Abstract
BACKGROUND AND OBJECTIVES:
Enumeration of hematopoietic progenitor cell (HPC) is vital to decide the time to initiate harvest (TTIH) and adequacy of harvest dose (AOHD). Standard of care used for HPC enumeration is flowcytometric CD34+ enumeration, but it is expensive, time-consuming and requires skilled staff to perform the test. Alternatively, HPC-count by advanced automated cell analyzer is cheaper, quicker, and easy-to-perform test. Our objective was to find a correlation of HPC count with CD34+ enumeration in leukapheresis.
MATERIALS AND METHODS:
An observational, prospective study was conducted in the year 2018–2019. A total of 126 samples were included in the study, the peripheral blood (PB) group comprised of 42samples and apheresis group of 84 samples. The samples were simultaneously tested for CD34+ expression and complete blood count which included the HPC count, white blood cells (WBC) count and multinational corporation (MNC) count and correlation analysis was performed with CD34+ flowcytometric count. The cut-off of PB HPC count for the target dose of 5 × 106 CD34+ cells/kg was established using Receiver Operator Curve.
RESULTS:
The correlation coefficient (r) of HPC with CD34+ count was 0.617 and 0.699 for PB group and apheresis group sample respectively, which was statistically significant. The correlation with MNC and WBC count was not very significant. A cut-off value of PB HPC was established to be 66 HPC/μl with a positive predictive value of 94.12%. The cost of CD34 + flow cytometric enumeration was six times that of HPC enumeration by analyzer.
CONCLUSION:
The HPC count is a cheaper, rapid and easy test and can be clinically applied to predict TTIH and AOHD but requires more studies to validate its efficacy in clinical use.
Keywords: Adequacy of harvest dose, CD34+, hematopoietic progenitor cell, leukapheresis, time to initiate harvest
Introduction
The most common indications for allogenic hematopoietic progenitor cell (HPC) collection by apheresis (HPC-A) are leukemias, lymphoproliferative disorders, and nonmalignant disorders, while the main indications for autologous HPC (A) are lymphoproliferative disorders, solid tumors and nonmalignant disorders.[1] HPC-A is the commonest source of HPC harvest because of easy acquisition, rapid engraftment, and minimum laboratory processing before infusion as compared to HPC harvest from Marrow (HPC-M). After mobilization of HPCs with granulocyte-colony stimulating factor (G-CSF), the total white cell count and CD34+ cell count are monitored and if adequate, HPC harvest is done by leukapheresis.[2]
HPC enumeration is essential to decide the time to initiate harvest (TTIH) by testing the peripheral blood (PB) for the concentration of HPC into circulation and to determine the adequacy of harvest dose (AOHD), in the apheresis collection during or after the procedure. A minimum of 10–20 cells/μl of CD34+ cells in PB was thought to be adequate to initiate leukapheresis.[3] AOHD estimation is crucial for efficient engraftment and a dose of 5 × 106 cells/kg patient’s body weight is desirable.[2]
The standard of care is the measurement of cell surface antigen CD34 which is used for identification and quantification of HPC typically by flowcytometer technique. However, flow cytometry is expensive, time-consuming, and requires trained staff for performing and analyzing the test. The availability of flowcytometer is also limited and very few centers have this equipment.
There is an alternative method of HPC-count enumeration, by newer advanced automated cell analyzers. It is inexpensive, provides quick results and does not require specially trained staff for performance. Automated analyzers are comparatively more widely available, in almost all the centers that perform HPC (A) in India, in comparison to the flow-cytometers.
The objective of our study was to find the usefulness of HPC count by automated analyzer in estimating the TTIH and AOHD in HPC-A setting as compared to the current standard-of-care testing by CD34 flowcytometry. In addition to this, the correlation of white blood cells (WBC) count and multinational corporation (MNC) count were also analyzed with respect to CD34 cell count.
Materials and Methods
Settings
An observational, prospective study involving 42 informed and consented donors (allogenic donors and autologous, i.e., patient-donors) posted for HPC (A), was conducted in a tertiary care hospital in North India over a period of 15 months (February 2018 to April 2019).
Indications for hematopoietic progenitor cell autologous leukapheresis
There were a total of 42 donors in the study, which included 29 (69%) allogenic donors and 13 (31%) patient-donors. The indications for allogenic leukapheresis (n = 29) were acute leukemia (n = 12), acquired aplastic anemia (n = 5) and Fanconi’s anemia (n = 2), hemoglobinopathies (n = 2) and other miscellaneous conditions (n = 8). The indications for autologous leukapheresis (n = 13) were Lymphoma (n = 6), multiple myeloma (n = 5), and other miscellaneous conditions (n = 2).
Donor demographics
The gender ratio (male: Female) in donor population (n = 42) was 2:1. The median age and weight of allogenic-donors was 21 years and 58 kg respectively; and that of the patient-donors was 50 years and 62 kg respectively. The distribution of blood Group O pos was more in our donor as well as patient population, followed by the blood Groups A positive and B positive. The allogenic HPC (A) group (n = 29) had variable ABO compatibility between donors and patients. Majority were ABO compatible (55%), followed by minor incompatible (31%), major incompatible (7%), and bidirectional incompatible group (7%).
Mobilization
The donors were mobilized with G-CSF (Grafeel, Dr. Reddy’s Laboratories Ltd., India) injected subcutaneously in a dose of 10ug/kg/day, in two divided doses for four consecutive days and a single dose on the 5th day morning before leukapheresis. On 4th day, the donors with PB CD34+ count of <20 cells/ul, were labeled as “poor mobilizers,” and were additionally mobilized using single dose of 0.24 mg/kg Plerixafor (Mozifor, Hetero Drugs Ltd., India) subcutaneously, a night prior to leukapheresis.
Leukapheresis
All the leukapheresis procedures were done on apheresis machine COM.TEC® (Fresenius Kabi, Germany). The P1YA kit was used, and the collection program was set to auto-mononuclear cells (auto-MNC). The vascular access used was hemodialysis-type double-lumen intravenous catheter in the jugular or femoral vein of 11 French units. The targeted dose of CD34+ cells was 5 × 106 cells/kg patient’s body weight.
Study Samples
Two (2) ml of sample was collected each time in an (Ethylenediaminetetraacetic acid) vacutainer from each donor at three-time intervals; the “preharvest” sample was collected from the PB before the procedure, the “mid-harvest” sample was from apheresis collection bag during the mid-procedure and the “postharvest” sample was taken at the end of HPC (A) collection from the apheresis collection bag. Thus, 126 samples of which 42 PB group samples (PB) and 84 Apheresis group samples (APH) were considered for statistical evaluation.
Enumeration
The sample was simultaneously analyzed on automated advanced cell analyzer (Sysmex XN-9112, Kobe, Japan) and flowcytometer (Becton, Dickinson and Company, New Jersey, U.S), for CD34+ enumeration. Flowcytometric CD34+ enumeration was considered the gold standard in this study.
Enumeration using automated advanced cell-analyzer
HPCs were identified using the Sysmex XN-9112 in Immature Myeloid information channel that uses the principle of radiofrequency and direct current to measure cell size and density. The cell analyzer uses Stromatolyser-IM (Sysmex, Kobe, Japan) which lyses mature leukocytes leaving behind the immature cells based on the difference in lipid content of these two cells. The fluorescence flow cytometer uses three dimensions to identify HPC and hence other cell populations, such as Nucleated red blood cell, myeloid progenitor cells or lymphocytes, which look morphologically similar to stem cells, do not interfere with the HPC as they have a different membrane composition.[4] The HPC count and complete blood count including WBC and MNC was obtained.
Enumeration of CD34+ cells (hematopoietic progenitor cell) using flowcytometer
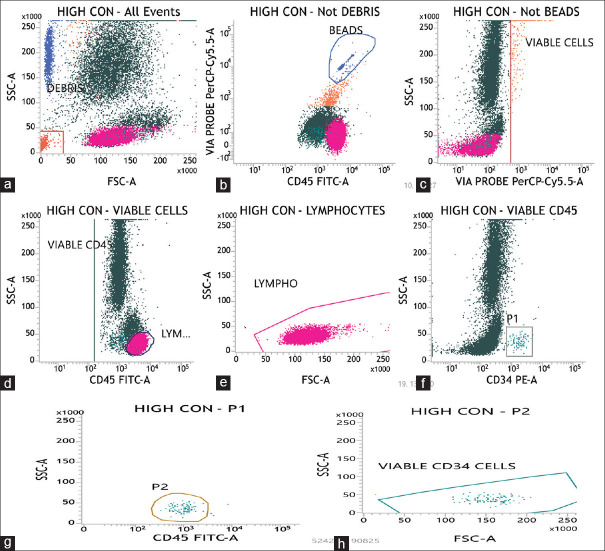
According to ISHAGE[5] (international society of hematotherapy and graft engineering) single platform protocol and BD software FACSuite v1.0.6 (Becton, Dickinson and Company, New Jersey, USA), stem cells were gated and enumerated. We used CD34 PE (Phycoerythrin) and CD45 FITC (Fluorescein isothiocyanate) from BD (Becton, Dickinson and Company, New Jersey, USA). The primary sample was diluted to achieve the final concentration of WBC in the test sample to 25 × 103 WBC/μl Three trucount tubes namely low control, high control, and test sample were labeled. 10μl of both CD34 PE and CD45 FITC antibodies were added to each tube. Then, 100 μL of test sample was added to each tube by reverse pipetting technique. The tubes were given a vortex mix and then incubated at 4°C for 20 min in the dark. After incubation, 2 ml of ×1 lysing buffer (Pharma Lyse, Becton, Dickinson and Company, New Jersey, USA) to lyse the RBC and 20μlof Viability Dye: 7-Amino Actinomycin D (7-AAD, Becton, Dickinson and Company, New Jersey, USA) to stain the nonviable cells was added to the each tube. Again these tubes were given a brief vortex mix and incubated for 10 min. 7-AAD, used in viability testing is membrane impermeant dye that is excluded from viable cells and helps differentiating viable and nonviable cells. The quality control of the flowcytometer was undertaken. A new CD34 assay was obtained, an example of the same is depicted in Figure 1. A threshold of total number of viable CD34+ cells was set to 200 according to our standard operating procedures. The tubes were acquired as low control, high control, and test sample.
Figure 1.
Flowcytometric enumeration of CD34+ cells using international society of hematotherapy and graft engineering single platform: (a) Debris are gated in FSC-A versus SSC-A plot (all events are considered). (b) Non-debris population is selected and beads are gated. (c) Then non-beads events are plotted with through probe PerCp cy 5.5 in FSC versus SSC-A, the viable cells are then gated. (d and e) Out of those viable cells CD45+ cells are taken and lymphocytes are gated to check the position of blasts cells i.e., dim for CD45+ but behind the lymphocytes. (f) Out of these viable CD45+ cells plot, another graph is made with CD34PE versus SSC-A and bright CD34+ cells are gated and named as P1. (g) The population of cells positive for CD45 cells is named as P2 cells. (h) These P1 cells, dim for CD45 (as seen in e) are then represented on FSC-A versus SSC-A plot and bright cluster is gated and includes viable CD34+ cells. * FSC = Forward Scatter, SSC = Side Scatter, (*Correlation is significant at 0.01 level (2-tailed))
The absolute numbers of viable CD34 cells are calculated using formula-
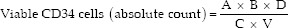
A = Number of CD34 cells acquired D = Dilution factor
B = Total bead count V = Volume of sample used
C = Number of beads acquired
Turn-around time
The Turn-around-time (TAT) was described as the time in minutes from moment the sample was received to the time the report was generated.
Cost-benefit analysis
The costing of the enumeration included the cost of the reagents (sheath fluid, CD45 FITC, and CD34 PE antibodies, forest advisory committee (FACs) lyse, FACs Clean, reagent for HPC enumeration, etc.,), consumables (vials, tips, falcon tube) and controls, calculated per test in Indian Rupee (INR; 1 INR = 0.014 US Dollar [$]).
Data compilation and statistical analysis
The donor demographics; HPC count, WBC count, MNC count, and IG index as well as CD34+ flowcytometric enumeration, pertinent to each HPC (A) procedure were analyzed using Microsoft Excel 2007 Microsoft Excel 2007 (v.12.0), (Microsoft Corporation, Redmond, WA)and SPSS (v. 23), SPSS (v.23) (Statistical Package for the Social Sciences; IBM Bengaluru, India). The analysis focused on estimating the correlation of HPC count, WBC count, MNC count, and IG index with CD34+ count using a flowcytometer (gold-standard). The targeted dose of CD34+ cells in apheresis collection bag was 5 × 106 cells/kg patient’s body weight according to institutional protocol. The receiver operator characteristic (ROC) curve was plotted to calculate the cut-off of PB HPC count for the targeted dose and the area under the curve (AUC) was analyzed. The ROC curve represents the trade-offs involved between a test’s sensitivity (Sn) and specificity (Sp) in a graphical form. Sn was defined as the probability of PB HPC to be more than the cut-off, to obtain the target dose of CD34 + yield and Sp, as the probability that the target dose was not achieved when the PB HPC count was less than the cut-off. Positive predictive value (PPV) was defined as the percentage chance of obtaining more than the target CD34+ dose when the HPC count was more than the cut-off and negative predictive value (NPV), as the percentage chance of not completing the target when the PB HPC was less than the cut-off.
Ethical approval
The study was approved by Institutional Review Board (MICR-849/2018).
Results
Study samples
A total of 42 PB and 84 APH samples from 29 allogenic and 13 autologous donors were analyzed.
3.2 Range, Mean and Correlation analysis of PB group and APH group samples.
The range and mean of WBC, MNC, IG, HPC, and CD34+ counts in PB group and APH group are detailed in the Table 1. The correlation between HPC count and CD34+ count was most significant, with a correlation coefficient, r = 0.617 in PB group samples (n = 42) and r = 0.699 in APH group samples (n = 84), depicted in Figure 2. The WBC, MNC, and IG counts also showed a positive correlation with CD34+ cell count. The correlation coefficients (r) are given in the table below:
Table 1.
Range, mean and correlation coefficient of peripheral blood and apheresis samples, considering CD34+as the gold standard
| Parameter (103*/µl) | PB | APH | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Range | Mean | Correlation coefficient (r) | Range | Mean | Correlation coefficient (r) | |
| WBC | 6.574-118.412 | 49.846 | 0.397* | 24.365-522.07 | 297.691 | 0.216 |
| MNC | 1.403-19.187 | 7.842 | 0.245 | 4.521-357.921 | 146.942 | 0.358* |
| HPC | 0.009-0.0187 | 0.058 | 0.617* | 0.087-10.176 | 1.712 | 0.699* |
| CD34 (gold standard) | 0.010-0.678 | 0.091 | 1 | 0.179-17.241 | 1.754 | 1 |
PB=Peripheral blood, APH=Apheresis, HPC=Hematopoietic progenitor cell, MNC=Mononuclear cell, WBC=White blood cell Range, Mean and correlation coefficient of peripheral blood (PB) and Apheresis samples (APH), considering CD34+ as the gold standard. (*Correlation is significant at 0.01 level (2-tailed))
Figure 2.
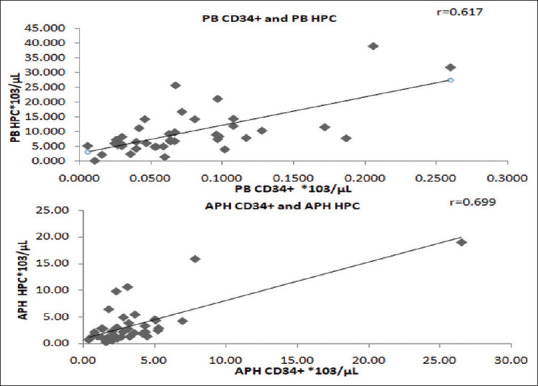
Scatter plot of correlation analysis of peripheral bloodhematopoietic progenitor cell and Apheresis blood (APH) hematopoietic progenitor cell with CD34 + count. *PB = Peripheral blood, APH = Apheresis sample, r = correlation coefficient
Correlation analysis of CD34+ yield with peripheral blood counts
TTIH, which depends on the PB counts, is an important determinant to obtain an adequate dose of CD34+ cells. CD34+ cell count in PB was observed to correlate well with the CD34+ cell yield (CD34+ cell count × 106/kg), r = 0.674. PB HPC count estimates TTIH fairly well; the correlation of PB HPC with CD34+ cell × 106/kg weight was observed as r = 0.444.
Cut-off point of peripheral blood hematopoietic progenitor cell count
ROC curve was analyzed to determine the Sn, Sp, PPV, and NPV at the range of cut-offs for PB HPC to obtain the target dose (5 × 106 CD34 cells/kg), given in Figure 3. The cut-off value of PB HPC was established to be 66 HPC/μl, with maximum Sp of 88.89%, Sn of 48.48%, and AUC of 0.631 [Table 2].
Figure 3.
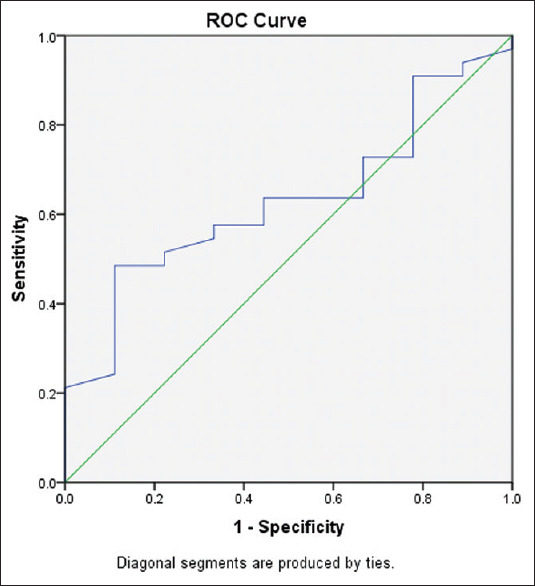
Receiver operator curve for peripheral blood hematopoietic progenitor cell count to obtain a dose of 5 × 106 CD34 cells/kg)
Table 2.
Sensitivity, specificity, negative predictive value, positive predictive value at cut-off ranges of peripheral blood hematopoietic progenitor cell
| Cut-off range for PB HPC count (/µL) | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) |
|---|---|---|---|---|
| 20-39 | 90.91 | 22.22 | 81.80 | 40 |
| 40-59 | 63.64 | 55.56 | 84 | 29.41 |
| 60-79 | 48.48 | 88.89 | 94.12 | 32 |
| >79 | 27.27 | 88.89 | 90 | 25 |
PB=Peripheral blood, HPC=Hematopoietic progenitor cell, PPV=Positive predictive value, NPV=Negative predictive value
Turn-around time
The TAT of flowcytometric CD34+ enumeration was 40–60 min (mean = 50 min) was around 30 times that of HPC count by analyzer, which was 60–120 s (mean = 90 s).
Cost-benefit analysis
The cost of CD34+ flow cytometric enumeration per sample was calculated as 2650 INR ($ 36.14), which was around six times more than HPC enumeration per sample, which was 450 INR ($ 6.14).
Discussion
Demographics
In the present study, leukapheresis was performed most commonly for allogenic HPC (A), mainly for hematological cancers and marrow failure states and hence our study population had a comparatively younger allogenic-donor (and patient) population. In the studies by Fatorova et al.[3] and Peerschke et al.[6] who have evaluated the correlation of HPC with flowcytometric CD34+ cell counts, the most common indication for leukapheresis was autologous HPC (A) for multiple myeloma and lymphoma, and therefore had a comparatively older patient-donors population.
Correlation of hematopoietic progenitor cell count in peripheral blood group and apheresis group samples group samples with CD34+ cell count
Our study evaluated the correlation of HPC count using an advanced automated cell analyzer (Sysmex-XN 9112) with CD34+ flowcytometric enumeration. Besides HPC, we also evaluated the correlation of certain other parameters such as WBC and MNC counts in our study with the CD34+ cell counts. Our results of correlation analysis of HPC and CD34+ counts were statistically significant with r = 0.617 in PB group and r = 0.699 in APH group, respectively. The correlation coefficient (r) of HPC and CD34+ cell count in various published studies ranges from 0.440 to 0.880 in PB group[7,8,9,10] and 0.460–0.930 in APH group.[3,6,11,12] In the present study, the correlation coefficient of WBC, MNC with CD34+ cell count was not significant and this was similar to other studies.[6,7,8,13,14]
Correlation of peripheral blood counts in predicting CD34+ yield
Following mobilization of HPCs, there is a transient increase in the HPC in PB. It is therefore essential to initiate leukapheresis at the appropriate time (TTIH) to achieve an adequate dose. The TTIH is usually determined by PB CD34 + cell counts by flowcytometry. Flowcytometric enumeration is expensive, time-consuming, and requires trained staff for performing the analysis. We analyzed the correlation coefficient of PB CD34+ counts and PB HPC counts with CD34+ yield, which was r = 0.674 and r = 0.444 respectively. Other transplant settings using PB CD34+ and PB HPC counts to predict the yield showed a significant correlation coefficient of CD34+ cell yield with PB CD34+ ranging from 0.847 to 0.921 and with PB HPC count ranging from 0.592 to 0.620.[7,14,15,16]
Peripheral blood hematopoietic progenitor cell cut-off for target dose of 5 × 106 cells/kg
In our study, we determined the cut-off of PB HPC for the target dose of 5 × 106 cells/kg body weight using the ROC curve, which revealed the cut-off of 66 PB HPC/μl for optimum Sn and Sp. This translated into the PPV of 94.12% and NPV of 32% as per Table 2. This is possibly for the first time that cut-off of PB HPC has been calculated for a dose of 5 × 106 cells/kg body weight of the patient. In previous studies, the cut-off for PB HPC has been calculated for a target dose of 1–2.5 × 106 cells/kg body weight.[7,9,15] The dose of 5 × 106 cells/kg is more relevant because of better engraftment kinetics and also there is loss of some viable cells during cryopreservation of leukapheresis product.[17,18,19]
Can hematopoietic progenitor cell replace CD34+ flowcytometric enumeration?
Considering the results of our study and the advantages of rapid, cheaper and technically easier way of HPC enumeration using an automated cell analyzer, we emphasize the use of PB HPC count as a supplementary test to CD34 + enumeration for determining the TTIH and AOHD. Considering our comparatively lower correlation as compared to other published data, we recommend more studies in our population setting to determine the efficacy of HPC count in replacing CD34 flowcytometric enumeration and for establishing a definite cut-off of PB HPC for adequate transplant.
Conclusion
Considering the correlation of HPC and CD34+ cell count of r = 0.617 in PB group and r = 0.699 in APH group, we recommend using HPC along with CD34 flowcytometric enumeration for adequate harvest dose.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Gratwohl A, Baldomero H, Gratwohl M, Aljurf M, Bouzas LF, Horowitz M, et al. Quantitative and qualitative differences in use and trends of hematopoietic stem cell transplantation:A Global Observational Study. Haematologica. 2013;98:1282–90. doi: 10.3324/haematol.2012.076349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fung MK, Grossman BJ, Hillyer CD, Westhoff CM, Koepsell SA, Jacob EK, et al. AABB Technical manual. 18th ed. USA: 2014. The collection and processing of hematopoietic stem cells. [Google Scholar]
- 3.Fatorova I, Blaha M, Lanska M, Vokurkova D, Rezacova V, Zak P. Timing of peripheral blood stem cell yield:Comparison of alternative methods with the classic method for CD34+cell determination. Biomed Res Int. 2014;2014:575368. doi: 10.1155/2014/575368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Erba Mannheim XN Stem Cells:The Technology Behind Automated Stem Cell Counting. [Last accessed on 2018 Mar 30]. Available from:https://erbamannheim.com/article/scientific-updates/165 .
- 5.Sutherland DR, Anderson L, Keeney M, Nayar R, Chin-Yee I. The ISHAGE guidelines for CD34+cell determination by flow cytometry. International Society of Hematotherapy and Graft Engineering. J Hematother. 1996;5:213–26. doi: 10.1089/scd.1.1996.5.213. [DOI] [PubMed] [Google Scholar]
- 6.Peerschke EI, Moung C, Pessin MS, Maslak P. Evaluation of new automated hematopoietic progenitor cell analysis in the clinical management of peripheral blood stem cell collections. Transfusion. 2015;55:2001–9. doi: 10.1111/trf.13078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yu J, Leisenring W, Fritschle W, Heimfeld S, Shulman H, Bensinger WI, et al. Enumeration of HPC in mobilized peripheral blood with the Sysmex SE9500 predicts final CD34+cell yield in the apheresis collection. Bone Marrow Transplant. 2000;25:1157–64. doi: 10.1038/sj.bmt.1702406. [DOI] [PubMed] [Google Scholar]
- 8.Gutensohn K, Magens M, Krüger W, Kröger N, Kühnl P. Comparison of flow cytometry vs. a haematology cell analyser-based method to guide the optimal time-point for peripheral blood stem cell apheresis. Vox Sang. 2006;90:53–8. doi: 10.1111/j.1423-0410.2005.00720.x. [DOI] [PubMed] [Google Scholar]
- 9.Tanaka H, Ishii A, Sugita Y, Shimizu R, Sato F, Sakuma Y, et al. Impact of hematopoietic progenitor cell count as an indicator for optimal timing of peripheral stem cell harvest in clinical practice. J Clin Exp Hematop. 2017;56:150–9. doi: 10.3960/jslrt.56.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mitani N, Yujiri T, Tanaka Y, Tanaka M, Fujii Y, Hinoda Y, et al. Hematopoietic progenitor cell count, but not immature platelet fraction value, predicts successful harvest of autologous peripheral blood stem cells. Journal of clinical apheresis. 2011;26(3):105–10. doi: 10.1002/jca.20275. [DOI] [PubMed] [Google Scholar]
- 11.Peng L, Yang J, Yang H, et al. Determination of peripheral blood stem cells by the sysmex SE-9500. Int J Lab Hematol. 2001;23:231–6. doi: 10.1046/j.1365-2257.2001.00390.x. [DOI] [PubMed] [Google Scholar]
- 12.Kumar K, Subash CS. A study to identify correlation of hematopoietic progenitor cell (XN-HPC) by sysmex cell counters with final yield of CD34+stem cells. Blood. 2017;130:5458. [Google Scholar]
- 13.Park KU, Kim SH, Suh C, Kim S, Lee SJ, Park JS, et al. Correlation of hematopoietic progenitor cell count determined by the SE-9000™automated hematology analyzer with CD34+cell count by flow cytometry in leukapheresis products. American journal of hematology. 2001 May;67(1):42–7. doi: 10.1002/ajh.1074. [DOI] [PubMed] [Google Scholar]
- 14.Chapple P, Prince HM, Quinn M, Bertoncello I, Juneja S, Wolf M, et al. Peripheral blood CD34+cell count reliably predicts autograft yield. Bone Marrow Transplant. 1998;22:125–30. doi: 10.1038/sj.bmt.1701308. [DOI] [PubMed] [Google Scholar]
- 15.Lee JL, Kim SB, Lee GW, Ryu MH, Kim EK, Kim S, et al. Clinical usefulness of the hematopoietic progenitor cell counts in predicting the optimal timing of peripheral blood stem cell harvest. J Korean Med Sci. 2003;18:27–35. doi: 10.3346/jkms.2003.18.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fontão-Wendel R, Lazar A, Melges S, Altobeli C, Wendel S. The absolute number of circulating CD34+cells as the best predictor of peripheral hematopoietic stem cell yield. J Hematother. 1999;8:255–62. doi: 10.1089/106161299320271. [DOI] [PubMed] [Google Scholar]
- 17.Weaver CH, Hazelton B, Birch R, Palmer P, Allen C, Schwartzberg L, et al. An analysis of engraftment kinetics as a function of the CD34 content of peripheral blood progenitor cell collections in 692 patients after the administration of myeloablative chemotherapy. Blood. 1995;86:3961–9. [PubMed] [Google Scholar]
- 18.Bensinger W, Appelbaum F, Rowley S, Storb R, Sanders J, Lilleby K, et al. Factors that influence collection and engraftment of autologous peripheral-blood stem cells. J Clin Oncol. 1995;13:2547–55. doi: 10.1200/JCO.1995.13.10.2547. [DOI] [PubMed] [Google Scholar]
- 19.Al-Anazi KA. Autologous hematopoietic stem cell transplantation for multiple myeloma without cryopreservation. Bone Marrow Res. 2012;2012:917361. doi: 10.1155/2012/917361. [DOI] [PMC free article] [PubMed] [Google Scholar]