Abstract
Purpose
Belinostat is an intravenous histone deacetylase inhibitor with approval for T-cell lymphomas. Adavosertib is a first in class oral Wee1 inhibitor. Preclinical studies of the combination demonstrated synergy in various human acute myeloid leukemia (AML) lines as well as AML xenograft mouse models.
Experimental design
This was a phase 1 dose-escalation study of belinostat and adavosertib in patients with relapsed/refractory AML and myelodysplastic syndrome (MDS). Patients received both drugs on days 1–5 and 8–12 of a 21-day cycle. Safety and toxicity were monitored throughout the study. Plasma levels of both drugs were measured for pharmacokinetic analysis. Response was determined by standard criteria including bone marrow biopsy.
Results
Twenty patients were enrolled and treated at 4 dose levels. A grade 4 cytokine release syndrome at dose level 4 (adavosertib 225 mg/day; belinostat 1000 mg/m2) qualified as a dose-limiting toxicity event. The most common non-hematologic treatment-related adverse events were nausea, vomiting, diarrhea, dysgeusia, and fatigue. No responses were seen. The study was terminated prior to maximum tolerated dose/recommended phase 2 dose determination.
Conclusions
The combination of belinostat and adavosertib at the tested dose levels was feasible but without efficacy signals in the relapsed/refractory MDS/AML population.
Keywords: Acute leukemia, Belinostat, Adavosertib, Myelodysplastic syndrome, Phase 1 clinical trial
Introduction
Acute myeloid leukemia (AML) remains a devastating disease. Over half of young adults and the majority of older adults still succumb to their disease. Outcomes for certain subgroups, including older patients, patients with therapy-related AML, or patients with antecedent hematologic disorders, such as myelodysplastic syndrome (MDS), remain particularly dismal. For patients with relapsed disease, particularly for those without a targetable mutation, there are limited treatment options. Clearly, novel therapies and treatment paradigms are urgently needed.
Adavosertib (AZD1775) is a potent and selective small-molecule Wee1 inhibitor. The Wee1 kinase plays a critical role in the DNA-damage response (DDR), consisting of checkpoint, repair, and survival-related events by negatively regulating the activity of the Cdc2 (CDK1)/cyclin A/B complex, and by extension, the G2M checkpoint [1]. Several lines of evidence suggest that AML is an appropriate target for an adavosertib-based regimen: (a) transformed cells in general exhibit defective checkpoints; (b) integrated genomic analysis identifies Wee1 as a target, critical for cell fate in AML; (c) evidence generated by the Cancer Genome Atlas Research Network indicates that AML is characterized by very frequent aberrations in cell cycle and DDR regulatory genes; and (d) cells with certain poor-prognostic mutations such as TP53 or FLT3-ITD mutations exhibit intrinsic defects in checkpoints or DNA repair [2-4].
Histone deacetylase inhibitors (HDACIs) are epigenetic agents approved in cutaneous T-cell lymphoma/peripheral T-cell lymphoma that modify chromatin structure and gene expression, selectively promoting neoplastic cell death, including AML cells. HDACI mechanisms of action are pleiotropic; more recently, attention has focused on their anti-tumor activity and promotion of DNA damage, particularly in AML cells. The HDACI belinostat has no significant single-agent activity in AML [5]. We recently reported a maximum tolerated dose (MTD) of 1000 mg/m2 on days 1–5 and 8–12 in combination with bortezomib in patients with relapsed/refractory AML or MDS with an exceptional responder [6].
Adavosertib is primarily metabolized by CYP3A4 with potential contributions by FMO3 and FMO5, and is a substrate of ABCB1, ABCG2, and OATP1A2 [7, 8]. It appears that a moderate CYP3A4 inhibitor can increase the exposure of adavosertib by 40% [7]. Belinostat is primarily metabolized by UDP-glucuronosyltransferase 1A1 (UGT1A1) and to a lesser extent by CYP2A6, CYP2C9, and CYP3A4, is known to inhibit CYP2C8 and CYP2C9, and is a substrate of P-glycoprotein (ABCB1) [9, 10]. A drug–drug interaction is unlikely to occur via the metabolic or drug transporter routes between adavosertib and belinostat.
The rationale for combining Wee1 and HDAC inhibition in AML was based on several considerations. We previously demonstrated that disruption of the DDR, e.g., by Chk1 inhibitors potentiates HDACI activity in AML both in vitro and in vivo [11]. We also showed that in human AML cells, Wee1 inhibition interacts reciprocally with HDACIs via multiple mechanisms including disruption of DNA-damage checkpoints and the mitotic spindle as well as induction of mitotic slippage [12]. Of note, co-administration of an HDACI blocked CDC2/CDK1 tyrosine Y15 as well as threonine T14 phosphorylation, leading to loss of the intra-S-phase checkpoint and premature mitotic entry. In preclinical models, interactions between adavosertib and an HDACI (vorinostat) were synergistic in both AML cell lines and primary AML specimens [12]. Enhanced cell death was observed in AML cells with either mutant or wild-type p53 or FLT3-ITD. The regimen was also active against CD34+CD38−CD123+ populations enriched for more primitive progenitors. Finally, in an AML mouse xenograft model, the combination significantly reduced tumor burden and prolonged survival with minimal toxicity [12]. Based on these findings, the current phase 1 study had as its aims determination of the safety and identification of the RP2D for a regimen involving two novel agents (a Wee1 and an HDACI) when administered in vivo to patients with relapsed or refractory AML or MDS.
Materials and methods
Drug supply
Belinostat and adavosertib were supplied by Acrotech Biopharma (and formerly by Spectrum Pharmaceuticals) and AstraZeneca, respectively, through the Cancer Therapy Evaluation Program by the Division of Cancer Treatment and Diagnosis, National Cancer Institute.
Eligibility criteria
Eligible patients were 18 years of age and older with a diagnosis of relapsed or refractory nonacute promyelocytic leukemia AML, MDS (International Prognostic Scoring System intermediate-2 or greater and previously treated with a hypomethylating agent), or chronic myeloid leukemia with blast crisis. Treatment-naïve patients 60 years of age or older with secondary or therapy-related AML were permitted if their disease did not have favorable cytogenetic/molecular features as per European LeukemiaNet recommendations [13].
Additional inclusion criteria included an Eastern Cooperative Oncology Group performance status 0 to 2 (Karnofsky Performance Scale Index ≥ 50%), creatinine within normal limits for the laboratory or glomerular filtration rate greater than or equal to 60 mL/min/1.73 m2, aspartate aminotransferase and alanine aminotransferase less than or equal to 2.5× the upper limits of normal (ULN), and serum total bilirubin less than or equal to 1.5× the ULN. Patients with prior allogeneic transplantation were eligible if the interval from transplant was at least 3 months.
Patients were excluded from the study if they had a clinical picture consistent with leukostasis or disseminated intravascular coagulopathy; a circulating blast count greater than 50 × 109/L within the week preceding enrollment; active CNS leukemia; other investigational agents within 3 weeks of starting study treatment; ongoing grade 2 or greater toxicity from prior therapy; uncontrolled infection; inability to swallow or tolerate oral medication; significant cardiovascular disease or history of ventricular arrhythmia; QTc equal to or greater than 450 ms or known risk factors for Torsades de pointes or medications associated with prolonged QTc, including second- or third-degree atrioventricular block or pretreatment ventricular heart rate less than 50 or greater than 120; treatment with atorvastatin, metformin, inhibitors of UGT1A1 or known UGT1A1 polymorphism, or strong inhibitors or inducers of CYP3A4. Pregnant or nursing women were ineligible. Patients who were candidates for potentially curative allogeneic transplant were ineligible unless they had declined the procedure. Concomitant anti-cancer therapy other than gonadotrophin-releasing hormone agonists for prostate cancer was not permitted except hydroxyurea, which was allowed through the 5th day of treatment to mitigate potential risk of tumor lysis syndrome. Prior diagnosis or treatment of other malignancy within 3 years of study was not allowed, with the exception of any in situ cancer or low-risk malignancies of the skin or prostate. All patients provided written informed consent. The study was approved and conducted in accordance with the policies of the NCI Central Institutional Review Board. This clinical trial is registered at clinicaltrials.gov, NCT02381548.
Study design
This was a phase 1 dose-escalation study designed to determine the recommended phase 2 dose for the combination of belinostat and adavosertib. Belinostat was administered by a 30-min intravenous (IV) infusion on days 1 through 5 and days 8 through 12 of each cycle; adavosertib was administered orally immediately after belinostat on days 1 through 5 and 8 through 12 of each cycle. The treatments were repeated on 21-day cycles until disease progression or unacceptable toxicity. There were 4 planned dose levels of the combination with 2 additional dose levels planned if a dose reduction was necessary (Table 2). The starting dose level for belinostat was 600 mg/m2; the starting dose level for adavosertib was 200 mg.
Table 2.
Dose levels and dose-limiting toxicities
| Dose level | Adavosertib (mg/day) D1–5, 8–12 Oral |
Belinostat (mg/ m2) D1–5, 8–12 IV |
#Treated/ #DLT evaluable |
DLT event | Median # of cycles (range) |
|---|---|---|---|---|---|
| 1 | 200 | 600 | 3/3 | 2 (2–4) | |
| 2 | 200 | 800 | 3/3 | 4 (3–4) | |
| 3 | 225 | 800 | 9/3 | 1 (1–7) | |
| 4 | 225 | 1000 | 5/3 | Gr4 CRS | 2 (1–4) |
CRS cytokine release syndrome, D day of the cycle, DLT dose-limiting toxicity, Gr grade of toxicity
All adverse events (AEs) were characterized in terms of attribution, severity, and relatedness to study treatment, and they were reported according to the NCI Common Terminology Criteria for Adverse Events (CTCAE) version 4.0. Dose-limiting toxicity (DLT) was defined as any of the following occurring during the first cycle of treatment and determined to be possibly, probably, or definitely related to study treatment: (a) any grade 3 or higher non-hematologic toxicity that did not improve to grade 1 or less with optimal medical management within 1 week excluding grade 3 fever, febrile neutropenia or infection or electrolyte abnormalities that, once corrected, could be maintained with oral repletion; (b) failure to recover neutrophil and/or platelet count by day 42 in patient with less than 5% blasts in the marrow; (c) any toxicity requiring discontinuation of either study drug; or (d) a toxicity resulting in a delay in starting cycle 2 by more than 14 days. For DLT evaluation, patients received greater than 80% of prescribed study drug doses in cycle 1 and were observed for DLT for up to 21 days from initiation of therapy. Patients who were not DLT evaluable were replaced.
To assess response to therapy, a bone marrow aspiration was performed after 2 cycles and again as clinically indicated. Treatment response was classified using the International Working Group for AML and European Leukemia Net (ELN) [14-16]. Morphologic complete remission (CR) required a normal bone marrow aspirate with less than 5% blast cells as well as count recovery with absolute neutrophil count (ANC) of 1000/μL or higher, platelet count of 100,000/μL or greater, absence of leukemia blasts in peripheral blood as well as red blood cell transfusion independence. Complete remission with incomplete blood count recovery (CRi) required fulfillment of CR criteria except for platelet count less than 100,000/μL or ANC less than 1000/μL. Partial remission (PR) was defined as decrease of bone marrow blasts to 5% to 24% and decrease of pretreatment blast percent by at least 50% with peripheral blood criteria for CR. Progressive disease (PD) was defined as greater than 50% increase in bone marrow or peripheral blood blasts from baseline or the development of extramedullary leukemia. Stable disease (SD) was assigned to patients who did not have CR, CRi, PR, or PD.
Pharmacodynamic analysis
Research samples were collected from consenting patients with ≥ 10% leukemic blasts in the peripheral blood. Specimens were obtained prior to and 24 h (± 6 h) following treatment. Whole blood was collected in tubes containing EDTA, after which peripheral blood mononuclear cells were isolated using Ficoll-Hypaque according to the manufacturer’s protocol, and subsequently cryopreserved at − 80 °C.
For flow cytometric analysis, a previously described method applicable for AML specimens was employed as previously described [17] with minor modifications. Specifically, isolated cells were fixed and permeabilized with True/Phos Perm Buffer (Biolegend), and stained with PE Cy7 conjugated CD45, APC-CD3, and APC Cy7-CD20 antibodies (Biolegend) in conjunction with PE-Bim, PE-p-Chk1, PE-p-cdc2 Y15, FITC-p-cdc2 T14, FITC-γH2AX (all Cell Signaling), and FITC-p-HH3 (Biolegend) antibodies as well as the appropriate isocontrol. Cells were analyzed using a BD FACSCanto flow cytometer. Analysis of biomarkers was conducted on the gated CD45dim SSlow CD3−CD20− population. The mean fluorescence intensity ratio of signal to isocontrol for pretreatment samples for each specimen was set at 100%.
Pharmacokinetic analysis
Pharmacokinetics were not assessed until dose levels 3 and 4, since a drug–drug interaction was not anticipated. Blood samples for measurement of plasma concentrations were collected on day 1 of cycle 1 for both belinostat and adavosertib. For belinostat, blood samples were collected in sodium heparin tubes pretreatment, 5 min prior to the end of the infusion, and 15 min, 30 min, 1 h, 2 h, 4 h, 6 h, 8 h, and 24 h after the end of the infusion. For adavosertib, blood samples were collected in potassium EDTA tubes pretreatment and 30 min, 1 h, 2 h, 4 h, 6 h, 8 h, and 24 h after oral administration. Plasma was cryopreserved at − 80 °C until analysis.
Concentrations of adavosertib were quantified over the range of 2 to 1000 ng/mL using a validated LC/MS/MS method at Covance. Concentrations of belinostat were determined using a validated liquid chromatography–tandem mass spectrometry (LC/MS/MS) method at the Analytical Pharmacology Core Laboratory at the Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins [18]. Belinostat glucuronide was measured indirectly by quantitation of the peak area at the retention time of belinostat glucuronide before and after a 2-h incubation at 37 °C with β-Glucuronidase from Helix pomatia, using the belinostat calibration curve as previously described for SN-38 glucuronide [19]. Concentrations of belinostat and belinostat glucuronide were quantified over the range of 30 to 5000 ng/mL with dilutions of up to 1:100 v/v being accurate. During the conduct of the trial, the accuracy and precision were within FDA guidance for bioanalytical methods [20].
Pharmacokinetic parameters for adavosertib, belinostat, and belinostat glucuronide were calculated from individual concentration–time data using standard noncompartmental methods as implemented in Phoenix WinNonlin version 8.2 (Pharsight A Certara Company, Cary, North Carolina). If more than two-thirds of the individual concentration values were greater than the limit of quantitation (30 ng/mL for belinostat and belinostat glucuronide; 2 ng/mL for adavosertib), then the pharmacokinetic parameters were deemed reportable. Pharmacokinetic parameters determined for adavosertib included maximum concentration (Cmax) and time to Cmax (Tmax), and for belinostat included Cmax, Tmax, area under the plasma concentration–time curve (AUC), and terminal half-life (T1/2). If the correlation coefficient (r2) for the terminal slope was less than 0.9, the T1/2 was not reported. If the extrapolated area was greater than 20%, the AUCINF was not reported.
Statistical considerations
Pharmacokinetic parameters were summarized using descriptive statistics. Differences between the pharmacokinetic parameters of belinostat and metabolite between dose levels were evaluated statistically by use of the Kruskal–Wallis test. Adavosertib and belinostat exposures were correlated with safety using Kruskal–Wallis test by ranks. Concomitant medications were reviewed and categorized from 0 to 5 according to QTc prolongation potential (using https://crediblemeds.org). Study patients’ composite QTc scores (i.e., the average of the individual QTc scores of their concomitant medications) were then compared across dose levels, belinostat exposure, and correlated with QTc prolongation events. All statistical tests were performed using JMP Statistical Discovery software (version 7; SAS Institute, Cary, NC). Statistical significance was set to the level 0.05.
Results
Patient characteristics
From August 2015 to June 2017, 20 patients were enrolled and treated on study at two participating centers (Massey Cancer Center, Richmond, VA and H. Lee Moffitt Cancer Center & Research Institute, Tampa, FL). The characteristics of the treated patients are reported in Table 1. The median age was 64 (range, 32–77) and 50% (10 of 20) were women. The majority of patients (18 of 20) had AML with intermediate or adverse cytogenetic risk stratification [15]. The median number of prior regimens was 2 (range, 1–5).
Table 1.
Patient characteristics (20 treated patients)
| Gender | |
| Female | 10 |
| Male | 10 |
| Race | |
| Asian | 1 |
| Black or African-American | 3 |
| White | 16 |
| Ethnicity | |
| Hispanic or Latino | 1 |
| Non-Hispanic | 19 |
| Age (years) | |
| Median | 64 |
| Range | 32–77 |
| Performance status | |
| 0 | 1 |
| 1 | 16 |
| 2 | 3 |
| Diagnosis | |
| AML | 18 |
| MDS | 2 |
| AML ELN risk stratification | |
| Intermediate | 7 |
| Adverse | 8 |
| Unknown | 3 |
| No. prior regimens | |
| Median | 2 |
| Range | 1–6 |
Safety and tolerability
Patients were enrolled to 4 planned dose levels. Details of patient enrollment and cycle administration are provided in Table 2. A summary of drug-related toxicity is provided in Table 3. Patients received a median of 2 cycles of study treatment with a range of 1 to 6 cycles. Eight patients were not evaluable for DLT. Two patients had insufficient drug exposure due to dose modifications (grade 3 diarrhea and grade 3 fatigue); 3 patients had insufficient drug exposure due to intercurrent illness; 3 patients were not followed for the full DLT evaluation period including a patient who had a grade 5 unrelated sepsis event that was attributed to underlying leukemia. The most common non-hematologic treatment-related AEs were nausea, vomiting, diarrhea, dysgeusia, and fatigue; much of the gastrointestinal toxicity was grade 1/2. The most common non-hematologic grade 3/4 treatment-related toxicities were nausea and fatigue.
Table 3.
Toxicity summary for treatment-related toxicities
| Category | Adverse event | All grades Number of patients (%) |
Grades 3–4 Number of patients (%) |
|---|---|---|---|
| Cardiac | Prolonged QTc | 8 (40) | 0 (0) |
| Gastrointestinal | Anorexia | 12 (60) | 0 (0) |
| Abdominal pain | 4 (20) | 0 (0) | |
| Diarrhea | 16 (80) | 3 (15) | |
| Mucositis | 4 (20) | 1 (5) | |
| Nausea | 16 (80) | 1 (5) | |
| Vomiting | 12 (60) | 1 (5) | |
| General | Fatigue | 10 (50) | 3 (15) |
| Hematologic | White blood cell decreased | 4 (20) | 4 (20) |
| Investigations | Weight loss | 9 (45) | 0 (0) |
| Metabolic | Dehydration | 9 (45) | 1 (5) |
| Musculoskeletal | Generalized muscle weakness | 6 (30) | 0 (0) |
| Neurologic | Dizziness | 4 (20) | 0 (0) |
| Dysgeusia | 10 (50) | 0 (0) | |
| Vascular | Flushing | 4 (20) | 0 (0) |
Includes toxicities with at least 20% incidence
There was one DLT event at dose level 4 described as grade 4 cytokine release syndrome (CRS) with hypotension, fever, and renal insufficiency after 1 day of study treatment (the only treatment day administered in cycle 1). All signs and symptoms related to CRS resolved within several days. Of note, the same patient also had grade 3 tumor lysis syndrome in cycle 2. Additional DLT assessment at dose level 4 was not completed due to study closure by the company collaborator and sponsor. No RP2D or MTD could be established.
Based on prior experience with HDACIs, QTc prolongation was closely monitored. There were 33 QTc prolongation events in 12 patients on study; 8 of the patients had treatment-related grade 1/2 QTc prolongation. Two patients had grade 3 QTc prolongation events that were not treatment related. No dose modifications were required for QTc events in the affected patients. Cardiac events are detailed in Table 4. To further characterize QTc prolongation and its attribution in study patients, concomitant medications were reviewed for QTc prolongation potential (using https://crediblemeds.org) and a composite QTc score assigned for each patient. There were no statistically significant differences in composite QTc scores across dose levels, nor with exposure to belinostat, nor with QTc prolongation events by Kruskal–Wallis (data not shown).
Table 4.
All (related and unrelated) cardiac adverse events
| Adverse event | All grades Number of patients (%) |
Grades 3–4 Number of patients (%) |
|---|---|---|
| Atrial fibrillation | 2 (10) | 1 (5) |
| Atrial flutter | 1 (5) | 0 (0) |
| Cardiac arrest | 2 (10) | 2 (10) |
| Non-specific ST and/or T wave changes | 3 (15) | 0 (0) |
| Cardiac troponin I increased | 2 (10) | 2 (10) |
| Cardiac troponin T increased | 1 (5) | 1 (5) |
| Chest pain, cardiac | 2 (10) | 1 (5) |
| Conduction disorder | 1 (5) | 0 (0) |
| Electrocardiogram QTc prolonged | 17 (85) | 2 (10) |
| Myocardial infarction | 1 (5) | 1 (5) |
| Pericardial effusion | 1 (5) | 0 (0) |
| Pericarditis | 1 (5) | 0 (0) |
| Sinus bradycardia | 2 (10) | 0 (0) |
| Sinus tachycardia | 11 (55) | 0 (0) |
| Ejection fraction decreased | 1 (5) | 0 (0) |
| Acute coronary syndrome | 1 (5) | 0 (0) |
| Pericardial tamponade | 1 (5) | 1 (5) |
Responses
There were no responses on study. Nine patients had SD, six patients had PD, and five were not evaluable due to not having had at least one post-treatment response evaluation (Supplemental Table 1). The mean duration of treatment was 7.9 weeks.
Pharmacokinetic analysis
Adavosertib and belinostat pharmacokinetic data were available for 11 patients enrolled on Dose Levels 3 and 4 (Table 5). Adavosertib Cmax and Tmax were unchanged between the two belinostat dose levels and comparable with previously reported Cmax values [7]. Due to incomplete sample collections (i.e., 8 or 24 h missing), the T1/2 and AUCINF are not reportable for the majority of patients. Belinostat and belinostat glucuronide maximum concentration (Cmax) and area under the curve (AUCINF) increased from 800 to 1000 mg/m2, similar to previously reported pharmacokinetic parameters [21-23]. There were no associations between exposure and toxicity. Notably, Cmax concentrations for belinostat (~ 200 nM) and adavosertib (~ 500 nM) approximated (belinostat) or were slightly above (adavosertib) those concentrations associated with HDAC/Wee1 inhibitor synergism in preclinical studies [12].
Table 5.
Plasma pharmacokinetic parameters
| Cmax (μM) | Tmax (h) | AUCINF (μM·h) | T1/2 (h) | Cl (L/h) | V (L) | |
|---|---|---|---|---|---|---|
| Belinostat | ||||||
| 800 mg/m2 | 124.8 ± 30.9 (6) | 0.4 (0.4–0.4; 6) | 66.7 ± 11.5 (6) | 0.77 ± 0.43 (6) | 38.6 ± 6.5 | 14.5 ± 5.1 |
| 1000 mg/m2 | 157.0 ± 36.9 (4) | 0.4 (0.4–0.4; 4) | 77.9, 81.7 (2) | 0.7, 1.89 (2) | 39.4 ± 1.3 | 13.9 ± 0.3 |
| Belinostat glucuronide | ||||||
| 800 mg/m2 | 148.9 ± 46.7 (6) | 0.8 (0.4–0.8; 6) | 396.0 ± 151.1 (6) | 3.51 ± 0.95 (6) | NR | NR |
| 1000 mg/m2 | 126.4 ± 24.5 (5) | 0.8 (0.8–1.0; 5) | 668.6 ± 452.2 (4) | 4.41 ± 1.30 (4) | NR | NR |
| Cmax (nM) | Tmax (h) | AUCINF (nM·h) | T1/2 (h) | Cl/F (L/h) | V/F (L) | |
| Adavosertib | ||||||
| 225 mg | 652 ± 221 (10) | 3 (2–4; 10) | 7409 (1) | 9.70 ± 1.93 | 57.0 ± 13.6 | 784.6 ± 204.3 |
Data are presented in the table as mean ± SD (n). Tmax is presented as median (range; n). If n < 3, the actual values are reported
AUCINF, area under the plasma concentration–time curve to infinity; Cl, systemic clearance; Cl/F, apparent systemic clearance; Cmax, peak plasma concentration; NR, not reportable; Tmax, time to peak concentration; T1/2 half-life; V volume of distribution; V/F apparent volume of distribution
Correlative studies
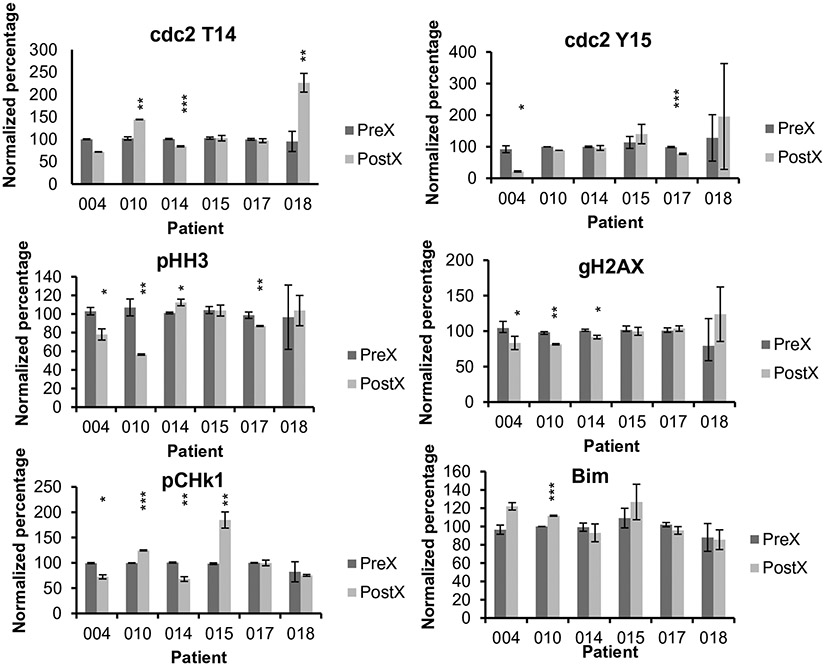
Consent for pre- and post-treatment peripheral blood samples for pharmacodynamic analysis was obtained from 6 patients whose specimens contained greater than 10% blasts. Analysis was performed to monitor pre- and post-treatment expression of p-Wee1 (at Ser642), γH2A.X, p-Chk1, p-HH3, Bim, and p-cdc2 (both at Tyr15 and at Thr14) (Fig. 1). In most cases, changes in expression of the various pharmacodynamic markers were relatively modest. Significant reductions in p-cdc2 T14 were observed in 1 patient and increases were observed in 2 patients, whereas 2 patients exhibited post-treatment declines in p-cdc2 Y15. Unexpectedly, post-treatment reductions in pHH3 were seen in 3 patient samples and an increase in one specimen. Reductions in γH2A.X were observed in 3 patients, and no increases were noted. Reductions in expression of pChk1 occurred in 2 patients, whereas increases were observed in 2 patients. Finally, increased expression of Bim was detected in only one patient sample. In view of the lack of clinical responses, no correlations could be made between clinical responses and post-treatment changes in the expression of pharmacodynamic indicators of responsiveness.
Fig. 1.
Flow cytometric analysis of pre- and post-treatment samples. PBMCs were isolated at baseline (Pre-) and 24 h after (Post*-) treatment. PBMC from each sample were analyzed for levels of Bim, p-cdc2 Y15, p-cdc2 T14, p-Chk1, gH2AX, and p-HH3. Analysis of biomarkers were performed on the CD45dim SSlow CD3-CD20-population. The mean fluorescence intensity (MFI) ratio of signal to its isocontrol for pretreatment samples from each patient was set as 100%. Results of analysis showed as bar charts displaying the relative levels of the assayed proteins in samples after averaging triplicate determinations, including standard deviation bars. Asterisks indicate significant post-treatment changes with * = significance with p values between 0.04 and 0.01; ** = significance with p values between 0.009 and 0.002; and *** = significance with p values between 0.0003 and 0.00007
Discussion
The phase 1 study of belinostat and adavosertib represents a novel combination strategy for the treatment of relapsed/refractory leukemia and MDS. Previous preclinical studies have shown that Wee1 inhibitors sensitize human AML cells to various genotoxic anti-metabolites, including in cells with TP53 deficiency [24]. In addition, synergism between adavosertib and inhibitors of homologous recombination, such as PARP antagonists, have been reported in AML cells [25]. Similar anti-leukemic interactions have been observed between adavosertib and inhibitors of the Wee1 kinase [12]. Notably, our group and others have described synergism between adavosertib and HDACIs in AML preclinical models [26]. The presumed mechanism underlying the latter interactions involves HDACI-mediated prevention of compensatory responses to Wee1-induced cell cycle disruption [12]. Significantly, this strategy was effective against primary AML blasts, spared normal hematopoietic cells, and prolonged the survival of NSG mice inoculated systemically with leukemic cells [12]. Collectively, these findings prompted testing of the Wee1/HDACI strategy in patients with relapsed/refractory AML. While the present study demonstrated the feasibility of this approach, there was no evidence for clinical activity at the doses utilized, and it would be difficult to justify further pursuit of this regimen in the current setting. Nevertheless, this trial provided information that may be of use in future studies.
With respect to toxicity, the combination of the two agents was feasible in a population that is challenging to treat. Gastrointestinal toxicity was most common and readily managed in most patients. During the study design, special attention was paid to QTc prolongation given prior experience with belinostat. Drugs that can cause QTc prolongation, such as quinolones and azoles, were not specifically excluded in an attempt to mimic real-world leukemia management. Specific guidance was included in the protocol regarding use of medications with known Torsades de pointes risk. Additionally, hypokalemia and hypomagnesemia were corrected prior to administration of belinostat. Study patients had electrocardiograms as part of eligibility and then pre- and post-treatment on days 4 and 11 with cycle 1. As noted, 8 patients had grade 1/2 QTc prolongation possibly, probably, or definitely related to study treatment. No dose modifications were necessary for grade 1/2 QTc prolongation. Per the belinostat package insert, 11% of patients in the registration trial had QTc prolongation, all grades [10]. A similar phenomenon has been seen with other HDACIs such as vorinostat. At the time of this study, there was not a full understanding of adavosertib toxicity, but QTc prolongation was not identified as an AE in the investigator brochure (subsequently confirmed). Review of concomitant medications for study patients did not demonstrate significant statistical correlations between composite QTc scores and QTc prolongation events. However, it is difficult to draw definitive conclusions from this particular analysis, given heterogeneity in drug formularies and clinical practice across institutions as well as limited information about patients’ exposures to the various concomitant medications surrounding the events. While QTc prolongation did not affect treatment administration or meet any predetermined DLT criteria, it contributed to the decision by the company collaborator to close the study before completion.
In this study, the preclinical data combining an HDACI and adavosertib did not translate into clinical responses. While drug exposure was comparable to previously reported results for adavosertib and another HDACI (vorinostat) [12], it is possible that by not reaching the MTD, drug dosing was insufficient to achieve objective response in patients, and that if tolerable, such doses may have been associated with greater clinical efficacy. The usual dosing for belinostat in T-cell lymphoma, its approved indication, is 1000 mg/m2 days 1–5 in a 21-day cycle. A dose and schedule for adavosertib was not defined at the time of study development. Of note, belinostat and adavosertib exposure was consistent with previously reported values [7, 21-23]. Mean adavosertib Cmax was 652 nM which exceeded the concentrations utilized by our group in preclinical experiments [12]. Another possibility is that while peak drug combinations comparable to those employed in preclinical studies were achieved, more sustained peak drug concentrations may be necessary to induce a degree of leukemic cell death required for objective responses.
The results of the pharmacodynamic findings were inconclusive, in part due to the modest changes in post-treatment expression of various proteins, as well as the absence of clinical responses. Notably, some of the changes observed in several specimens were contrary to preclinical findings, e.g., diminished rather than increased dephosphorylation of cdc2 T14 or Y15, or reduced expression of pHH3 and γH2A.X. We cannot exclude the possibility that the failure to recapitulate all of the pharmacodynamic changes observed in preclinical models may have contributed to the lack of clinical efficacy. In any case, the present results document the feasibility of employing a flow cytometric technique based on staining of intracellular proteins which is suitable for analyzing specimens with relatively low percentages of blasts. For example, in a previous AML study in which Western blot analysis was used to monitor post-treatment protein expression, only samples containing ≥ 65% blasts were used to avoid problems related to heterogeneity [6]. As a result, only a small percentage of specimens met this criterion and could be analyzed.
A question arises regarding the optimal HDACI to employ in a study involving adavosertib in AML. In AML cell lines, belinostat exhibits anti-leukemic effect by promoting cell cycle arrest, inhibiting cell proliferation and inducing apoptosis. However, as a single agent, belinostat has limited clinical activity in AML [5, 27]. Of note, while the preclinical studies upon which this trial was based employed the HDACI vorinostat, in the clinical study described here, belinostat was employed. In this context, we have previously reported our experience with a phase 2 study of belinostat and bortezomib, in which an exceptional responder was described [6]. An ongoing study of belinostat and pevonedistat (NCT03772925), an NEDD8-activating enzyme inhibitor, was based on preclinical evidence of synergistic interactions in AML cells independent of TP53 or FLT3-ITD status [28]. Single-agent panobinostat, an oral HDACI approved for the treatment of multiple myeloma, also exhibits anti-leukemia activity in AML cell lines, primary leukemia cells from patients, and improves survival of mice with AML. However, as a single agent, it had very modest activity in AML patients with low response rates [29]. Panobinostat was subsequently combined with a number of active agents in AML but with disappointing results [30]. It is possible that an HDACI with single-agent AML activity (e.g., pracinostat) [31] might warrant consideration in this setting. On the other hand, it is possible that the class of HDACIs may be sub-optimal partners for combination with Wee1 antagonists in AML due to the lack of single-agent activity and limited genotoxic activity. Finally, it is conceivable that in vitro results and xenograft models may not provide sufficient evidence for potential objective responses in the clinic. Newer models such as patient-derived xenografts may be necessary to identify drugs/combinations with superior clinical efficacy.
In summary, the results of the phase 1 study of adavosertib and belinostat in patients with MDS/AML were feasible but with no efficacy signals in the limited population studied. Aside from the issue of failure to replicate pharmacodynamic events underlying synergism in preclinical studies, it is possible that HDACIs may be insufficiently genotoxic to benefit from concomitant administration of a Wee1 inhibitor, at least in the setting of AML. Alternatively, combinations with other classes of agents with established activity in AML, e.g., hypomethylating agents and venetoclax, or with more pronounced DNA-damaging activity, may hold more promise Given that both HDAC and Wee1 inhibitors are known to potentiate the anti-leukemic actions of genotoxic agents [24, 32], it is conceivable that a regimen combining the Wee1/HDACI regimen with such agents, if tolerable, could yield superior activity. Preclinical studies designed to test these hypotheses are underway.
Supplementary Material
Funding
The study was supported by the following grants from the National Institutes of Health (NIH): UH2TR001373 (SG), Experimental Therapeutics Clinical Trials Network (grants UM1CA186644 [SG] and U24CA247648 [MAR]), and the Clinical Pharmacology Training Program Grant (T32GM066691 [supporting ABK]). The project described was also supported by the Analytical Pharmacology Core of the Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins (NIH grants P30CA006973 [MAR], UL1TR003098 [MAR], the Shared Instrument Grant S10OD020091 [MAR], and Massey CCSG P30 CA016059 [DP]). The project described was also supported by grant number UL1TR003098 from the National Center for Advancing Translational Sciences (NCATS), a component of the NIH, and the NIH Roadmap for Medical Research [MAR]. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of the NCATS or NIH.
Footnotes
Supplementary Information The online version contains supplementary material available at https://doi.org/10.1007/s00280-023-04511-0.
Conflicts of interest No potential conflicts of interest were disclosed by any of the authors.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
- 1.Do K, Doroshow JH, Kummar S (2013) Wee1 kinase as a target for cancer therapy. Cell Cycle 12(19):3159–3164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Porter CC, Kim J, Fosmire S, Gearheart CM, van Linden A, Baturin D et al. (2012) Integrated genomic analyses identify WEE1 as a critical mediator of cell fate and a novel therapeutic target in acute myeloid leukemia. Leukemia 26(6):1266–1276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cancer Genome Atlas Research N, Ley TJ, Miller C, Ding L, Raphael BJ, Mungall AJ et al. (2013) Genomic and epigenomic landscapes of adult de novo acute myeloid leukemia. N Engl J Med 368(22):2059–2074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Seedhouse C, Grundy M, Shang S, Ronan J, Pimblett H, Russell N et al. (2009) Impaired S-phase arrest in acute myeloid leukemia cells with a FLT3 internal tandem duplication treated with clofarabine. Clin Cancer Res 15(23):7291–7298 [DOI] [PubMed] [Google Scholar]
- 5.Kirschbaum MH, Foon KA, Frankel P, Ruel C, Pulone B, Tuscano JM et al. (2014) A phase 2 study of belinostat (PXD101) in patients with relapsed or refractory acute myeloid leukemia or patients over the age of 60 with newly diagnosed acute myeloid leukemia: a California Cancer Consortium Study. Leuk Lymphoma 55(10):2301–2304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Holkova B, Shafer D, Yazbeck V, Dave S, Bose P, Tombes MB et al. (2021) Phase 1 study of belinostat (PXD-101) and bortezomib (Velcade, PS-341) in patients with relapsed or refractory acute leukemia and myelodysplastic syndrome. Leuk Lymphoma 62(5):1187–1194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leijen S, van Geel RM, Pavlick AC, Tibes R, Rosen L, Razak AR et al. (2016) Phase I study evaluating WEE1 inhibitor AZD1775 as monotherapy and in combination with gemcitabine, cisplatin, or carboplatin in patients with advanced solid tumors. J Clin Oncol 34(36):4371–4380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li J, Wu J, Bao X, Honea N, Xie Y, Kim S et al. (2017) Quantitative and mechanistic understanding of AZD1775 penetration across human blood-brain barrier in glioblastoma patients using an IVIVE-PBPK modeling approach. Clin Cancer Res 23(24):7454–7466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang LZ, Ramirez J, Yeo W, Chan MY, Thuya WL, Lau JY et al. (2013) Glucuronidation by UGT1A1 is the dominant pathway of the metabolic disposition of belinostat in liver cancer patients. PLoS ONE 8(1):e54522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.BELEODAQ® (belinostat) for injection, for intravenous use Prescribing Information. Acrotech Biopharma LLC. East Windsor, NJ. Revised Apr 2022 [Google Scholar]
- 11.Dai Y, Chen S, Kmieciak M, Zhou L, Lin H, Pei XY et al. (2013) The novel Chk1 inhibitor MK-8776 sensitizes human leukemia cells to HDAC inhibitors by targeting the intra-S checkpoint and DNA replication and repair. Mol Cancer Ther 12(6):878–889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou L, Zhang Y, Chen S, Kmieciak M, Leng Y, Lin H et al. (2015) A regimen combining the Wee1 inhibitor AZD1775 with HDAC inhibitors targets human acute myeloid leukemia cells harboring various genetic mutations. Leukemia 29(4):807–818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dohner H, Estey E, Grimwade D, Amadori S, Appelbaum FR, Buchner T et al. (2017) Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood 129(4):424–447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dohner H, Estey EH, Amadori S, Appelbaum FR, Buchner T, Burnett AK et al. (2010) Diagnosis and management of acute myeloid leukemia in adults: recommendations from an international expert panel, on behalf of the European LeukemiaNet. Blood 115(3):453–474 [DOI] [PubMed] [Google Scholar]
- 15.Cheson BD, Bennett JM, Kopecky KJ, Buchner T, Willman CL, Estey EH et al. (2003) Revised recommendations of the International Working Group for Diagnosis, standardization of response criteria, treatment outcomes, and reporting standards for therapeutic trials in acute myeloid leukemia. J Clin Oncol 21(24):4642–4649 [DOI] [PubMed] [Google Scholar]
- 16.Cheson BD, Greenberg PL, Bennett JM, Lowenberg B, Wijermans PW, Nimer SD et al. (2006) Clinical application and proposal for modification of the International Working Group (IWG) response criteria in myelodysplasia. Blood 108(2):419–425 [DOI] [PubMed] [Google Scholar]
- 17.Ito S, Ishida Y, Murai K, Kuriya S (2001) Flow cytometric analysis of aberrant antigen expression of blasts using CD45 blast gating for minimal residual disease in acute leukemia and high-risk myelodysplastic syndrome. Leuk Res 25(3):205–211 [DOI] [PubMed] [Google Scholar]
- 18.Kiesel BF, Parise RA, Tjornelund J, Christensen MK, Loza E, Tawbi H et al. (2013) LC-MS/MS assay for the quantitation of the HDAC inhibitor belinostat and five major metabolites in human plasma. J Pharm Biomed Anal 81–82:89–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Bruijn P, Willems EW, Loos WJ, Verweij J, Sparreboom A (2004) Indirect determination of the irinotecan metabolite 7-ethyl-10-O-glucuronyl-camptothecin in human samples. Anal Biochem 328(1):84–86 [DOI] [PubMed] [Google Scholar]
- 20.U. S. Food and Drug Administration Center for Drug Evaluation and Research (2001) Guidance for industry bioanalytical method validation
- 21.Lassen U, Molife LR, Sorensen M, Engelholm SA, Vidal L, Sinha R et al. (2010) A phase I study of the safety and pharmacokinetics of the histone deacetylase inhibitor belinostat administered in combination with carboplatin and/or paclitaxel in patients with solid tumours. Br J Cancer 103(1):12–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Agarwal N, McPherson JP, Bailey H, Gupta S, Werner TL, Reddy G et al. (2016) A phase I clinical trial of the effect of belinostat on the pharmacokinetics and pharmacodynamics of warfarin. Cancer Chemother Pharmacol 77(2):299–308 [DOI] [PubMed] [Google Scholar]
- 23.Bailey H, McPherson JP, Bailey EB, Werner TL, Gupta S, Batten J et al. (2016) A phase I study to determine the pharmacokinetics and urinary excretion of belinostat and metabolites in patients with advanced solid tumors. Cancer Chemother Pharmacol 78(5):1059–1071 [DOI] [PubMed] [Google Scholar]
- 24.Van Linden AA, Baturin D, Ford JB, Fosmire SP, Gardner L, Korch C et al. (2013) Inhibition of Wee1 sensitizes cancer cells to antimetabolite chemotherapeutics in vitro and in vivo, independent of p53 functionality. Mol Cancer Ther 12(12):2675–2684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Garcia TB, Snedeker JC, Baturin D, Gardner L, Fosmire SP, Zhou C et al. (2017) A small-molecule inhibitor of WEE1, AZD1775, synergizes with olaparib by impairing homologous recombination and enhancing DNA damage and apoptosis in acute leukemia. Mol Cancer Ther 16(10):2058–2068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Qi W, Zhang W, Edwards H, Chu R, Madlambayan GJ, Taub JW et al. (2015) Synergistic anti-leukemic interactions between panobinostat and MK-1775 in acute myeloid leukemia ex vivo. Cancer Biol Ther 16(12):1784–1793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schlenk RF, Krauter J, Raffoux E, Kreuzer KA, Schaich M, Noens L et al. (2018) Panobinostat monotherapy and combination therapy in patients with acute myeloid leukemia: results from two clinical trials. Haematologica 103(1):e25–e28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhou L, Chen S, Zhang Y, Kmieciak M, Leng Y, Li L et al. (2016) The NAE inhibitor pevonedistat interacts with the HDAC inhibitor belinostat to target AML cells by disrupting the DDR. Blood 127(18):2219–2230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morabito F, Voso MT, Hohaus S, Gentile M, Vigna E, Recchia AG et al. (2016) Panobinostat for the treatment of acute myelogenous leukemia. Expert Opin Investig Drugs 25(9):1117–1131 [DOI] [PubMed] [Google Scholar]
- 30.Garcia-Manero G, Sekeres MA, Egyed M, Breccia M, Graux C, Cavenagh JD et al. (2017) A phase 1b/2b multicenter study of oral panobinostat plus azacitidine in adults with MDS, CMML or AML with 30% blasts. Leukemia 31(12):2799–2806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Abaza YM, Kadia TM, Jabbour EJ, Konopleva MY, Borthakur G, Ferrajoli A et al. (2017) Phase 1 dose escalation multicenter trial of pracinostat alone and in combination with azacitidine in patients with advanced hematologic malignancies. Cancer 123(24):4851–4859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xie C, Drenberg C, Edwards H, Caldwell JT, Chen W, Inaba H et al. (2013) Panobinostat enhances cytarabine and daunorubicin sensitivities in AML cells through suppressing the expression of BRCA1, CHK1, and Rad51. PLoS ONE 8(11):e79106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.