Abstract
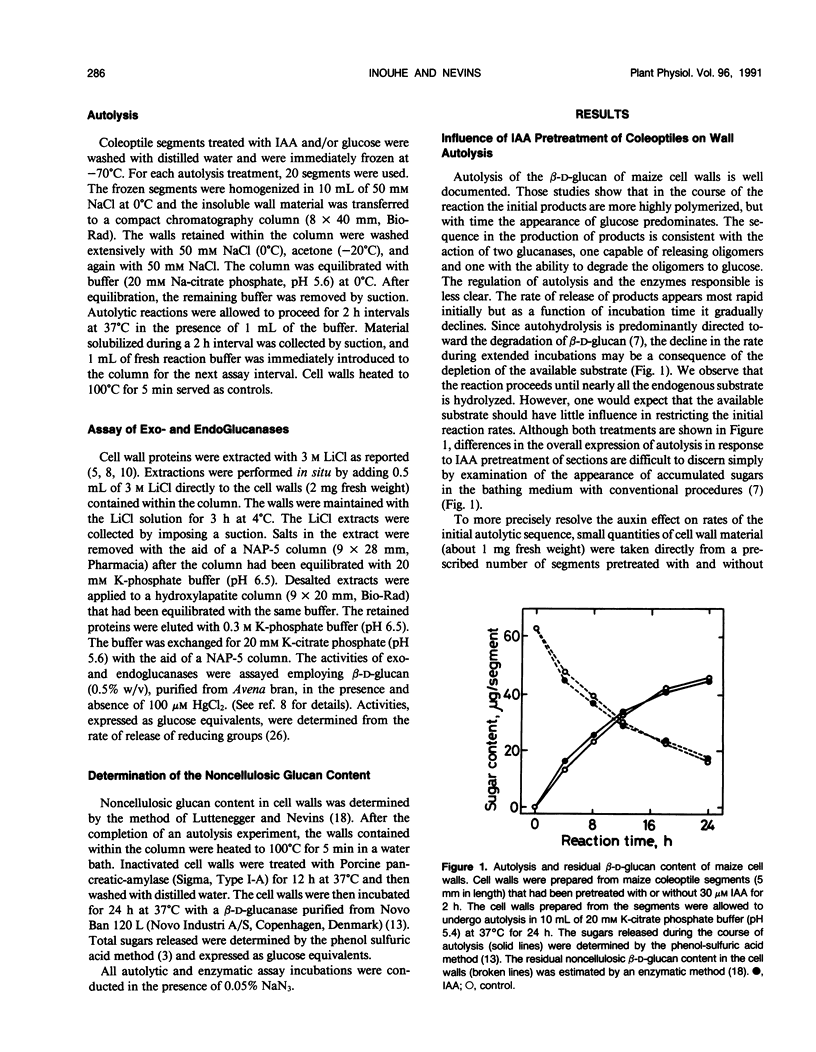
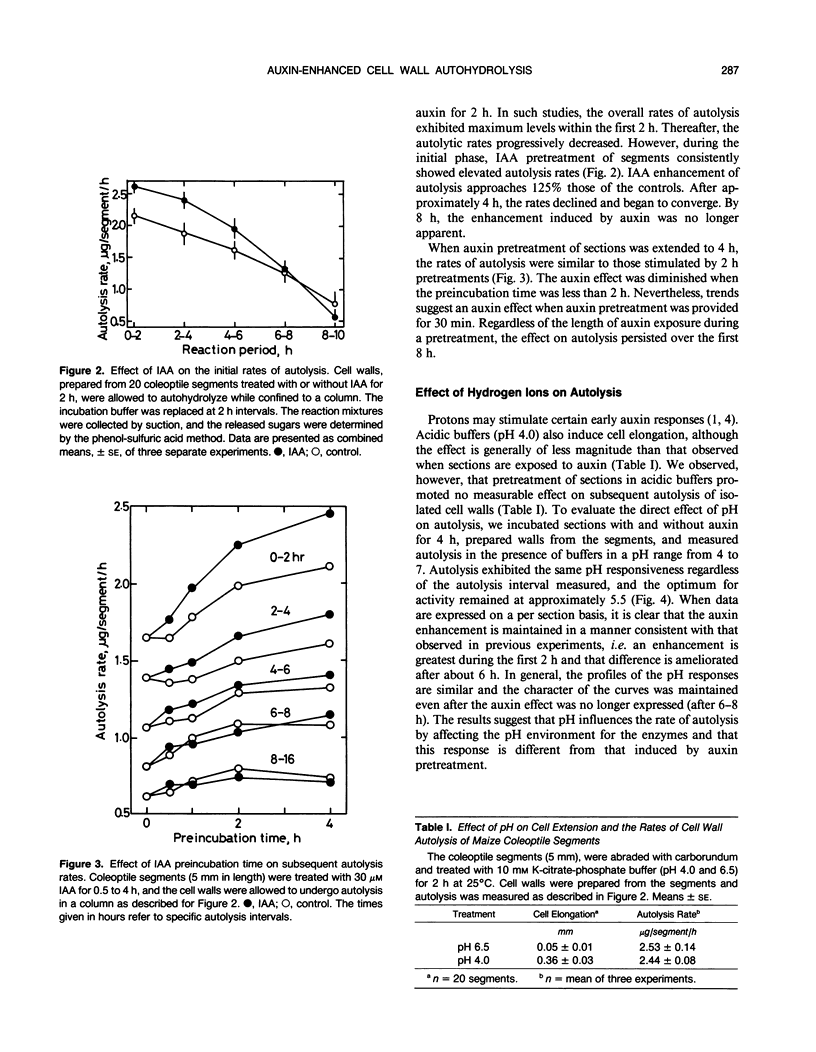
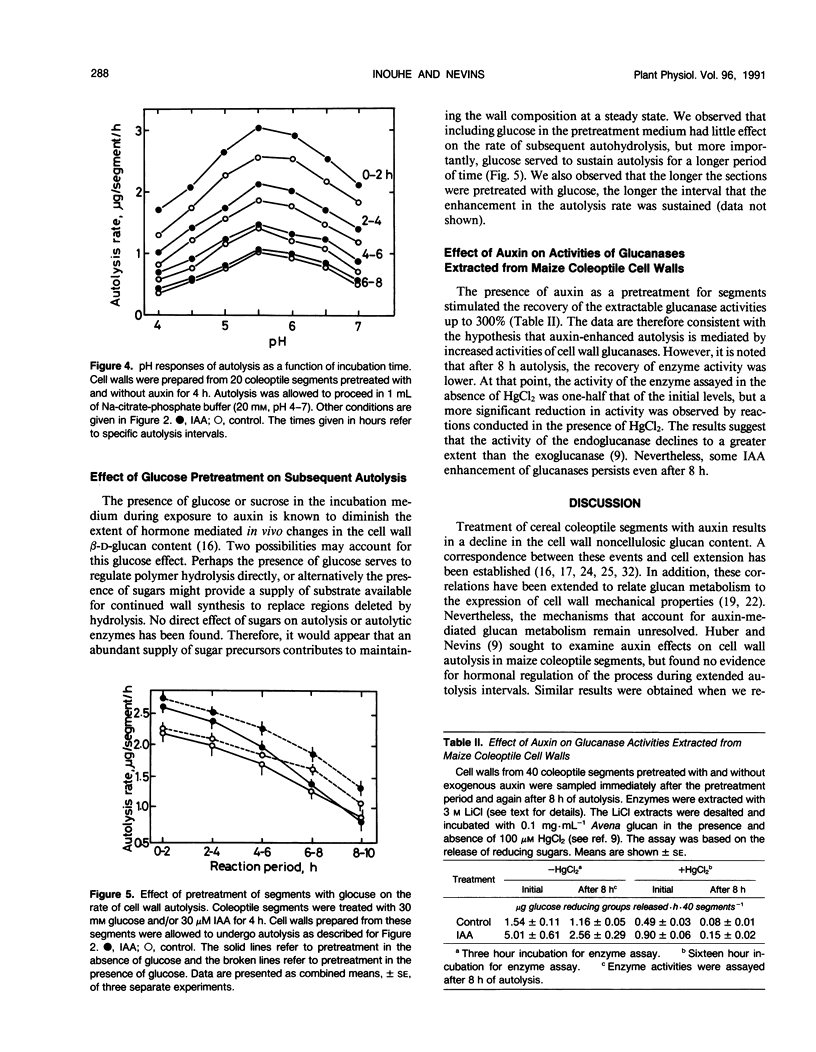
Cell walls isolated from auxin-pretreated maize (Zea mays L.) coleoptile segments were assayed to disclose evidence for the existence of enhanced autolysis. To improve the sensitivity of the measurements and to facilitate kinetic analysis, isolated cell walls were consolidated within a small column, and the autolysis rate was directly determined from the sugar content of the effluent. This protocol revealed that the maximum rate of autohydrolysis of walls prepared from segments occurs within the first 2 hours and a steady decline commences almost immediately. Walls from indoleacetic acid pretreated segments (0.5-4 hours) released sugar at a higher rate initially (110-125% of controls) and the enhanced rate of autolysis continued for 6 to 8 hours, but then it became equivalent to that of the controls. Pretreatment of the segments at acidic pH had no effect on the measurable rates of autolysis. The (1→3), (1→4)-β-d-glucan content of the walls and the extractable glucanase activities support the hypothesis that temporal enhancement of autohydrolysis is a function of auxin on enzyme activity. The progressive decline in autolysis during prolonged incubations is consistent with the decrease in the quantity of the β-d-glucan in the wall. The relationship between glucan content and autolysis rate is supported by the observation that while glucose pretreatment of segments had only a small effect on initial autolysis rates, the presence of the sugar during pretreatment served to extend the interval over which higher rates of autolysis could be sustained. The results demonstrate that autolysis is related to auxin-induced wall metabolism in maize coleoptiles.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Hoson T., Nevins D. J. beta-d-Glucan Antibodies Inhibit Auxin-Induced Cell Elongation and Changes in the Cell Wall of Zea Coleoptile Segments. Plant Physiol. 1989 Aug;90(4):1353–1358. doi: 10.1104/pp.90.4.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber D. J., Nevins D. J. beta-d-Glucan Hydrolase Activity in Zea Coleoptile Cell Walls. Plant Physiol. 1980 May;65(5):768–773. doi: 10.1104/pp.65.5.768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson K. D., Daniels D., Dowler M. J., Rayle D. L. Activation of Avena coleoptile cell wall glycosidases by hydrogen ions and auxin. Plant Physiol. 1974 Feb;53(2):224–228. doi: 10.1104/pp.53.2.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato Y., Nevins D. J. Enzymic Dissociation of Zea Shoot Cell Wall Polysaccharides : II. Dissociation of (1 --> 3),(1 --> 4)-beta-d-Glucan by Purified (1 --> 3),(1 --> 4)-beta-d-Glucan 4-Glucanohydrolase from Bacillus subtilis. Plant Physiol. 1984 Jul;75(3):745–752. doi: 10.1104/pp.75.3.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz M., Ordin L. A cell wall polysaccharide-hydrolyzing enzyme system in Avena sativa L. coleoptiles. Biochim Biophys Acta. 1967 Jun 13;141(1):126–134. doi: 10.1016/0304-4165(67)90251-6. [DOI] [PubMed] [Google Scholar]
- Labavitch J. M., Ray P. M. Relationship between Promotion of Xyloglucan Metabolism and Induction of Elongation by Indoleacetic Acid. Plant Physiol. 1974 Oct;54(4):499–502. doi: 10.1104/pp.54.4.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loescher W. H., Nevins D. J. Turgor-dependent Changes in Avena Coleoptile Cell Wall Composition. Plant Physiol. 1973 Sep;52(3):248–251. doi: 10.1104/pp.52.3.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loescher W., Nevins D. J. Auxin-induced Changes in Avena Coleoptile Cell Wall Composition. Plant Physiol. 1972 Nov;50(5):556–563. doi: 10.1104/pp.50.5.556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luttenegger D. G., Nevins D. J. Transient Nature of a (1 --> 3), (1 --> 4)-beta-d-Glucan in Zea mays Coleoptile Cell Walls. Plant Physiol. 1985 Jan;77(1):175–178. doi: 10.1104/pp.77.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda Y., Yamamoto R. Effect of auxin on beta-1, 3-glucanase activity in Avena coleoptile. Dev Growth Differ. 1970 Mar;11(4):287–296. doi: 10.1111/j.1440-169x.1970.00287.x. [DOI] [PubMed] [Google Scholar]
- SMOGYI M. Notes on sugar determination. J Biol Chem. 1952 Mar;195(1):19–23. [PubMed] [Google Scholar]


