Abstract
Objective:
To examine the differences in participation, life satisfaction, and psychosocial outcomes among individuals with traumatic brain injury (TBI) endorsing current, past, or no chronic pain.
Setting:
Community
Participants:
3,804 TBI Model Systems (TBIMS) participants 1- to 30-years post-injury classified into one of three groups based on their pain experience: current pain, past pain, no pain completed a pain survey at their usual follow-up appointment which on average was approximately 8 years post-injury.
Design:
Multi-site, cross-sectional observational cohort study.
Main Outcome Measure(s):
Sociodemographic and injury characteristics, and psychosocial outcomes (i.e., satisfaction with life, depression, anxiety, posttraumatic stress disorder – PTSD, sleep quality, community participation).
Results:
Persons with current chronic pain demonstrated higher scores on measures of PTSD, anxiety, and depression, and the lower scores on measures of sleep quality, community participation and satisfaction with life. Those with resolved past pain had mean scores for these outcomes that were all between the current and no chronic pain groups, but always closest to the no pain group. After adjusting for sociodemographic and function in multivariate analysis, having current chronic pain was associated with more negative psychosocial outcomes. The largest effect sizes (in absolute value) were observed for the PTSD, depression, anxiety, and sleep quality measures (|ES| = 0.52 to 0.81) when comparing current pain to past or no pain, smaller effect sizes were observed for life satisfaction (|ES| = 0.22 to 0.37) and out and about participation (|ES| = 0.16 to 0.18). When comparing past and no pain groups, adjusted effect sizes were generally small for life satisfaction, PTSD, depression, anxiety, and sleep quality (|ES| = 0.10 to 0.23) and minimal for participation outcomes (|ES| = 0.02 to 0.06).
Conclusions:
Chronic pain is prevalent among individuals with TBI and is associated with poorer psychosocial outcomes, especially for PTSD, depression, anxiety, and sleep disturbance. The results from this study highlight the presence of modifiable comorbidities among those with chronic pain and TBI. Persons who experience persistent pain following TBI may be at greater risk for worse psychosocial outcomes.
Keywords: Traumatic Brain Injury, Chronic Pain
Introduction
Traumatic brain injury (TBI) is associated with a variety of poor psychosocial health outcomes, including low rates of participation (employment, social involvement, and community activities),1–4 low life satisfaction,4,5 mental health difficulties,6–8 and poor sleep quality.9,10 Chronic pain is a common comorbidity in individuals with TBI, occurring in 1/3 to 3/4 of individuals with TBI living in the community.11 The biopsychosocial model is the prevailing framework for understanding the chronic pain experience, recognizing pain and disability as a multidimensional, dynamic interaction among physiological, psychological and social factors that are positioned to reciprocally influence one another.12 Chronic pain itself has been linked to a variety of poor psychosocial health outcomes in the general population and in other medical populations,13–17 and thus has the potential to adversely impact outcomes following TBI.
Prior literature in understanding the relationship between chronic pain and psychosocial outcomes post-TBI has focused on mild injury, relied on small circumscribed sample sets, or lacked relevant comparison groups such as those with similar severity of injury without chronic pain. For instance, in persons with mild TBI, headache pain severity has been shown to be a risk factor for posttraumatic stress disorder (PTSD)18 and posttraumatic headache has been shown to be a risk factor for depression and anxiety relative to healthy controls.19,20 Among thirty-eight individuals with TBI, neuropathic pain severity was associated with increased anxiety, as well as higher rates of depression, PTSD and overall greater affective distress compared to those with lower or no neuropathic pain.21 Following moderate-severe TBI, depressive symptoms are positively associated with report of pain at one year post-injury in both cross-sectional and longitudinal investigations.22
Few studies have investigated the relationship of chronic pain to participation or life satisfaction outcomes for individuals with TBI.23,24 In a sample of 146 individuals with moderate to severe TBI who received inpatient rehabilitation, Hoffman and colleagues found that chronic pain at one year after injury was associated with lower community participation; however, this relationship was moderated by depression, and chronic pain was no longer significant when depression was entered into the model.23 Dawson and colleagues investigated the relationship of pain with productive activity (return to school or work) at four years after TBI in those with mild to severe injuries.24 Results showed pain severity and maladaptive coping with pain were associated with less productivity. These remained significant in a hierarchical linear regression model that also included injury severity, neuropsychological test performance, and depression. Noyes and colleagues investigated the relationship of medical comorbidities to life satisfaction and mental health at 2 (n=225) and 5 (n=283) years post-injury in a sample of veterans in the VA Polytrauma Rehabilitation TBI Model Systems database.25 They found chronic pain was associated with less life satisfaction and with greater depression at both 2 and 5 years. Overall, these smaller studies indicate that the impact of chronic pain after TBI is dependent on a number of interacting factors, which in turn affect overall psychosocial functioning. More in depth evaluation of these complex relationships has yet to be assessed however.
Understanding differences in chronic pain experiences following TBI may aid in the development of interventions that address multimorbidity needs in this population, as well as increase recognition of the importance of chronic pain as a probable factor in psychosocial health outcomes. Given how prevalent chronic pain in is in the population of those with moderate-severe TBI, and how little is known about the complex inter-relationships between pain and psychosocial health outcomes in this special population, the purpose of this manuscript is to characterize how these important outcomes differ among those with current pain, past pain, and no pain in a large, multi-site sample. This is an important initial step preliminary to establishing causal models to describe the complex associations between pain and psychosocial health after TBI.
Methods
Participants
Participants in the present study were previously enrolled in the National Institute on Disability Independent Living and Rehabilitation Research (NIDILRR)-funded Traumatic Brain Injury Model Systems (TBIMS) who were invited to additionally take part in a secondary study on chronic pain. TBIMS eligibility criteria include: TBI designated as an external mechanical force to the head with (a) loss of consciousness greater than 30 minutes, (b) post-traumatic amnesia (PTA) greater than 24 hours, (c) Glasgow Coma Scale (GCS) score in the Emergency Department of less than 13, or (d) trauma-related intracranial abnormalities or neuroimaging abnormalities.26 TBIMS participants are routinely interviewed at years 1, 2, 5, and every 5 years thereafter post-injury. After completing their regularly scheduled TBIMS follow-up interview, the TBIMS participants26–28 at 18 sites (16 currently funded model systems, 1 model system follow-up center, and 1 VA medical center site) were invited to complete the Pain Survey regarding their experience with chronic pain and3,804 individuals completed the survey. Individuals who completed more than one TBIMS follow-up interview during the time of the pain study were only asked to complete the pain survey once. Eligibility for the current study required that participants had completed their TBIMS follow-up interview in English and without support of a proxy. Individuals were given eight weeks to complete the Pain Survey and offered three survey modalities: phone interview, mailed packet, or online via secure email link.
Three separate Pain Surveys were constructed, based on participant endorsement of 1) currently experiencing chronic pain, 2) had experienced chronic pain since their TBI, but not currently experiencing chronic pain, or 3) had no chronic pain since their TBI. Chronic pain was defined for participants as “persistent or recurring pain that lasts longer than 3 months. It includes headaches or pain anywhere in the body which occurs more than half of the days over a three-month period.” To determine the appropriate Pain Survey, participants were asked “Are you experiencing chronic pain?” If they answered Yes, the participant completed the Current Chronic Pain Survey. If they responded No, the participant was asked: “Did you have chronic pain for some period of time after your TBI, but then it stopped/resolved/improved?” If they answered Yes, the participant completed the Past Chronic Pain Survey. If the participant answered No, they completed the No Pain Survey. All centers received approval from their institutional review board.
Measures
Psychosocial Outcomes
Life satisfaction was measured using the Satisfaction with Life Scale (SWLS).29 Each of the 5 items is rated on a 7-point Likert scale (1=strongly disagree to 7=strongly agree) and item scores are summed to produce a total score (range 5–35), with higher scores representing greater overall life satisfaction. Depression symptomology was measured using the Patient Health Questionnaire-9 (PHQ-9)30 which assesses the frequency of the 9 DSM-IV depression criteria during the past 2 weeks. The 9 items are rated on a 4-point Likert scale (0=not at all to 3=nearly every day) and item scores are summed to produce a total score (range 0–27), with higher scores indicating more depression symptomology. Anxiety symptomology was measured using the General Anxiety Disorder-7 (GAD-7)31 which assesses the frequency of the 7 DSM-IV anxiety criteria over the past 2 weeks. The 7 items are rated on a 4-point Likert scale (0=not at all to 3=nearly every day) and item scores are summed to produce a total score (range 0–21), with higher scores indicating more anxiety symptomology. Community participation was measured using the 17-item Participation Assessment with Recombined Tools – Objective (PART-O),32 which assesses participation in life roles at the societal level. Items are averaged within the domains (Productivity, Social, Out and About) to produce domain and total (Summary) scores that each range from 0 to 5, with higher scores indicating more participation. All psychosocial outcome measures were collected during the TBIMS follow-up interview, with the exception of the Posttraumatic Stress Disorder Checklist for DSM-5 (PCL-5) and the Pittsburgh Sleep Quality Index (PSQI) which were included in the Pain Survey. PTSD symptomology was measured using the PCL-5. Each of the 20 items are rated on a 5-point Likert scale (0= not at all to 4=extremely) and item scores are summed to produce a total score (range 0–80), with higher scores indicating more severe PTSD symptomology. Sleep quality was measured using the 19-item PSQI33 which measures sleep quality and disturbances over a 1-month period. Seven component scores (subjective sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbances, use of sleeping medication, and daytime dysfunction) are generated which are summed to produce a total score (range 0–21), with higher scores representing poorer sleep quality.
A basic set of covariates were considered for adjustment in statistical analyses for all psychosocial outcome models. Sociodemographic characteristics included years post-injury, sex, race (White, Black, Other), Hispanic ethnicity, and age at the time of TBIMS follow-up, marital status, years of education, and employment status. Functional outcome measures included FIM™ Motor and Cognitive scores at the time of the TBIMS follow-up. Injury characteristics included cause of injury, time (in days) to follow-commands (TFC), and days of post-traumatic amnesia (PTA) were summarized for this sample. Sociodemographic and concurrent functional outcome measures are all standard variables collected during TBIMS follow-up interviews; recoding of classification variables and additional details on measures and data collection can be found at https://hub.tbindsc.org/tbimsdatadictionary/Home.26
Statistical Analysis
All statistical analyses were conducted using SAS v.9.4 (© 2016 SAS Institute Inc., Cary, NC) assuming a 5% level of significance unless otherwise stated. The sociodemographic, injury, and functional outcome characteristics were summarized by chronic pain group (current, past, and no pain) using means, standard deviations (SDs), and percentiles for continuous variables, and frequency counts and percentages for categorical variables. Differences among groups in these characteristics were assessed using ANOVA, Kruskal-Wallis, and chi-square tests. For continuous variables with considerable skewness (time since injury, PTA, TFC, FIM Motor and Cognitive) the median (50th percentile) and interquartile range (25th-75th percentiles) are preferred as measures of central tendency and spread and the Kruskal-Wallis tests for comparing groups.
The psychosocial outcomes were summarized using descriptive statistics and compared across the chronic pain groups with ANOVA. Pairwise differences in outcomes among groups were estimated, and effect sizes were derived as the pairwise difference in means divided by the ANOVA model root mean square error (RMSE). Next, general linear models (GLM) were used to assess for differences in outcome among the pain groups after controlling for covariates and follow-up period (categorized as 1, 2, 5, 10, 15+ years post-injury), age, sex, race, Hispanic ethnicity, marital status, education, employment, and FIM Motor and Cognitive scores; models for overall and productivity participation did not include employment status as this information is part of the outcome measure. These models were used to estimate and test for pairwise differences in outcomes among the groups and compute associated adjusted effect sizes. In addition, the overall percentage of variability (R2) in the outcome explained by the covariates, the covariates plus group, and the increase in R2 due to group was determined from the GLMs.
Results
Sample Characteristics by Chronic Pain Group
Sample characteristics for each of the chronic pain groups (current, past, and no pain) are summarized in Table 1. Overall, the sample was predominantly male (70–78%), White race (75–78%), middle-aged (mean current age 45–48 years) participants injured primarily in motor vehicle accidents (53–56%) or from falls (20–26%). Differences in sociodemographic, injury, and current functional outcomes among the pain groups were detailed in Harrison-Felix, PhD et al (unpublished data, 2023).
Table 1:
Subject Characteristics by Chronic Pain Group
| Current Pain (N = 1172) | Past Pain (N = 525) | No Pain (N = 1517) | p-value | ||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Mean (SD) | Percentiles | Mean (SD) | Percentiles | Mean (SD) | Percentiles | ||
|
| |||||||
| Follow Up Time Point (years) | 8.7 (7.1) | [0.8, 2.1, 5.4, 14.6, 30.5] | 8.7 (7.0) | [0.8, 2.1, 5.5, 14.6, 30.4] | 8.8 (7.4) | [0.8, 1.9, 5.4, 14.7, 30.5] | 0.8085 b |
|
| |||||||
| Age (years) | 46.4 (15.1) | [18, 35, 45, 57, 94] | 44.8 (15.9) | [18, 33, 41.5, 57, 89] | 47.7 (17.6) | [17, 33, 45, 62, 96] | 0.0011 a |
|
| |||||||
| PTA (days) | 22.4 (27.9) | [0, 3, 16, 31, 441] | 24.7 (25.9) | [0, 6, 19, 35, 213] | 27.0 (29.0) | [0, 7, 19, 37, 282] | <0.0001 b |
|
| |||||||
| GCS | 9.6 (4.6) | [3, 5, 10, 14, 15] | 9.4 (4.5) | [3, 5.5, 9, 14, 15] | 9.4 (4.6) | [3, 5, 10, 14, 15] | 0.4886 b |
|
| |||||||
| Time to Follow Commands (days) | 5.4 (11.0) | [0, .5, 1, 5, 135] | 5.9 (9.8) | [0, 0.5, 1, 7, 69] | 7.1 (12.1) | [0, 0.5, 2, 8, 130] | <0.0001 b |
|
| |||||||
| FIM Motor | 85.0 (9.4) | [13, 83, 88, 91, 91] | 88.5 (5.8) | [30, 88, 91, 91, 91] | 88.3 (6.9) | (15, 89, 91, 91, 91] | <0.0001 b |
|
| |||||||
| FIM Cognitive | 31.0 (3.6) | [14, 29, 32, 34, 35] | 32.6 (2.5) | [18, 32, 33, 34, 35] | 32.7 (2.8) | [14, 32, 34, 35, 35] | <0.0001 b |
|
| |||||||
|
| |||||||
| Count | Percentage | Count | Percentage | Count | Percentage | p-value | |
|
| |||||||
| Follow-up period | 0.0893 c | ||||||
| 1 Year post-TBI | 298 | 16.9% | 88 | 16.8% | 298 | 19.6% | |
| 2 Years post-TBI | 229 | 13.0% | 62 | 11.8% | 181 | 11.9% | |
| 5 Years post-TBI | 383 | 21.7% | 114 | 21.7% | 310 | 20.4% | |
| 10 Years post-TBI | 341 | 19.4% | 112 | 21.3% | 251 | 16.5% | |
| 15+ Years post-TBI | 511 | 29.0% | 149 | 28.4% | 477 | 31.4% | |
|
| |||||||
| Male Sex | 1284 | 72.9% | 369 | 70.3% | 1179 | 77.8% | 0.0004 c |
|
| |||||||
| White Race † | 1322 | 75.3% | 401 | 76.7% | 1173 | 77.8% | 0.2426 c |
| Black Race † | 329 | 18.7% | 95 | 18.2% | 245 | 16.3% | 0.1827 c |
| Other Race † | 119 | 6.8% | 23 | 4.4% | 92 | 6.1% | 0.1380 c |
| Asian | 41 | 2.3% | 10 | 1.9% | 55 | 3.7% | 0.0305 c |
| American Indian / Alaskan Native | 72 | 4.1% | 12 | 2.3% | 33 | 2.2% | 0.0039 c |
| Native Hawaiian / Pacific Islander | 20 | 1.1% | 4 | 0.8% | 15 | 1.0% | 0.7476 c |
|
| |||||||
| Hispanic, Latino, or Spanish Ethnicity † | 209 | 11.9% | 60 | 11.5% | 146 | 9.7% | 0.1191 c |
|
| |||||||
| Cause of Injury | <0.0001 c | ||||||
| Vehicular | 939 | 53.4% | 295 | 56.3% | 801 | 52.9% | * |
| Fall | 357 | 20.3% | 123 | 23.5% | 396 | 26.2% | |
| Violence | 263 | 15.0% | 53 | 10.1% | 132 | 8.7% | * |
| Sports / Other | 200 | 11.4% | 53 | 10.1% | 185 | 12.2% | |
|
| |||||||
| GCS Group | 0.6089 c | ||||||
| Mild | 489 | 41.3% | 143 | 37.6% | 424 | 38.9% | |
| Moderate | 177 | 14.9% | 61 | 16.1% | 160 | 14.7% | |
| Severe | 519 | 43.8% | 176 | 46.3% | 507 | 46.5% | |
|
| |||||||
| Marital Status | 0.0047 c | ||||||
| Single / Other | 619 | 35.2% | 216 | 41.1% | 562 | 37.1% | * |
| Married | 676 | 38.4% | 180 | 34.3% | 620 | 40.9% | * |
| Separated / Divorced / Widowed | 464 | 26.4% | 129 | 24.6% | 333 | 22.0% | * |
|
| |||||||
| Level of Education | <0.0001 c | ||||||
| Less than High School | 285 | 16.2% | 69 | 13.1% | 178 | 11.8% | * |
| High School / Other | 422 | 24.0% | 100 | 19.0% | 339 | 22.4% | * |
| Some College / Associate’s | 654 | 37.2% | 182 | 34.7% | 461 | 30.5% | * |
| Bachelor’s or Higher | 396 | 22.5% | 174 | 33.1% | 535 | 35.4% | * |
|
| |||||||
| Employment Status | <0.0001 c | ||||||
| Employed / Student | 687 | 39.1% | 293 | 55.8% | 766 | 50.6% | * |
| Retired | 719 | 40.9% | 159 | 30.3% | 501 | 33.1% | * |
| Unemployed | 258 | 14.7% | 53 | 10.1% | 175 | 11.6% | * |
| Other | 92 | 5.2% | 20 | 3.8% | 73 | 4.8% | |
SD = standard deviation; Percentiles = 0th (minimum), 25th, 50th (median), 75th, 100th (maximum)
Participants may self-report more than one race; PTA = post-traumatic amnesia; GCS = Glasgow Coma Scale; TFC = time to follow commands
ANOVA test
Kruskal-Wallis test
chi-square test; bold indicates statistically significant differences at α = 0.05
indicates categorical levels with large (>2) cell chi-squares contributing to global differences
Psychosocial Outcomes across Chronic Pain Groups
The psychosocial outcomes are summarized for each pain group in Table 2. For all outcomes assessed, there were significant differences in the mean outcome among the three pain groups (all p-values <0.0001). The current pain group had significantly poorer psychosocial outcomes than both the past pain and no pain groups. In addition, the past pain group had significantly poorer life satisfaction, PTSD, depression, anxiety, and sleep quality, and one domain of participation (productivity) than the no pain group; no significant differences were found between past and no pain for overall participation or the other two domains (out and about, social). Pairwise (unadjusted) effect sizes for each outcome assessing current vs past pain, current vs no pain, and past vs no pain is summarized in Table 3 (left half). The largest effect sizes (in absolute value) were observed for the PTSD, depression, anxiety, and sleep quality measures (ES = 0.64–0.94) when comparing current pain to past or no pain.
Table 2:
Psychosocial Outcomes by Chronic Pain Group
| Current Pain (N = 1172) | Past Pain (N = 525) | No Pain (N = 1517) | |||||
|---|---|---|---|---|---|---|---|
| Mean (SD) | Percentiles | Mean (SD) | Percentiles | Mean (SD) | Percentiles | p-value | |
| SWLS (Life Satisfaction) | 20.4 (8.2) | [5, 14, 21, 27, 35] | 23.2 (7.5) | [5, 18, 24, 29, 35] | 24.4 (7.4) | [5, 18, 24, 29, 35] | <0.0001 a |
| PCL-5 (PTSD Symptoms) | 22.9 (17.9) | [0, 8, 19, 34, 80] | 13.3 (13.2) | [0, 3, 9, 19, 68] | 9.1 (11.4) | [0, 3, 9, 19, 68] | <0.0001 b |
| PHQ-9 (Depression Symptoms) | 8.0 (6.2) | [0, 3, 7, 12, 27] | 4.5 (4.8) | [0, 1, 3, 7, 27] | 3.3 (4.1) | [0, 1, 3, 7, 27] | <0.0001 b |
| GAD-7 (Anxiety Symptoms) | 6.5 (5.8) | [0, 2, 5, 10, 21] | 3.4 (4.3) | [0, 0, 2, 5, 21] | 2.5 (3.8) | [0, 0, 2, 5, 21] | <0.0001 b |
| PSQI (Sleep Quality) | 8.6 (4.6) | [0, 5, 8, 12, 21] | 5.8 (4.0) | [0, 3, 5, 8, 20] | 4.8 (3.5) | [0, 3, 5, 8, 20] | <0.0001 b |
| PART-O Summary (Participation) | 1.8 (0.7) | [0, 1.2, 1.8, 2.3, 3.5] | 2.0 (0.6) | [0, 1.5, 2.0, 2.5, 3.5] | 1.9 (0.7) | [0, 1.5, 2.0, 2.5, 3.5] | <0.0001 b |
| PART-O Out and About (Participation) | 1.4 (0.7) | [0, 0.9, 1.4, 1.9, 3.6] | 1.6 (0.6) | [0, 1.2, 1.6, 2.0, 3.4] | 1.6 (0.7) | [0, 1.2, 1.6, 2.0, 3.4] | <0.0001 b |
| PART-O Productivity (Participation) | 1.5 (1.0) | [0, 0.7, 1.3, 2.3, 4.7] | 1.7 (1.0) | [0, 1.0, 2.0, 2.3, 4.0] | 1.6 (1.0) | [0, 0.7, 1.7, 2.3, 4.7] | <0.0001 b |
| PART-O Social (Participation) | 2.5 (1.0) | [0, 1.7, 2.6, 3.3, 5.0] | 2.6 (1.0) | [0, 1.9, 2.7, 3.3, 4.9] | 2.6 (1.0) | [0, 1.9, 2.7, 3.4, 5.0] | <0.0001 b |
SD = standard deviation; Percentiles = 0th (minimum), 25th, 50th (median), 75th, 100th (maximum)
ANOVA test
Kruskal-Wallis test
SWLS = Satisfaction with Life Scale; PCL-5 = Posttraumatic Stress Disorder Checklist for DSM-5; PHQ-9 = Patient Health Questionnaire; GAD-7 = General Anxiety Disorder Questionnaire; PSQI = Pittsburgh Sleep Quality Index; PART-O = Participation Assessment with Recombined Tools – Objective
Table 3:
Effect Sizes Comparing Psychosocial Outcomes across Pain Groups
| Unadjusted Effect Sizes | Adjusted Effect Sizes | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Current vs Past Pain | Current vs No Pain | Past vs No Pain | Current vs Past Pain | Current vs No Pain | Past vs No Pain | |
|
| ||||||
| SWLS (Life Satisfaction) | −0.36 | −0.51 | −0.15 | −0.22 | −0.37 | −0.15 |
| PCL-5 (PTSD Symptoms) | 0.64 | 0.92 | 0.28 | 0.54 | 0.78 | 0.23 |
| PHQ-9 (Depression Symptoms) | 0.66 | 0.89 | 0.24 | 0.52 | 0.71 | 0.19 |
| GAD-7 (Anxiety Symptoms) | 0.64 | 0.81 | 0.17 | 0.55 | 0.65 | 0.10 |
| PSQI (Sleep) | 0.68 | 0.94 | 0.25 | 0.60 | 0.81 | 0.21 |
| PART-O Summary (Participation) | −0.30 | −0.26 | 0.04 | −0.10 | −0.05 | 0.05 |
| PART-O Out and About (Participation) | −0.34 | −0.34 | 0.00 | −0.18 | −0.16 | 0.02 |
| PART-O Productivity (Participation) | −0.26 | −0.16 | 0.10 | −0.02 | 0.04 | 0.06 |
| PART-O Social (Participation) | −0.13 | −0.15 | −0.02 | −0.02 | −0.01 | 0.02 |
Effect sizes (ES) were derived as the pairwise difference in means divided by the model root mean square error (RMSE). Bold values indicate statistically significant pairwise differences among groups (α = 0.05). Shading represents ES of 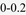 ,
,  ,
,  ,
,  ,
,  with darker shading used for larger effect sizes. Adjusted models control for follow-up period (categorical), current age, sex, White race, Black race, Other race, Hispanic ethnicity, current marital status, current level of education, current employment status, and current FIM Motor and Cognitive scores; PART-O Productivity and Summary Models do not include employment status as a covariate; PCL-5 = Posttraumatic Stress Disorder Checklist for DSM-5; SWLS = Satisfaction with Life Scale; PHQ-9 = Patient Health Questionnaire; GAD-7 = General Anxiety Disorder Questionnaire; PSQI = Pittsburgh Sleep Quality Index; PART-O = Participation Assessment with Recombined Tools – Objective
with darker shading used for larger effect sizes. Adjusted models control for follow-up period (categorical), current age, sex, White race, Black race, Other race, Hispanic ethnicity, current marital status, current level of education, current employment status, and current FIM Motor and Cognitive scores; PART-O Productivity and Summary Models do not include employment status as a covariate; PCL-5 = Posttraumatic Stress Disorder Checklist for DSM-5; SWLS = Satisfaction with Life Scale; PHQ-9 = Patient Health Questionnaire; GAD-7 = General Anxiety Disorder Questionnaire; PSQI = Pittsburgh Sleep Quality Index; PART-O = Participation Assessment with Recombined Tools – Objective
Differences in Psychosocial Outcomes across Chronic Pain Groups Adjusted for Covariates
The covariates (follow-up period, age, sex, race, Hispanic ethnicity, marital status, education, employment, and FIM Motor and Cognitive scores) and chronic pain group together accounted for 19.6%–42.9% of variability in psychosocial outcomes, with the increase in variance due to chronic pain group (R2 increase) ranging from 0.0% to 10.3% (See Table 4). There were statistically significant differences among the groups (all p-values < 0.0001) after controlling for covariates for the life satisfaction (R2 increase = 2.0%), PTSD (R2 increase = 8.4%), depression (R2 increase = 7.2%), anxiety (R2 increase = 6.6%), sleep quality (R2 increase = 10.3%), and out and about participation (R2 increase = 0.5%) outcome measures; overall, productivity, and social participation were not significantly different among groups when controlling for covariates (R2 increase <0.1% to 0.5%, p-values > 0.11). The adjusted pairwise effect sizes for each outcome assessing current vs past pain, current vs no pain, and past vs no pain are summarized in Table 3 (right half). The largest effect sizes (in absolute value) were observed for the PTSD, depression, anxiety, and sleep quality measures (|ES| = 0.52 to 0.81) when comparing current pain to past or no pain, smaller effect sizes were observed for life satisfaction (|ES| = 0.22 to 0.37) and out and about participation (|ES| = 0.16 to 0.18). When comparing past and no pain groups, adjusted effect sizes were generally small for life satisfaction, PTSD, depression, anxiety, and sleep quality (|ES| = 0.10 to 0.23) and minimal for participation outcomes (|ES| = 0.02 to 0.06). Complete adjusted model results for all outcomes can be found in supplemental material.
Table 4.
Model R2 without and with pain group in the model, and increase in R2 due to group
| R2 without group | R2 with group | R2 Increase | |
|---|---|---|---|
|
| |||
| SWLS | 0.204 | 0.224 | 0.020 |
| PCL5 | 0.222 | 0.306 | 0.084 |
| PHQ9 | 0.226 | 0.298 | 0.072 |
| GAD7 | 0.190 | 0.255 | 0.066 |
| PSQI | 0.125 | 0.228 | 0.103 |
| PART Summary | 0.429 | 0.429 | 0.001 |
| PART Out and About | 0.191 | 0.196 | 0.005 |
| PART Productivity | 0.348 | 0.349 | <0.001 |
| PART Social | 0.367 | 0.367 | <0.001 |
SWLS = Satisfaction with Life Scale; PCL-5 = Posttraumatic Stress Disorder Checklist for DSM-5; PHQ-9 = Patient Health Questionnaire; GAD-7 = General Anxiety Disorder Questionnaire; PSQI = Pittsburgh Sleep Quality Index; PART-O = Participation Assessment with Recombined Tools – Objective
Discussion
This study is the first large-scale investigation of the relationship between chronic pain and psychosocial health outcomes among individuals who have sustained complicated mild to severe TBI. It is the only investigation examining these differences across those who experience chronic pain, have experienced noticeable resolution or improvement in their pain since TBI, and those who have not experienced chronic pain at any time since injury. In unadjusted bivariate analysis, all psychosocial measures assessed differed across chronic pain groups. Individuals with current chronic pain demonstrated the highest levels of PTSD, anxiety, and depression, and the lowest levels of sleep quality, community participation and satisfaction with life. The past pain group demonstrated mean scores for these outcomes that were all between the current and no chronic pain groups, but were consistently most similar to the no pain group. In multivariable analysis, adjusting for sociodemographic and concurrent motor and cognitive function, having current chronic pain was associated with more negative outcomes in psychological distress symptoms, life satisfaction, sleep quality, and involvement in community activities. This indicates that chronic pain is an important factor in psychosocial outcomes after TBI and highlights the important need for clinical interventions to address pain following TBI, as well as pain’s inclusion in research investigating TBI outcomes.
In examining effect sizes between chronic pain groups in the covariate-adjusted model, the largest were observed for current compared to no pain groups, with medium-large effect sizes that ranged from 0.65 to 0.81 for sleep quality, PTSD, depression and anxiety. Although not as large, current chronic pain compared to past pain still indicated moderate effect sizes (0.52 to 0.60). In covariate-adjusted analysis the group endorsing chronic pain at some point after their index TBI, but not currently (past pain), was again found to be similar to the no pain group. Similarities between the no pain and past pain groups on the psychosocial measures indicate that these important outcomes for persons with TBI could potentially be improved with effective treatment of current chronic pain, or that addressing psychosocial morbidity may aid in the adjustment to and improvement in chronic pain. The direction of the relationships between psychosocial health outcomes and chronic pain is likely reciprocal and complex, warranting further study.
In adjusted models the strongest effect was seen in differences between current and no pain on sleep quality, with those in the current pain group having a mean score above the cutoff for identifying “poor sleepers.” The presence of clinically significant sleep disturbance is consistent with an established literature of sleep disorder comorbidity in the years post-injury.34,35 Sleep disturbances and pain share a bidirectional and mutually exacerbating relationship, although the underlying pathophysiological mechanisms have not been fully elucidated.36,37 Pain itself is inherently disruptive to sleep through discomfort and at the time of injury is associated with a system-wide inflammatory cascade and disruption to neural circuitry that contributes directly to both sleep disturbance as well as setting individuals on a path that raises their risk for developing chronic pain.35,38–40 Sleep disturbance contributes to the experience of pain via physiological pathways such as intermittent hypoxia, impaired immunity, reduced glymphatic waste clearance, and disruption of endogenous pain modulation.41–43 Additionally, psychological and behavioral pathways are implicated, such as dysfunctional beliefs about the pain-sleep relationship,44 psychiatric comorbidity (e.g., depression, PTSD) and maladaptive coping responses (e.g., increased time spent in bed).45,46
Consistent with prior literature that has found PTSD and associated symptoms to be highly comorbid with chronic pain,47,48 a large effect was also seen on PTSD symptoms between the current and no pain groups. Mean scores on measures of PTSD severity were over twice as high among those in the current pain group compared to those in the no pain group. Although mean scores in the current pain group were below the accepted cutoff to indicate probable PTSD diagnosis, subclinical PTSD symptoms may still be associated with levels of distress and impaired functioning approximating that of a full PTSD diagnosis.49,50 These symptoms are modifiable targets responsive to intervention51 that are associated with pain potentiating inflammatory stress response.52,53 PTSD symptoms have been directly implicated in pain sensitivity, with some evidence indicating a mediating role in central sensitization and centralized pain experiences.47,54 The presence of PTSD following TBI is associated with poorer pain coping 55 while reductions in PTSD severity have been shown to improve physical activity and engagement in daily activities in persons with chronic pain.56 Conversely, chronic pain may act as a persistent reminder of psychological trauma serving as a cue for hypervigilance and avoidance of pain-related activity which limits daily functioning.48,57
A symptom cluster of pain, PTSD and TBI sequelae has been labeled the polytrauma triad, although it has seen limited exploration outside of military and Veteran samples, where blast related injury has been the focal mechanism.58 The current study features a predominantly civilian sample with cause of injury largely represented by motor vehicle accidents (MVA) and falls, yet the large effect size seen in comparing those with and without current pain suggests this constellation of symptoms is not specific to military and Veteran samples and warrants further investigation within civilian samples. Peixoto and colleagues examined the presence of the polytrauma clinical triad in a Canadian community sample of patients referred for pain management following MVA, finding that over half met criteria for experiencing this group of symptoms (i.e., TBI, chronic pain, PTSD). Those patients who met criteria also experienced worse pain and poorer sleep quality outcomes.59
Both depression and anxiety, while significantly different reflect less prominent clinical differences across pain groups in the current study, as mean scores on measures met threshold for only “minimal” elevations in symptoms for the current chronic pain group. Nonetheless, the differences observed are consistent with a reciprocal relationship between depression and chronic pain,60 whereby improvement in the severity of either symptom (e.g., depression) predicts subsequent severity of the other symptom (e.g., chronic pain).61
This study has several limitations. It is cross-sectional, self-reported survey which asks participants to recall pain experiences. Interpretation for cross-sectional designs is limited and causality cannot be inferred, i.e., the direction of relationships with pain and PTSD, sleep and depression are unknown. Self-reported data is susceptible to recall bias, social desirability bias, and information is limited to the questions that were asked to participants (e.g., possible restricted response options or confounding variables that were unintentionally omitted). Specific to individuals with TBI, length of time since injury may also contribute to recalling pain histories, and the ability for introspective self-assessment may be reduced. Results from this project have limited generalizability, and is only representative for people with TBI who received inpatient rehabilitation, live in the community, speak English, and were able to complete the survey without a proxy.
Conclusions
Chronic pain is prevalent among individuals with TBI and is associated with poorer psychosocial outcomes, and include symptoms of PTSD, depression, anxiety, and sleep disturbance. These findings in a well-defined, large-scale sample of individuals previously hospitalized for TBI, demonstrate a strong triadic association between brain injury, psychological distress, and chronic pain in a predominantly civilian sample. These findings underscore the presence of modifiable comorbidities among those with chronic pain in the years following TBI. Individuals who experience persistent pain following TBI may be at greater risk for worse psychosocial outcomes. Without intervention, these symptoms are likely to continue influencing the experience of and adjustment to chronic pain and consequently the development of co-occurring psychiatric conditions. Addressing chronic pain is an important priority for the psychosocial well-being and long-term rehabilitation outcome after injury. The direction of the relationships between psychosocial functioning and pain is likely reciprocal, complex, and requires further study with more complex modeling. We observed more favorable psychosocial outcomes in those with a history of chronic pain that had remitted by the time of the interview indicating that some of the negative psychosocial impact of pain may be mitigated by successful recognition and pain treatment. This finding offers a very promising area for future treatment development and research.
Supplementary Material
Acknowledgements
James A. Haley Veteran’s Administration: The views expressed in this manuscript are those of the authors and do not necessarily represent the official policy or position of the Defense Health Agency, Department of Defense, or any other U.S. government agency. This work was prepared under Contract HT0014–22-C-0016 with DHA Contracting Office (CO-NCR) HT0014 and, therefore, is defined as U.S. Government work under Title 17 U.S.C.§101. Per Title 17 U.S.C.§105, copyright protection is not available for any work of the U.S. Government. For more information, please contact dha.TBICOEinfo@health.mil.
Conflict of Interest and Source of Funding
The authors have no conflicts of interest. Research reported in this article was funded through the National Institute on Disability, Independent Living, and Rehabilitation (NIDILRR), a Center within the Administration for Community Living (ACL), Department of Health and Human Services (HHS) Collaborative Grant Award (90DPTB0017) which leveraged the infrastructure of the NIDILRR and the Department of Veterans Affairs (VA) TBI Model Systems programs of research (James A. Haley Veterans Hospital TBI Model Systems, IRB PR00000094; see additional acknowledgement, Characterization and Treatment of Chronic Pain after TBI, 90DPTB0017, IRB PR00039496; Craig Hospital, Rocky Mountain Regional TBI Model System, 90DPTB007 (2017–2022) and 90DPTB0020 (2022–2027), IRB 231579, Characterization and Treatment of Chronic Pain after TBI, 90DPTB0017, IRB 1335849; Craig Hospital TBI Model Systems National Data and Statistical Center, 90DP0084 (2016–2021) and 90DPTB0018 (2021–2026), IRB 231626; University of Washington School of Medicine, University of Washington TBI Model System, 90DPTB0008 (2017–2022) and 90DPTB0024 (2022–2027), IRB STUDY00001788; Indiana University School of Medicine, Indiana TBI Model System, 90DPTB0002 (2017–2022) and 90DPTB0022 (2022–2027), IRB 1211010085R006; Spaulding Rehabilitation Hospital, Harvard Medical School, Spaulding-Harvard TBI Model System, 90DPTB0011 (2017–2022) and 90DPTB0027 (2022–2027), IRB 2012P002476; Wayne State University School of Medicine, Southeastern Michigan TBI System, 90DPTB006 (2017–2022) and 90DPTB0030 (2022–2027), IRB 102908B3E; Mayo Clinic College of Medicine and Science, Mayo Clinic TBI Model System, 90DPTB0012 (2017–2022) and 90DPTB0031 (2022–2027), IRB 69–03; NYU Langone Health, Rusk Rehabilitation TBI Model System at NYU and Bellevue, 90DPTB0010 (2017–2022) and 90DPTB0034 (2022–2027), IRB 13–00056; Baylor Scott and White Institute for Rehabilitation, North Texas TBI Model System, 90DPTB0013 (2017–2022) and 90DPTB0023 (2022–2027), IRB 002–212; Virginia Commonwealth University, The Virginia TBI Model System (VTBIMS), 90DP0033 (2017–2022) and 90DBTB0021 (2022–2027), IRB PR00039496; Thomas Jefferson University, Moss Rehabilitation Research Institute, Moss TBI Model Systems 90DPTB0004 (2017–2022) and 90DPTB001 (2022–2027), IRB; University of Alabama at Birmingham, UAB TBI Model System, 90DPTB0015 (2017–2022) and 90DPTB0029 (2022–2027), IRB 980904002; TIRR Memorial Hermann, Texas TBI Model System at TIRR, 90DPTB0016 (2017–2022) and 90DPTB0025 (2022–2027), IRB H-21935; The Ohio State University College of Medicine, Ohio Regional TBI Model System, 90DPTB0001 (2017–2022) and 90DPTB0026 (2022–2027), IRB 1993H0142; Kessler Foundation, Northern New Jersey TBI System, 90DPTB0003 (2017–2022) and 90DPTB0032 (2022–2027), IRB R-597–07; Carolinas Rehabilitation, TBI Model System Follow-Up Center, TBI Model Systems National Data and Statistical Center, 90DP0084 (2017–2022) and 90DPTB0018 (2021–2026), IRB PR00022242; Icahn School of Medicine at Mount Sinai, The New York TBI Model System at Mount Sinai, 90DPTB0009 (2017–2022) and 90DPTB0028 (2022–2027), IRB 11–01799, IRB 22–0700; Hackensack Meridian JFK Johnson Rehabilitation, TBI Model System Projects at JFK Johnson Rehabilitation 90DPTB0014 (2017–2022), IRB CR00005522.) The views expressed in this manuscript are those of the authors and do not necessarily represent the official policy or position of NIDILRR, ACL, HHS; Defense Health Agency, Department of Army/Navy/Air Force, Department of Defense (DOD); Veterans Health Administration (VHA), or any other U.S. government agency. No official endorsement should be inferred.
Contributor Information
Robin Hanks, Department of Physical Medicine and Rehabilitation, Wayne State University School of Medicine, Detroit, MI
Jessica M. Ketchum, Craig Hospital Research Department, Englewood, CO
Mackenzie Peckham, Craig Hospital Research Department, Englewood, CO
Mitch Sevigny, Craig Hospital Research Department, Englewood, CO
Angelle M. Sander, H. Ben Taub Department of Physical Medicine and Rehabilitation, Baylor College of Medicine and Brain Injury Research Center, TIRR Memorial Hermann, Houston, TX
Aaron M. Martin, Mental Health and Behavioral Science Service, James A. Haley Veterans Hospital; Department of Psychiatry and Behavioral Neurosciences, University of South Florida, Tampa, FL
Stephanie Agtarap, Craig Hospital Research Department, Englewood, CO
Cynthia L. Beaulieu, Department of Physical Medicine and Rehabilitation, The Ohio State University College of Medicine, Columbus, OH
Libby Callender, Baylor Scott and White Institute for Rehabilitation, Dallas, TX
Flora M. Hammond, Department of Physical Medicine and Rehabilitation, Indiana University School of Medicine & Rehabilitation Hospital of Indiana, Indianapolis, IN
Jeannie Lengenfelder, Department of Physical Medicine Rehabilitation, Rutgers-New Jersey Medical School, Newark, NJ; Kessler Foundation, East Hanover, NJ
Amanda R. Rabinowitz, Department of Physical Medicine and Rehabilitation, Moss Rehabilitation Research Institute, Elkins Park, PA
William C. Walker, Department. of Physical Medicine and Rehabilitation, School of Medicine, Virginia Commonwealth University, Richmond, VA
Jeanne M. Hoffman, Department of Rehabilitation Medicine, University of Washington School of Medicine, Seattle, WA
Cynthia Harrison-Felix, Craig Hospital Research Department, Englewood, CO
Risa Nakase-Richardson, MHBS/Polytrauma, James A. Haley Veterans Hospital, Tampa, FL; Sleep and Pulmonary Division, Department of Internal Medicine, University of South Florida, Tampa, FL; Defense Health Agency Traumatic Brain Injury Center of Excellence, Tampa, FL
References
- 1.Erler KS, Juengst SB, Whiteneck GG, et al. The Association of Rehospitalization With Participation 5 Years After Traumatic Brain Injury. The Journal of head trauma rehabilitation. 2018;33(6):E77–E84. doi: 10.1097/htr.0000000000000386 [DOI] [PubMed] [Google Scholar]
- 2.Cuthbert JP, Pretz CR, Bushnik T, et al. Ten-Year Employment Patterns of Working Age Individuals After Moderate to Severe Traumatic Brain Injury: A National Institute on Disability and Rehabilitation Research Traumatic Brain Injury Model Systems Study. Archives of Physical Medicine and Rehabilitation. 2015/December/01/ 2015;96(12):2128–2136. doi: 10.1016/j.apmr.2015.07.020 [DOI] [PubMed] [Google Scholar]
- 3.Cuthbert JP, Harrison-Felix C, Corrigan JD, Bell JM, Haarbauer-Krupa JK, Miller AC. Unemployment in the United States after traumatic brain injury for working-age individuals: prevalence and associated factors 2 years postinjury. The Journal of head trauma rehabilitation. May-Jun 2015;30(3):160–74. doi: 10.1097/htr.0000000000000090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Corrigan JD, Cuthbert JP, Harrison-Felix C, et al. US Population Estimates of Health and Social Outcomes 5 Years After Rehabilitation for Traumatic Brain Injury. The Journal of head trauma rehabilitation. 2014;29(6):E1–E9. doi: 10.1097/htr.0000000000000020 [DOI] [PubMed] [Google Scholar]
- 5.Juengst SB, Adams LM, Bogner JA, et al. Trajectories of life satisfaction after traumatic brain injury: Influence of life roles, age, cognitive disability, and depressive symptoms. Rehabilitation psychology. 2015;60:353–364. doi: 10.1037/rep0000056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heath LM, Kidwai MR, Colella B, et al. Predictors and Functional Outcomes Associated With Longitudinal Trajectories of Anxiety and Depression from 2 to ≥36 Months After Moderate to Severe Traumatic Brain Injury. Journal of Neurotrauma. 2023;doi: 10.1089/neu.2023.0003 [DOI] [PubMed] [Google Scholar]
- 7.Neumann D, Juengst SB, Bombardier CH, et al. Anxiety Trajectories the First 10 Years After a Traumatic Brain Injury (TBI): A TBI Model Systems Study. Archives of Physical Medicine and Rehabilitation. 2022/November/01/ 2022;103(11):2105–2113. doi: 10.1016/j.apmr.2022.07.002 [DOI] [PubMed] [Google Scholar]
- 8.Carmichael J, Hicks AJ, Gould KR, Ponsford J, Spitz G. Ten-Year Cohort Study of Emotional Distress Trajectories After Moderate-Severe Traumatic Brain Injury. Archives of Physical Medicine and Rehabilitation. 2023/March/04/ 2023;doi: 10.1016/j.apmr.2023.02.008 [DOI] [PubMed] [Google Scholar]
- 9.Lequerica AH, Weber E, Dijkers MP, et al. Factors associated with the remission of insomnia after traumatic brain injury: a traumatic brain injury model systems study. Brain Injury. 2020;34(2):187–194. doi: 10.1080/02699052.2019.1682193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cantor JB, Bushnik T, Cicerone K, et al. Insomnia, Fatigue, and Sleepiness in the First 2 Years After Traumatic Brain Injury: An NIDRR TBI Model System Module Study. The Journal of head trauma rehabilitation. 2012;27(6):E1–E14. doi: 10.1097/HTR.0b013e318270f91e [DOI] [PubMed] [Google Scholar]
- 11.Irvine K-A, Clark JD. Chronic Pain After Traumatic Brain Injury: Pathophysiology and Pain Mechanisms. Pain Medicine. 2017;19(7):1315–1333. doi: 10.1093/pm/pnx153 [DOI] [PubMed] [Google Scholar]
- 12.Meints S, Edwards R. Evaluating psychosocial contributions to chronic pain outcomes. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2018;87:168–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goyal AK, Mohanty SK. Association of pain and quality of life among middle-aged and older adults of India. BMC Geriatrics. 2022;22(1)doi: 10.1186/s12877-022-03480-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dong H-J, Larsson B, Dragioti E, Bernfort L, Levin L-Å, Gerdle B. <p>Factors Associated with Life Satisfaction in Older Adults with Chronic Pain (PainS65+)</p>. Journal of Pain Research. 2020;Volume 13:475–489. doi: 10.2147/jpr.s234565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Suso-Ribera C, Yakobov E, Carriere JS, García-Palacios A. The impact of chronic pain on patients and spouses: Consequences on occupational status, distribution of household chores and care-giving burden. 10.1002/ejp.1616. European Journal of Pain. 2020/October/01 2020;24(9):1730–1740. doi: 10.1002/ejp.1616 [DOI] [PubMed] [Google Scholar]
- 16.Herrera-Escobar JP, Seshadri AJ, Stanek E, et al. Mental health burden after injury: it’s about more than just posttraumatic stress disorder. Annals of surgery. 2021;274(6):e1162–e1169. [DOI] [PubMed] [Google Scholar]
- 17.Koffel E, Kroenke K, Bair MJ, Leverty D, Polusny MA, Krebs EE. The bidirectional relationship between sleep complaints and pain: Analysis of data from a randomized trial. Health Psychology. 2016;35(1):41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu C, Li Q, Gao Y, Huo H, Zhang W. Changes and Influencing Factors of Stress Disorder in Patients with Mild Traumatic Brain Injury Stress Disorder. BioMed research international. 2022/September/26 2022;2022:9082946. doi: 10.1155/2022/9082946 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 19.Ashina H, Al-Khazali HM, Iljazi A, et al. Psychiatric and cognitive comorbidities of persistent post-traumatic headache attributed to mild traumatic brain injury. The Journal of Headache and Pain. 2021/July/26 2021;22(1):83. doi: 10.1186/s10194-021-01287-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Phillips KM, Clark ME, Gironda RJ, et al. Pain and psychiatric comorbidities among two groups of Iraq-and Afghanistan-era Veterans. Journal of Rehabilitation Research & Development. 2016; [DOI] [PubMed] [Google Scholar]
- 21.Robayo LE, Govind V, Vastano R, et al. Multidimensional pain phenotypes after Traumatic Brain Injury. Front Pain Res (Lausanne). 2022;3:947562. doi: 10.3389/fpain.2022.947562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kumar RG, Gao S, Juengst SB, Wagner AK, Fabio A. The effects of post-traumatic depression on cognition, pain, fatigue, and headache after moderate-to-severe traumatic brain injury: a thematic review. Brain Injury. 2018/March/21 2018;32(4):383–394. doi: 10.1080/02699052.2018.1427888 [DOI] [PubMed] [Google Scholar]
- 23.Hoffman JM, Pagulayan KF, Zawaideh N, Dikmen S, Temkin N, Bell KR. Understanding Pain After Traumatic Brain Injury: Impact on Community Participation. American Journal of Physical Medicine & Rehabilitation. 2007;86(12):962–969. doi: 10.1097/PHM.0b013e31815b5ee5 [DOI] [PubMed] [Google Scholar]
- 24.Dawson DR, Schwartz ML, Winocur G, Stuss DT. Return to productivity following traumatic brain injury: Cognitive, psychological, physical, spiritual, and environmental correlates. Disability and Rehabilitation. 2007/January/01 2007;29(4):301–313. doi: 10.1080/09638280600756687 [DOI] [PubMed] [Google Scholar]
- 25.Noyes ET, Tang X, Sander AM, et al. Relationship of medical comorbidities to psychological health at 2 and 5 years following traumatic brain injury (TBI). Rehabilitation psychology. 2021;66:107–117. doi: 10.1037/rep0000366 [DOI] [PubMed] [Google Scholar]
- 26.Traumatic Brain Injury Model Systems National Database. Traumatic Brain Injury Model System National Data and Statistical Center. doi: 10.17605/OSF.IO/A4XZB Accessed March 18, 2023. http://www.tbindsc.org [DOI]
- 27.Dijkers MP, Harrison-Felix C, Marwitz JH. The Traumatic Brain Injury Model Systems: History and Contributions to Clinical Service and Research. The Journal of head trauma rehabilitation. 2010;25(2):81–91. doi: 10.1097/HTR.0b013e3181cd3528 [DOI] [PubMed] [Google Scholar]
- 28.Lamberty GJ, Nakase-Richardson R, Farrell-Carnahan L, et al. Development of a Traumatic Brain Injury Model System Within the Department of Veterans Affairs Polytrauma System of Care. The Journal of head trauma rehabilitation. 2014;29(3):E1–E7. doi: 10.1097/HTR.0b013e31829a64d1 [DOI] [PubMed] [Google Scholar]
- 29.Diener E, Emmons RA, Larsen RJ, Griffin S. The Satisfaction With Life Scale. Journal of personality assessment. 1985/February/01 1985;49(1):71–75. doi: 10.1207/s15327752jpa4901_13 [DOI] [PubMed] [Google Scholar]
- 30.Kroenke K, Spitzer RL, Williams JBW. The PHQ-9. Journal of General Internal Medicine. 2001;16(9):606–613. doi: 10.1046/j.1525-1497.2001.016009606.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Spitzer RL, Kroenke K, Williams JBW, Löwe B. A Brief Measure for Assessing Generalized Anxiety Disorder: The GAD-7. Archives of Internal Medicine. 2006;166(10):1092–1097. doi: 10.1001/archinte.166.10.1092 [DOI] [PubMed] [Google Scholar]
- 32.Bogner J, Bellon K, Kolakowsky-Hayner SA, Whiteneck G. Participation Assessment With Recombined Tools–Objective (PART-O). The Journal of head trauma rehabilitation. 2013;28(4):337–339. doi: 10.1097/HTR.0b013e31829af969 [DOI] [PubMed] [Google Scholar]
- 33.Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh sleep quality index: A new instrument for psychiatric practice and research. Psychiatry Research. 1989/May/01/ 1989;28(2):193–213. doi: 10.1016/0165-1781(89)90047-4 [DOI] [PubMed] [Google Scholar]
- 34.Kempf J, Werth E, Kaiser PR, Bassetti CL, Baumann CR. Sleep–wake disturbances 3 years after traumatic brain injury. Journal of Neurology, Neurosurgery & Psychiatry. 2010;81(12):1402–1405. doi: 10.1136/jnnp.2009.201913 [DOI] [PubMed] [Google Scholar]
- 35.Barshikar S, Bell KR. Sleep Disturbance After TBI. Current Neurology and Neuroscience Reports. 2017/September/20 2017;17(11):87. doi: 10.1007/s11910-017-0792-4 [DOI] [PubMed] [Google Scholar]
- 36.Finan PH, Goodin BR, Smith MT. The Association of Sleep and Pain: An Update and a Path Forward. The Journal of Pain. 2013;14(12):1539–1552. doi: 10.1016/j.jpain.2013.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Charokopos A, Card ME, Gunderson C, Steffens C, Bastian LA. The association of obstructive sleep apnea and pain outcomes in adults: a systematic review. Pain Medicine. 2018;19(suppl_1):S69–S75. [DOI] [PubMed] [Google Scholar]
- 38.Kanefsky R, Motamedi V, Mithani S, Mysliwiec V, Gill JM, Pattinson CL. Mild traumatic brain injuries with loss of consciousness are associated with increased inflammation and pain in military personnel. Psychiatry research. 2019;279:34–39. [DOI] [PubMed] [Google Scholar]
- 39.Gironda RJ, Clark ME, Ruff RL, et al. Traumatic brain injury, polytrauma, and pain: challenges and treatment strategies for the polytrauma rehabilitation. Rehabilitation psychology. 2009;54(3):247. [DOI] [PubMed] [Google Scholar]
- 40.Werner JK Jr, Baumann CR. TBI and Sleep–Wake Disorders: Pathophysiology, Clinical Management, and Moving towards the Future. Thieme Medical Publishers; 2017:419–432. [DOI] [PubMed] [Google Scholar]
- 41.Herrero Babiloni A, De Koninck BP, Beetz G, De Beaumont L, Martel MO, Lavigne GJ. Sleep and pain: recent insights, mechanisms, and future directions in the investigation of this relationship. Journal of neural transmission. 2020;127:647–660. [DOI] [PubMed] [Google Scholar]
- 42.Wickwire EM, Williams SG, Roth T, et al. Sleep, sleep disorders, and mild traumatic brain injury. What we know and what we need to know: findings from a national working group. Neurotherapeutics. 2016;13:403–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Piantino JA, Iliff JJ, Lim MM. The bidirectional link between sleep disturbances and traumatic brain injury symptoms: A role for glymphatic dysfunction? Biological psychiatry. 2022;91(5):478–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Afolalu EF, Moore C, Ramlee F, Goodchild CE, Tang NK. Development of the pain-related beliefs and attitudes about sleep (PBAS) scale for the assessment and treatment of insomnia comorbid with chronic pain. Journal of clinical sleep medicine. 2016;12(9):1269–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Balba NM, Elliott JE, Weymann KB, et al. Increased sleep disturbances and pain in veterans with comorbid traumatic brain injury and posttraumatic stress disorder. Journal of clinical sleep medicine. 2018;14(11):1865–1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.de la Vega R, Miró J, Esteve R, Ramírez-Maestre C, López-Martínez AE, Jensen MP. Sleep disturbance in individuals with physical disabilities and chronic pain: The role of physical, emotional and cognitive factors. Disability and health journal. 2019;12(4):588–593. [DOI] [PubMed] [Google Scholar]
- 47.Kind S, Otis JD. The Interaction Between Chronic Pain and PTSD. Current Pain and Headache Reports. 2019/November/28 2019;23(12):91. doi: 10.1007/s11916-019-0828-3 [DOI] [PubMed] [Google Scholar]
- 48.Lumley MA, Yamin JB, Pester BD, Krohner S, Urbanik CP. Trauma matters: psychological interventions for comorbid psychosocial trauma and chronic pain. PAIN. 2022;163(4):599–603. doi: 10.1097/j.pain.0000000000002425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Blevins CA, Weathers FW, Davis MT, Witte TK, Domino JL. The Posttraumatic Stress Disorder Checklist for DSM-5 (PCL-5): Development and Initial Psychometric Evaluation. Journal of traumatic stress. 2015;28(6):489–498. doi: 10.1002/jts.22059 [DOI] [PubMed] [Google Scholar]
- 50.Bovin MJ, Marx BP, Weathers FW, et al. Psychometric properties of the PTSD checklist for diagnostic and statistical manual of mental disorders–fifth edition (PCL-5) in veterans. Psychological assessment. 2016;28(11):1379. [DOI] [PubMed] [Google Scholar]
- 51.Korte KJ, Allan NP, Gros DF, Acierno R. Differential treatment response trajectories in individuals with subclinical and clinical PTSD. Journal of Anxiety Disorders. 2016;38:95–101. doi: 10.1016/j.janxdis.2016.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Song Y, Zhou D, Wang X. Increased serum cortisol and growth hormone levels in earthquake survivors with PTSD or subclinical PTSD. Psychoneuroendocrinology. 2008/September/01/ 2008;33(8):1155–1159. doi: 10.1016/j.psyneuen.2008.05.005 [DOI] [PubMed] [Google Scholar]
- 53.Wessa M, Karl A, Flor H. Central and peripheral psychophysiological responses to trauma-related cues in subclinical posttraumatic stress disorder: a pilot study. Experimental Brain Research. 2005/November/01 2005;167(1):56–65. doi: 10.1007/s00221-005-0007-0 [DOI] [PubMed] [Google Scholar]
- 54.McKernan LC, Johnson BN, Crofford LJ, Lumley MA, Bruehl S, Cheavens JS. Posttraumatic Stress Symptoms Mediate the Effects of Trauma Exposure on Clinical Indicators of Central Sensitization in Patients With Chronic Pain. The Clinical Journal of Pain. 2019;35(5):385–393. doi: 10.1097/ajp.0000000000000689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Aase DM, Babione JM, Proescher E, et al. Impact of PTSD on post-concussive symptoms, neuropsychological functioning, and pain in post-9/11 veterans with mild traumatic brain injury. Psychiatry Research. 2018/October/01/ 2018;268:460–466. doi: 10.1016/j.psychres.2018.08.019 [DOI] [PubMed] [Google Scholar]
- 56.Bourn LE, Sexton MB, Porter KE, Rauch SAM. Physical Activity Moderates the Association Between Pain and PTSD in Treatment-Seeking Veterans. Pain Medicine. 2016;17(11):2134–2141. doi: 10.1093/pm/pnw089 [DOI] [PubMed] [Google Scholar]
- 57.Powell MA, Corbo V, Fonda JR, Otis JD, Milberg WP, McGlinchey RE. Sleep Quality and Reexperiencing Symptoms of PTSD Are Associated With Current Pain in U.S. OEF/OIF/OND Veterans With and Without mTBIs. Journal of traumatic stress. 2015;28(4):322–329. doi: 10.1002/jts.22027 [DOI] [PubMed] [Google Scholar]
- 58.Lew HL, Otis JD, Tun C, Kerns RD, Clark ME, Cifu DX. Prevalence of chronic pain, posttraumatic stress disorder, and persistent postconcussive symptoms in OIF/OEF veterans: polytrauma clinical triad. J Rehabil Res Dev. 2009;46(6):697–702. [DOI] [PubMed] [Google Scholar]
- 59.Peixoto C, Hyland L, Buchanan DM, Langille E, Nahas R. The polytrauma clinical triad in patients with chronic pain after motor vehicle collision. Journal of pain research. 2018:1927–1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lerman SF, Rudich Z, Brill S, Shalev H, Shahar G. Longitudinal associations between depression, anxiety, pain, and pain-related disability in chronic pain patients. Psychosomatic medicine. 2015;77(3):333–341. [DOI] [PubMed] [Google Scholar]
- 61.Kroenke K, Wu J, Bair MJ, Krebs EE, Damush TM, Tu W. Reciprocal relationship between pain and depression: a 12-month longitudinal analysis in primary care. The Journal of Pain. 2011;12(9):964–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


