Abstract
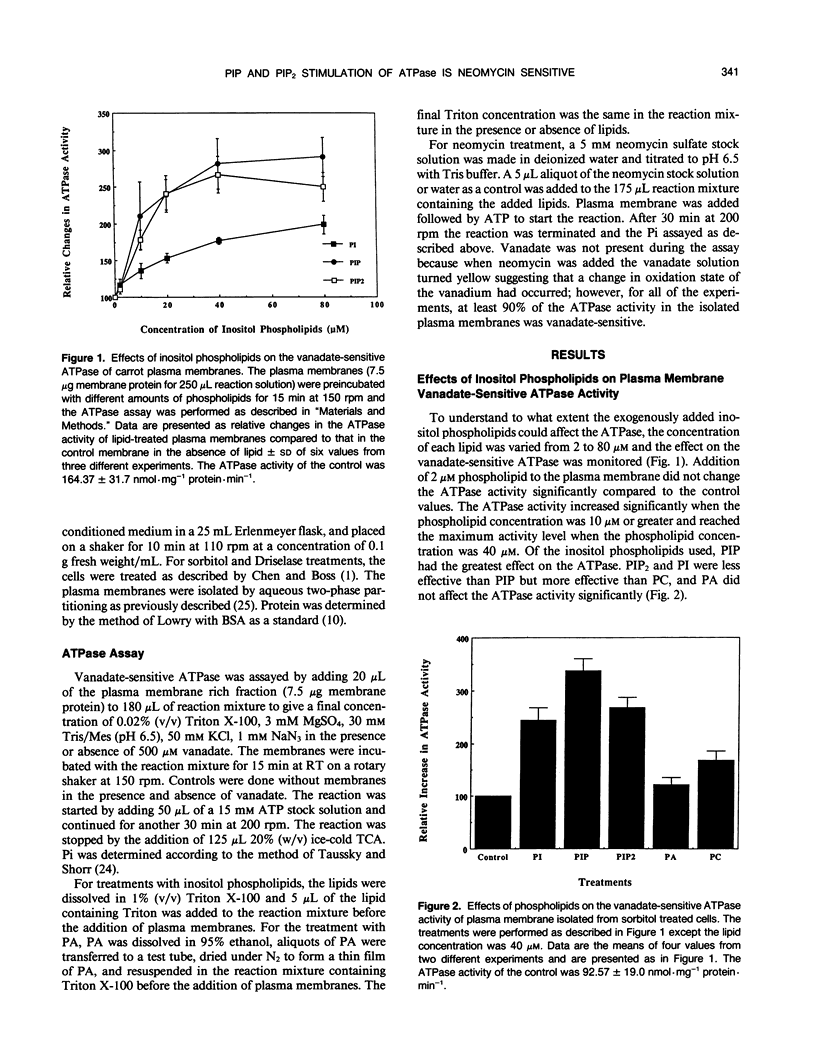
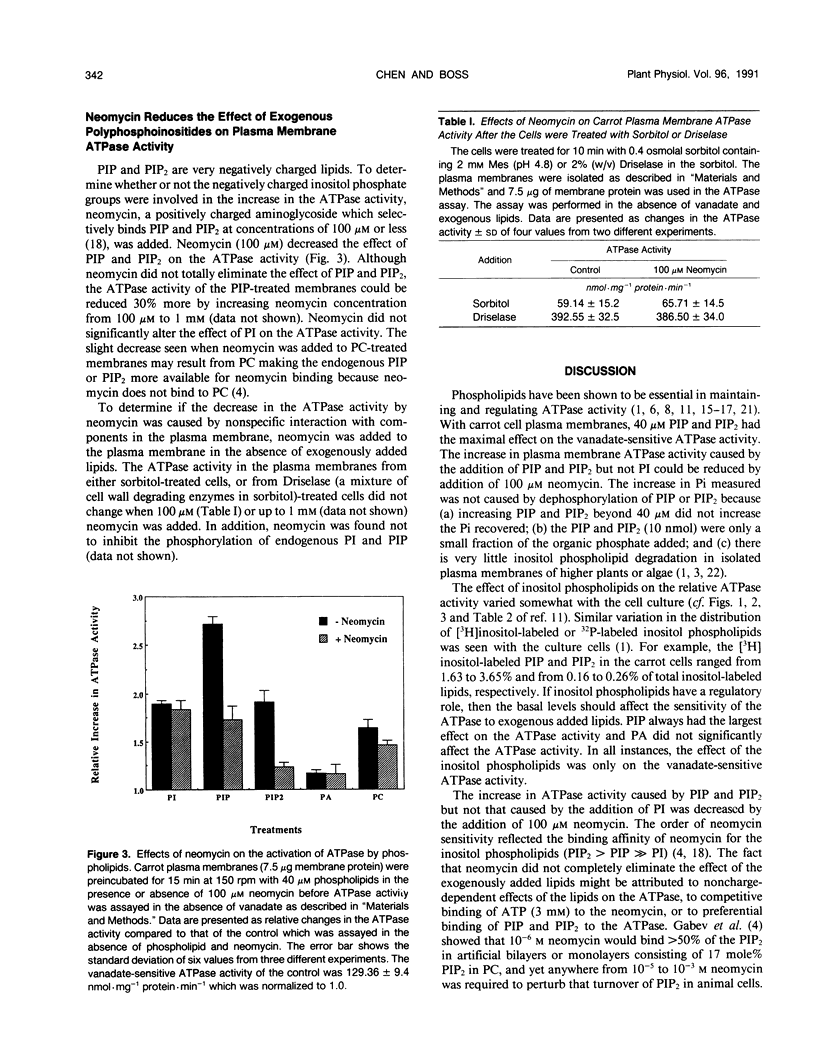
The inositol phospholipids, phosphatidylinositol monophosphate (PIP) and phosphatidylinositol bisphosphate (PIP2), have been shown to increase the vanadate-sensitive ATPase activity of plant plasma membranes (AR Memon, Q Chen, WF Boss [1989] Biochem Biophys Res Commun 162: 1295-1301). In this paper, we show the effect of various concentrations of phosphatidyinositol, PIP, and PIP2 on the plasma membrane vanadate-sensitive ATPase activity. PIP and PIP2 at concentrations of 10 nanomoles per 30 microgram membrane protein per milliliter of reaction mixture caused a twofold and 1.8-fold increase in the ATPase activity, respectively. The effect of these negatively charged phospholipids on the ATPase activity was inhibited by adding the positively charged aminoglycoside, neomycin. Neomycin did not affect the endogenous plasma membrane ATPase activity in the absence of exogenous lipids.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chen Q., Boss W. F. Short-term treatment with cell wall degrading enzymes increases the activity of the inositol phospholipid kinases and the vanadate-sensitive ATPase of carrot cells. Plant Physiol. 1990 Dec;94(4):1820–1829. doi: 10.1104/pp.94.4.1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einspahr K. J., Peeler T. C., Thompson G. A. Phosphatidylinositol 4,5-Bisphosphate Phospholipase C and Phosphomonoesterase in Dunaliella salina Membranes. Plant Physiol. 1989 Jul;90(3):1115–1120. doi: 10.1104/pp.90.3.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabev E., Kasianowicz J., Abbott T., McLaughlin S. Binding of neomycin to phosphatidylinositol 4,5-bisphosphate (PIP2). Biochim Biophys Acta. 1989 Feb 13;979(1):105–112. doi: 10.1016/0005-2736(89)90529-4. [DOI] [PubMed] [Google Scholar]
- Gallagher S. R., Leonard R. T. Effect of vanadate, molybdate, and azide on membrane-associated ATPase and soluble phosphatase activities of corn roots. Plant Physiol. 1982 Nov;70(5):1335–1340. doi: 10.1104/pp.70.5.1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imbrie C. W., Murphy T. M. Solubilization and partial purification of ATPase from a rose cell plasma membrane fraction. Plant Physiol. 1984 Mar;74(3):611–616. doi: 10.1104/pp.74.3.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiehl R., Varsányi M., Neumann E. Phosphorylation of phosphatidylinositol associated with the nicotinic acetylcholine receptor of Torpedo californica. Biochem Biophys Res Commun. 1987 Sep 30;147(3):1251–1258. doi: 10.1016/s0006-291x(87)80205-x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lipsky J. J., Lietman P. S. Neomycin inhibition of adenosine triphosphatase: evidence for a neomycin-phospholipid interaction. Antimicrob Agents Chemother. 1980 Oct;18(4):532–535. doi: 10.1128/aac.18.4.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodhi S., Weiner N. D., Schacht J. Interactions of neomycin and calcium in synaptosomal membranes and polyphosphoinostide monolayers. Biochim Biophys Acta. 1976 Apr 5;426(4):781–785. doi: 10.1016/0005-2736(76)90147-4. [DOI] [PubMed] [Google Scholar]
- Memon A. R., Boss W. F. Rapid light-induced changes in phosphoinositide kinases and H(+)-ATPase in plasma membrane of sunflower hypocotyls. J Biol Chem. 1990 Sep 5;265(25):14817–14821. [PubMed] [Google Scholar]
- Memon A. R., Chen Q. Y., Boss W. F. Inositol phospholipids activate plasma membrane ATPase in plants. Biochem Biophys Res Commun. 1989 Aug 15;162(3):1295–1301. doi: 10.1016/0006-291x(89)90814-0. [DOI] [PubMed] [Google Scholar]
- Monk B. C., Montesinos C., Leonard K., Serrano R. Sidedness of yeast plasma membrane vesicles and mechanisms of activation of the ATPase by detergents. Biochim Biophys Acta. 1989 Jun 6;981(2):226–234. doi: 10.1016/0005-2736(89)90032-1. [DOI] [PubMed] [Google Scholar]
- Palmgren M. G., Sommarin M. Lysophosphatidylcholine stimulates ATP dependent proton accumulation in isolated oat root plasma membrane vesicles. Plant Physiol. 1989 Jul;90(3):1009–1014. doi: 10.1104/pp.90.3.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandstrom R. P., Deboer A. H., Lomax T. L., Cleland R. E. Latency of Plasma Membrane H-ATPase in Vesicles Isolated by Aqueous Phase Partitioning : Increased substrate Accessibility or Enzyme Activation. Plant Physiol. 1987 Nov;85(3):693–698. doi: 10.1104/pp.85.3.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacht J., Lodhi S., Weiner N. D. Effects of neomycin on polyphosphoinositides in inner ear tissues and monomolecular films. Adv Exp Med Biol. 1977;84:191–208. doi: 10.1007/978-1-4684-3279-4_9. [DOI] [PubMed] [Google Scholar]
- Schäfer M., Behle G., Varsányi M., Heilmeyer L. M., Jr Ca2+ regulation of 1-(3-sn-phosphatidyl)-1D-myo-inositol 4-phosphate formation and hydrolysis on sarcoplasmic-reticular Ca2+-transport ATPase. A new principle of phospholipid turnover regulation. Biochem J. 1987 Nov 1;247(3):579–587. doi: 10.1042/bj2470579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAUSSKY H. H., SHORR E. A microcolorimetric method for the determination of inorganic phosphorus. J Biol Chem. 1953 Jun;202(2):675–685. [PubMed] [Google Scholar]
- Wheeler J. J., Boss W. F. Polyphosphoinositides are present in plasma membranes isolated from fusogenic carrot cells. Plant Physiol. 1987 Oct;85(2):389–392. doi: 10.1104/pp.85.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]


