Summary
Background:
Progression to cirrhosis in non-alcoholic steatohepatitis (NASH) is associated with a decrease in liver fat. However, the prognostic significance of liver fat content in NASH-related significant fibrosis and cirrhosis is unclear.
Aim:
To investigate the risk of decompensation, hepatocellular carcinoma (HCC) and mortality stratified by liver fat content in NASH-related significant fibrosis and cirrhosis.
Methods:
In this meta-analysis of individual participant data, 456 patients with both magnetic resonance elastography (MRE) and MRI-derived protein density fat fraction (MRI-PDFF) were enrolled, and 296 patients with longitudinal follow-up were analysed. MRE combined with fibrosis-4 (MEFIB-index), and MRI-PDFF were used to measure liver fibrosis and fat, respectively. MEFIB-negative, MEFIB-positive+ MRI-PDFF ≥5% and MEFIB-positive+ MRI-PDFF <5% were defined as no significant liver fibrosis, NASH with significant fibrosis and higher liver fat content, and NASH with significant fibrosis and low liver fat content groups, respectively. The primary outcome was hepatic decompensation, HCC and death.
Results:
The rates of decompensation, HCC and mortality were highest in the NASH with significant fibrosis and low liver fat group (33%, 17% and 17%, respectively), followed by the NASH with significant fibrosis and higher liver fat group (18%, 13% and 13% respectively), and lowest in the no significant fibrosis (MEFIB-negative) group (0%, 1% and 2% respectively). In multivariable-adjusted analysis, low liver fat content was strongly associated (HR = 42.2 [95% CI: 7.5–235.5, p < 0.0001]) with HCC, decompensation and death. Sensitivity analyses for patients with cirrhosis (MRE ≥5 kPa) determined consistent findings.
Conclusions:
Low liver fat content in patients with burnt-out NASH-related significant fibrosis and cirrhosis is associated with an increase in hepatic decompensation, HCC and mortality.
1 |. INTRODUCTION
Non-alcoholic fatty liver disease (NAFLD) is currently the most common type of liver disease, with an estimated global prevalence of 37.8% in 2016 or later.1 It represents a broad spectrum of liver conditions, from simple hepatic steatosis to non-alcoholic steatohepatitis (NASH), significant fibrosis, cirrhosis and NAFLD-related hepatocellular carcinoma (HCC). The prognosis of patients with NAFLD worsens with the progression of liver fibrosis, with a reported all-cause mortality of 0.89 and 1.76 deaths per 100 person years in patients with stages F3 and F4 fibrosis, respectively.2
Numerous studies have identified increased liver fat content, cellular injury and hepatic inflammation as the key drivers associated with fibrosis progression in NASH patients.3 On the other hand, a recent study has suggested that there is a causal relationship between liver fat accumulation and fibrosis progression, independent of disease activity.4 However, such histologic hallmarks of NASH, including steatosis, lobular inflammation and hepatocellular ballooning have been reported to diminish when patients have further progressed to advanced liver fibrosis or cirrhosis, also termed burnt-out NASH.5 Furthermore, it has been recently reported that lower liver fat content was associated with a higher incidence of HCC in chronic hepatitis B (CHB) patients with advanced fibrosis, suggesting fat storage capacity as a function of hepatocytes that is lost in advanced liver disease across the spectrum of aetiologies leading to cirrhosis.6
Liver biopsy is considered the gold standard for the diagnosis of NASH and the staging of liver fibrosis. However, due to its limitations, such as the invasive nature of the procedure, cost, potential complications and inter- and intra-observer variability in histological assessments, several noninvasive tests (NITs) have been studied and are currently being used in real-world clinical practice to risk stratify liver disease.7 Magnetic resonance elastography (MRE) combined with the fibrosis-4 (FIB-4) (MEFIB) index is a recently introduced MR-based NIT with a high positive predictive value for the detection of stage 2 liver fibrosis or higher in NAFLD patients.8 Also, magnetic resonance imaging-derived proton density fat fraction (MRI-PDFF) has been reported to provide an accurate and reproducible quantitative assessment of the liver fat content.9
The prognostic significance of liver fat content in NASH-related significant fibrosis and cirrhosis is unclear and has not been systematically assessed. Therefore, we aimed to investigate the risk of decompensation, HCC and mortality stratified by liver fat content in NASH-related significant fibrosis and cirrhosis.
2 |. MATERIALS AND METHODS
2.1 |. Patients
A collaborative systematic review and meta-analysis of individual participants (IPDMA) was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. An experienced medical librarian performed a systematic search of literature from several databases from inception to 24 April, 2023, and identified all articles related to this study. A total of 765 records were screened through database searching, cross-referencing and additional identification after discussion with experts in the field. After the exclusion of 721 records, 44 full-text articles were reviewed for eligibility, and four studies that met the inclusion criteria were finally selected. The inclusion criteria of this study were: (1) assessment of liver stiffness by MRE; (2) assessment of liver fat content by MRI-PDFF; (3) completed assessment for hepatic decompensation, HCC and death and (4) adult NAFLD patients of age ≥18 years. In the four cohorts, there were 456 patients with both MRE and MRI-PDFF available. Twenty-five patients with prevalent decompensation or HCC, and 135 patients with no longitudinal follow-up were excluded, and 296 patients were analysed (Figure 1). Institutional review board (IRB) approval for this study was obtained from the University of California at San Diego office of the IRB administration (approval number 111298).
FIGURE 1.
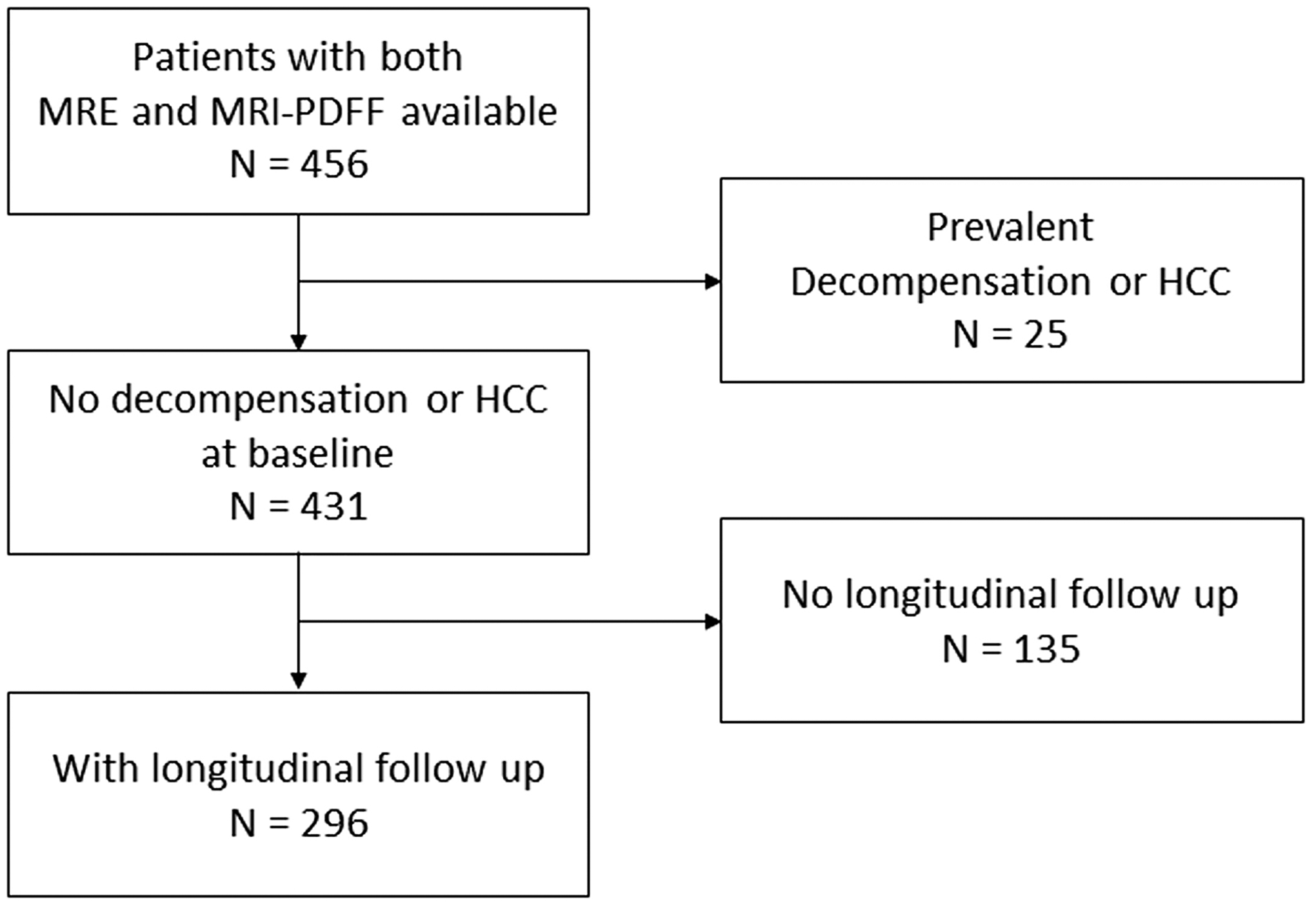
Patient flow diagram.
2.2 |. Assessment
All charts were reviewed retrospectively for data collection and follow-up assessments. Detailed baseline patient data, including age, sex, race/ethnicity, comorbidities, complete blood count, body mass index (BMI), blood chemistry, FIB-4, MRE and MRI-PDFF were collected. Patients with other diseases such as viral hepatitis, human immunodeficiency virus infection or alcoholic liver disease were excluded. The date of the first MRI was defined as the starting date, and participants with recorded follow-up time were followed until death or the last clinical encounter. The primary outcome included the rate of hepatic decompensation, HCC and death.
MRE uses propagating mechanical shear waves to probe the mechanical properties of tissues.10 Then these waves are processed to yield images with a special MRI sequence, and the wave information are used to generate elastograms that provide quantitative measures of liver stiffness.11 We obtained the mean liver stiffness values in Kilopascal (kPa) units from MRE. The MRI-PDFF technique measures the fraction of the liver proton density attributable to liver fat, and is a reliable and standardised tool to quantify fat content in the liver.12 We acquired the mean values of liver fat content using MRI-PDFF.
2.3 |. Definitions
Significant liver fibrosis of stage 2 (F2) or higher was defined non-invasively using the MEFIB index as previously published.8 The MEFIB index is a combination of MRE ≥3.3 kPa, and FIB-4 ≥1.6 and it has previously reported a very high positive predictive value of 91.0% to 97.1% for the detection of stage F2 or higher.8,13 Cirrhosis was defined as MRE ≥5 kPa based on the NAFLD practice guidance criteria.7 Liver fat content was also defined non-invasively using MRI-PDFF. Lower liver fat content was defined as MRI-PDFF <5%, and higher liver fat content was defined as MRI-PDFF ≥5% in accordance with the previous study that demonstrated a good correlation between histological steatosis grade 0 and MRI-PDFF <5%.9 MEFIB index and MRI-PDFF are both recommended as NITs for NAFLD patients in the American Association for the Study of the Liver (AASLD) guidelines.7
The patients were divided into three groups stratified by MEFIB and MRI-PDFF for comparative analysis. The MEFIB-negative patients were defined as no significant liver fibrosis group; MEFIB-positive and MRI-PDFF ≥5% patients were defined as NASH-related significant fibrosis with a higher liver fat content group, and MEFIB-positive and MRI-PDFF <5% patients were defined as having NASH-related significant fibrosis with a low liver fat content group, respectively. For sensitivity analysis stratified by the presence of cirrhosis, MRE <5 kPa patients were defined as no cirrhosis group, MRE ≥5 kPa and MRI-PDFF ≥5% patients were defined as cirrhosis with a higher liver fat content group, and MRE ≥5 kPa and MRI-PDFF <5% patients were defined as cirrhosis with a low liver fat content group, respectively.
The primary outcome was defined as the composite outcome of HCC, decompensation and all-cause mortality. Hepatic decompensation was defined as the development of ascites, hepatic encephalopathy and variceal haemorrhage. Ascites was defined using imaging tests, history taking and physical examination according to the clinical guidelines.14 Hepatic encephalopathy was defined as a brain dysfunction caused by liver insufficiency and/or portosystemic shunting and diagnosed using clinical criteria.14 Variceal haemorrhage was defined according to the American College of Gastroenterology/AASLD guidelines.15 HCC was defined using the Liver Imaging Reporting and Data System (LI-RADS 5) imaging criteria or pathologic confirmation.16 NAFLD was diagnosed based on the NAFLD practice guidance criteria.7
2.4 |. Statistical analysis
For the comparison of the continuous and categorical variables, independent T-tests, Kruskal–Wallis tests and chi-squared tests were used. For the analysis of the cumulative incidence of decompensation, HCC and death, the outcome variables were estimated using the Kaplan-Meier method. A comparison of hazard ratios (HRs) between the groups was carried out using the Cox proportional hazard model to evaluate the association between outcomes and each group after adjustments for age, sex, BMI and race/ethnicity. A sensitivity analysis was performed in patients with cirrhosis, defined as MRE ≥5 kPa. SAS software V.9.4 (SAS Institute) was used for analyses. p value <0.05 was considered to be statistically significant.
3 |. RESULTS
3.1 |. Patient characteristics
The mean (±SD) age of patients in the NASH-related significant fibrosis with a low liver fat content group was 66.9 (±7.3) years, which was higher compared to 65.1 (±8.4) years in the NASH-related significant fibrosis with a higher liver fat content group and 52.8 (±13.5) years in the no significant liver fibrosis group, respectively (Table 1). Baseline characteristics, including demographics, co-morbidities and liver disease-related parameters, are detailed in Table 1, stratified by liver fat content and MEFIB status. The markers of fibrosis, including FIB-4 and MRE, were 4.08 and 5.92 kPa in the low liver fat group, which were higher than 3.06 and 5.37 kPa in the higher liver fat group, and 1.08 and 2.90 kPa in the no significant fibrosis group, respectively. The mean MRE value of 5.50 in MEFIB-positive patients indicated that the majority of the MEFIB-positive patients in this study were cirrhotic based upon the AASLD NAFLD practice guidance.7 The mean (±SD) MRI-PDFF value in the NASH-related significant fibrosis and low liver fat group was 3.18 (±0.87), which was significantly lower than the 12.07 (±6.19) of the NASH-related significant fibrosis with higher liver fat group and 13.15 (±8.09) in the no significant fibrosis group, respectively.
TABLE 1.
Baseline characteristics.
| Male, n (%) | 86 (44.6) | 34 (43.0) | 11 (45.8) | 0.9613 |
| Diabetes, n (%) | 85 (44.0) | 57 (72.2) | 19 (82.6) | <0.0001 |
| Hypertension, n (%) | 62 (32.1) | 36 (45.6) | 16 (66.7) | 0.0015 |
| Dyslipidaemia, n (%) | 106 (55.5) | 45 (57.0) | 9 (37.5) | 0.2160 |
| BMI (kg/m2), mean (SD) | 30.1 (6.0) | 28.4 (5.1) | 28.9 (4.1) | 0.0740 |
| Race, n (%) | ||||
| White | 72 (37.3) | 19 (24.1) | 12 (50.0) | 0.1285 |
| Hispanic | 25 (13.0) | 10 (12.7) | 4 (16.7) | |
| Asian | 91 (47.2) | 49 (62.0) | 8 (33.3) | |
| Others | 5 (2.6) | 1 (1.3) | 0 (0) | |
| AST (U/L), median (IQR) | 29.0 (18.5) | 47.0 (36.0) | 34.5 (30.5) | <0.0001 |
| ALT (U/L), median (IQR) | 41.0 (36.5) | 42.0 (41.0) | 29.0 (25.0) | 0.1324 |
| Total bilirubin (mg/dL), median (IQR) | 0.40 (0.32) | 0.60 (0.51) | 0.92 (0.93) | <0.0001 |
| Albumin (g/dL), median (IQR) | 4.5 (0.4) | 4.3 (0.5) | 4.2 (0.5) | <0.0001 |
| Creatinine (mg/dL), median (IQR) | 0.77 (0.16) | 0.80 (0.26) | 0.69 (0.38) | 0.0251 |
| Triglycerides (mg/dL), median (IQR) | 169.0 (111.0) | 163.0 (92.0) | 142.0 (88.5) | 0.1046 |
| HDL (mg/dL), median (IQR) | 48.0 (11.5) | 47.0 (13.0) | 45.4 (18.0) | 0.2395 |
| LDL (mg/dL), median (IQR) | 129.5 (40.0) | 120.0 (35.0) | 101.0 (45.0) | 0.0003 |
| HbA1c (%), median (IQR) | 6.1 (0.9) | 6.5 (1.7) | 6.5 (1.4) | 0.0111 |
| Platelet count (109/L), median (IQR) | 249.0 (81.5) | 150.0 (87.0) | 123.5 (59.0) | <0.0001 |
| PT INR, median (IQR) | 1.00 (0.06) | 1.04 (0.11) | 1.13 (0.23) | <0.0001 |
| FIB-4, median (IQR) | 1.08 (0.75) | 3.06 (2.02) | 4.08 (2.28) | <0.0001 |
| MRE (kPa), mean (SD) | 2.90 (1.09) | 5.37 (1.51) | 5.92 (1.64) | <0.0001 |
| MRI-PDFF, mean (SD) | 13.15 (8.09) | 12.07 (6.19) | 3.18 (0.87) | <0.0001 |
Abbreviations: AST, aspartate aminotransferase; ALT, alanine aminotransferase; BMI, body mass index; HbA1c, Haemoglobin A1c; HDL, high-density lipoprotein; IQR, interquartile range; LDL, low-density lipoprotein; FIB-4, fibrosis index based on the 4 factor; MEFIB, MRI combined with FIB-4; MRE, magnetic resonance elastography; MRI-PDFF, magnetic resonance imaging derived proton density fat fraction; PT INR, prothrombin time international normalised ratio; SD, standard deviation.
3.2 |. Decompensation, HCC and death
There were a total of 32 incident events (11%) (Table 2). Twenty-two patients (7%) experienced decompensation, 15 patients (5%) developed HCC and 17 patients (6%) expired during follow-up. In the no significant fibrosis group, none experienced decompensation, one patient (1%) developed HCC, three patients (2%) expired and four patients (2%) experienced at least one of the three events. In the higher liver fat group, 14 patients (18%) experienced decompensation, 10 patients (13%) developed HCC, 10 patients (13%) expired and 18 patients (23%) experienced at least one event. In the low liver fat group, eight patients (33%) experienced decompensation, four patients (17%) developed HCC, four patients (17%) expired and 10 patients (42%) experienced at least one event. In terms of time-to-event, the incidence rate of decompensation was 0, 3.84 and 13.81 per 100 person-years in the no significant fibrosis group, the higher liver fat group and the low liver fat group, respectively. The incidence rate of HCC was 0.12, 2.81 and 6.31 per 100 person-years in the no significant fibrosis group, the higher liver fat group and the low liver fat group, respectively. The incidence rate of death was 0.35, 2.61 and 6.18 per 100 person-years in the no significant fibrosis group, the higher liver fat group and the low liver fat group, respectively. The incidence rate of experiencing at least one event was 0.47, 5.26 and 17.55 per 100 person-years in the no significant fibrosis group, the higher liver fat group and the low liver fat group, respectively. Ascites was the most prevalent decompensation event, followed by hepatic encephalopathy and variceal haemorrhage. The Kaplan–Meier analysis indicated that the frequency of decompensation, HCC and death stratified by MEFIB and MRI-PDFF was significantly higher in the NASH-related significant fibrosis with low liver fat group, followed by the NASH-related significant fibrosis with higher liver fat group, and the no significant fibrosis group served as the referent group (Figure 2) (p < 0.0001). Also, for the additional verification of our hypothesis, we performed analysis excluding the events that occurred within the first 6 months, and the results remained constant; the highest frequency of composite outcome was observed in the NASH-related significant fibrosis with low liver fat group, followed by the NASH-related significant fibrosis with higher liver fat group, with the no significant fibrosis group as the referent group (Figure S1).
TABLE 2.
Frequency of decompensation, HCC and death stratified by MEFIB and MRI-PDFF.
| Incident events (%) | MEFIB-negative, N = 193 (65%) | MEFIB+ and MRI-PDFF ≥5%, N = 79 (27%) | ||
|---|---|---|---|---|
| Ascites | 19 (6) | 0 | 12 (15) | 7 (29) |
| Hepatic encephalopathy | 13 (4) | 0 | 9 (11) | 4 (17) |
| Variceal haemorrhage | 3 (1) | 0 | 1 (1) | 2 (8) |
| Hepatocellular carcinoma, N (%) | 15 (5) | 1 (1) | 10 (13) | 4 (17) |
| Death, N (%) | 17 (6) | 3 (2) | 10 (13) | 4 (17) |
| Any of abovea, N (%) | 32 (11) | 4 (2) | 18 (23) | 10 (42) |
Abbreviations: HCC, hepatocellular carcinoma; MEFIB, MRI combined with FIB-4; MRI-PDFF, magnetic resonance imaging-derived proton density fat fraction.
Patients with any of following—decompensation, HCC or death.
FIGURE 2.
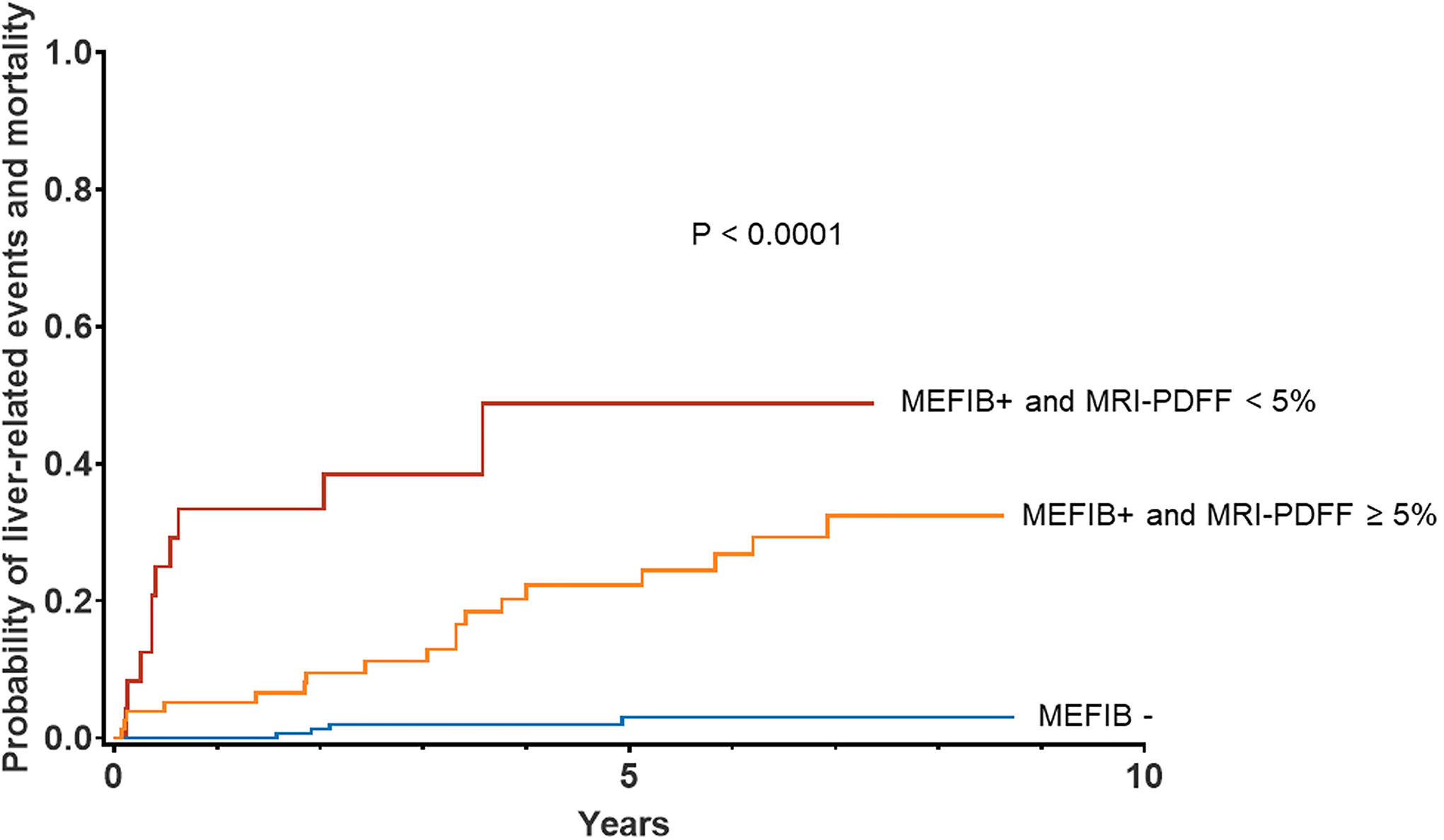
Cumulative incidence of composite outcome (decompensation, HCC and death) stratified by MEFIB and MRI-PDFF.
3.3 |. Association between ‘burnt-out’ NASH-related significant fibrosis, cirrhosis and increased risk of hepatic decompensation, HCC and death
The Cox-proportional hazards model was used for univariable and multivariable-adjusted analyses after adjusting for age, sex, BMI and race/ethnicity (Table 3), with no significant fibrosis group as the referent group. In univariable analysis, NASH-related significant fibrosis with a higher liver fat content group was associated with a probability of decompensation, HCC and death (HR = 11.9 [95% CI: 4.0–35.2, p < 0.0001]) compared to the no significant fibrosis group. Low liver fat content was associated with the highest probability of the primary outcome (HR = 28.8 [95% CI: 8.9–93.5, p < 0.0001]). The results remained consistent and statistically and clinically significant even after multivariable-adjusted analyses, with an increased risk in the NASH-related significant fibrosis with a higher liver fat content group (HR = 20.0 [95% CI: 4.1–98.2, p = 0.0002]), and the highest risk in the NASH-related significant fibrosis with a low liver fat content group (HR = 42.2 [95% CI: 7.5–235.5, p < 0.0001]) compared to no significant fibrosis as the referent group. Among patients with cirrhosis, multivariable-adjusted analyses determined the highest risk of hepatic decompensation, HCC and death in cirrhotic patients with low liver fat content (HR = 10.1 [95% CI: 3.1–33.1, p = 0001]), followed by cirrhotic patients with higher liver fat content (HR = 8.1 [95% CI: 3.0–21.8, p < 0.0001]) compared to patients without cirrhosis (Table 4). Also, after the exclusion of events that occurred within 6 months following enrolment, the results were still significant after multivariable-adjusted analyses, with an increased risk in the NASH-related significant fibrosis with a higher liver fat content group (HR = 14.54 [95% CI: 2.83–74.79, p = 0.0014]) and the highest risk in the NASH-related significant fibrosis with a low liver fat content group (HR = 17.71 [95% CI: 2.45–128.13, p = 0.0044]) compared to no significant fibrosis as the referent group (Table S1).
TABLE 3.
Factors associated with the composite outcome (decompensation, HCC and death).
| Hazard ratio (95% CI) | p-value | Hazard ratio (95% CI) | ||
|---|---|---|---|---|
| MEFIB+ and MRI-PDFF ≥5% | 11.9 (4.0–35.2) | <0.0001 | 20.0 (4.1–98.2) | 0.0002 |
| MEFIB+ and MRI-PDFF <5% | 28.8 (8.9–93.5) | <0.0001 | 42.2 (7.5–235.5) | <0.0001 |
Abbreviations: CI, confidence interval; HCC, hepatocellular carcinoma; MEFIB, MRI combined with FIB-4; MRI-PDFF, magnetic resonance imaging-derived proton density fat fraction.
Adjusted for age, sex, BMI and race/ethnicity.
TABLE 4.
Sensitivity analysis on the factors associated with the composite outcome (decompensation, HCC and death) stratified by the presence of cirrhosis.
| Hazard ratio (95% CI) | p-value | Hazard ratio (95% CI) | ||
|---|---|---|---|---|
| MRE ≥5 kPa and MRI-PDFF ≥5% | 6.9 (3.0–15.6) | <0.0001 | 8.1 (3.0–21.8) | <0.0001 |
| MRE ≥5 kPa and MRI-PDFF <5% | 12.9 (4.8–34.7) | <0.0001 | 10.1 (3.1–33.1) | 0.0001 |
Abbreviations: CI, confidence interval; HCC, hepatocellular carcinoma; kPa, kilopascal; MRE, magnetic resonance elastography; MRI-PDFF, magnetic resonance imaging-derived proton density fat fraction.
Adjusted for age, sex, BMI and race/ethnicity.
4 |. DISCUSSION
4.1 |. Main findings
In this IPDMA, we assessed the significance of liver fat content in NASH-related significant fibrosis and cirrhosis using non-invasive, advanced MR-based diagnostic modalities. NASH-related significant fibrosis and cirrhosis with lower liver fat content (also known as ‘burnt-out’ NASH-fibrosis or cirrhosis) were associated with a higher probability of hepatic decompensation, HCC and death compared to patients with higher liver fat content. These findings remained consistent even after adjusting for age, sex, BMI and race/ethnicity, and in a sensitivity analysis stratified by the presence of cirrhosis.
4.2 |. In context with current literature
Previously, studies have shown that the clinical features of NAFLD, such as obesity, dyslipidaemia and diabetes, were predominantly observed in the majority of patients with cryptogenic cirrhosis.17,18 Therefore, although these patients had low liver fat content, NAFLD was suggested to be the most common aetiology of patients with cryptogenic cirrhosis. These results indirectly indicated that liver fat loss may occur with disease progression in patients with NASH-related significant fibrosis and cirrhosis. Furthermore, liver fat loss has been reported in patients with NASH-related cirrhosis in studies that investigated the natural history of NAFLD.19 However, the association between the prognosis of patients with NASH-related significant fibrosis and cirrhosis and liver fat content has not been systematically studied using advanced MRI-PDFF and MRE in this patient population.
On the other hand, the association between the degree of liver fat and prognosis in patients with viral hepatitis has been investigated in a few studies. In patients with combined CHB and fatty liver, there are controversial results, with a few reports suggesting that fatty liver was associated with a higher probability of HCC,20,21 and others reporting the contrary, that fatty liver was associated with a lower risk of HCC.6,22–24 These discordant results may be attributed to the complex relationships between hepatic steatosis and hepatitis B virus (HBV) infection, such as the direct suppression of HBV viral activity by steatosis,25 which may have affected the outcome, and the inclusion of heterogeneous CHB patients with or without antiviral therapy or different adherence to antiviral drugs. However, in an analysis of only fully virologically suppressed patients but with advanced chronic liver disease (≥10 kPa), a lower degree of liver fat content was shown to be significantly associated with a higher risk of HCC, which corresponds to the results shown in our study.6
The underlying mechanisms behind decreased liver fat content in progressive NASH patients remain an area of investigation in the field. One potential explanation is the diminished exposure to insulin and lipoproteins, which are known to generate fat and lipid-storing signals.26 The changes in portal circulation with the progression of liver disease, such as reduced portal blood flow, portosystemic shunting and loss of sinusoidal fenestrations, may incur weaker interactions among hepatocytes, insulin and lipoproteins and therefore result in reduced liver fat.27 Another hypothesis to explain this phenomenon is the elevation of serum adiponectin. Adiponectin is an insulin-sensitising molecule, and its augmentation has been reported to be associated with anti-steatotic changes, and reduced liver function.28,29 Furthermore, hypoadiponectinaemia has been reported to be closely associated with an increase in the hepatic steatosis grade and necroinflammation, hence aggravation from simple steatosis to NASH.28,30,31 Therefore, it may be speculated that adiponectin is augmented in advanced NASH patients with liver dysfunction and plays a role in augmenting the fat storage capacity of hepatocytes as liver disease progresses.29
More recently, somatic mutagenesis in metabolism genes has been extensively analysed and this has provided novel insights regarding the underlying mechanism of decreased liver fat content in patients with advanced NASH.32 Convergent somatic mutations in three genes that regulate lipid processing and storage, out of 1590 genomes, were observed in association with the catabolism of lipid droplets in the liver. The mutations were observed in FOXO1, which is a major transcription factor in insulin signalling; CIDEB, which regulates the fusion of intracellular lipid droplets; and GPAM, which catalyses the rate-limiting step in triacylglycerol synthesis. It was suggested that the mutations in these genes were possible results of clonal selection for the protection of hepatocytes against chronic lipotoxic exposure, and therefore, to some extent, reduced liver fat in advanced NASH patients may be regarded as a natural protective response against prolonged liver damage.33
4.3 |. Strengths and limitations
The strength of our study was that we gathered individual patient data from three different regions with different race/ethnicities, which enhances the generalisability of the findings. In addition, the patients underwent a long-term follow-up of median (IQR) 4.2 (5.0) years. To our knowledge, this is the first study that investigated the prognosis of NASH-related significant fibrosis and cirrhosis stratified by liver fat content and fibrosis using NITs, which provides an additional advantage compared to histologic assessment in terms of patient accessibility. The limitation of this study was that it was carried out retrospectively, which may have caused recall or observer biases. Also, the wide range of confidence intervals in the adjusted HR would likely be due to the relatively small number of events. Larger, prospective studies are needed to better establish causality.
In conclusion, low liver fat content in patients with NASH-related significant fibrosis and cirrhosis is associated with an increase in HCC, hepatic decompensation and mortality. Further prospective data are required to validate the results of this study.
Supplementary Material
ACKNOWLEDGEMENTS
Declaration of personal interests: RL serves as a consultant to Aardvark Therapeutics, Altimmune, Anylam/Regeneron, Amgen, Arrowhead Pharmaceuticals, AstraZeneca, Bristol-Myer Squibb, CohBar, Eli Lilly, Galmed, Gilead, Glympse bio, Hightide, Inipharma, Intercept, Inventiva, Ionis, Janssen Inc., Madrigal, Metacrine, Inc., NGM Biopharmaceuticals, Novartis, Novo Nordisk, Merck, Pfizer, Sagimet, Theratechnologies, 89 bio, Terns Pharmaceuticals and Viking Therapeutics. In addition, his institutions received research grants from Arrowhead Pharmaceuticals, Astrazeneca, Boehringer-Ingelheim, Bristol-Myers Squibb, Eli Lilly, Galectin Therapeutics, Galmed Pharmaceuticals, Gilead, Intercept, Hanmi, Intercept, Inventiva, Ionis, Janssen, Madrigal Pharmaceuticals, Merck, NGM Biopharmaceuticals, Novo Nordisk, Merck, Pfizer, Sonic Incytes and Terns Pharmaceuticals. He is the co-founder of LipoNexus Inc.
Funding information
National Center for Advancing Translational Sciences, Grant/Award Number: 5UL1TR001442; National Heart, Lung, and Blood Institute, Grant/Award Number: P01HL147835; National Institute of Diabetes and Digestive and Kidney Diseases, Grant/Award Number: K23DK119460, P30DK120515, R01DK106419, R01DK121378, R01DK124318, U01DK061734 and U01DK130190
FUNDING INFORMATION
RL received funding support from NCATS (5UL1TR001442), NHLBI (P01HL147835), and NIDDK (U01DK061734, U01DK130190, R01DK106419, R01DK121378, R01DK124318, P30DK120515). VA is supported by NIDDK (K23DK119460).
Footnotes
AUTHOR CONTRIBUTIONS
Sung Won Lee: Data curation (lead); formal analysis (equal); writing – original draft (lead); writing – review and editing (lead). Daniel Q. Huang: Data curation (equal); writing – original draft (equal); writing – review and editing (equal). Ricki Bettencourt: Formal analysis (lead); writing – review and editing (equal). Veeral Ajmera: Data curation (equal); writing – review and editing (equal). Monica Tincopa: Data curation (equal); writing – review and editing (equal). Nabil Noureddin: Data curation (equal); writing – review and editing (equal). Maral Amangurbanova: Data curation (equal); writing – review and editing (equal). Harris Siddiqi: Data curation (equal); writing – review and editing (equal). Egbert Madamba: Data curation (equal); writing – review and editing (equal). Abdul M. Majzoub: Data curation (equal); writing – review and editing (equal). Tarek Nayfeh: Data curation (equal); writing – review and editing (equal). Nobuharu Tamaki: Data curation (equal); writing – review and editing (equal). Namiki Izumi: Data curation (equal); writing – review and editing (equal). Atsushi Nakajima: Data curation (equal); writing – review and editing (equal). Masato Yoneda: Data curation (equal); writing – review and editing (equal). Ramzan Idilman: Data curation (equal); writing – review and editing (equal). Mesut Gumussoy: Data curation (equal); writing – review and editing (equal). Digdem Kuru Oz: Data curation (equal); writing – review and editing (equal). Ayse Erden: Data curation (equal); writing – review and editing (equal). Rohit Loomba: Conceptualization (lead); supervision (lead); data curation (lead); formal analysis (equal); writing - original draft (equal); writing – review and editing (lead).
SUPPORTING INFORMATION
Additional supporting information will be found online in the Supporting Information section.
REFERENCES
- 1.Riazi K, Azhari H, Charette JH, Underwood FE, King JA, Afshar EE, et al. The prevalence and incidence of NAFLD worldwide: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2022;7:851–61. [DOI] [PubMed] [Google Scholar]
- 2.Sanyal AJ, van Natta M, Clark J, Neuschwander-Tetri BA, Diehl A, Dasarathy S, et al. Prospective study of outcomes in adults with nonalcoholic fatty liver disease. N Engl J Med. 2021;385:1559–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hammerich L, Tacke F. Hepatic inflammatory responses in liver fibrosis. Nat Rev Gastroenterol Hepatol. 2023;20:633–46. [DOI] [PubMed] [Google Scholar]
- 4.Dongiovanni P, Stender S, Pietrelli A, Mancina RM, Cespiati A, Petta S, et al. Causal relationship of hepatic fat with liver damage and insulin resistance in nonalcoholic fatty liver. J Intern Med. 2018;283:356–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yoshioka Y, Hashimoto E, Yatsuji S, Kaneda H, Taniai M, Tokushige K, et al. Nonalcoholic steatohepatitis: cirrhosis, hepatocellular carcinoma, and burnt-out NASH. J Gastroenterol. 2004;39:1215–8. [DOI] [PubMed] [Google Scholar]
- 6.Oh JH, Lee HW, Sinn DH, Park JY, Kim BK, Kim SU, et al. Controlled attenuation parameter value and the risk of hepatocellular carcinoma in chronic hepatitis B patients under antiviral therapy. Hepatol Int. 2021;15:892–900. [DOI] [PubMed] [Google Scholar]
- 7.Rinella ME, Neuschwander-Tetri BA, Siddiqui MS, Abdelmalek MF, Caldwell S, Barb D, et al. AASLD practice guidance on the clinical assessment and management of nonalcoholic fatty liver disease. Hepatology. 2023;77:1797–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jung J, Loomba RR, Imajo K, Madamba E, Gandhi S, Bettencourt R, et al. MRE combined with FIB-4 (MEFIB) index in detection of candidates for pharmacological treatment of NASH-related fibrosis. Gut. 2021;70:1946–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Caussy C, Reeder SB, Sirlin CB, Loomba R. Noninvasive, quantitative assessment of liver fat by MRI-PDFF as an endpoint in NASH trials. Hepatology. 2018;68:763–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Muthupillai R, Lomas DJ, Rossman PJ, Greenleaf JF, Manduca A, Ehman RL. Magnetic resonance elastography by direct visualization of propagating acoustic strain waves. Science. 1995;269:1854–7. [DOI] [PubMed] [Google Scholar]
- 11.Venkatesh SK, Yin M, Ehman RL. Magnetic resonance elastography of liver: technique, analysis, and clinical applications. J Magn Reson Imaging. 2013;37:544–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reeder SB, Cruite I, Hamilton G, Sirlin CB. Quantitative assessment of liver fat with magnetic resonance imaging and spectroscopy. J Magn Reson Imaging. 2011;34:729–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yip TC, Lyu F, Lin H, et al. Non-invasive biomarkers for liver inflammation in non-alcoholic fatty liver disease: present and future. Clin Mol Hepatol. 2023;29:S171–s83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Biggins SW, Angeli P, Garcia-Tsao G, Ginès P, Ling SC, Nadim MK, et al. Diagnosis, evaluation, and management of ascites, spontaneous bacterial peritonitis and hepatorenal syndrome: 2021 practice guidance by the American Association for the Study of Liver Diseases. Hepatology. 2021;74:1014–48. [DOI] [PubMed] [Google Scholar]
- 15.Garcia-Tsao G, Sanyal AJ, Grace ND, Carey WD, the Practice Guidelines Committee of the American Association for the Study of Liver Diseases and the Practice Parameters Committee of the American College of Gastroenterology. Prevention and management of gastroesophageal varices and variceal hemorrhage in cirrhosis. Am J Gastroenterol. 2007;102:2086–102. [DOI] [PubMed] [Google Scholar]
- 16.Singal AG, Llovet JM, Yarchoan M, Mehta N, Heimbach JK, Dawson LA, et al. AASLD practice guidance on prevention, diagnosis, and treatment of hepatocellular carcinoma. Hepatology. 2023. [Online ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ratziu V, Bonyhay L, di Martino V, Charlotte F, Cavallaro L, Sayegh-Tainturier MH, et al. Survival, liver failure, and hepatocellular carcinoma in obesity-related cryptogenic cirrhosis. Hepatology. 2002;35:1485–93. [DOI] [PubMed] [Google Scholar]
- 18.Bugianesi E, Leone N, Vanni E, Marchesini G, Brunello F, Carucci P, et al. Expanding the natural history of nonalcoholic steatohepatitis: from cryptogenic cirrhosis to hepatocellular carcinoma. Gastroenterology. 2002;123:134–40. [DOI] [PubMed] [Google Scholar]
- 19.Powell EE, Cooksley WG, Hanson R, Searle J, Halliday JW, Powell LW. The natural history of nonalcoholic steatohepatitis: a follow-up study of forty-two patients for up to 21 years. Hepatology. 1990;11:74–80. [DOI] [PubMed] [Google Scholar]
- 20.Chan AW, Wong GL, Chan HY, Tong JH, Yu YH, Choi PC, et al. Concurrent fatty liver increases risk of hepatocellular carcinoma among patients with chronic hepatitis B. J Gastroenterol Hepatol. 2017;32:667–76. [DOI] [PubMed] [Google Scholar]
- 21.van Kleef LA, Choi HSJ, Brouwer WP, Hansen BE, Patel K, de Man RA, et al. Metabolic dysfunction-associated fatty liver disease increases risk of adverse outcomes in patients with chronic hepatitis B. JHEP Rep. 2021;3:100350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hsueh RC, Wu WJ, Lin CL, Liu CJ, Huang YW, Hu JT, et al. Impact of PNPLA3 p.I148M and hepatic steatosis on long-term outcomes for hepatocellular carcinoma and HBsAg Seroclearance in chronic hepatitis B. J Hepatocell Carcinoma. 2022;9:301–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li J, Yang HI, Yeh ML, le MH, le AK, Yeo YH, et al. Association between fatty liver and cirrhosis, hepatocellular carcinoma, and hepatitis B surface antigen Seroclearance in chronic hepatitis B. J Infect Dis. 2021;224:294–302. [DOI] [PubMed] [Google Scholar]
- 24.Mak LY, Hui RW, Fung J, Liu F, Wong DK, Li B, et al. Reduced hepatic steatosis is associated with higher risk of hepatocellular carcinoma in chronic hepatitis B infection. Hepatol Int. 2021;15:901–11. [DOI] [PubMed] [Google Scholar]
- 25.Huang SC, Liu CJ. Chronic hepatitis B with concurrent metabolic dysfunction-associated fatty liver disease: challenges and perspectives. Clin Mol Hepatol. 2023;29:320–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Caldwell SH, Crespo DM. The spectrum expanded: cryptogenic cirrhosis and the natural history of non-alcoholic fatty liver disease. J Hepatol. 2004;40:578–84. [DOI] [PubMed] [Google Scholar]
- 27.Matsui O, Kadoya M, Takahashi S, Yoshikawa J, Gabata T, Takashima T, et al. Focal sparing of segment IV in fatty livers shown by sonography and CT: correlation with aberrant gastric venous drainage. Am J Roentgenol. 1995;164:1137–40. [DOI] [PubMed] [Google Scholar]
- 28.Tietge UJ, Böker KH, Manns MP, Bahr MJ. Elevated circulating adiponectin levels in liver cirrhosis are associated with reduced liver function and altered hepatic hemodynamics. Am J Physiol Endocrinol Metab. 2004;287:E82–9. [DOI] [PubMed] [Google Scholar]
- 29.van der Poorten D, Samer CF, Ramezani-Moghadam M, Coulter S, Kacevska M, Schrijnders D, et al. Hepatic fat loss in advanced nonalcoholic steatohepatitis: are alterations in serum adiponectin the cause? Hepatology. 2013;57:2180–8. [DOI] [PubMed] [Google Scholar]
- 30.Hui JM, Hodge A, Farrell GC, Kench JG, Kriketos A, George J. Beyond insulin resistance in NASH: TNF-alpha or adiponectin? Hepatology. 2004;40:46–54. [DOI] [PubMed] [Google Scholar]
- 31.Targher G, Bertolini L, Rodella S, Zoppini G, Scala L, Zenari L, et al. Associations between plasma adiponectin concentrations and liver histology in patients with nonalcoholic fatty liver disease. Clin Endocrinol (Oxf). 2006;64:679–83. [DOI] [PubMed] [Google Scholar]
- 32.Ng SWK, Rouhani FJ, Brunner SF, Brzozowska N, Aitken SJ, Yang M, et al. Convergent somatic mutations in metabolism genes in chronic liver disease. Nature. 2021;598:473–8. [DOI] [PubMed] [Google Scholar]
- 33.Valenti L, Romeo S, Pajvani U. A genetic hypothesis for burnt-out steatohepatitis. Liver Int. 2021;41:2816–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


