Abstract
OBJECTIVE:
To describe the rationale, principles, and dosage calculations for continuous-infusion β-lactam antibiotics to treat multidrug-resistant bacteria in patients undergoing continuous venovenous hemofiltration (CVVH).
DATA SOURCES:
A MEDLINE search (1968–November 2008) of the English-language literature was performed using the terms continuous infusion and Pseudomonas or Acinetobacter, hemofiltration or CVVH or hemodiafiltration or CVVHDF or continuous renal replacement therapy or pharmacokinetics; and terms describing different β-lactam antibiotics.
STUDY SELECTION AND DATA EXTRACTION:
In vitro, in vivo, and human studies were evaluated that used continuous-infusion β-lactam antibiotics to treat Pseudomonas aeruginosa and Acinetobacter baumannii infections. Studies were reviewed that described the pharmacokinetics of β-lactam antibiotics during CVVH as well as other modalities of continuous renal replacement therapy.
DATA SYNTHESIS:
Continuous infusion of β-lactam antibiotics, maintaining drug concentrations 4–5 times higher than the minimum inhibitory concentration, is a promising approach for managing infections caused by P. aeruginosa and A. baumannii. Safe yet effective continuous infusion therapy is made difficult by the occurrence of acute renal failure and the need for renal replacement therapy. Case series and pharmacokinetic properties indicate that several β-lactam antimicrobials that have been studied for continuous infusion, such as cefepime, ceftazidime, piperacillin, ticarcillin, clavulanic acid, and tazobactam, are significantly cleared by hemofiltration. Methodology and formulas are provided that allow practitioners to calculate dosage regimens and reach target drug concentrations for continuous β-lactam antibiotic infusions during CVVH based on a literature review, pharmacokinetic principles, and our experience at the National Institutes of Health Clinical Center.
CONCLUSIONS:
Continuous infusion of β-lactam antibiotics may be a useful treatment strategy for multidrug-resistant gram-negative infections in the intensive care unit. Well-established pharmacokinetic and pharmacodynamic principles can be used to safely reach and maintain steady-state target concentrations of β-lactam antibiotics in critical illness complicated by acute renal failure requiring CVVH.
Keywords: Acinetobacter baumannii, β-lactam, continuous infusion, continuous venovenous hemofiltration, Pseudomonas aeruginosa
EXTRACTO
OBJETIVO:
Describir los fundamentos, principios, y cálculos de las dosis de infusión continua de antibióticos β-lactamicos para el tratamiento de bacterias resistentes a múltiples medicamentos en pacientes siendo sometidos a antibióticos hemofiltración veno-venosa continua (HVVC).
FUENTES DE INFORMACIÓN:
Se realizó una búsqueda en MEDLINE (1968–noviembre 2008) de la literatura en idioma Inglés usando los términos como “continous infusión” y Pseudomonas o Acinetobacter; “hemofiltration” o “CVVH”, o “hemodiafiltration” o “CVVHDF”, o “continuous renal replacement therapy”, o “pharmacokinetics”, y los términos que describen los diferentes antibióticos β-lactámicos.
SELECCIÓN DE ESTUDIOS Y EXTRACCIÓN DE DATOS:
Se evaluaron los estudios in vitro, in vivo, y en humanos que utilizaron infusión continua de antibióticos β-lactámicos para el tratamiento de infecciones causadas por Pseudomonas aeruginosa y Acinetobacter baumannii. Se repasaron los estudios que describieron la farmacocinética de los antibióticos β-lactámicos durante HCCV, así como otras modalidades de terapia de reemplazo renal continua.
SÍNTESIS DE DATOS:
La infusión continua de antibióticos β-lactámicos y el mantenimiento de los niveles del medicamento de 4 a 5 veces superior a la concentración mínima inhibitoria, es un enfoque prometedor en el manejo de las infecciones causadas por P. Aeruginosa y A. baumannii. La seguridad y eficacia de la terapia de infusión continua se ve dificultado por la ocurrencia de insuficiencia renal aguda y la necesidad de terapia de reemplazo renal. Series de casos y propiedades farmacocinéticas indican que varios de los antibióticos β-lactámicos, que han sido estudiados por medio de infusión continua, tales como cefepime, ceftazidima, piperacilina, ticarcilina, ácido clavulánico y tazobactam, son significativamente depurados por medio de la hemofiltración. Se proveen la metodología y las fórmulas que permiten los practicantes calcular regímenes de dosificación para alcanzar concentraciones optimas del medicamento por medio de infusiones continuas de antibióticos β-lactámicos durante HCCV y basados en un repaso de la literatura, principios farmacocinéticos, y nuestra experiencia en el NIH Clinical Center.
CONCLUSIONES:
La infusión continua de antibióticos β-lactámicos puede ser una estrategia de tratamiento útil para infecciones gram negativas resistentes a múltiples medicamentos en la unidad de cuidado intensivo. Principios farmacocinéticos y farmacodinámicos bien establecidos pueden ser utilizados para alcanzar y mantener con seguridad el estado de equilibrio en los niveles de los antibióticos beta lactámicos en enfermedad grave y complicada por insuficiencia renal aguda que requiere HCCV.
RÉSUMÉ
OBJECTIF:
Décrire le raisonnement, les principes, et les calculs de dosage pour l’administration en perfusion intraveineuse continue d’antibiotiques β-lactames pour traiter une bactérie multi-résistante chez des patients subissant une hémofiltration veino-veineuse continue (CVVH).
SOURCES DE DONNÉES:
Une recherche MEDLINE (1968–novembre 2008) de la documentation scientifique de langue anglaise a été effectuće avec les termes perfusion intraveineuse continue et Pseudomonas ou Acinetobacter; hémofiltration ou CVVH ou hémofiltration ou CVVHDF ou thérapie de remplacement rénale continue ou pharmacocinétique et des termes décrivant différents antibiotiques du groupe des β-lactames.
SÉLECTION DES ÉTUDES ET EXTRACTION DES DONNÉES:
Des études in vitro, in vivo et humaines ont été évaluées lorsque l’administration d’antibiotiques du groupe des β-lactames en perfusion intraveineuse continue était utilisée pour traiter les infections à Pseudomonas aeruginosa et Acinetobacter baumannii. Les énudes retenues décrivaient la pharmacocinétique des antibiotiques du groupe des β-lactames durant une CVVH de même que pour d’autres modalités thérapeutiques de remplacement rénal continu.
SYNTHÈSE DES DONNÉES:
L’administration en perfusion intraveineuse continue d’antibiotiques β-lactames, tout en maintenant des niveaux de médicament de 4 à 5 fois supérieurs à la concentration minimale inhibitrice, constitue une approche prometteuse pour la prise en charge des infections causées par le P. aeruginosa et l’A. baumannii. Quoique sécuritaire et efficace, le traitement par perfusion intraveineuse continue devient difficile suite à l’apparition d’insuffisance rénale aiguë et la nécessité d’une thérapie de remplacement rénale. Des érudes de cas et les caractéristiques pharmacocinétiques indiquent que plusieurs antibiotiques du groupe des β-lactames, qui ont été étudiés en perfusion intraveineuse continue tels que le céfépime, la ceftazidime, la pipéracilline, la ticarcilline, l’acide clavulanique et le tazobactam, sont éliminés de façon significative par l’hémofiltration. Une méthodologie et des formules sont fournies pour permettre aux cliniciens de calculer les posologies et atteindre des concentrations médicamenteuses cibles pour les perfusions intraveineuses continues de β-lactames durant l’hémofiltration veino-veineuse continue basé sur une revue de la documentation scientifique, les principes de pharmacocinétique et l’expérience au Centre Clinique NIH.
CONCLUSIONS:
La perfusion intraveineuse continue d’antibiotiques du groupe des β-lactames peut être une stratégie de traitement utile pour des infections à Gram négatif multi-résistantes dans les unités de soins intensifs. Des principes de pharmacocinétique et pharmacodynamique bien établis peuvent être utilisés pour atteindre de façon sécuritaire et maintenir des niveaux d’antibiotiques β-lactames à l’équilibre cibles durant une maladie critique compliquée d’une insuffisance rénale aiguë nécessitant une CVVH.
Recent years have witnessed a striking increase in the incidence of multidrug-resistant (MDR) gram-negative bacteria including Pseudomonas aeruginosa, Acinetobacter baumannii, and organisms that produce extended-spectrum β-lactamases, type 1 β-lactamases, and metaloenzymes.1–3 Many of the infections caused by these bacteria commonly occur in intensive care units (ICUs) among critically ill patients who have a high risk of acute kidney injury. Strategies to treat these resistant bacterial infections have relied on new agents, the use of older antibiotics associated with greater toxicity, and administration of existing drugs in novel ways. Unfortunately, there has been a decline in research and development of new antibiotics with activity against resistant gram-negative bacteria.1,2 The Infectious Diseases Society of America has highlighted these concerns in several position statements.1,4 An alternative treatment strategy is exploiting innovative administration methods of existing agents, such as continuous infusion, to maximize the pharmacodynamics of β-lactam antibiotics.
The objective of this review is to provide a rational approach to dosing continuous-infusion β-lactam antibiotics in patients undergoing continuous venovenous hemofiltration (CVVH) for the treatment of MDR gram-negative bacteria. We focus on P. aeruginosa and Acinetobacter spp. because these organisms have emerged as life-threatening pathogens with the potential for resistance against multiple classes of antimicrobial agents. Although other gram-negative pathogens, such as extended-spectrum β-lactamase–producing Escherichia coli and type 1 β-lactamase–producing Enterobacter spp., are also important, multidrug resistance is less frequent and there is a paucity of data on continuous infusion of β-lactam antibiotics for these organisms. While there is limited published literature, recommendations are based on well-established pharmacokinetic and pharmacodynamic principles of both β-lactam antibiotics and of hemofiltration. We have used these methods to treat several patients infected with MDR gram-negative bacteria in the medical ICU of the National Institutes of Health Clinical Center. The following case illustrates this method.
Clinical Scenario
A 35-year-old male with a history of severe aplastic anemia and profound neutropenia developed septic shock and was transferred to the ICU. The patient developed acute respiratory failure requiring intubation and acute renal failure, with urine output 5 mL/h or less. The microbiology laboratory reported growth of P. aeruginosa from both blood and a tracheal aspirate culture. The isolates were intermediately resistant to ceftazidime (minimum inhibitory concentration [MIC] = 16 mg/L) and amikacin (MIC = 32 mg/L) but fully resistant to piperacillin, ticarcillin, imipenem, meropenem, aztreonam, ciprofloxacin, levofloxacin, gentamicin, and tobramycin. The patient was started on CVVH with the following settings: AN69 M100 filter, blood flow rate 200 mL/min, predilution replacement fluid rate 2100 mL/h, and ultrafiltration rate 2100 mL/h, with no net fluid removal. The patient’s weight was 70 kg and height was 170 cm. The infectious diseases consultants recommend amikacin, colistin, and continuous-infusion ceftazidime. They page you, the ICU pharmacist, for drug dosing recommendations for continuous-infusion ceftazidime. What do you recommend? (Recommendation from clinical scenario is shown in Appendix I.)
Overview of Continuous-Infusion β-Lactam Antibiotics
PRINCIPLES AND THEORY
β-Lactam pharmacodynamics are described by time-dependent killing (ie, bacterial killing is dependent on the amount of time that the drug concentration is above the MIC of the causative bacteria).5,6 Time-kill studies in aggregate indicate that maximal bactericidal activity occurs for most β-lactam antibiotics when drug concentrations are 4 times the MIC, with no additional effect at higher concentrations.7–11 Continuous-infusion β-lactam antibiotics can ensure time above MIC within the range of safely achievable concentrations for the entire dosing interval. This may be particularly critical for the treatment of MDR bacteria, as intermittent dosing may allow drug concentrations to fall below the MIC of the organism, thereby permitting survival and regrowth. While continuous infusion of β-lactam antibiotics has been shown to be effective in a number of clinical trials,12,13 there are limited data in patients receiving continuous renal replacement therapy (CRRT) and for the treatment of infections caused by P. aeruginosa and Acinetobacter spp. The following sections summarize the pharmacodynamic rationale of continuous infusion of β-lactam antibiotics for the treatment of P. aeruginosa infections. This rationale is based upon in vitro and in vivo studies as well as the current literature for treatment of P. aeruginosa infections in humans. Although in vitro, in vivo, and clinical data for Acinetobacter spp. are more limited, similar concepts may also be applied for the treatment of these organisms with continuous-infusion β-lactam antibiotics.
FORMULAS
A continuous infusion dosing regimen for β-lactam antibiotics that follows linear pharmacokinetics can be calculated from the following equation14,15:
| Eq. 1 |
| Eq. 2 |
| Eq. 3 |
| Eq. 4 |
where target peak concentration; = target mean steady-state concentration; total body clearance; elimination rate constant half-life; and volume of distribution.
The dosing regimen consists of an initial loading dose to rapidly achieve therapeutic concentrations (Equation 1). The loading dose is calculated from the and of the β-lactam antibiotic. The continuous infusion rate, which maintains the target concentration, is calculated from the Css and the of the drug (Equation 2).
PSEUDOMONAS SPP. AND ACINETOBACTER SPP.
In Vitro and In Vivo Studies
Several in vitro and in vivo studies of continuous-infusion β-lactam antibiotics suggest that maximal bactericidal activity against P. aeruginosa occurs when drug concentrations are 4–5 times the MIC (Table 1).16–23 An in vitro study by Mouton et al.16 compared intermittent with continuous infusion of ceftazidime for the treatment of P. aeruginosa infections. At 32 hours for strains with MICs of 1 mg/L and 4 mg/L, respectively, bacterial concentrations decreased approximately 101 cfu/mL and 101 cfu/mL with intermittent dosing and 103 cfu/mL and 104 cfu/mL with continuous infusion. The authors concluded that continuous infusion of ceftazidime was more effective than intermittent dosing if concentrations were maintained at greater than or equal to 4 times MIC. Tessier et al.18 also suggested a target concentration of 4 times MIC for continuous-infusion cefepime. In this study there was a reduction of approximately 102 cfu/mL for the P. aeruginosa strains treated with continuous-infusion cefepime alone at 24 hours. Other studies are summarized in Table 1.17,19
Table 1.
Selected In Vitro and In Vivo Studies of Continuous-Infusion β-Lactam Antibiotics for the Treatment of P. aeruginosa and A. baumannii
| Microorganism | Drug (model) | MICa (mg/L) | Dosage Regimen | Drug Concentrations | Conclusion | Target Drug Concentration as Multiple of MICb | Reference |
|---|---|---|---|---|---|---|---|
|
| |||||||
| P. aeruginosa | ceftazidime (in vitro, dialyzer unit model) | ceftazidime: 1, 4, 16 | 300 mg/L/24 h by intermittent dosing (every 8 h) or continuous infusion for 36 h | intermittent dosing peak: 92.3 mg/L trough: 1.4 mg/L continuous infusion 19.8 mg/L concentrations at 24 h |
continuous infusion more effective (in reduction log10 cfu/mL) than intermittent dosing with concentrations ≥4 times MIC | ceftazidime 4–5 times MIC | 16 |
| P. aeruginosa | ceftazidime ± amikacin (in vitro pharmacodynamic model) | ceftazidime: 1.56, 50 | simulated dosing of ceftazidime: 2 g iv q12h or q8h or continuous infusion (loading dose 2 g, then concentrations of 5 mg/L, 10 mg/L, or 20 mg/L) for 48 h simulated dosing of amikacin: 15 mg/kg/day |
ceftazidime intermittent dosing troughs: 2 g hr every 12 h: 2.6 mg/L 2 g iv every 8 h: 9.8 mg/L ceftazidime continuous infusion expected: observed 5 mg/L: 5.6 mg/L 10 mg/L: 11.9 mg/L 20 mg/L: 30.8 mg/L (concentrations in central compartment at 24 h) |
continuous infusion 10 and 20 mg/L as effective (in reduction log10 cfu/mL) as 2 g iv every 12 h and 2 g iv every 8 h, respectively, for sensitive strain amikacin synergistic with all regimens with sensitive strain and synergistic with ceftazidime 20 mg/L or 2 g iv q12 or q8h with resistant strain |
ceftazidime 10–20 times MIC | 17 |
| P. aeruginosa | cefepime ± tobramycin (in vitro pharmacodynamic model) | cefepime: 2.8 | simulated dosing of cefepime: 1 g iv q12h or continuous infusion (loading dose 1 g, then 2 g/24 h) for 48 h simulated dosing of once-daily tobramycin: peak 10 mg/L |
intermittent dosing: peak 106.6 mg/L continuous infusion: 11.48 mg/L |
continuous infusion more effective (in reduction log10 cfu/mL) than intermittent dosing addition of tobramycin to continuous infusion cefepime increased bactericidal activity |
cefepime 4 times MIC | 18 |
| P. aeruginosa | ceftazidime (in vitro, computer-controlled model) | ceftazidime: 8 16 (N = 2) 32 |
simulated dosing of 2 g iv q8h or continuous infusion (loading dose 1 g, then 6 g/24 h) for 32 h | intermittent dosing: peak: 119.97 mg/L trough: 9.17 mg/L continuous infusion: 40.38 mg/L |
continuous infusion and intermittent dosing produced ≥103 reduction in cfu/mL up to 32 h for strains with MIC 8 and 16 mg/L | 19 | |
| A. baumannii | CMS ± ceftazidime (in vitro pharmacodynamic model) | colistin sulfate: 0.5 ceftazidime: ≥64 |
regimen 1: CMS bolus for concentrations of 3, 6, 12, or 24 mg/L at time zero; in 1 experiment, additional 24 mg/L CMS bolus at 12 h regimen 2: CMS bolus of 24 mg/L + continuous-infusion ceftazidime (concentration 50 mg/L) for 24 h; 3 experiments with different antibiotic starting times: CMS 0 h, ceftazidime 2 h CMS 2 h, ceftazidime 0 h ceftazidime 0 h |
not determined | CMS bolus with continuous-infusion ceftazidime produced a 103 reduction in cfu/mL, prevented bacterial regrowth, and prevented the development of resistance to colistin sulfate | 20 | |
| P. aeruginosa | ceftazidime, amikacin, sulbactam (in vivo, rabbit endocarditis model) | ceftazidime: 16 sulbactam: >256 |
continuous infusion over 24 h starting 12 h or 48 h after infection: ceftazidime 800 mg/kg, amikacin 400 mg/kg ceftazidime 800 mg/kg + amikacin 400 mg/kg ceftazidime 800 mg/kg + sulbactam 400 mg/kg ceftazidime 800 mg/kg + amikacin 400 mg/kg + sulbactam 400 mg/kg |
ceftazidime continuous infusion: 127 mg/L | continuous-infusion ceftazidime effective (101 decrease in cfu/g) when started 12 h after infection continuous-infusion ceftazidime effective when started 48 h after infection only with combination therapy with amikacin, sulbactam, or both drugs |
21 | |
| P. aeruginosa | ceftazidime ± amikacin (in vivo, rabbit endocarditis model) | ceftazidime: 1 8 (oxacillinase producer) 4 (penicillinase producer) 8 (cephalosporinase producer) |
simulated dosing of ceftazidime: 2 g iv every 8 h or continuous infusion (4, 6, or 8 g/24 h) for 24 h amikacin 15 mg/kg/day |
ceftazidime intermittent dosing peak: 160 mg/L ceftazidime continuous infusion 4 g: 22.7 mg/L 6g: 34.8 mg/L 8 g: 79.6 mg/L |
continuous-infusion effective as intermittent dosing with concentrations ≥4 times MIC, but efficacy depends on bacterial strain no effect with addition of amikacin to continuous-infusion ceftazidime |
ceftazidime 4–5 times MIC | 22 |
| P. aeruginosa | imipenem or cefepime ± tobramycin (in vivo, rabbit endocarditis model) | imipenem: 2 cefepime: 1 |
continuous-infusion imipenem (10, 15, 25, 100 mg/kg/day) over 24 h continuous-infusion cefepime (10, 25, 40, 100 mg/kg/day) over 24 h simulated dosing of tobramycin 3 mg/kg/day |
imipenem continuous infusionc 15 mg/kg/day: 0.5 mg/L 25 mg/kg/day: 0.8 mg/L 100 mg/kg/day: 2.3 mg/L cefepime continuous infusion 10 mg/kg/day: 1.6 mg/L 25 mg/kg/day: 3.8 mg/L 40 mg/kg/day: 5.7 mg/L 100 mg/kg/day: 15.1 mg/L |
lowest effective steady-state concentration (102 decrease in cfu/g) with concentrations 3–4 times MIC with cefepime and 0.25 times MIC for imipenem no effect with addition of tobramycin |
cefepime 4–6 times MIC | 23 |
CMS = colistin methanesulfonate; MIC = minimum inhibitory concentration.
Unless otherwise noted, each study used one strain.
Author’s conclusion, see reference.
The clinical significance and mechanism of subinhibitory concentrations of imipenem in this study are uncertain.
Ceftazidime intermittent infusion was compared with continuous infusion with or without amikacin in a rabbit model of P. aeruginosa endocarditis.22 Continuous infusion was as effective as intermittent dosing if concentrations were 4 or more times MIC, and there was no additional effect of amikacin. The authors recommended a target concentration of 4–5 times MIC when using continuous-infusion ceftazidime. In another study of P. aeruginosa endocarditis in rabbits, Navas et al.23 compared the effect of continuous-infusion imipenem or cefepime with or without tobramycin. A decrease in 102 cfu/g of P. aeruginosa within the vegetations occurred with concentrations 3–4 times MIC for cefepime and 0.25 times MIC for imipenem, with no additional effect of tobramycin. However, the clinical significance and mechanism of subinhibitory concentrations of imipenem in this study are uncertain. Cefepime concentrations 4–6 times MIC were suggested as a reasonable goal.
A critical issue when treating patients with continuous-infusion β-lactam antibiotics is whether concentrations 4–5 times the MIC are actually bactericidal against intermediate and resistant strains of P. aeruginosa. The bactericidal activity of ceftazidime against sensitive, intermediate, and resistant strains of P. aeruginosa has been studied in a time-kill assay.7 Reductions of greater than or equal to 103 cfu/mL occurred in 11 of 13 strains and 12 of 13 strains exposed to ceftazidime at 4 times and 8 times MIC, respectively. There was no increase in bactericidal activity at concentrations 8 times MIC. Bactericidal activity against intermediate strains of P. aeruginosa has also been shown in vitro with continuous-infusion ceftazidime. In a study by Alou et al.,19 continuous-infusion ceftazidime (mean steady-state concentration 40.38 mg/L) produced reductions greater than or equal to 103 cfu/mL in 2 strains of P. aeruginosa (MIC = 16 mg/L). However, the effectiveness of continuous-infusion β-lactam antibiotic treatment may depend on factors other than the MIC of the bacterial strain and therefore may not be entirely dependent on just achieving appropriate drug concentrations. In the study by Robaux et al.,22 continuous-infusion ceftazidime was not bactericidal at concentrations up to 79.6 mg/L against a cephalosporinase-producing strain of P. aeruginosa (MIC = 8 mg/L). Likewise, in a time-kill study by Xiong et al.,21 ceftazidime (concentration 64 mg/L) failed to achieve bactericidal activity against a cephalosporinase-producing strain of P. aeruginosa (MIC = 16 mg/L). In a rabbit model of endocarditis, this same strain of P. aeruginosa was reduced by only 101 cfu/g in vegetations by continuous-infusion ceftazidime (concentration of 127 mg/L) started 12 hours after infection.21
In comparison with continuous-infusion β-lactam antibiotic studies with P. aeruginosa, in vitro and in vivo studies with A. baumannii are limited (Table 1). In a study by Kroeger et al.,20 the combination of colistin methanesulfonate and continuous-infusion ceftazidime produced a 103 cfu/mL reduction in bacterial load and prevented regrowth of A. baumannii for 24 hours.
Clinical Studies
Only a few studies have been published in which continuous-infusion β-lactam antibiotics were used for the treatment of infections caused by P. aeruginosa (Table 2).24–31 Continuous-infusion ceftazidime appears to be effective for treating pulmonary infections in cystic fibrosis and skin lesions in neutropenic patients.24–28 In addition, one case report described the use of continuous-infusion meropenem for the treatment of MDR P. aeruginosa pneumonia.29
Table 2.
Selected Studies of Continuous-Infusion β-Lactam Antibiotics for the Treatment of P. aeruginosa and A. baumannii in Humans
| Microorganism | Drug | MIC (mg/L) | Dosage Regimen | Drug Concentrations | Pts. | Type of Infection | Conclusiona | Reference |
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| P. aeruginosa | ceftazidime | NR | pt. 1 LD: 2 g iv MD: 6 g iv per day infused over 24 h pt.2 LD: 2 g iv MD: NR |
NR | 30-y-old male, aplastic anemia, neutropenia | persistent skin lesions following bacteremia | continuous-infusion ceftazidime may be more effective than intermittent dosing in pseudomonal infections in granulocytopenic pts. | 24 |
| 28 mg/L | 46-y-old male, leukemia, neutropenia | |||||||
| P. aeruginosa | ceftazidime | NR | regimen A LD: 10 mg/kg iv MD: 4.5 mg/kg/h for 7 days regimen B LD: 7.5 mg/kg iv MD: 3.4 mg/kg/h for 7 days |
regimen A (mean) 38.3 mg/L, (n = 3) regimen B (mean) 21.3 mg/L (n = 2) concentrations on day 7, at 8–9 h |
cystic fibrosis, 9–25 y old, (n = 6) | pulmonary infection | pts. improved on continuous-infusion ceftazidime | 25 |
| P. aeruginosa | ceftazidime | NR | 101.5 mg/kg/day infused over 24 h (average dose) | 28.4 mg/L (mean) (n = 10) | cystic fibrosis, 15–52 y old (N = 12) | exacerbation of acute lower respiratory tract | home iv therapy with continuous-infusion ceftazidime clinically effective | 26 |
| P. aeruginosa | ceftazidime + tobramycin | 1.5–6 | ceftazidime 50 mg/kg iv every 8 h (maximum 6 g) for 10 days ceftazidime continuous infusion 6.6 times MIC (maximum 6 g, average 78 mg/kg/day) for 10 days |
NR | cystic fibrosis, 19–32 y old (N = 5) | exacerbation of acute lower respiratory tract | continuous infusion as effective as intermittent dosing with changes in WBC count, sputum density, and pulmonary function test | 27 |
| P. aeruginosa | ceftazidime + amikacin | 0.5–4 | ceftazidime 200 mg/kg/day 3 divided doses for 14 days ceftazidime continuous infusion 100 mg/kg/day × 14 days amikacin 20 mg/kg/day |
ceftazidime intermittent dosing trough (mean) 6.1 mg/L ceftazidime continuous infusion (mean) day 3: 29.7 mg/L day 10: 27.4 mg/L |
cystic fibrosis 5–16.8 y old (N = 14) | chronic pulmonary colonization | continuous infusion as effective as intermittent dosing with changes in pulmonary, inflammatory, and nutritional status | 28 |
| P. aeruginosa | meropenem | 32 | LD: 2 g iv MD: 8 g iv per day infused over 24 h |
NR | 58-y-old male, emphysema, double lung transplant | pneumonia | MDR P. aeruginosa pneumonia successfully treated with continuous-infusion meropenem | 29 |
| P. aeruginosa | aztreonam + tobramycin | 4 | aztreonam continuous infusion 200 mg/kg/day tobramycin 5 mg/kg iv every 12 h |
aztreonam continuous infusion 160 mg/L | cystic fibrosis, 3-mo-old female | tracheobronchial colonization | P. aeruginosa successfully eradicated with continuous-infusion aztreonam and tobramycin | 30 |
| A. baumannii | imipenem + amikacin | NR | imipenem 1 g iv per day infused over 24 h amikacin 500 mg iv per day |
NR | 40-y-old male, motorcycle accident | pneumonia | MDR A. baumannii pneumonia successfully treated with continuous-infusion imipenem after rapid desensitization procedure | 31 |
LD = loading dose; MD = maintenance dose; MDR = multidrug resistant; MIC = minimum inhibitory concentration; NR = not reported; WBC = white blood cell.
Author’s conclusion; see reference.
Only a single case report described the use of continuous-infusion β-lactam antibiotics for the treatment of infections caused by A. baumannii (Table 2).31 Continuous-infusion imipenem was reported to successfully treat A. baumannii ventilator-associated pneumonia. However, that regimen was employed to prevent allergic reactions after a rapid desensitization protocol and not specifically to provide a constant level of bactericidal activity.
Overview of Continuous Renal Replacement Therapy
A number of excellent articles on drug dosing in CRRT have been published; we recommend that the reader consult these references for a more in-depth review.32–43 Here, we briefly describe the different modes of CRRT and review critical concepts related to drug dosing in hemofiltration.
The common modes of CRRT include slow continuous ultrafiltration, CVVH, continuous venovenous hemodialysis (CVVHD), and continuous venovenous hemodiafiltration (CVVHDF).44,45 Slow continuous ultrafiltration and CVVH are forms of hemofiltration, CVVHD is a form of hemodialysis, and CVVHDF is a combination of hemofiltration and hemodialysis. In hemofiltration, solutes are removed by convection (ie, solutes move with water flow). In hemodialysis, solutes are removed by diffusion (ie, solutes move from an area of high concentration to an area of low concentration). Slow continuous ultrafiltration is used only for fluid removal (solute removal is negligible), while CVVH, CVVHD, and CVVHDF are used to remove solutes as well as fluid.
A diagram of a typical CVVH system is illustrated in Figure 1.32,35,36,42,43 Both access and return lines are connected to a double-lumen catheter that, for adults, is commonly inserted into the femoral or internal jugular vein. Computerized external pumps control the blood flow rate and the replacement fluid rate, which can be given prefilter and/or postfilter. The ultrafiltration rate is the sum of the replacement fluid rate and any additional fluid removed from the patient.
Figure 1.
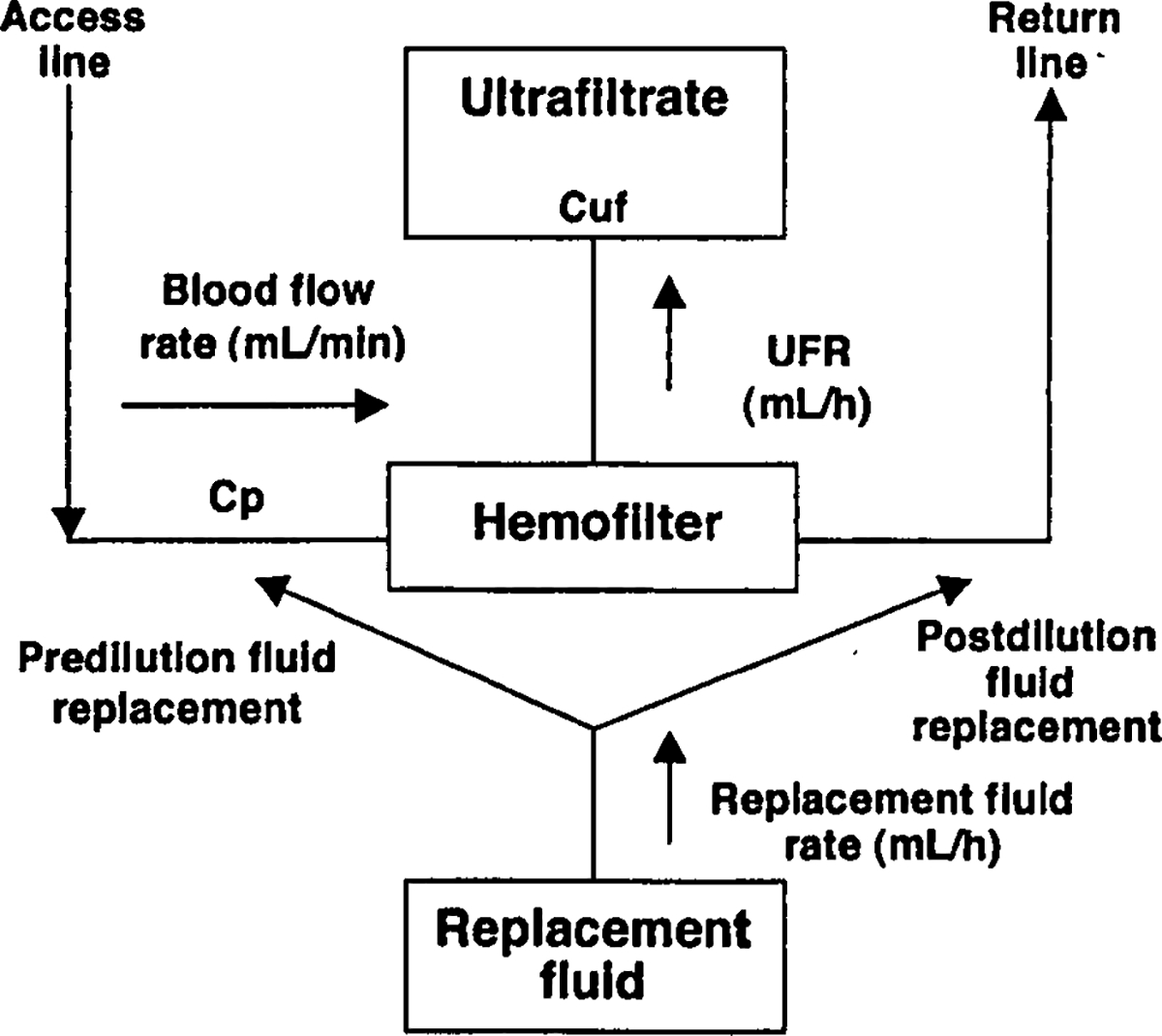
Schematic of a CVVH system.32,35,36,12,43
1.
2. (if all replacement fluid is postfilter).
3. .
4. .
5. .
drug concentration in plasma; drug concentration in ultrafiltrate; continuous venovenous hemofiltration; extracorporeal clearance; hemotiltration clearance; nonrenal clearance; renal clearance; total body clearance; fractional extracorporeal clearance; sleving coefficient; UFR ultrafiltration rate.
Two important pharmacokinetic concepts related to drug dosing in hemofiltration include the sieving coefficient and hemofiltration clearance (Figure 1). The sieving coefficient is calculated by dividing the solute concentration in the ultrafiltrate (Cuf) by the solute concentration in plasma (Cp) (Figure 1, Equation 1). A sieving coefficient of 0 indicates that a drug does not pass across the hemofilter, while a sieving coefficient of 1 indicates that a drug freely passes across the hemofilter.35,36,42 The primary factor that affects the sieving coefficient is protein binding and there is a reasonable correlation between the free fraction and the sieving coefficient of a drug.36–38,42,43 Hemofiltration clearance is calculated from the ultrafiltration rate (UFR) and the sieving coefficient (Figure 1; Equations 2 and 3). Clearance is affected by the location of fluid replacement, as prefilter administration dilutes the solute concentration entering the hemofilter, thereby decreasing solute clearance.46 The correction factor for the effect of predilution on hemofiltration clearance is the extracorporeal blood flow rate divided by the blood flow rate plus the prefilter replacement fluid rate (Figure 1; Equation 3).
Hemofiltration clearance is thought to be clinically significant, requiring a change in maintenance dose if the fractional extracorporeal clearance of a drug is greater than 25–30% (Figure 1; Equation 5).42 The major drug properties affecting removal during hemofiltration include , protein binding, and molecular weight. A less than 1 L/kg, protein binding less than 70–80%, and molecular weight below the cutoff of the hemofilter suggest that a drug will be removed by hemofiltration.
Continuous-Infusion β-Lactam Antibiotics and CVVH
PHARMACOKINETICS
The pharmacokinetic properties of selected β-lactam antibiotics are summarized in Table 3.47–69 In general, these agents have low molecular weights, ranging from 237.3 to 636.6 daltons, low around 0.3–0.4 L/kg, and relatively low protein binding varying between 10% and 68%. With the exception of ampicillin, aztreonam, and penicillin G, these antibiotics are excreted primarily unchanged by the kidney via glomerular filtration (GFR) with a small, clinically insignificant component of tubular secretion.
Table 3.
Pharmacokinetic Properties of Selected β-Lactam Antibiotics in Healthy Volunteers and Patients with Renal Failure
| Drug | Molecular Weight (daltons) | Vd (L/kg) | Protein Binding (%) | t1/2 (h) | Percent Excreted Unchanged by Kidney | Cltotal (mL/min) | Clrenal (mL/min) | Clnonrenal (mL/min) | Notes | Assays Lab/Type | Suggested Maximum Tolerated Concentrations (mg/L) |
|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||
| Ampicillin | 371.3947 | 0.28–0.3348 | 2847 | 147 | 7549 | 203–31948 | glomerular filtration and tubular secretion48 | ampicillin | ampicillin | ||
| 218.650 | 158.550 | 6050 | Focus BA | 100 | |||||||
| 3150,a | 0.3850,a | 30.650,a | Mayo HPLC | ||||||||
| Sulbactam | 255.2247 | 0.24–0.448 | 3847 | 147 | 8949 | 169–20448 | glomerular filtration and tubular secretion48 | ||||
| 75.551 | 216.650 | 167.750 | 5050 | ||||||||
| 266.351 | 203.851 | 65.351 | |||||||||
| 45.350,a | 0.550,a | 44.850,a | |||||||||
| Aztreonam | 435.4452 | 12.6L52 | 5652 | 1.752 | 6853 | 9152 | 5652 | glomerular filtration and tubular secretion49 | Focus BA | 128 | |
| 0.2254 | 10754 | 26.7554 | |||||||||
| 2954,b | |||||||||||
| Cefepime | 571.5155 | 18L56 | 2056 | 256 | 82.957 | 12056 | glomerular filtration49 | Mayo HPLC | 100 | ||
| 0.2858 | 13157 | 11057 | 20.257 | ||||||||
| 18.757,a | 4.2257,a | 16.957,a | |||||||||
| Ceftazidime | 636.659 | 0.18–0.3148 | <1059 | 1.959 | 80–9059 | 11559 | 10059 | glomerular filtration49 | Focus BA | 128 | |
| 0.2360 | 130 mL/min/1,73 m2 60 | 103.9 mL/min/1,73 m2 60 | |||||||||
| 6.8 mL/min/1.73 m2 60,a | |||||||||||
| Penicillin Gd | 372.4861 | 0.53–0.6748 | 45–6848 | 0.4–0.948 | 60–8562 | 42.763,a | not widely reported | not widely reported | glomerular filtration and tubular secretion61 | Focus BA | 20 |
| Mayo HPLC | |||||||||||
| Piperacillin | 539.564 | 0.14–0.3148 | 3064 | 0.7–1.264 | 6864 | 153–297 mL/min/1.73 m2 48 | glomerular filtration and tubular secretion64 | piperacillin Focus BA | piperacillin | ||
| 100 | |||||||||||
| 22565 | 16865 | 5765 | |||||||||
| 62.865,a | 6265,a | ||||||||||
| Tazobactam | 322.364 | 0.2162 | 3064 | 0.7–1.264 | 8064 | 21965 | 14865 | 7265 | glomerular filtration and tubular secretion64 | ||
| 25.165,a | 2565,a | ||||||||||
| Ticarcillin | 428.455 | 0.167–0.17348 | 4566 | 1.166 | 80–9348 | 132–25348 | glomerular filtration and tubular secretion49,67 | Focus BA | ticarcillinc | ||
| 78.267 | 97.867 | 78.267 | 19.767 | 100 | |||||||
| 15.868,b | 1.668,b | ||||||||||
| Clavulanic acid | 237.369 | 0.315–0.34248 | 2566 | 1.166 | 5049 | 21067 | 10267 | 10867 | extensively metabolized, glomerular filtration48 | ||
| 47.567 | 51.568,b | 0.568,b | |||||||||
BA = bioassay; Clnonrenal = nonrenal clearance; Clrenal = renal clearance; Cltotal = total body clearance; Focus = Focus Diagnostics; HPLC = high-performance liquid chromatography; Mayo = Mayo Medical Laboratories; t1/2 = half-life; Vd = volume of distribution.
Anuric patients or on maintenance dialysis.
CrCl <10 mL/min.
Although ticarcillin concentrations are not currently measured at the NIH Clinical Center, we would suggest 100 mg/L as a maximum therapeutic concentration.
Penicillin G included in paper as an example of a β-lactam antibiotic that undergoes extensive tubular secretion.
Clinical Laboratory Improvement Amendment–approved drug concentration assays are available by high-performance liquid chromatography (HPLC) for ampicillin, cefepime, and penicillin G from Mayo Clinic Medical Laboratories (Rochester, MN), while the concentrations of other β-lactam antibiotics are available by bioassay from Focus Diagnostics (Cypress, CA). Unfortunately, bioassays are not reliable when other antibiotics are being used concurrently and the number of drug concentration assays available by HPLC has declined over the past several years, making it more difficult to monitor concentrations. Our suggested maximum recommended drug concentrations for the β-lactam antibiotics based on our clinical experience and review of the literature are listed in Table 3. We recommend using total rather than unbound drug concentrations for several reasons: the measurement of unbound drug concentrations is difficult and has not been standardized; serum protein levels vary considerably in ICU patients70; and unbound versus bound drug is unpredictable.
The low , low protein binding, low molecular weight, and high renal clearance of the β-lactam antibiotics shown in Table 3 suggest that they will be removed by hemofiltration. As noted in the previous section, hemofiltration clearance is clinically significant when extracorporeal clearance exceeds 25–30% of total clearance .42 Therefore, cefepime, ceftazidime, piperacillin, ticarcillin, clavulanic acid, and tazobactam (which have a ) would all require dosage adjustments when CVVH or other forms of renal replacement therapy are initiated for acute renal failure (Table 4).3,38,71–78 There are limited pharmacokinetic data describing the effects of hemofiltration on ampicillin, aztreonam, and penicillin G clearance.79–81 The pharmacokinetics of sulbactam have been reported only with CVVHD; ranged from 22.9% to 46.4%.82
Table 4.
Sieving Coefficient and Fractional Extracorporeal Clearance for Selected β-Lactam Antibiotics
| Drug | CRRT Type | Clinical Setting (pts.) | S | Filter | UFR | Cltotal | ClCRRT | FrEC (%) | Reference |
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Ampicillin | CAVH | 069 | Amicon (polysulfone) | 35 | |||||
| Cefepime | 0.72 | 38 | |||||||
| CVVH | adult ICU (N = 5) | 0.86 | Hospal Multiflow 60 (AN69) | 16 mL/min | 36 mL/min | 13 mL/min | 40 | 71 | |
| CVVH | adult ICU (N = 2) | pt. 1 0.76 (Cr Cl 29 mL/min) |
Prisma M100 (AN69) | 35 mL/min | 70.4 mL/min | 29.1 mL/min | 41.3 | 72 | |
| pt. 3 0.47 (CrCl not determined) |
16.7 mL/min | 172.2 mL/min | 7.8 mL/min | 4.5 | |||||
| Ceftazidime | 0.9 | 38 | |||||||
| CVVH | adult ICU (N = 2) | pt. 3 1.01 (CrCl 80 mL/min) |
Prisma M100 (AN69) | 16.7 mL/min | 333.8 mL/min | 16.8 mL/min | 5 | 73 | |
| pt. 4 0.91 (CrCl 75 mL/min) |
16.7 mL/min | 154.3 mL/min | 17.4 mL/min | 11.3 | |||||
| CVVH | adult ICU (N = 7) | 0.87 | CT-190G (cellulose triacetate) | 25 mL/kg/h | 74 | ||||
| CVVH | adult ICU (N = 12) | 0.69 | Diafilter-30 (polysulphone) | 47 mL/min | 98.7 mL/min | 32.1 mL/min | 32.5a | 75 | |
| CVVH | adult non-ICU, end-stage renal disease (N = 8) | 0.8 | Filtryzer B1–2.1U (PMMA) | 76 | |||||
| 0.97 | Hospal Multiflow 60 (AN69) | ||||||||
| 0.97 | Fresenius F40 (polysulfone) | ||||||||
| Penicillin G | 0.68 | 38 | |||||||
| Piperacillin | 0.82 | 38 | |||||||
| CVVH | adult ICU (N = 14) | 0.42 (CrCl <10 mL/min) | Prisma M100 (AN69) | 27.1 mL/min | 50 mL/min | 11.45 mL/min | 37 | 77 | |
| 0.38 (CrCl 10–50 mL/min) | 30.3 mL/min | 90.6 mL/min | 12.2 mL/min | 12.7 | |||||
| 0.23 (CrCl > 50 mL/min) | 20 mL/min | 265.2 mL/min | 4.8 mL/min | 2.8 | |||||
| Tazobactam | 0.76 (CrCl <10 mL/min) | 27.1 mL/min | 50.4 mL/min | 20.9 mL/min | 62.5 | 77 | |||
| 0.73 (CrCl 10–50 mL/min) | 30.3 mL/min | 68.2 mL/min | 21.9 mL/min | 35.4 | |||||
| 0.86 (CrCl >50 mL/min) | 20 mL/min | 180.1 mL/min | 19.6 mL/min | 13.1 | |||||
| Ticarcillin | 0.83 | 38 | |||||||
| CVVH | pediatric ICU (N = 3) | 0.83 | Amicon D-20 | 890 mL/h | 0.038 L/kg/h | 0.022 L/kg/h | 57.9a | 78 | |
| Clavulanic acid | 1.69 | 0.184 L/kg/h | 0.049 L/kg/h | 26.6a | 78 | ||||
CAVH = continuous arteriovenous hemofiltration; ClCRRT = CRRT clearance; Cltotal = total body clearance; CrCl = creatinine clearance; CRRT = continuous renal replacement therapy; CVVH = continuous venovenous hemofiltration; FrEC = fractional extracorporeal clearance; ICU = intensive care unit; S = sieving coefficient; UFR = ultrafiltration rate.
FrEC calculated from clearance data in sstudy.
Studies investigating the removal of piperacillin by hemofiltration are conflicting. In a study by Capellier et al.83 a minimal amount of piperacillin was found in ultrafiltrate, suggesting that the drug was not significantly removed by hemofiltration. In this study, hemofiltration clearance and the sieving coefficient were not reported. However, in a study by Arzuaga et al.,77 piperacillin was removed by hemofiltration, with an of 37% in patients with creatinine clearance less than 10 mL/min. Notably, the mean sieving coefficient was only 0.34, while the mean unbound drug fraction was 78.8%. The reason for this discrepancy is not clear, as the molecular weight of piperacillin suggests that unbound drug should freely cross hemofilter membranes.
Results of studies investigating the removal of tazobactam by hemofiltration are also conflicting. Two studies suggest that tazobactam may accumulate during CVVH, but the authors did not report hemofiltration clearance and the sieving coefficient.84,85 In the study by Arzuaga et al.,77 tazobactam was significantly cleared by CVVH, with an of 62.5% and 34.5% in patients with creatinine clearance values of less than 10 mL/min and between 10 and 50 mL/min, respectively. No accumulation of tazobactam was reported. These conflicting findings underscore the importance of monitoring concentrations of β-lactam antibiotics in serum of patients receiving CVVH.
STUDIES WITH CONTINUOUS-INFUSION β-LACTAM ANTIBIOTICS IN CRRT
Only 2 studies have been published investigating the pharmacokinetics of continuous-infusion β-lactam antibiotics during CRRT. In a study by Mariat et al.,86 7 adults in ICU received a ceftazidime loading dose of 2 g, followed by a continuous infusion of 3 g/day for 72 hours. The CVVHDF settings were a blood flow rate of 150 mL/min, dialysis flow rate of 1 L/h, UFR of 1.5 L/h, and prefilter fluid replacement. The ceftazidime dosing calculations were based on a of 0.3 L/kg, of 4 hours, and estimated target concentration of 30–40mg/L. The mean steady-state concentration with continuous-infusion ceftazidime was 33.5 mg/L.
The pharmacokinetics of continuous infusion and intermittent dosing of meropenem on CVVHDF were compared in a randomized crossover study.87 Six adults in ICU received either (1) meropenem loading dose of 0.5 g followed by a continuous infusion of 2 g /day for 2 days or (2) meropenem 1 g intravenously every 12 hours for 2 days. After crossover to the opposite arm of the study, patients continued to receive the study drug for another 2 days. The reported CVVHDF settings were a blood flow rate of 150 mL/min and UFR of 25 mL/kg/h. The median meropenem concentration was 19.1 mg/L with continuous infusion; with intermittent dosing, the median peak was 62.8 mg/L and the median trough was 8.2 mg/L.
CALCULATING A DOSAGE REGIMEN DURING CVVH
Methods for calculating a continuous-infusion dosage regimen during CVVH for β-lactam antibiotics with either renal clearance by GFR alone or renal clearance by GFR and tubular secretion, are listed below. Estimating or even measuring residual creatinine clearance is difficult and often inaccurate in ICU patients with changing renal function. Furthermore, nonrenal clearance for drugs metabolized by the liver is difficult to determine in ICU patients.
Vigilant monitoring is required, as changes in CRRT settings (UFR, location of fluid replacement, blood flow rate) as well as system down-times due to procedures and filter clotting, may require major dose adjustments. Frequent drug concentrations obtained at or near steady-state should be measured if available (Table 3). Prior to collecting samples for drug concentrations of β-lactam antibiotics, clinicians should note any interruptions in the infusions, infusion rate changes, and the administration of repeat loading doses.
The following 2 methods for dosage calculation during CVVH are recommended.
-Lactam Antiblotics with by Glomerular Filtration (Cefepime, Ceftazidime, Ticarcillin)
Obtain MIC of bacteria from microbiology laboratory.
Select target mean Css concentration at least 4 times MIC (Table 3).
Calculate residual CrCl with a timed urine collection, if possible, before initiation of CVVH. During the course of CVVH, a decline in urinary output, often to an anuric state, may occur. In such instances, residual CrCl = 0 mL/min.
Calculate (Figure 1).
- Calculate estimated clearance.
- Calculate maintenance infusion rate on CVVH
- Adjust maintenance infusion rate based on drug concentrations
-Lactam Antibiotics with by Glomerular Filtration and Substantial Tubular Secretion (Ampicillin, Aztreonam, Penicillin G, Piperacillin)
The calculations below should only be used in patients with anuria.
-
1–2.
Same as above.
-
3.
Calculate (Figure 1).
- 4.
- 5.
-
6.Calculate maintenance infusion rate on CVVH.
-
7.Adjust maintenance infusion rate based on drug concentrations.
Summary
While the incidence of antibiotic resistance in gram-negative bacteria is increasing, there has been a decline in the development and approval of new antibacterial agents. ICU practitioners and their infectious diseases consultants commonly need to treat patients infected with MDR gram-negative bacteria, such as P. aeruginosa and A. baumannii, that are resistant to the majority of available antibiotics and who are at high risk for acute renal failure requiring renal replacement therapy. Diligent monitoring of the CRRT system, as well as monitoring of drug concentrations, is recommended when following the concepts described in this paper. Although the number of β-lactam antibiotic concentration assays available by HPLC has declined over the past several years, we hope that this article will stimulate a renewed interest in the measurement and availability of these concentrations.
Financial disclosure:
This work was supported in part by the intramural research program of the National Institutes of Health.
Appendix I. Recommendation from Clinical Scenario
| You call the infectious diseases consultants with suggested drug dosing recommendations for continuous-infusion ceftazidime. |
| 1. P. aeruginosa MIC = 16 mg/L |
| 2. Target mean Css concentration = 64 mg/L |
| 3. Residual CrCl = 0 mL/min |
| 4. ClHF = UFR × S × [blood flow rate/(blood flow rate + replacement fluid rate)] |
| ClHF = 2100 mL/h × 0.9 × [200 mL/min/(200 mL/min + 35 mL/min)] |
| ClHF = 1608.5 mL/h × 1 h/60 min = 26.8 mL/min |
| 5. Clestimated = ClHF + residual CrCl |
| Clestimated = 26.8 mL/min + 0 mL/min = 26.8 mL/min |
| 6. Loading dose = Cpeak (mg/L) × Vd (L/kg) × weight (kg) |
| loading dose = 64 mg/L × 0.3 L/kg × 70 kg = 1344 mg |
| 7. Maintenance infusion rate for a patient with normal renal function |
| Ke = 0.693/t1/2 (h) |
| Cltotal = Ke (h−1) × Vd (L/kg) × weight (kg) |
| Maintenance infusion rate = Css (mg/L) × Cltotal (L/h) |
| Ke = 0.693/1.9 h = 0.3647 h−1 |
| Cltotal = 0.3647 h−1 × 0.3 L/kg × 70 kg = 7.659 L/h |
| Maintenance infusion rate = 64 mg/L × 7.659 L/h = 490.21 mg/h |
| 8. Maintenance infusion rate during CVVH = (maintenance infusion rate from equation 7) × (Clestimated/100) |
| Maintenance infusion rate = 490.21 mg/h × (26.8 mL/min/100 mL/min) = 131 mg/h |
Cpeak = peak concentration; Css = steady-state concentration; Clestimated = estimated clearance; ClHF = hemofiltration clearance; Cltotal = total clearance; CrCl = creatinine clearance; CVVH = continuous venovenous hemofiltration; Ke = elimination rate constant; MIC = minimum inhibitory concentration; S = sieving coefficient; UFR = ultrafiltration rate; Vd = volume of distribution.
Footnotes
Estimates of lean body weight and dry body weight should be considered in calculation of these dosages. Clinical judgment should be used in determining the dosing weight in patients with morbid obesity.
Table 3 provides data for renal and nonrenal clearance for β-lactam antibiotics. Nonrenal clearance in this table may vary substantlally in ICU patients. Caution should be taken in using nonrenal clearance, as this may overestimale actual clearance in critically ill patients.
Contributor Information
Brad Moriyama, Pharmacy Department, National Institutes of Health (NIH) Clinical Center, Bethesda, MD.
Stacey A Henning, Pharmacy Department, NIH Clinical Center.
Melinda M Neuhauser, US Department of Veteran Affairs, Pharmacy Benefits Management Services, Hines, IL.
Robert L Danner, Critical Care Medicine Department, NIH Clinical Center.
Thomas J Walsh, Immunocompromised Host Section, Pediatric Oncology Branch, National Cancer Institute, Bethesda.
References
- 1.Talbot GH, Bradley J, Edwards JE Jr, Gilbert D, Scheld M, Bartlett JG. Bad bugs need drugs: an update on the development pipeline from the Antimicrobial Availability Task Force of the Infectious Diseases Society of America. Clin Infect Dis 2006;42:657–68. [DOI] [PubMed] [Google Scholar]
- 2.O’Neill AJ. New antibacterial agents for treating infections caused by multi-drug resistant gram-negative bacteria. Expert Opin Investig Drugs 2008;17:297–302. [DOI] [PubMed] [Google Scholar]
- 3.Nicasio AM, Kuti JL, Nicolau DP. The current state of multidrug-resistant gram-negative bacilli in North America. Pharmacotherapy 2008;28:235–49. [DOI] [PubMed] [Google Scholar]
- 4.Spellberg B, Guidos R, Gilbert D, et al. The epidemic of antibiotic-resistant infections: a call to action for the medical community from the Infectious Diseases Society of America. Clin Infect Dis 2008;46:155–64. [DOI] [PubMed] [Google Scholar]
- 5.Lodise TP, Lomaestro BM, Drusano GL; Society of Infectious Diseases Pharmacists. Application of antimicrobial pharmacodynamic concepts into clinical practice: focus on β-lactam antibiotics: insights from the Society of Infectious Diseases Pharmacists. Pharmacotherapy 2006;26:1320–32. [DOI] [PubMed] [Google Scholar]
- 6.DeRyke CA, Lee SY, Kuti JL, Nicolau DP. Optimising dosing strategies of antibacterials utilising pharmacodynamic principles: impact on the development of resistance. Drugs 2006;66:1–14. [DOI] [PubMed] [Google Scholar]
- 7.Piccoli L, Larosa M, Marchetti F. Time-kill curves as a tool for targeting ceftazidime serum concentration during continuous infusion. J Antimicrob Chemother 2003;52:1047–8. [DOI] [PubMed] [Google Scholar]
- 8.Craig WA. Pharmacokinetic/pharmacodynamic parameters: rationale for antibacterial dosing of mice and men. Clin Infect Dis 1998;26:1–12. [DOI] [PubMed] [Google Scholar]
- 9.Craig WA, Ebert SC. Killing and regrowth of bacteria in vitro: a review. Scand J Infect Dis Suppl 1991;74:63–70. [PubMed] [Google Scholar]
- 10.Gerber AU, Feller-Segessenmann C. In-vivo assessment of in-vitro killing patterns of Pseudomonas aeruginosa. J Antimicrob Chemother 1985;15(suppl A):201–6. [DOI] [PubMed] [Google Scholar]
- 11.Gerber AU, Feller C, Brugger HP. Time course of the pharmacological response to beta-lactam antibiotics in vitro and in vivo. Eur J Clin Microbiol 1984;3:592–7. [DOI] [PubMed] [Google Scholar]
- 12.Mouton JW, Vinks AA. Continuous infusion of beta-lactams. Curr Opin Crit Care 2007;13:598–606. [DOI] [PubMed] [Google Scholar]
- 13.Kasiakou SK, Sermaides GJ, Michalopoulos A, Soteriades ES, Falagas ME. Continuous versus intermittent intravenous administration of antibiotics: a meta-analysis of randomised controlled trials. Lancet Infect Dis 2005;5:581–9. [DOI] [PubMed] [Google Scholar]
- 14.Bauer LA. Applied clinical pharmacokinetics. New York: McGraw-Hill, 2001:39–41 [Google Scholar]
- 15.Mouton JW, Vinks AA. Is continuous infusion of β-lactam antibiotics worthwhile? Efficacy and pharmacokinetic considerations. J Antimicrob Chemother 1996;38:5–15. [DOI] [PubMed] [Google Scholar]
- 16.Mouton JW, den Hollander JG. Killing of Pseudomonas aeruginosa during continuous and intermittent infusion of ceftazidime in an in vitro pharmacokinetic model. Antimicrob Agents Chemother 1994;38:931–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cappelletty DM, Kang SL, Palmer SM, Rybak MJ. Pharmacodynamics of ceftazidime administered as continuous infusion or intermittent bolus alone and in combination with single daily-dose amikacin against Pseudomonas aeruginosa in an in vitro infection model. Antimicrob Agents Chemother 1995;39:1797–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tessier PR, Nicolau DP, Onyeji CO, Nightingale CH. Pharmacodynamics of intermittent- and continuous-infusion cefepime alone and in combination with once-daily tobramycin against Pseudomonas aeruginosa in an in vitro infection model. Chemotherapy 1999;45:284–95. [DOI] [PubMed] [Google Scholar]
- 19.Alou L, Aguilar L, Sevillano D, et al. Is there a pharmacodynamic need for the use of continuous versus intermittent infusion with ceftazidime against Pseudomonas aeruginosa? An in vitro pharmacodynamic model. J Antimicrob Chemother 2005;55:209–13. [DOI] [PubMed] [Google Scholar]
- 20.Kroeger LA, Hovde LB, Mitropoulos IF, Schafer J, Rotschafer JC. Colistin methanesulfonate against multidrug-resistant Acinetobacter baumannii in an in vitro pharmacodynamic model. Antimicrob Agents Chemother 2007;51:3431–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xiong YQ, Caillon J, Zhou XY, et al. Treatment of experimental rabbit infective endocarditis due to a multidug-resistant Pseudomonas aeruginosa with high-dose ceftazidime alone and combined with amikacin or sulbactam or both. J Antimicrob Chemother 1995;35:697–706. [DOI] [PubMed] [Google Scholar]
- 22.Robaux MA, Dube L, Caillon J, et al. In vivo efficacy of continuous infusion versus intermittent dosing of ceftazidime alone or in combination with amikacin relative to human kinetic profiles in a Pseudomonas aeruginosa rabbit endocarditis model. J Antimicrob Chemother 2001;47:617–22. [DOI] [PubMed] [Google Scholar]
- 23.Navas D, Caillon J, Gras-Le Guen C, et al. Comparison of in vivo intrinsic activity of cefepime and imipenem in a Pseudomonas aeruginosa rabbit endocarditis model: effect of combination with tobramycin simulating human serum pharmacokinetics. J Antimicrob Chemother 2004;54:767–71. [DOI] [PubMed] [Google Scholar]
- 24.Daenen S, de Vries-Hospers H. Cure of Pseudomonas aeruginosa infection in neutropenic patients by continuous infusion of ceftazidime (letter). Lancet 1988;1:937. [DOI] [PubMed] [Google Scholar]
- 25.Kuzemko J, Crawford C. Continuous infusion of ceftazidime in cystic fibrosis (letter). Lancet 1989;2:385. [DOI] [PubMed] [Google Scholar]
- 26.Vinks AA, Brimicombe RW, Heijerman HG, Bakker W. Continuous infusion of ceftazidime in cystic fibrosis patients during home treatment: clinical outcome, microbiology and pharmacokinetics. J Antimicrob Chemother 1997;40:125–33. [DOI] [PubMed] [Google Scholar]
- 27.Bosso JA, Bonapace CR, Flume PA, White RL. A pilot study of the efficacy of constant-infusion ceftazidime in the treatment of endobronchial infections in adults with cystic fibrosis. Pharmacotherapy 1999;19:620–6. [DOI] [PubMed] [Google Scholar]
- 28.Rappaz I, Decosterd LA, Bille J, Pilet M, Bélaz N, Roulet M. Continuous infusion of ceftazidime with a portable pump is as effective as thrice-a-day bolus in cystic fibrosis children. Eur J Pediat 2000;159:919–25. [DOI] [PubMed] [Google Scholar]
- 29.Domenig C, Traunmuller F, Kozek S, et al. Continuous beta-lactam antibiotic therapy in a double-lung transplanted patient with a multidug-resistant Pseudomonas aeruginosa infection. Transplantation 2001;71:744–5. [DOI] [PubMed] [Google Scholar]
- 30.Hayes D Jr, Kanga JF, Anstead MI, Kuhn RJ. Novel approach to the eradication of Pseudomonas aeruginosa in an infant with CF after outpatient treatment failure. Pediatr Pulmonol 2008;43:511–3. [DOI] [PubMed] [Google Scholar]
- 31.Gorman SK, Zed PJ, Dhingra VK, Ronco JJ. Rapid imipenem/cilastatin desensitization for multidrug-resistant Acinetobacter pneumonia. Ann Pharmacother 2003;37:513–6. DOI 10.1345/aph.1C315 [DOI] [PubMed] [Google Scholar]
- 32.Bohler J, Donauer J, Keller F. Pharmacokinetic principles during continuous renal replacement therapy: drugs and dosage. Kidney Int Suppl 1999;72:S24–8. [PubMed] [Google Scholar]
- 33.Bressolle F, Kinowski JM, de la Coussaye JE, Wynn N, Eledjam JJ, Galtier M. Clinical pharmacokinetics during continuous haemofiltration. Clin Pharmacokinet 1994;26:457–71. [DOI] [PubMed] [Google Scholar]
- 34.Bugge JF. Pharmacokinetics and drug dosing adjustments during continuous venovenous hemofiltration or hemodiafiltration in critically ill patients. Acta Anaesthesiol Scand 2001;45:929–34. [DOI] [PubMed] [Google Scholar]
- 35.Golper TA, Wedel SK, Kaplan AA, Saad AM, Donta ST, Paganini EP. Drug removal during continuous arteriovenous hemofiltration: theory and clinical observations. Int J Artif Organs 1985;8:307–12. [PubMed] [Google Scholar]
- 36.Golper TA. Drug removal during continuous hemofiltration or hemodialysis. Contrib Nephrol 1991;93:110–6. [DOI] [PubMed] [Google Scholar]
- 37.Golper TA, Marx MA. Drug dosing adjustments during continuous renal replacement therapies. Kidney Int Suppl 1998;66:S165–8. [PubMed] [Google Scholar]
- 38.Golper TA. Update on drug sieving coefficients and dosing adjustments during continuous renal replacement therapies. Contrib Nephrol 2001;132:349–53. [DOI] [PubMed] [Google Scholar]
- 39.Joy MS, Matzke GR, Armstrong DK, Marx MA, Zarowitz BJ. A primer on continuous renal replacement therapy for critically ill patients. Ann Pharmacother 1998;32:362–75. DOI 10.1345/aph.17105 [DOI] [PubMed] [Google Scholar]
- 40.Keller F, Böhler J, Czock D, Zellner D, Mertz AK. Individualized drug dosage in patients treated with continuous hemofiltration. Kidney Int Suppl 1999;56:S29–31. [PubMed] [Google Scholar]
- 41.Reetze-Bonorden P, Böhler J, Keller E. Drug dosage in patients during continuous renal replacement therapy. Pharmacokinetic and therapeutic considerations. Clin Pharmacokinet 1993;24:362–79. [DOI] [PubMed] [Google Scholar]
- 42.Schetz M, Ferdinande P, Van den Berghe G, Verwaest C, Lauwers P. Pharmacokinetics of continuous renal replacement therapy. Intensive Care Med 1995;21:612–20. [DOI] [PubMed] [Google Scholar]
- 43.Schetz M Drug dosing in continuous renal replacement therapy: general rules. Curr Opin Crit Care 2007;13:645–51. [DOI] [PubMed] [Google Scholar]
- 44.Manns M, Sigler MH, Teehan BP. Continuous renal replacement therapies: an update. Am J Kidney Dis 1998;32:185–207. [DOI] [PubMed] [Google Scholar]
- 45.Kellum JA, Mehta RL, Angus DC, Palevsky P, Ronco C; ADQI Work-group. The first international consensus conference on continuous renal replacement therapy. Kidney Int 2002;62:1855–63. [DOI] [PubMed] [Google Scholar]
- 46.Brunet S, Leblanc M, Geadah D, Parent D, Courteau S, Cardinal J. Diffusive and convective solute clearances during continuous renal replacement therapy at various dialysate and ultrafiltration flow rates. Am J Kidney Dis 1999;34:486–92. [DOI] [PubMed] [Google Scholar]
- 47.Product information. Unasyn (ampicillin/sulbactam). New York, NY: Pfizer Roerig, September 2003. [Google Scholar]
- 48.McEvoy GK, Snow ED, eds. AHFS: drug information. Bethesda, MD: American Society of Health-System Pharmacists, 2008. [Google Scholar]
- 49.Kucers A, Crowe SM, Grayson ML, et al. The use of antibiotics—a clinical review of antibacterial, antifungal and antiviral drugs. Sth ed. Oxford, England: Butterworth-Heinemann, 1997. [Google Scholar]
- 50.Blum RA, Kohli RK, Harrison NJ, Schentag JJ. Pharmacokinetics of ampicillin (2.0 grams) and sulbactam (1.0 gram) coadministered to subjects with normal and abnormal renal function and with end-stage renal disease on hemodialysis. Antimicrob Agents Chemother 1989;33:1470–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Foulds G, Stankewich JP, Marshall DC, et al. Pharmacokinetics of sulbactam in humans. Antimicrob Agents Chemother 1983;23:692–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Product information. Azactam (aztreonam). Princeton, NJ: Bristol-Myers Squibb Co., January 2007. [Google Scholar]
- 53.Swabb EA, Sugerman AA, Platt TB, Pilkiewicz FG, Frantz M. Single-dose pharmacokinetics of the monobactam aztreonam (SQ 26,776) in healthy subjects. Antimicrob Agents Chemother 1982;21:944–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mihindu JCL, Scheld WM, Bolton ND, Spyker DA, Swabb EA, Bolton WK. Pharmacokinetics of aztreonam in patients with various degrees of renal dysfunction. Antimicrob Agents Chemother 1983;24:252–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.O’Neil MJ, Smith A, Heckelman PE, et al. The Merck index. 13th ed. Whitehouse Station, NJ: Merck & Co., Inc., 2001. [Google Scholar]
- 56.Product information. Maxipime (cefepime). Princeton, NJ: Bristol-Myets Squibb Co., January 2007. [Google Scholar]
- 57.Barthaiya RH, Knupp CA, Forgue ST, Matzke GR, Guay DRP, Pittman KA. Pharmacokinetics of cefepime in subjects with renal insufficiency. Clin Pharmacol Ther 1990;48:268–76. [DOI] [PubMed] [Google Scholar]
- 58.Tam VH, McKinnon PS, Akins RL, Drusano GL, Rybak MJ. Pharmacokinetics and pharmacodynamics of cefepime in patients with various degrees of renal function. Antimicrob Agents Chemother 2003;47:1853–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Product information. Fortaz (ceftazidime). Research Triangle Park, NC: GlaxoSmithKline, February 2007. [Google Scholar]
- 60.Leroy A, Leguy F, Borsa F, Spencer GR, Fillastre JP, Humbert G. Pharmacokinetics of ceftazidime in normal and uremic subjects. Antimicrob Agents Chemother 1984;25:638–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Product information. Pfizerpen (penicillin G potassium). New York, NY: Pfizer Roerig, December 2005. [Google Scholar]
- 62.Aronoff GR, Berns JS, Brier ME, et al. Drug prescribing in renal failure. 4th ed. Philadelphia, PA: American College of Physicians, 1999:51. [Google Scholar]
- 63.Bryan CS, Stone WJ. “Comparably massive” penicillin G therapy in renal failure. Ann Intern Med 1975;82:189–95. [DOI] [PubMed] [Google Scholar]
- 64.Product information. Zosyn (piperacillin/tazobactam). Philadelphia, PA: Wyeth Pharmaceutical Inc., August 2006. [Google Scholar]
- 65.Johnson CA, Halstenson CE, Kelloway JS, et al. Single-dose pharmacokinetics of piperacillin and tazobactam in patients with renal disease. Clin Pharmacol Ther 1992;51:32–41. [DOI] [PubMed] [Google Scholar]
- 66.Product information. Timentin (ticarcillin/clavulanate). Research Triangle Park, NC: GlaxoSmithKline, December 2004. [Google Scholar]
- 67.Jungbluth GL, Cooper DL, Doyle GD, Chudzik GM, Jusko WJ. Pharmacokinetics of ticarcillin and clavulanic acid (Timentin) in relation to renal function. Antimicrob Agents Chemother 1986;30:896–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dalet F, Amado E, Cabrera E, Donate T, del Rio G. Pharmacokinetics of the combination of ticarcillin with clavulanic acid in renal insufficiency. J Antimicrob Chemother 1986;17(suppl C):57–64. [DOI] [PubMed] [Google Scholar]
- 69.Sweetman SC, ed. Martindale. 33rd ed. London, England: Royal Pharmaceutical Society of Great Britain, 2002. [Google Scholar]
- 70.Kuang D, Verbine A, Ronco C. Pharmacokinetics and antimicrobial dosing adjustment in critically ill patients during continuous renal replacement therapy. Clin Nephrol 2007;67:267–84. [DOI] [PubMed] [Google Scholar]
- 71.Malone RS, Fish DN, Abraham E, Teitelbaum I. Pharmacokinetics of cefepime during continuous renal replacement therapy in critically ill patients. Antimicrob Agents Chemother 2001;45:3148–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Isla A, Gascón AR, Maynar J, Arzuaga A, Toral D, Pedraz JL. Cefepime and continuous renal replacement therapy (CRRT): in vitro permeability of two CRRT membranes and pharmacokinetics in four critically ill patients. Clin Ther 2005;27:599–608. [DOI] [PubMed] [Google Scholar]
- 73.Isla A, Gascón AR, Maynar J, Arzuaga A, Sánchez-lzquierdo JA, Pedraz JL. In vitro AN69 and polysulphone membrane permeability to ceftazidime and in vivo pharmacokinetics during continuous renal replacement therapies. Chemotherapy 2007;53:194–201. [DOI] [PubMed] [Google Scholar]
- 74.Bouman CS, van Kan HJ, Koopmans RP, Korevaar JC, Schultz MJ, Vroom MB. Discrepancies between observed and predicted continuous venovenous hemofiltration removal of antimicrobial agents in critically ill patients and the effects on dosing. Intensive Care Med 2006;32:2013–9. [DOI] [PubMed] [Google Scholar]
- 75.Traunmüller F, Schenk P, Mittermeyer C, Thalhammer-Scherrer R, Ratheiser K, Thalhammer F. Clearance of ceftazidime during continuous venovenous haemofiltration in critically ill patients. J Antimicrob Chemother 2002;49:129–34. [DOI] [PubMed] [Google Scholar]
- 76.Matzke GR, Frye RF, Joy MS, Palevsky PM. Determinants of ceftazidime clearance by continuous venovenous hemofiltration and continuous venovenous hemodialysis. Antimicrob Agents Chemother 2000;44:1639–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Arzuaga A, Maynar J, Gascón AR, et al. Influence of renal function on the pharmacokinetics of piperacillin/tazobactam in intensive care unit patients during continuous venovenous hemofiltration. J Clin Pharmacol 2005;45:168–76. [DOI] [PubMed] [Google Scholar]
- 78.Lindsay CA, Bawdon R, Quigley R. Clearance of ticarcillin-clavulanic acid by continuous venovenous hemofiltration in three critically ill children, two with and one without concomitant extracorporeal membrane oxygenation. Pharmacotherapy 1996;16:458–62. [PubMed] [Google Scholar]
- 79.Rumpf KW, Rieger J, Doht B, Ansorg R, Scheler F. Drug elimination by hemofiltration. J Dial 1977;1:677–8. [DOI] [PubMed] [Google Scholar]
- 80.Kraft D, Lode H. Elimination of ampicillin and gentamicin by hemofiltration. Klin Wochenschr 1979;57:195–6. [DOI] [PubMed] [Google Scholar]
- 81.Joos B, Schmidli M, Keusch G. Pharmacokinetics of antimicrobial agents in anuric patients during continuous venovenous haemofiltration. Nephrol Dial Transplant 1996;11:1582–5. [PubMed] [Google Scholar]
- 82.Rohde B, Werner U, Hickstein H, Ehmcke H, Drewelow B. Pharmacokinetics of mezlocillin and sulbactam under continuous veno-venous hemodialysis (CVVHD) in intensive care patients with acute renal failure. Eur J Clin Pharmacol 1997;53:111–5. [DOI] [PubMed] [Google Scholar]
- 83.Capellier G, Comette C, Boillot A, et al. Removal of piperacillin in critically ill patients undergoing continuous venovenous hemofiltration. Crit Care Med 1998;26:88–91. [DOI] [PubMed] [Google Scholar]
- 84.Valtonen M, Tiula E, Takkunen O, Backman JT, Neuvonen PJ. Elimination of the piperacillin/tazobactam combination during continuous ven-ovenous haemofiltration and haemodiafiltration in patients with acute renal failure. J Antimicrob Chemother 2001;48:881–5. [DOI] [PubMed] [Google Scholar]
- 85.van der Werf TS, Mulder PO, Zijlstra JG, Uges DR, Stegeman CA. Pharmacokinetics of piperacillin and tazobactam in critically ill patients with renal failure, treated with continuous veno-venous hemofiltration (CVVH). Intensive Care Med 1997;23:873–7. [DOI] [PubMed] [Google Scholar]
- 86.Mariat C, Venet C, Jehl F, et al. Continuous infusion of ceftazidime in critically ill patients undergoing continuous venovenous haemodiafiltration: pharmacokinetic evaluation and dose recommendation. Crit Care 2006;10:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Langgartner J, Vasold A, Glück T, Reng M, Kees F. Pharmacokinetics of meropenem during intermittent and continuous intravenous application in patients treated by continuous renal replacement therapy. Intensive Care Med 2008;34:1091–6. [DOI] [PubMed] [Google Scholar]


