Abstract
Background and Aims:
Acute kidney injury (AKI) in cirrhosis is morbid, but the incidence rates of different etiologies of AKI are not well described in United States patients. We compared incidence rates, practice patterns, and outcomes across etiologies of AKI in cirrhosis.
Methods:
Retrospective cohort study of 11 hospital networks of consecutive adult patients admitted in 2019 with AKI and cirrhosis. Etiology of AKI was adjudicated based on pre-specified clinical definitions (prerenal/hypovolemic AKI, hepatorenal syndrome [HRS-AKI], acute tubular necrosis [ATN], other).
Results:
2,063 patients were included (median age 62 [IQR 54, 69] years, 38.3% female, median MELD-Na score 26 [19, 31]). The most common AKI etiology was prerenal AKI (44.3%), followed by ATN (30.4%) and HRS-AKI (12.1%); 6.0% had other AKI, and 7.2% were unable to classify. 8.1% patients received a liver transplant, 36.5% died by 90-days. Patients with prerenal AKI had the lowest rate of death (22.2%; p <0.001) whereas patients with HRS-AKI and ATN were higher, but not significantly different from each other (49.0% vs. 52.7%; p = 0.42). Using prerenal AKI as reference, the adjusted sHR for 90-day mortality was higher for HRS-AKI (sHR 2.78 [95% CI 2.18–3.54]; p <0.001) and ATN (sHR 2.83 [2.36–3.41]; p <0.001). In adjusted analysis, higher AKI stage and lack of complete response to treatment was associated with an increased risk of 90-day mortality (p <0.001 for all).
Conclusion:
AKI is a severe complication of cirrhosis. HRS-AKI is uncommon and has similar outcomes to ATN. Etiology of AKI, AKI stage/severity, and non-response to treatment were associated with mortality. Further optimization of vasoconstrictors for HRS-AKI and supportive therapies for ATN are needed.
Keywords: liver failure, acute on chronic liver failure, renal failure, liver transplant, vasoconstrictor
Graphical Abstract
Introduction
Acute kidney injury (AKI) is a common complication of cirrhosis and acute-on-chronic liver failure (ACLF) and is associated with high morbidity and mortality.[1, 2] Treatment for AKI in cirrhosis is based on its etiology. Hypovolemic or pre-renal AKI is treated with volume resuscitation, hepatorenal syndrome (HRS-AKI) can be reversed with intravenous (IV) albumin infusion and splanchnic vasoconstrictors, and acute tubular necrosis (ATN) is generally managed supportively.[3] Earlier treatment of AKI is associated with greater chance of reversal of kidney injury and decreased mortality.[4, 5] Therefore, prompt and accurate identification of AKI severity (i.e., AKI stage based on relative change in serum creatinine [SCr]) and etiology is vital to optimize outcomes.
Current data on the incidence, outcomes, and practice patterns in United States (U.S.) patients with AKI and cirrhosis are limited to single center studies,[6, 7] which may be hard to generalize, or payer databases, which are larger but have incomplete clinical data.[8–10] As a result, application and analysis of the impact of current clinical guidelines are largely extrapolated from non-U.S. centers, where there are different standards of care and allocation systems for liver transplantation (LT) compared to the U.S. For example, terlipressin (a splanchnic-specific V1a-receptor agonist) is routinely used as the first-line vasoconstrictor for treatment of HRS-AKI in numerous countries but has only just been approved in the U.S. and is not yet in wide clinical use there.[3, 4, 11] A more comprehensive database of U.S. patients with AKI and cirrhosis is needed to characterize the demographics and natural history of this population, and establish a baseline to determine how terlipressin’s impending adoption into practice will affect outcomes.
In this manuscript, we provide a comprehensive and granular analysis of a large, consecutive series of patients across multiple academic centers to identify current practice patterns, define the incidence of AKI etiologies in cirrhosis at-large, and examine the scope of this problem as it affects U.S. patients.
Methods
Study Population and Setting
Consecutive adult patients age >18 years hospitalized with AKI and cirrhosis between January 1, 2019 and December 31, 2019 were eligible for this retrospective analysis. Patients from 11 different hospital network systems (11 LT centers, 15 total hospitals) were included (see Table S1 for a list of sites). 2019 was chosen as a complete calendar year to provide up-to-date secular trends while avoiding obfuscation by the coronavirus-19 pandemic.
Potential patients were identified via local centralized clinical data warehouse tools using a validated list of International Code of Disease version 10 (ICD-10) codes for cirrhosis (see Table S2).[12] Patients with at least one cirrhosis ICD-10 code and an inpatient SCr >1.5 mg/dL proceeded to a manual chart review to determine the accuracy of the diagnostic codes for cirrhosis and AKI (see Definitions). SCr >1.5 mg/dL was chosen as an AKI threshold given its previous validation as a significant determinant of prognosis and diagnostic relevance in this population.[13] Patients were excluded if they had liver disease without cirrhosis, did not meet criteria for AKI (e.g., SCr >1.5 mg/dL due to chronic kidney disease), had a prior liver or kidney transplant, were on renal replacement therapy (RRT) at the time of admission, had AKI only after LT, or had incomplete clinical data. For patients with repeat admissions with AKI during the study period, only their first admission with AKI was included. Similarly, if a patient had recurrent episodes of AKI during the index hospitalization, only the first episode was considered. All data were obtained via review of electronic health records, United Network for Organ Sharing (UNOS) records, and if needed, by search of online obituaries and/or the Social-Security Death-Index.
Definitions
The etiology of cirrhosis was determined based on the clinical determination of the treating hepatologist and cirrhosis was confirmed by radiologic evidence, presence of complications related to cirrhosis, liver biopsy (if available) and/or endoscopic evidence of portal hypertension. Patients were classified as “listed” for LT if they were active on the UNOS waiting list during the index admission. AKI was defined using the 2015 International Club of Ascites (ICA)-AKI criteria, which uses relative change in SCr to define AKI stages.[3, 14, 15] Outpatient baseline SCr was recorded as the most recent outpatient value within 1-year of index admission. The relative difference between peak pre-transplant SCr, discharge SCr, and outpatient baseline SCr (where available) were used to stage AKI during admission.
Etiology of AKI was divided into 4 categories based on a priori definitions derived from previous literature[7, 16] and agreed upon by the principal investigators at each study site. AKI was considered (1) prerenal or hypovolemic if clinical history was consistent with volume depletion and there was improvement in AKI stage within 48-hours of volume resuscitation; (2) HRS-AKI if the patient met 2015 ICA HRS-AKI definition;[3] (3) ATN if clinical history was consistent with ischemic or nephrotoxic AKI and did not improve with volume resuscitation; and (4) other AKI, which included causes such as interstitial nephritis, glomerulonephritis, obstructive nephropathy, or abdominal compartment syndrome. Each patient had two independent adjudicators to agree on etiology of AKI, with a third adjudicator providing a tie-breaking diagnosis, if needed. Patients were deemed “unable to be classified” if there was disagreement between three adjudicators or there was insufficient clinical information available to determine etiology of AKI.
De-novo chronic kidney disease (CKD) was defined by Kidney Disease Improving Global Outcomes as the persistence of eGFR <60 ml/min per 1.73 m2 for ≥3 months from the time of AKI event in patients who did not have pre-existing CKD prior to admission. [17] The Chronic Kidney Disease Epidemiology Collaboration equation was chosen to calculate eGFR. [18]
Data Collection and Management
Study data were collected and managed using REDCap electronic data capture tools hosted at each participating institution using a standard variable template across all sites.[19] Laboratory values were recorded at the time of admission unless otherwise noted. Model for End Stage Liver Disease-Sodium (MELD-Na) score and Chronic Liver Failure-Consortium (CLIF-C) ACLF score were calculated at the time of admission.[20–22] Patients were followed for 90 days from their date of admission for death, transplant, readmission, and the need for RRT. Vasoconstrictors were categorized into two categories: (1) those used for the treatment of HRS-AKI (i.e., midodrine and octreotide, norepinephrine) and (2) vasopressors used for shock (i.e., vasopressin, norepinephrine, epinephrine) in the intensive care unit (ICU).
Outcomes and Statistical Analysis
The primary outcome for this study was 90-day mortality. Secondary outcome was rate of AKI response at discharge (defined as per the ICA guidelines, where a complete response denoted an improvement in SCr to within 0.3 mg/dL of baseline, partial response denoted improvement in at least 1 AKI stage with SCr > 0.3 mg/dL above baseline, and overall response combines complete and partial response).[3] The main exposure of interest was etiology of AKI as a predictor of outcomes, with devoted 3-way modeling comparing prerenal AKI, HRS-AKI, and ATN. Survival curves for outcomes were estimated by AKI etiology, AKI stage, and response to therapy using Kaplan Meier method and compared using log-rank test. Patients who demonstrated improvement with volume resuscitation (and thus were classified as prerenal AKI) but later suffered worsening of SCr by discharge were captured as AKI nonresponders. Univariate data were compared using Chi-square, Fisher exact, Student’s t-test, or Wilcoxon rank sum testing, as appropriate. Pre-specified multivariable regression models were used to evaluate the association between exposure variables and outcomes using an empiric scientific selection process[23] adjusting for age, race, gender, transplant listing status, and MELD-Na score, using Fine and Gray analysis to account for competing risks of transplant.[24] Sensitivity analyses were performed using alternative models, including a previously derived model from the European literature,[7] multivariable model using variables from a proportional hazards stepwise selection algorithm (p <0.1 for entry, p <0.05 for final selection), stratified analysis by transplant listing status, and removal of patients with disagreement between adjudicators of etiology of AKI. All models were adjusted for study center as a categorical variable. All covariates used in multivariable models had <3.0% missing data, these values were imputed using single imputation methodology. Continuous data were presented as median (interquartile range). SAS version 9.4 (Cary, NC) was used for analysis and R studio version 1.4 (Vienna, Austria) was used to generate figures.
Ethics
This study was approved by each site’s institutional review board. Each site abides by the guidelines set forth by the Declaration of Helsinki and Istanbul. The need for informed consent was waived.
Results
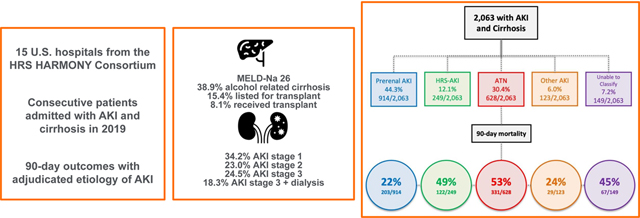
Of 6,596 patients screened, 2,063 met inclusion criteria and were included for analysis. (Figure S1). The median age was 62 [54, 69] years and the majority had ascites (77.9%). The median MELD-Na and CLIF-C ACLF scores were 26.0 [19.0, 31.0] and 48.8 [42.4, 55.7], respectively. Alcohol was the most common etiology of cirrhosis (38.9%); 14.7% met National Institute on Alcohol Abuse and Alcoholism criteria for alcohol-associated hepatitis.[25] 30.3% had documented CKD (eGFR <60 mL/min) at least 3 months prior to admission. 15.4% were listed for LT, 46.7% required ICU admission and the median inpatient length of stay was 9 [4, 16] days.
Patient Demographics and Clinical Characteristics
Comparison of patient demographics and clinical characteristics by etiology of AKI are presented in Table 1. 44.3% had prerenal AKI, 30.4% ATN, 12.1% HRS-AKI, 6.0% other AKI, and 7.2% were unable to be classified (Figure 1). 1844/2063 (89%) had full agreement between adjudicators. Among all patients, 34.2% had AKI stage I, 23.0% had AKI stage II, 24.5% had AKI stage III without RRT, and 18.3% had stage III requiring RRT. Comparisons of AKI etiology stratified by AKI stages can be found in Table S3. Patients with AKI stages 1 and 2 were more likely to have to prerenal AKI, 64.1% and 53.5%, respectively, whereas patients with stage 3 AKI were more likely to have ATN (47.5%) and HRS-AKI (17.5%). When stratified by ACLF grade, prerenal AKI was the most common etiology in ACLF grades 0, 1, and 2 (69.6%, 45.3%, and 47.1%, respectively), whereas ATN was the most common in ACLF grade 3 (41.5%, p <0.001; Table S4). HRS-AKI was uncommon across all ACLF grades, but the proportion was highest in ACLF grade 3 (13.7%) compared to those with ACLF grade 0 (2.4%).
Table 1:
Demographics and clinical characteristics by etiology of acute kidney injury
| All Patients (n = 2063) |
Prerenal AKI (n = 914) |
Hepatorenal Syndrome (n = 249) |
Acute Tubular Necrosis (n = 628) |
P Value | Other AKI (n = 123) |
Unable to Classify (n = 149) |
|
|---|---|---|---|---|---|---|---|
| Age (years) | 62 [54, 69] | 62 [55, 70] | 61 [53, 66] | 61 [51, 68] | <0.001 | 65 [57, 71.5] | 61 [52,68] |
| Female sex (%) | 790 (38.3) | 334 (36.6) | 99 (39.8) | 250 (39.8) | 0.38 | 50 (40.7) | 57 (38.3) |
| White race (%) | 1676 (81.2) | 761 (83.3) | 212 (85.1) | 494 (78.7) | 0.03 | 96 (78.0) | 113 (75.8) |
| Hispanic ethnicity (%) | 180 (8.7) | 90 (9.8) | 24 (9.4) | 38 (6.0) | 0.05 | 9 (7.3) | 19 (12.7) |
| Etiology of cirrhosis (%) | <0.001 | ||||||
| Alcohol | 803 (38.9) | 337 (36.9) | 112 (45.0) | 260 (41.4) | 33 (26.8) | 61 (40.9) | |
| Hepatitis C | 233 (11.3) | 114 (12.5) | 25 (10.0) | 63 (10.0) | 16 (13.0) | 15 (10.1) | |
| Non-alcoholic steatohepatitis | 444 (21.5) | 201 (22.0) | 71 (28.5) | 112 (17.8) | 27 (22.0) | 33 (22.1) | |
| Multifactorial | 191 (9.3) | 94 (10.3) | 14 (5.6) | 61 (9.7) | 13 (10.6) | 9 (6.0) | |
| Other | 392 (19.0) | 168 (18.4) | 27 (10.8) | 132 (21.0) | 34 (27.6) | 31 (20.8) | |
| Complications of cirrhosis (%) | |||||||
| Ascites | 1607 (77.9) | 643 (70.4) | 242 (97.2)*** | 512 (81.5) | <0.001 | 89 (72.4) | 121 (81.2) |
| Encephalopathy | 1212 (58.8) | 497 (54.4) | 174 (69.9) | 405 (64.6) | <0.001 | 45 (36.6) | 91 (61.1) |
| Gastrointestinal bleeding | 712 (34.5) | 303 (33.2) | 80 (32.1) | 253 (40.4) | 0.007 | 33 (26.8) | 43 (28.9) |
| Spontaneous bacterial peritonitis | 289 (14.0) | 111 (12.2) | 50 (20.1) | 102 (16.3) | 0.003 | 10 (8.2) | 16 (10.7) |
| Hepatocellular carcinoma | 236 (11.4) | 106 (11.6) | 33 (13.3) | 68 (10.8) | 0.60 | 11 (8.9) | 18 (12.1) |
| Characteristics of admission | |||||||
| Admission mean arterial pressure (mmHg) | 69.0 [60.7, 77.0] | 70.0 [62.3, 78.0] | 69.0 [61.3, 76.0] | 64.8 [56.7, 74.0] | <0.001 | 73.3 [66.0, 82.3] | 71.7 [64.3, 79.3] |
| Vasoconstrictors for HRS (%) | 788 (38.2) | 196 (21.5) | 192 (77.1) | 307 (49.0) | <0.001 | 25 (20.3) | 68 (45.6) |
| Vasopressors for shock (%) | 614 (29.8) | 139 (15.2) | 83 (33.6)**** | 331 (52.7) | <0.001 | 23 (18.7) | 38 (25.5) |
| Albumin given during admission (%) | 1370 (66.5) | 503 (55.1) | 235 (94.8) | 480 (76.7) | <0.001 | 54 (43.9) | 98 (65.8) |
| Total albumin given during admission in g | 122 [75, 175] | 101 [50, 175] | 144 [100, 200] | 100 [63, 175] | <0.001 | 125 [75, 175] | 100 [50, 184] |
| Intensive care admission (%) | 963 (46.7) | 267 (29.2) | 132 (53.2) | 464 (74.0) | <0.001 | 39 (31.7) | 61 (40.9) |
| Mechanical ventilation (%) | 551 (26.7) | 130 (14.2) | 68 (27.2) | 304 (48.4) | <0.001 | 18 (14.6) | 31 (20.9) |
| Renal replacement therapy (%) | 374 (18.2) | 25 (2.7) | 65 (26.1) | 234 (37.3) | <0.001 | 8 (6.5) | 42 (28.4) |
| Medications prior to admission (%) | |||||||
| Loop diuretic | 1295 (62.8) | 621 (68.0) | 155 (62.2) | 357 (56.9) | <0.001 | 74 (60.2) | 88 (59.1) |
| Mineralocorticoid receptor antagonist | 951 (46.2) | 466 (51.2) | 116 (46.6) | 256 (40.8) | 0.001 | 57 (46.3) | 56 (37.6) |
| Beta blockers | 831 (40.3) | 415 (45.4) | 75 (30.1) | 238 (37.9) | <0.001 | 55 (44.7) | 48 (32.2) |
| MELD-Na score | 26 [19, 31] | 24 [18, 29] | 30 [25, 34] | 28 [21, 34] | <0.001 | 22 [17, 26] | 26 [19, 34] |
| CLIF-C ACLF score | 48.8 [42.4, 55.7] | 45.3 [40.5, 51.2] | 51.3 [45.0, 56.2] | 54.0 [47.3, 60.9] | <0.001 | 47.2 [40.1, 51.7] | 49.1 [41.2, 57.4] |
| Laboratory values | |||||||
| Sodium (mEq/L) | 134 [130, 138] | 135 [131, 138] | 131 [127, 136] | 134 [130, 138] | <0.001 | 135 [131, 139] | 135 [129, 139] |
| Outpatient baseline creatinine* (mg/dL) | 1.10 [0.87, 1.37] | 1.11 [0.90, 1.35] | 1.10 [0.90, 1.45] | 1.05 [0.79, 1.32] | 0.01 | 1.24 [0.99, 1.49] | 1.11 [0.86, 1.50] |
| Admission creatinine (mg/dL) | 1.84 [1.50, 2.48] | 1.84 [1.50, 2.48] | 2.32 [1.67, 3.26] | 2.02 [1.34, 3.27] | <0.001 | 1.86 [1.52, 2.64] | 2.10 [1.37, 3.33] |
| Peak creatinine (mg/dL) | 2.55 [1.93, 3.66] | 2.08 [1.72, 2.70] | 3.36 [2.42, 4.58] | 3.24 [2.34, 4.71] | <0.001 | 2.43 [1.90, 3.30] | 2.94 [2.12, 4.60] |
| White blood count (K/uL) | 8.6 [6.0, 12.9] | 8.0 [5.6, 11.6] | 9.6 [6.1, 14.3] | 9.8 [6.6, 14.7] | <0.001 | 7.3 [5.1, 10.2] | 8.7 [6.1, 12.9] |
| Hematocrit (%) | 29.6 [25.0, 34.9] | 30.9 [25.7 36.3] | 28.4 [23.9, 32.2] | 28.9 [24.3, 33.7] | <0.001 | 31.0 [27.5, 35.7] | 28.2 [24.6, 33.4] |
| Platelets (K/uL) | 114 [73, 172] | 116 [76, 177] | 106 [70, 153] | 112 [69, 168] | 0.05 | 141 [85, 191] | 122 [75, 164] |
| Albumin (g/dL) | 2.90 [2.50, 3.60] | 3.00 [2.60, 3.60] | 2.80 [2.40, 3.20] | 2.80 [2.30, 3.20] | <0.001 | 3.10 [2.70, 3.40] | 2.90 [2.50, 3.40] |
| International normalized ratio (INR) | 1.60 [1.30, 2.10] | 1.50 [1.21, 1.90] | 1.70 [1.40, 2.22] | 1.80 [1.40, 2.50] | <0.001 | 1.40 [1.20, 1.88] | 1.60 [1.30, 2.10] |
| Total bilirubin (mg/dL) | 2.6 [1.1, 7.0] | 2.0 [0.9, 4.4] | 4.2 [1.8, 11.5] | 3.5 [1.6, 11.0] | <0.001 | 1.4 [0.7, 2.5] | 3.2 [1.2, 9.0] |
| Fractional excretion of sodium** (%) | 0.35 [0.17, 0.91] | 0.33 [0.13, 0.91] | 0.25 [0.17, 0.53] | 0.41 [0.17, 1.18] | 0.001 | 0.60 [0.19, 1.99] | 0.39 [0.14, 0.94] |
Key: AKI (acute kidney injury), MELD-Na (Model for End Stage Liver Disease-Sodium), CLIF-C ACLF (CLIF Consortium Organ Failure Acute on Chronic Liver Failure Score). MELD and CLIF-C ACLF score were taken at hospital admission. All laboratory values were taken at admission unless otherwise noted.
Continuous variables given as median [interquartile range]. P values represent a three-way comparison between prerenal AKI, hepatorenal syndrome, and ATN, calculated by Chi square (categorical variables), Student’s t test (parametric continuous variables) or Wilcoxon rank sum (non-parametric continuous variables).
Available in 1700 patients.
Available in 1149 patients.
Presence of ascites is required for the diagnosis of HRS-AKI. Complications of cirrhosis were recorded at the time of admission, and thus, some patients were found to have ascites after admission, but before the diagnosis of HRS-AKI.
Patients with HRS-AKI may have developed shock requiring vasopressors after the diagnosis of HRS-AKI was made.
Figure 1: Distribution and outcomes of patients by etiology of acute kidney injury.
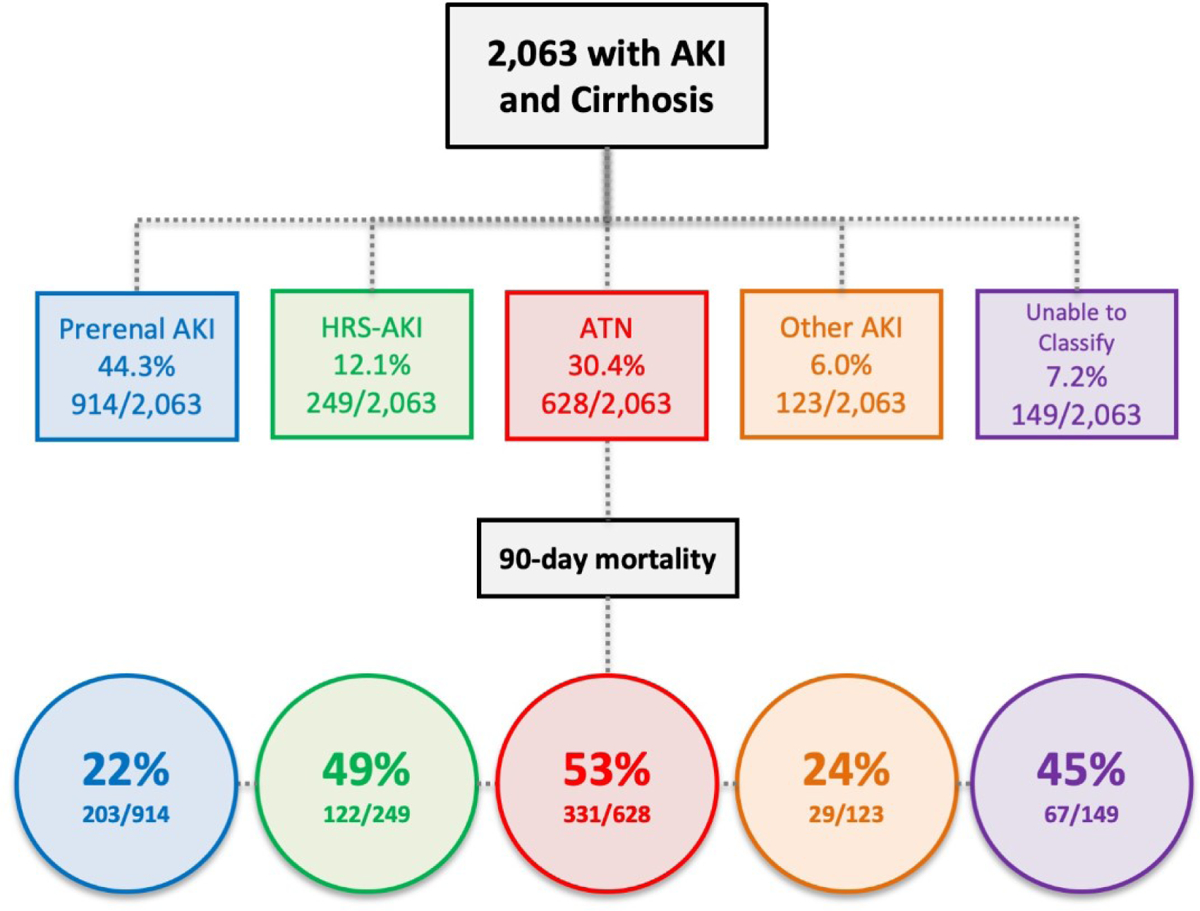
Key: AKI (acute kidney injury), HRS (hepatorenal syndrome), ATN (acute tubular necrosis)
Overall, 66.5% patients were treated with IV albumin during their admission. Patients with HRS-AKI received more IV albumin (144 [100, 200] g) compared to prerenal AKI (101 [50, 175] g) and ATN (100 [63, 175] g; p <0.001). Vasoconstrictors for HRS were used most in HRS-AKI (77.1%) compared to prerenal AKI (21.5%) and ATN (49.0%; p <0.001). Vasopressors for shock were used most commonly in ATN (52.7%) compared to prerenal AKI (14.2%) and HRS-AKI (33.6%; p <0.001). For the latter, vasopressor use occurred after the diagnosis of HRS-AKI was made. RRT was most common in patients with ATN (62.6%), followed by HRS-AKI (17.4%), unclassifiable (11.3%), prerenal AKI (6.7%) and other AKI (1.9%; p <0.001).
Etiology of AKI and 90-Day Mortality
Patient characteristics and significant univariate predictors of 90-day mortality are displayed in Table 2. Patients who died had significantly higher MELD-Na (p <0.001) and CLIF-C (p <0.001) scores compared to patients who were alive. Correspondingly, patients who died also had higher rates of ICU admission, vasopressor use for shock, and mechanical ventilation (p <0.001 for all). In the entire cohort, in-hospital, 30, 60, and 90-day mortality were 19.3%, 24.7%, 32.5%, and 36.5%, respectively. Loss to follow-up rates were low at each time point: 0%, 7.7%, 9.8%, and 11.4%, respectively.
Table 2:
Patient characteristics by vital status at 90 days
| > | Alive (n = 1311) |
Dead (n = 752) |
P value |
|---|---|---|---|
| Age (years) | 62 [54, 69] | 62 [53, 69] | 0.97 |
| Female sex (%) | 496 (37.9) | 294 (39.1) | 0.61 |
| White race (%) | 1071 (81.7) | 605 (80.5) | 0.52 |
| Hispanic ethnicity (%) | 132 (10.1) | 46 (6.1) | 0.003 |
| Complications of cirrhosis (%) | |||
| Ascites | 962 (73.4) | 645 (85.8) | <0.001 |
| Encephalopathy | 686 (52.4) | 526 (69.9) | <0.001 |
| Spontaneous bacterial peritonitis | 168 (12.9) | 121 (16.1) | 0.05 |
| Hepatocellular carcinoma | 118 (9.0) | 118 (15.7) | <0.001 |
| Alcohol-associated hepatitis | 177 (13.5) | 126 (16.8) | 0.05 |
| Characteristics of admission | |||
| Beta blocker prior to admission (%) | 582 (44.4) | 249 (33.1) | <0.001 |
| Admission mean arterial pressure (mmHg) | 70.0 [62.3, 78.0] | 66.3 [57.7, 74.3] | <0.001 |
| Vasoconstrictors for hepatorenal syndrome (%) | 402 (30.7) | 386 (51.3) | <0.001 |
| Vasopressors for shock (%) | 268 (20.5) | 346 (46.0) | <0.001 |
| Intensive care admission (%) | 469 (35.8) | 494 (65.8) | <0.001 |
| Mechanical ventilation (%) | 225 (17.2) | 326 (43.5) | <0.001 |
| Renal replacement therapy (%) | 155 (11.5) | 223 (29.7) | <0.001 |
| Received liver transplant (%) | 165 (12.6) | 2 (0.3) | <0.001 |
| MELD-Na score | 24 [18, 30] | 28 [23, 34] | <0.001 |
| CLIF-C ACLF score | 46.0 [40.5, 52.5] | 53.4 [47.5, 59.6] | <0.001 |
| Laboratory values | |||
| Sodium (mEq/L) | 135 [131, 138] | 134 [129, 138] | 0.001 |
| Outpatient baseline creatinine* (mg/dL) | 1.14 [0.90, 1.40] | 1.00 [0.80, 1.30] | <0.001 |
| Admission creatinine (mg/dL) | 1.92 [1.51, 2.70] | 2.00 [1.41, 3.03] | 0.29 |
| Peak creatinine (mg/dL) | 2.33 [1.81, 3.34] | 2.99 [2.22, 4.25] | <0.001 |
| White blood count (K/uL) | 8.1 [5.7, 12.1] | 9.6 [6.6, 14.3] | <0.001 |
| Hematocrit (%) | 30.5 [25.3, 35.6] | 28.7 [24.4, 33.4] | <0.001 |
| Albumin (g/dL) | 3.0 [2.6, 3.5] | 2.7 [2.3, 3.2] | <0.001 |
| International normalized ratio (INR) | 1.50 [1.21, 2.00] | 1.80 [1.50, 2.50] | <0.001 |
| Total bilirubin (mg/dL) | 2.0 [0.9, 4.8] | 4.2 [1.9, 10.8] | <0.001 |
| Fractional excretion of sodium** (%) | 0.38 [0.17, 1.13] | 0.31 [0.16, 0.85] | 0.03 |
Key: AKI (acute kidney injury), MELD-Na (Model for End Stage Liver Disease-Sodium), CLIF-C ACLF (CLIF Consortium Organ Failure Acute on Chronic Liver Failure Score). All laboratory values were taken at admission unless otherwise noted. Continuous variables given as median [interquartile range]. P values calculated by Chi square (categorical variables), Student’s t test (parametric continuous variables) or Wilcoxon rank sum (non-parametric continuous variables).
Available in 1713 patients.
Available in 1149 patients.
In unadjusted analysis, etiology of AKI was associated with 90-day mortality (Figure 2; p <0.001). Absolute rates of mortality at 30, 60, and 90-days by etiology of AKI are presented in Table 3. In pairwise analysis, patients with prerenal AKI had the lowest rate of mortality at 90 days (22.2%; p <0.001) whereas patients with HRS-AKI and ATN were not significantly different from each other (49.0% vs. 52.7%; p = 0.42). In multivariable regression analysis, using prerenal AKI as reference, the adjusted sHR for 90-day mortality was higher for HRS-AKI (sHR 2.78 [95% CI 2.18–3.54]; p <0.001) and ATN (sHR 2.83 [2.36–3.41]; p <0.001). Similar results were found in sensitivity analysis using alternative multivariable models, which can be found in Table S5.
Figure 2: Ninety-day survival probability by etiology of acute kidney injury.
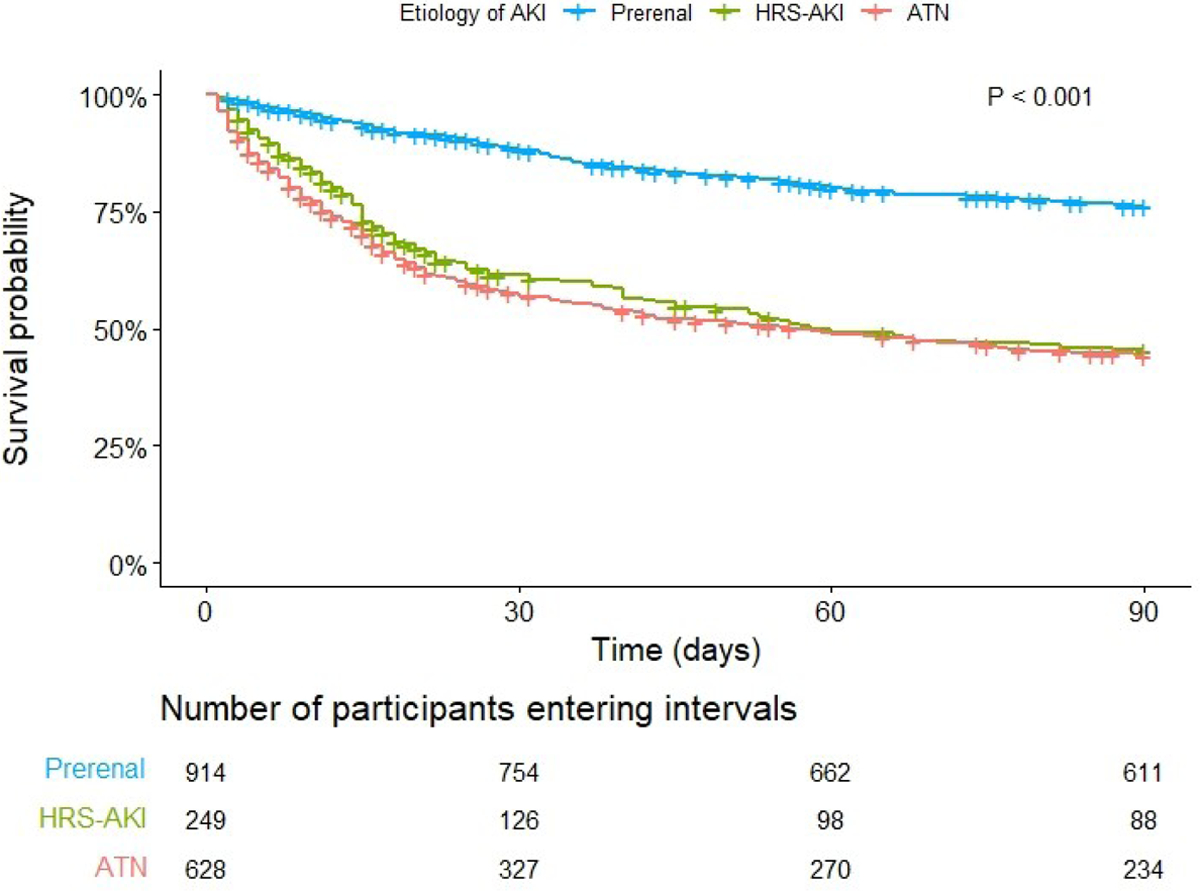
Key: AKI (acute kidney injury, HRS (hepatorenal syndrome), ATN (acute tubular necrosis). P <0.001 (Log rank)
Table 3:
Rates of mortality while in hospital and at 30, 60, and 90-days by etiology of acute kidney injury
| In Hospital | 30-Day | 60-Day | 90-Day | Sub-Hazard Ratio (95% CI) | P value | |
|---|---|---|---|---|---|---|
| Type of AKI | ||||||
| Prerenal AKI | 6.1% | 10.9% | 18.6% | 22.2% | Reference | --- |
| Hepatorenal syndrome | 24.5% | 36.1% | 45.4% | 49.0% | 2.78 2.18–3.54) | <0.001 |
| Acute tubular necrosis | 37.6% | 40.9% | 48.4% | 52.7% | 2.83 (2.36–3.41) | <0.001 |
| Other | 6.5% | 10.6% | 20.3% | 23.6% | 1.04 (0.71–1.51) | 0.85 |
| Unable to classify | 26.2% | 33.6% | 39.6% | 45.0% | 2.33 (1.75–3.09) | <0.001 |
| AKI Stage | ||||||
| Stage I | 5.1% | 11.0% | 17.9% | 21.7% | Reference | --- |
| Stage II | 11.3% | 18.9% | 28.0% | 31.6% | 1.36 (1.08–1.71) | 0.008 |
| Stage III without renal replacement therapy | 22.6% | 32.3% | 39.3% | 43.7% | 1.71 (1.38–2.13) | <0.001 |
| Stage III with renal replacement therapy | 51.1% | 46.5% | 55.6% | 59.6% | 3.63 (2.86–4.61) | <0.001 |
| AKI Response | ||||||
| Complete response | 2.2% | 5.7% | 13.1% | 17.3% | Reference | --- |
| Partial response | 11.3% | 19.3% | 29.3% | 34.3% | 2.04 (1.59–2.61) | <0.001 |
| No response | 38.5% | 44.7% | 52.3% | 55.6% | 4.85 (4.03–5.84) | <0.001 |
| Transplant Listing Status | ||||||
| Listed for liver transplant | 9.6% | 8.5% | 14.5% | 16.7% | Reference | --- |
| Not listed for liver transplant | 21.2% | 27.7% | 35.8% | 40.1% | 4.04 (3.00–5.45) | <0.001 |
Key: AKI (acute kidney injury), HRS (hepatorenal syndrome), ATN (acute tubular necrosis). Absolute rates of death at in hospital, 30, 60, and 90 days represent unadjusted mortality rates at their respective intervals. Sub-Hazard ratios and P values are derived from multivariable models using Fine and Gray analysis for 90-day mortality, adjusted for age, race, gender, transplant listing status, center, and MELD-Na score.
Associations between AKI Stage, Response to Therapy, and 90-Day Mortality
Compared to stage I AKI, stage II and III AKI were associated with an increased risk of 90-day mortality in unadjusted (Figure 3; p <0.001) and adjusted analyses (stage II sHR 1.36 [1.08–1.71], p = 0.008; stage III without dialysis sHR 1.71 [1.38–2.13], p <0.001; stage III with dialysis sHR 3.63 [2.86–4.61], p <0.001); Table 3).
Figure 3: Ninety-day survival probability by acute kidney injury stage.
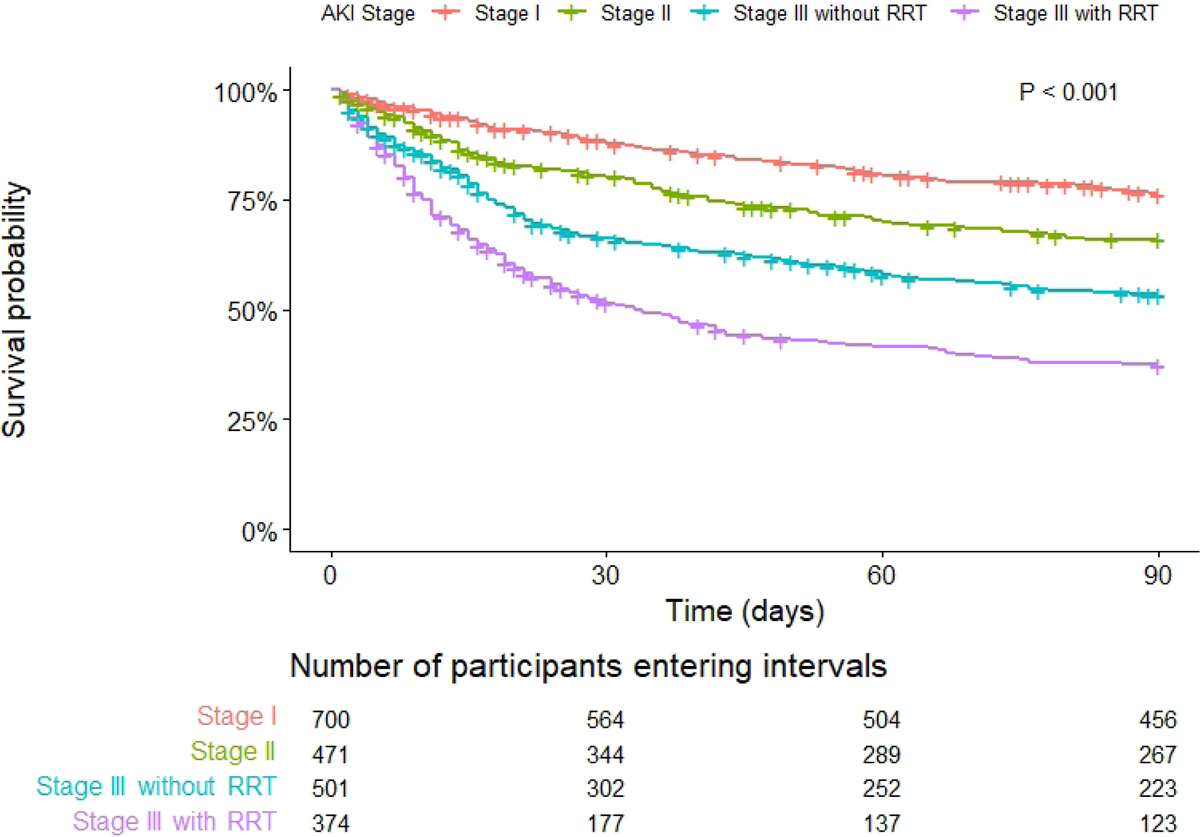
Key: AKI (acute kidney injury), RRT (renal replacement therapy). P <0.001 (Log rank)
Factors associated with overall AKI response are presented in Table S6. 41.8% patients had complete response, 14.5% had partial response, and 42.8% had no response. Rates of overall response varied by etiology of AKI (76.1% prerenal AKI, 36.5% HRS-AKI, 39.8% ATN, 64.2% other AKI, 31.5% unable to classify, p <0.001). Patients who experienced complete response of their AKI had the lowest adjusted risk of 90-day mortality compared to those with partial response (sHR 2.04 [1.59–2.61], p <0.001) and those with no response (sHR 4.85 [4.03–5.84], p <0.001); Table 3 and Figure 4). Factors associated with overall AKI response among patients treated with vasoconstrictors (n = 788), are presented in Table S7.
Figure 4: Ninety-day survival probability by acute kidney injury response.
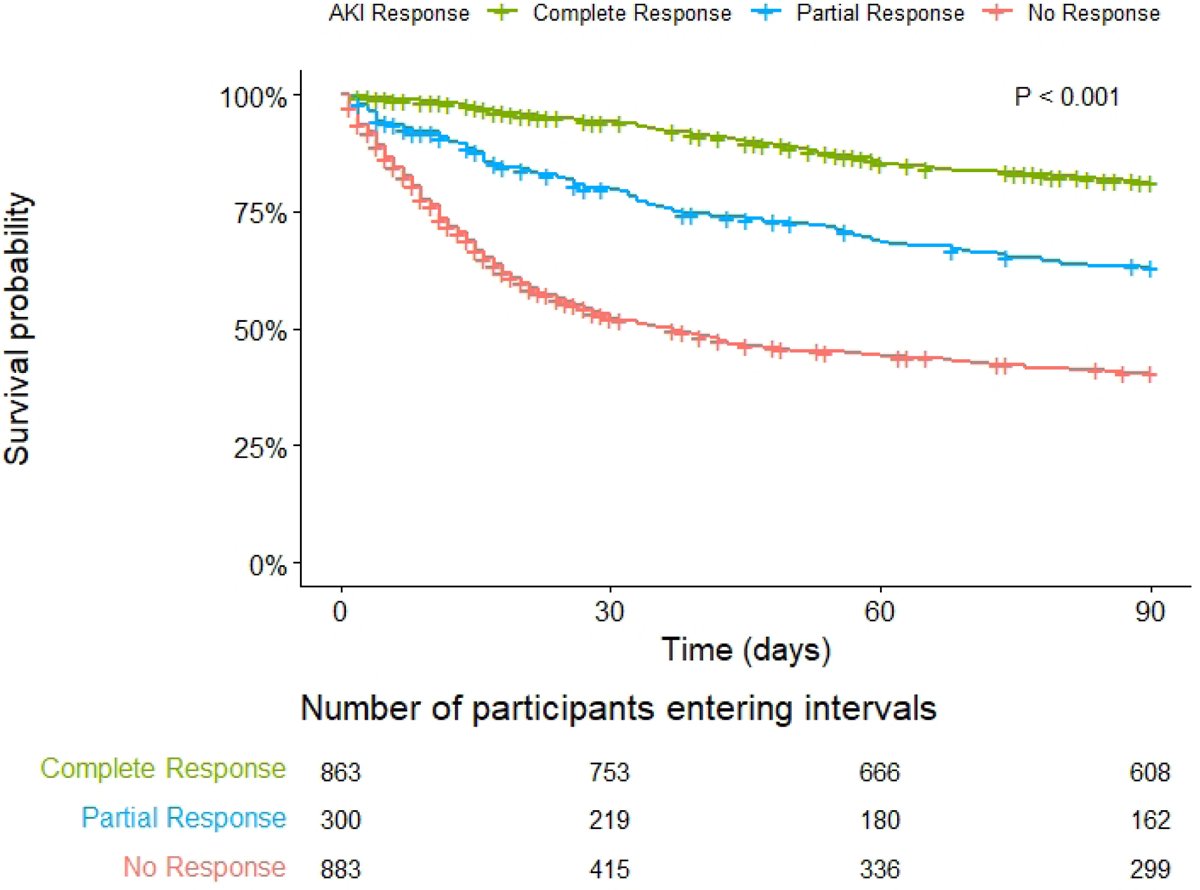
Key: AKI (acute kidney injury). P <0.001 (Log rank)
Complete response requires an improvement in SCr to within 0.3 mg/dL of baseline, partial response requires improvement in at least 1 AKI stage
Outcomes by Liver Transplant Status
8.1% of patients received a LT by 90 days (including 1.3% who received a simultaneous kidney transplant [SLKT]). Prerenal AKI was the most common etiology of AKI among patients who received a LT alone (39.2%) and SLKT (46.2%), followed by HRS-AKI (28.6% and 26.9%, respectively), then ATN (23.6% and 23.1% respectively). Patients not listed for transplant had a higher absolute risk of 90-day mortality compared to listed patients (40.1% vs. 16.7%, sHR 4.04 [3.00–5.45], p <0.001; Table 3). When examining etiology of AKI as a risk factor for death in stratified adjusted analysis, HRS-AKI and ATN had increased risk of 90-day mortality compared to prerenal AKI among 1,746 non-listed patients, but only ATN was associated with death among 317 listed patients (Table S5). When examining AKI response among the 167 patients who received a LT, complete response occurred in 35.9%, partial response in 10.8%, no response in 53.5% (including 20.3% who required RRT).
Other Outcomes
90-day survival probability by AKI etiology in patients without ascites and with ascites is visualized in Figure S2 and S3, respectively. We observed similar trends in mortality in both groups, with the ascites subgroup demonstrating higher absolute mortality relative to the group without ascites. 90-days after discharge, 58/157 (36.9%) patients who were at risk developed de-novo CKD after their index AKI episode. Rates of de-novo CKD were similar across etiologies of AKI (prerenal AKI 31.2%, HRS-AKI 52.6%, ATN 38.4%, other AKI 50.0%, unable to classify 42.9%; p = 0.34).
Discussion
In this study, we report the largest, and first consecutive, fully adjudicated cohort study of patients hospitalized with AKI and cirrhosis in a U.S. population, using the updated 2015 HRS-AKI criteria. We demonstrate distinguishing characteristics of the three main etiologies of AKI in this population – prerenal/hypovolemic AKI, HRS-AKI, and ATN. While etiology of AKI clearly influences practices patterns and outcomes, the overall short-term mortality between HRS-AKI and ATN was similar.
Our manuscript corroborates the incidence rates and mortality trends of HRS-AKI seen in other epidemiology studies. A recent analysis by Singal et al. of 2016–2019 National Inpatient Sample admissions demonstrated similar incidence of HRS (16.5% of admissions for AKI and cirrhosis using billing codes) to our study (12.1% fully adjudicated cases).[26] They also found nearly identical inhospital mortality rate to ours (24.5% vs. 25.8%). While billing data is not able to capture prerenal AKI or ATN reliably in this population, these findings suggest that the code for HRS provides a reasonable approximation for incidence of HRS-AKI. Singal’s paper also notes a decrease in mortality over time in HRS. Our 90-day survival in HRS-AKI (51%) was better than historic 90-day survival rates from the European literature (15%), which likely reflects overall improvements in patient care as well as changes to HRS definitions across eras.[7]
One notable aspect of current practice patterns in our study was the variability in the use of IV albumin and HRS vasoconstrictors across different etiologies of AKI. Although guidelines recommend an IV albumin challenge on presentation for all AKI and cirrhosis, only 67% of all patients received it during their admission, with HRS-AKI at the highest rate (95%) and total volume (144 grams). It is difficult to determine why up to 1/3 of patients did not receive IV albumin. Some patients may have received it prior to admission (in the case of hospital transfer to a study center), some may have received other colloids like blood or crystalloids in its place, and some treating teams may have felt it not to be appropriate based on clinical examination. However, it is also likely that guidelines for treating AKI in cirrhosis have not been fully embraced across centers, suggesting a need for ongoing education on management of this population.[27] This opportunity may also extend to discontinuation of potential precipitant medications to prevent AKI, such as diuretics and beta blockers, which were prescribed at high rates across all patients. Similarly, while patients with HRS-AKI received HRS vasoconstrictors at the highest rate (77%), patients with pre-renal AKI (22%) and ATN (49%) received them regularly as well, despite not being subgroups where one would expect benefit nor where they are guideline-recommended. Interestingly, HRS vasoconstrictor use (which was predominantly midodrine and octreotide, as terlipressin was not yet available in the U.S. during this study period), was associated with worse survival, highlighting the ineffectiveness of off-label vasoconstrictors in current U.S. practice. We would caution against overinterpretation of this finding, as this is mere association, rather than a causal link. We did not record dose, time to initiation, or duration of these medications, thus providers may have been prescribing HRS vasoconstrictors as a “salvage therapy” after a patient’s kidney function had already declined beyond the point of reversal. Importantly, multiple guidelines recommend terlipressin as the first-line splanchnic vasoconstrictor for HRS-AKI,[3, 28–30] and given terlipressin’s recent FDA approval,[11] it is likely to become a prominent therapeutic option for HRS-AKI.[4] However, terlipressin must be used judiciously given its side effect profile (most notably, ischemia and respiratory failure).[31–33] It should only be prescribed in confirmed cases of HRS-AKI where its benefits outweigh its risks. Further study is needed to determine whether novel biomarkers may aid in establishing an HRS-AKI diagnosis and/or the likelihood of responding to vasoconstrictors.[16, 34–36]
We identified several risks factors for poor outcomes, many of which recapitulate findings in the literature and support that established clinical predictors of mortality remain excellent prognostic tools in AKI and cirrhosis. First, common prognostic models of liver disease (and their individual components), such as MELD-Na and CLIF-ACLF score, were strong predictors of survival.[20–22] Similarly, markers of critical illness and/or advanced liver disease (admission to the ICU, intubation, advanced encephalopathy, hepatocellular carcinoma), were also strongly associated with mortality.[7, 37–39] Peak AKI stage and response to treatment also correlate linearly with survival, emphasizing the importance of early identification and intervention to reverse AKI in this population.[4, 5, 32] Patients requiring RRT had the highest mortality (60%) compared to AKI without RRT (stage I 22%, stage II 32%, stage III 44%), though interestingly, 90-day mortality for those requiring RRT was lower than some previous studies (63–80%).[38, 40, 41] Given the growing body of literature of poor outcomes among non-LT candidates who require RRT, we may be seeing a shift towards more conservative and/or palliative approaches, and a more selective approach to RRT in this group.[42]
The interplay between AKI and CKD in cirrhosis remains complicated. While 30.3% of the study population had pre-admission CKD, among the remaining patients at risk, 36.9% subsequently developed de-novo CKD 3 months after discharge, with similar rates across etiologies of AKI. This is consistent with prior literature outside of cirrhosis where any AKI event (regardless of it causing functional or parenchymal injury) predisposes a patient to future CKD, and warrants further study in patients with cirrhosis.[43] It is important to note that only eGFR at the time of transplant, not etiology of AKI/CKD, currently factors into allocation of SLKT, which may explain why prerenal AKI represented 46.2% of all SLKT. 5 of the 12 patients who underwent SLKT with prerenal AKI had pre-admission CKD, which suggests that their prerenal AKI during the index admission was one of a series of acute and chronic insults leading to a progressive decrease in eGFR, which for some, necessitated future SLKT. We may also hypothesize that listed patients with prerenal AKI were less likely to be deemed “status 7” or inactive due to illness, compared to those with HRS-AKI or ATN, adding to the high rate of SLKT in this subgroup.
The availability of LT continues to drive management decisions and influence outcomes in this population. We accounted for this competing risk in our mortality analyses. Additionally, in stratified analysis by transplant listing status, we saw similar results, though associations were weaker in the listed group due to a smaller sample size of 317 patients. Overall, we know that among listed patients, pattern/duration of kidney injury (e.g. acute or chronic) influences mortality.[44] Our analysis suggests that etiology of AKI should also be considered when modeling outcomes for this population.
These data should be interpreted in the context of their limitations. This study was retrospective, thus all findings should be viewed as associations without a causal link. While geographically diverse, nearly all sites were LT centers, which may influence referral patterns and overall demographics. Only the admission’s peak AKI episode was captured during the study period, which limits bias related to repeated measures, but does not capture recurrent episodes of AKI for each patient, which may contribute to the development of CKD and mortality during follow-up. The high mortality rates in this study could have been influenced by the exclusion of patients with less severe AKI (SCr <1.5 mg/dL), who have better prognosis compared to other stages of AKI.[45] Furthermore, given the lack of gold-standard kidney biopsy data in this population,[46, 47] etiology of AKI is defined by a clinical syndrome, though definitions were agreed upon by a multidisciplinary expert group, and sensitivity analyses excluding patients without adjudicator agreement showed consistent results with the primary models.
In conclusion, AKI is a severe complication of cirrhosis. HRS-AKI is uncommon and has similar outcomes to ATN. Etiology of AKI, AKI stage/severity, and non-response to treatment are associated with mortality. These data establish a baseline for U.S. patients as we anticipate optimization and implementation of newly available vasoconstrictors in this population.
Supplementary Material
Highlights:
Acute kidney injury is common and deadly in cirrhosis, but incidence and outcomes based on the etiology/cause are not well described in United States patients.
Hypovolemic or prerenal acute kidney injury is the most common (44%), followed by acute tubular necrosis (30%) then hepatorenal syndrome (12%).
Acute tubular necrosis and hepatorenal syndrome have similar outcomes (~50% mortality at 90 days).
Acute kidney injury in cirrhosis is common, but hepatorenal syndrome is uncommon. New treatments for persistent and severe AKI in cirrhosis, such as further optimization of vasoconstrictors for hepatorenal syndrome and novel supportive therapies for acute tubular necrosis, are needed.
Impact and Implications:
Acute kidney injury (AKI) in cirrhosis carries high morbidity, and management is determined by etiology of the injury. However, a large and well-adjudicated multicenter database from U.S. centers that uses updated AKI definitions is lacking. Our findings demonstrate that acute tubular necrosis and hepatorenal syndrome have similar outcomes (~50% mortality at 90 days), though hepatorenal syndrome is uncommon (12% of all AKI cases). These findings represent practice patterns at U.S. transplant/tertiary centers and can be used as a baseline prior to the U.S. adoption of terlipressin.
Acknowledgements:
We appreciate the providers and support staff at all participating centers for their clinical care of this population. We thank the HRS-HARMONY collaborative members not listed as authors for feedback on the data/results.
Financial Support:
JAN is supported by NIH awards R01DK128208, U01DK129989, P30DK079337. RTC is supported by institutional research grants from Gilead, Abbvie, Merck, BMS, Boehringer, Janssen, GSK, Roche. NNU is supported by a Clinical, Translational and Outcomes Research Award from the American Association for the Study of Liver Disease and an institutional MGH Physician Scientist Development Award. PMD and EMP are supported by NIH award T32DK007191. AHF is supported by NIH award K23DK128562. MLB is supported by NIH award T32GR103472. ASA is supported by an institutional research grant from Mallinckrodt Pharmaceuticals and NIH award K23 DK128567.
Disclosures:
HW reports consultant fees from Mallinckrodt Pharmaceuticals. MN reports consultant fees from Mallinckrodt Pharmaceuticals. KRR reports consultant fees from Mallinckrodt Pharmaceuticals. JMB reports consultant fees from Mallinckrodt Pharmaceuticals. JCQV reports consultant fees from Mallinckrodt Pharmaceuticals, Bayer Pharmaceuticals, Travere Therapeutics, and Calliditas. JAN reports consultant fees from Baxter and Leadiant Biosciences. ESO reports consultant fees from Biovie. ASA reports consultant fees from Mallinckrodt Pharmaceuticals, Ocelot Bio, and Cymabay Therapeutics.
Listing of Abbreviations:
- AKI
acute kidney injury
- HRS-AKI
hepatorenal syndrome acute kidney injury
- ATN
acute tubular necrosis
- MELD-Na
Model for End Stage Liver Disease-Sodium
- ACLF
acute on chronic liver failure
- IV
intravenous
- SCr
serum creatinine
- LT
liver transplant
- ICD-10
International Code of Disease version 10
- RRT
renal replacement therapy
- UNOS
United Network for Organ Sharing
- ICA
International Club of Ascites
- CLIF-C
Chronic Liver Failure-Consortium
- ICU
intensive care unit
- SLKT
simultaneous liver kidney transplant
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Publisher's Disclaimer: This is a PDF file of an article that has undergone enhancements after acceptance, such as the addition of a cover page and metadata, and formatting for readability, but it is not yet the definitive version of record. This version will undergo additional copyediting, typesetting and review before it is published in its final form, but we are providing this version to give early visibility of the article. Please note that, during the production process, errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Data Sharing: The data that support the findings of this study are available upon request. Restrictions apply to the availability of this data.
References
- [1].Cullaro G, Kanduri SR, Velez JCQ. Acute Kidney Injury in Patients with Liver Disease. Clin J Am Soc Nephrol 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Garcia-Tsao G, Parikh CR, Viola A. Acute kidney injury in cirrhosis. Hepatology (Baltimore, Md) 2008;48:2064–2077. [DOI] [PubMed] [Google Scholar]
- [3].Angeli P, Gines P, Wong F, Bernardi M, Boyer TD, Gerbes A, et al. Diagnosis and management of acute kidney injury in patients with cirrhosis: revised consensus recommendations of the International Club of Ascites. Gut 2015;64:531–537. [DOI] [PubMed] [Google Scholar]
- [4].Moore K, Jamil K, Verleger K, Luo L, Kebede N, Heisen M, et al. Real-world treatment patterns and outcomes using terlipressin in 203 patients with the hepatorenal syndrome. Aliment Pharmacol Ther 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Fagundes C, Barreto R, Guevara M, Garcia E, Sola E, Rodriguez E, et al. A modified acute kidney injury classification for diagnosis and risk stratification of impairment of kidney function in cirrhosis. Journal of hepatology 2013;59:474–481. [DOI] [PubMed] [Google Scholar]
- [6].Allegretti AS, Ortiz G, Wenger J, Deferio JJ, Wibecan J, Kalim S, et al. Prognosis of Acute Kidney Injury and Hepatorenal Syndrome in Patients with Cirrhosis: A Prospective Cohort Study. International journal of nephrology 2015;2015:108139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Martin-Llahi M, Guevara M, Torre A, Fagundes C, Restuccia T, Gilabert R, et al. Prognostic importance of the cause of renal failure in patients with cirrhosis. Gastroenterology 2011;140:496.e494. [DOI] [PubMed] [Google Scholar]
- [8].Cullaro G, Rubin JB, Fortune BE, Crawford CV, Verna EC, Hsu CY, et al. Association Between Kidney Dysfunction Types and Mortality Among Hospitalized Patients with Cirrhosis. Dig Dis Sci 2022;67:3426–3435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Rice JB, White AG, Galebach P, Korenblat KM, Wagh A, Lovelace B, et al. The burden of hepatorenal syndrome among commercially insured and Medicare patients in the United States. Curr Med Res Opin 2017;33:1473–1480. [DOI] [PubMed] [Google Scholar]
- [10].Jamil K, Huang X, Hayashida D, Lodaya K. The Hepatorenal Syndrome Patient Pathway: Retrospective Analysis of Electronic Health Records. Curr Ther Res Clin Exp 2022;96:100663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].FDA approves treatment to improve kidney function in adults with hepatorenal syndrome. 2022. [cited 2022 September 21]; Available from: https://www.fda.gov/drugs/news-events-human-drugs/fdaapproves-treatment-improve-kidney-function-adults-hepatorenal-syndrome
- [12].Hayward KL, Johnson AL, McKillen BJ, Burke NT, Bansal V, Horsfall LU, et al. ICD-10-AM codes for cirrhosis and related complications: key performance considerations for population and healthcare studies. BMJ Open Gastroenterol 2020;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Huelin P, Piano S, Sola E, Stanco M, Sole C, Moreira R, et al. Validation of a Staging System for Acute Kidney Injury in Patients With Cirrhosis and Association With Acute-on-Chronic Liver Failure. Clin Gastroenterol Hepatol 2017;15:438–445 e435. [DOI] [PubMed] [Google Scholar]
- [14].Section 2: AKI Definition. Kidney international supplements 2012;2:19–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, et al. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care 2007;11:R31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Allegretti AS, Parada XV, Endres P, Zhao S, Krinsky S, St Hillien SA, et al. Urinary NGAL as a Diagnostic and Prognostic Marker for Acute Kidney Injury in Cirrhosis: A Prospective Study. Clin Transl Gastroenterol 2021;12:e00359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Inker LA, Astor BC, Fox CH, Isakova T, Lash JP, Peralta CA, et al. KDOQI US commentary on the 2012 KDIGO clinical practice guideline for the evaluation and management of CKD. Am J Kidney Dis 2014;63:713–735. [DOI] [PubMed] [Google Scholar]
- [18].Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009;150:604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. Journal of Biomedical Informatics 2009;42:377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Kim WR, Biggins SW, Kremers WK, Wiesner RH, Kamath PS, Benson JT, et al. Hyponatremia and mortality among patients on the liver-transplant waiting list. The New England journal of medicine 2008;359:1018–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Wiesner R, Edwards E, Freeman R, Harper A, Kim R, Kamath P, et al. Model for end-stage liver disease (MELD) and allocation of donor livers. Gastroenterology 2003;124:91–96. [DOI] [PubMed] [Google Scholar]
- [22].Moreau R, Jalan R, Gines P, Pavesi M, Angeli P, Cordoba J, et al. Acute-on-chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis. Gastroenterology 2013;144:9. [DOI] [PubMed] [Google Scholar]
- [23].Lee PH. Should we adjust for a confounder if empirical and theoretical criteria yield contradictory results? A simulation study. Sci Rep 2014;4:6085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Jepsen P, Vilstrup H, Andersen PK. The clinical course of cirrhosis: The importance of multistate models and competing risks analysis. Hepatology 2015;62:292–302. [DOI] [PubMed] [Google Scholar]
- [25].Crabb DW, Bataller R, Chalasani NP, Kamath PS, Lucey M, Mathurin P, et al. Standard Definitions and Common Data Elements for Clinical Trials in Patients With Alcoholic Hepatitis: Recommendation From the NIAAA Alcoholic Hepatitis Consortia. Gastroenterology 2016;150:785–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Singal AK, Kuo YF, Reddy KR, Bataller R, Kwo P. Healthcare burden and outcomes of hepatorenal syndrome among cirrhosis-related hospitalisations in the US. Aliment Pharmacol Ther 2022. [DOI] [PubMed] [Google Scholar]
- [27].Patidar KR, Adibuzzaman M, Naved MA, Rodriquez D, Slaven JE, Grama A, et al. Practice patterns and outcomes associated with intravenous albumin in patients with cirrhosis and acute kidney injury. Liver Int 2022;42:187–198. [DOI] [PubMed] [Google Scholar]
- [28].European Association for the Study of the Liver. Electronic address eee, European Association for the Study of the L. EASL Clinical Practice Guidelines for the management of patients with decompensated cirrhosis. J Hepatol 2018;69:406–460. [DOI] [PubMed] [Google Scholar]
- [29].Bajaj JS, O’Leary JG, Lai JC, Wong F, Long MD, Wong RJ, et al. Acute-on-Chronic Liver Failure Clinical Guidelines. Am J Gastroenterol 2022;117:225–252. [DOI] [PubMed] [Google Scholar]
- [30].Biggins SW, Angeli P, Garcia-Tsao G, Ginès P, Ling SC, Nadim MK, et al. Diagnosis, Evaluation, and Management of Ascites, Spontaneous Bacterial Peritonitis and Hepatorenal Syndrome: 2021 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology 2021;74:1014–1048. [DOI] [PubMed] [Google Scholar]
- [31].Allegretti AS, Subramanian RM, Francoz C, Olson JC, Cárdenas A. Respiratory events with terlipressin and albumin in hepatorenal syndrome: A review and clinical guidance. Liver Int 2022;42:2124–2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Wong F, Pappas SC, Curry MP, Reddy KR, Rubin RA, Porayko MK, et al. Terlipressin plus Albumin for the Treatment of Type 1 Hepatorenal Syndrome. N Engl J Med 2021;384:818–828. [DOI] [PubMed] [Google Scholar]
- [33].Belcher JM, Parada XV, Simonetto DA, Juncos LA, Karakala N, Wadei HM, et al. Terlipressin and the Treatment of Hepatorenal Syndrome: How the CONFIRM Trial Moves the Story Forward. Am J Kidney Dis 2022;79:737–745. [DOI] [PubMed] [Google Scholar]
- [34].Huelin P, Sola E, Elia C, Sole C, Risso A, Moreira R, et al. Neutrophil Gelatinase-Associated Lipocalin for Assessment of Acute Kidney Injury in Cirrhosis: A Prospective Study. Hepatology 2019;70:319–333. [DOI] [PubMed] [Google Scholar]
- [35].Gambino C, Piano S, Stenico M, Tonon M, Brocca A, Calvino V, et al. Diagnostic and prognostic performance of urinary Neutrophil Gelatinase-associated Lipocalin in patients with cirrhosis and AKI. Hepatology 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Allegretti AS, Sola E, Gines P. Clinical Application of Kidney Biomarkers in Cirrhosis. Am J Kidney Dis 2020;76:710–719. [DOI] [PubMed] [Google Scholar]
- [37].Bahirwani R, Ghabril M, Forde KA, Chatrath H, Wolf KM, Uribe L, et al. Factors that predict short-term intensive care unit mortality in patients with cirrhosis. Clin Gastroenterol Hepatol 2013;11:1194–1200.e1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Allegretti AS, Parada XV, Eneanya ND, Gilligan H, Xu D, Zhao S, et al. Prognosis of Patients with Cirrhosis and AKI Who Initiate RRT. Clin J Am Soc Nephrol 2018;13:16–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Patidar KR, Peng JL, Pike F, Orman ES, Glick M, Kettler CD, et al. Associations Between Mean Arterial Pressure and Poor ICU Outcomes in Critically Ill Patients With Cirrhosis: Is 65 The Sweet Spot? Crit Care Med 2020;48:e753–e760. [DOI] [PubMed] [Google Scholar]
- [40].Wang PL, Silver SA, Djerboua M, Thanabalasingam S, Zarnke S, Flemming JA. Recovery From Dialysis-Treated Acute Kidney Injury in Patients With Cirrhosis: A Population-Based Study. Am J Kidney Dis 2022;80:55–64.e51. [DOI] [PubMed] [Google Scholar]
- [41].Saraiva IE, Ortiz-Soriano VM, Mei X, Gianella FG, Woc WS, Zamudio R, et al. Continuous renal replacement therapy in critically ill patients with acute on chronic liver failure and acute kidney injury: A retrospective cohort study. Clin Nephrol 2020. [DOI] [PubMed] [Google Scholar]
- [42].Tandon P, Walling A, Patton H, Taddei T. AGA Clinical Practice Update on Palliative Care Management in Cirrhosis: Expert Review. Clin Gastroenterol Hepatol 2021;19:646–656.e643. [DOI] [PubMed] [Google Scholar]
- [43].Chawla LS, Kimmel PL. Acute kidney injury and chronic kidney disease: an integrated clinical syndrome. Kidney Int 2012;82:516–524. [DOI] [PubMed] [Google Scholar]
- [44].Cullaro G, Verna EC, Lai JC. Association Between Renal Function Pattern and Mortality in Patients With Cirrhosis. Clin Gastroenterol Hepatol 2019;17:2364–2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Huelin P, Piano S, Sola E, Stanco M, Sole C, Moreira R, et al. Validation of a Staging System for Acute Kidney Injury in Patients With Cirrhosis and Association With Acute-on-Chronic Liver Failure. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association 2016. [DOI] [PubMed] [Google Scholar]
- [46].Wadei HM, Abader P, Alsaad AA, Croome K, Cortese C, Geiger XJ, et al. Arterial Blood Pressure at Liver Transplant Evaluation Predicts Renal Histology in Candidates With Renal Dysfunction. Liver Transpl 2019;25:1756–1767. [DOI] [PubMed] [Google Scholar]
- [47].Wadei HM, Heckman MG, Rawal B, Taner CB, Mai ML, Cortese C, et al. Renal outcomes of liver transplant recipients who had pretransplant kidney biopsy. Transplantation 2014;98:1323–1330. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


