De Castilhos BB, et al. Analysis of the association of IL1B (C+3954T) and IL1RN (intron 2) polymorphisms with dental implant loss in a Brazilian population. Clin Oral Implants Res 2009; 20: 208. doi: 10.1111/j.1600-0501.2008.01629.x. [DOI] [PubMed] [Google Scholar]
- 20.Lechner J. Validation of dental X-ray by cytokine RANTES – comparison of X-ray findings with cytokine overexpression in jawbone. Clin Cosmet Investig Dent 2014; 6: 71–79. doi: 10.2147/CCIDE.S69807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bouquot J, Martin W, Wrobleski G. Computer-based thru-transmission sonography (CTS) imaging of ischemic osteonecrosis of the jaws – a preliminary investigation of 6 cadaver jaws and 15 pain patients. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2001; 92: 550. [Google Scholar]
- 22.Lechner J, Zimmermann B, Schmidt M, et al. Ultrasound sonography to detect focal osteoporotic jawbone marrow defects: clinical comparative report with corresponding Hounsfield units and RANTES/CCL5 expression. Clin Cosmet Investig Dent 2020; 12: 205–216. doi: 10.2147/CCIDE.S247345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Al-Nawas B, Grotz KA, Kann P. Ultrasound transmission velocity of the irradiated jaw bone in vivo. Clin Oral Investig 2001; 5: 266–268. doi: 10.1007/s00784-001-0133-4. [DOI] [PubMed] [Google Scholar]
- 24.Klein MO, Grotz KA, Manefeld B, et al. Ultrasound transmission velocity for non-invasive evaluation of jaw bone quality in vivo prior to dental implantation. Ultrasound Med Biol 2008; 34: 1966–1971. doi: 10.1016/j.ultrasmedbio.2008.04.016. [DOI] [PubMed] [Google Scholar]
- 25.Lechner J, Zimmermann B, Schmidt M. Focal bone-marrow defects in the jawbone determined by ultrasonography—validation of new Trans-alveolar ultrasound technique for measuring jawbone density in 210 participants. Ultrasound Med Biol 2021; 47: 3135–3146. doi: 10.1016/j.ultrasmedbio.2021.07.012. [DOI] [PubMed] [Google Scholar]
- 26.Tadinada A, Jalali E, Jadhav A, et al. Artifacts in cone beam computed tomography image volumes: an illustrative depiction. J Mass Dent Soc 2015; 64: 12–15. [PubMed] [Google Scholar]
- 27.Schulze RKW, Berndt D, D'Hoedt B. On cone-beam computed tomography artifacts induced by titanium implants. Clin Oral Implants Res 2010; 21: 100–107. doi: 10.1111/j.1600-0501.2009.01817.x. [DOI] [PubMed] [Google Scholar]
- 28.Schulze R, Heil U, Gross D, et al. Artefacts in CBCT: a review. Dentomaxillofac Radiol 2011; 40: 265–273. doi: 10.1259/dmfr/30642039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sancho-Puchades M, Hämmerle CH, Benic GI. In vitro assessment of artifacts induced by titanium, titanium-zirconium and zirconium dioxide implants in cone-beam computed tomography. Clin Oral Implants Res 2015; 26: 1222–1228. doi: 10.1111/clr.12438. [DOI] [PubMed] [Google Scholar]
- 30.Albrektsson T, Chrcanovic B, Jacobsson M, et al. Osseointegration of implants– a biological and clinical overview. JSM Dent Surg 2017; 2: 1022. [Google Scholar]
- 31.Balkwill FR, Burke F. The cytokine network. Immunol Today 1989; 10: 299–304. doi: 10.1016/0167-5699(89)90085-6. [DOI] [PubMed] [Google Scholar]
- 32.Oishi Y, Manabe I. Macrophages in inflammation, repair and regeneration. Int Immunol 2018; 30: 511–528. doi: 10.1093/intimm/dxy054. [DOI] [PubMed] [Google Scholar]
- 33.Tsukasaki M, Takayanagi H. Osteoimmunology: evolving concepts in bone-immune interactions in health and disease. Nat Rev Immunol 2019; 19: 626–642. doi: 10.1038/s41577-019-0178-8. [DOI] [PubMed] [Google Scholar]
- 34. Deutsche Akkreditierungsstelle GmbH; accredited to DIN EN ISO/IEC 17025:2005 and DIN. En ISO 2007; 15189. [Google Scholar]
- 35.Lechner J, Von Baehr V, Schick F. RANTES/CCL5 signaling from Jawbone cavitations to epistemology of multiple sclerosis – research and case studies. Degener Neurol Neuromuscul Dis 2021; 11: 41–50. doi: 10.2147/DNND.S315321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lechner J, Schulz T, Lejeune B, et al. Jawbone cavitation expressed RANTES/CCL5: case studies linking silent inflammation in the jawbone with epistemology of breast cancer. Breast Cancer (Dove Med Press) 2021; 13: 225–240. doi: 10.2147/BCTT.S295488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brånemark PI, Breine U, Adell R, et al. Intraosseous anchorage of dental prostheses. I. Experimental findings. Scand J Plast Reconstr Surg 1969; 3: 81–100. doi: 10.3109/02844316909036699. [DOI] [PubMed] [Google Scholar]
- 38.Masaki C, Schneider GB, Zaharias R, et al. Effects of implant surface microtopography on osteoblast gene expression. Clin Oral Implants Res 2005; 16: 650–656. doi: 10.1111/j.1600-0501.2005.01170.x. [DOI] [PubMed] [Google Scholar]
- 39.Wennerberg A, Albrektsson T. Effects of titanium surface topography on bone integration: a systematic review. Clin Oral Implants Res 2009; 20: 172–184. doi: 10.1111/j.1600-0501.2009.01775.x. [DOI] [PubMed] [Google Scholar]
- 40.Guizzardi S, Galli C, Martini D, et al. Different titanium surface treatment influences human mandibular osteoblast response. J Periodontol 2004; 75: 273–282. doi: 10.1902/jop.2004.75.2.273. [DOI] [PubMed] [Google Scholar]
- 41.Franchi M, Bacchelli B, Giavaresi G, et al. Influence of different implant surfaces on peri-implant osteogenesis: histomorphometric analysis in sheep. J Periodontol 2007; 78: 879–888. doi: 10.1902/jop.2007.060280. [DOI] [PubMed] [Google Scholar]
- 42.Slaets E, Carmeliet G, Naert I, et al. Early trabecular bone healing around titanium implants: a histologic study in rabbits. J Periodontol 2007; 78: 510–517. doi: 10.1902/jop.2007.060183. [DOI] [PubMed] [Google Scholar]
- 43.Berglundh T, Abrahamsson I, Lang NP, et al. De novo alveolar bone formation adjacent to endosseous implants: a model study in the dog. Clin Oral Implants Res 2003; 14: 251–262. doi: 10.1034/j.1600-0501.2003.00972.x. [DOI] [PubMed] [Google Scholar]
- 44.Linder L, Carlsson A, Marsal L, et al. Clinical aspects of osseointegration in joint replacement. A histological study of titanium implants. J Bone Joint Surg Br 1988; 70: 550–555. doi: 10.1302/0301-620X.70B4.3403596. [DOI] [PubMed] [Google Scholar]
- 45.Sennerby L, Thomsen P, Ericson LE. Early tissue response to titanium implants inserted in rabbit cortical bone. Part I. Light microscopic observations. J Mater Sci: Mater Med 1993; 4: 240–250. doi: 10.1007/BF00122275. [Google Scholar]
- 46.Zarb GA, Albrektsson T. Nature of implant attachments. In: Branemark PI, Zarb GA, Albrektsson T. (eds) Tissue-Integrated Prostheses: Osseointegration in Clinical Dentistry. Chicago: Quintessence Publishing Co, 1985, pp.88–98. [Google Scholar]
- 47.Zarb GA, Albrektsson T. Osseointegration: a requiem for periodontal ligament? Int J Periodontal Restor Dent 1991; 11: 88–91. [Google Scholar]
- 48.Lichtinger TK, Müller RT, Schürmann N, et al. Osseointegration of titanium implants by addition of recombinant bone morphogenetic protein 2 (rhBMP-2). Mat-Wiss u Werkstofftech 2001; 32: 937–941. doi: 10.1002/1521-4052. [Google Scholar]
- 49.Lechner J, Noumbissi S, Von Baehr V. Titanium implants and silent inflammation in jawbone – a critical interplay of dissolved titanium particles and cytokines TNF-a and RANTES/CCL5 on overall health? EPMA Journal 2018; 9: 331–343. doi: 10.1007/s13167-018-0138-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gruica B, Wang HY, Lang NP, et al. Impact of IL-1 genotype and smoking status on the prognosis if osseointegrated implants. Clin Oral Implants Res 2004; 15: 393–400. doi: 10.1111/j.1600-0501.2004.01026.x. [DOI] [PubMed] [Google Scholar]
- 51.Jacobi-Gresser E, Huesker K, Schütt S. Genetic and immunological markers predict titanium implant failure: a retrospective study. Int J Oral Maxillofac Surg 2013; 42: 537. doi: 10.1016/j.ijom.2012.07.018. [DOI] [PubMed] [Google Scholar]
- 52.Stolzer C, Müller M, Gosau M, et al. Do titanium dioxide particles stimulate macrophages to release proinflammatory cytokines and increase the risk for peri-implantitis? J Oral Maxillofac Surg 2023; 81: 308–317. doi: 10.1016/j.joms.2022.10.019. [DOI] [PubMed] [Google Scholar]
- 53.Soria G, Ben-Baruch A. The inflammatory chemokines CCL2 and CCL5 in breast cancer. Cancer Lett 2008; 267: 271–285. doi: 10.1016/j.canlet.2008.03.018. [DOI] [PubMed] [Google Scholar]
- 54.Eichbaum C, Meyer AS, Wang N, et al. Breast cancer cell-derived cytokines, macrophages and cell adhesion: implications for metastasis. Anticancer Res 2011; 31: 3219–3228. [PubMed] [Google Scholar]
- 55.Zischek C, Niess H, Ischenko I, et al. Targeted stem cell based RANTES/TK suicide gene-therapy in a murine pancreatic cancer tumour model. Deutsche Gesellschaft Für Chirurgie 2009; 38: 3–5. doi: 10.1007/978-3-642-00625-8_2 [Google Scholar]
- 56.Ransohoff RM. The chemokine system in neuroinflammation: an update. J Infect Dis 2002; 186: S152–S156. doi: 10.1086/344266. [DOI] [PubMed] [Google Scholar]
- 57.Ubogu E, Callahan M, Tucky B, et al. Determinants of CCL5-driven mononuclear cell migration across the blood–brain barrier. Implications for therapeutically modulating neuroinflammation. J Neuroimmunol 2006; 179: 132–144. doi: 10.1016/j.jneuroim.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 58.Schick F, Lechner J, Notter F. Linking dentistry and chronic inflammatory autoimmune diseases – can oral and Jawbone stressors affect systemic symptoms of atopic dermatitis? A case report. Int Med Case Rep J 2022; 15: 323–338. doi: 10.2147/IMCRJ.S367434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lechner J, Schmidt M, Von Baehr V, et al. Undetected jawbone marrow defects as inflammatory and degenerative signaling pathways: chemokine RANTES/CCL5 as a possible link between the jawbone and systemic interactions? J Inflamm Res 2021; 14: 1603–1612. doi: 10.2147/JIR.S307635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Feloutzis A, Lang NP, Tonetti MS, et al. IL-1 gene polymorphism and smoking as risk factors for peri-implant bone loss in a well-maintained population. Clin Oral Implants Res 2003; 14: 10–17. doi: 10.1034/j.1600-0501.2003.140102.x. [DOI] [PubMed] [Google Scholar]
- 61.Muraoka H, Hirahara N, Ito K, et al. Dental implants: a potential cause of bone marrow edema in the jaw—preliminary report. Int J Implant Dent 2021; 7: 25. doi: 10.1186/s40729-021-00306-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Albrektsson T, Tengvall P, Amengual L, et al. Osteoimmune regulation underlies oral implant osseointegration and its perturbation. Front Immunol 2023; 13: 1056914. doi:10.3389/fimmu1056914. [DOI] [PMC free article] [PubMed] [Google Scholar]