Abstract
Objective
Osteoarthritis (OA) is a common disease with complex and unclear pathogenesis. Ferroptosis is a new cell death mode, which is proved to be involved in different kinds of disease. We hypothesized that ferroptosis contributes to the progress of human OA.
Design
Chondrocytes were extracted from waste cartilage of total knee arthroplasty, and stimulated with interleukin-1β (IL-1β). Then, we detected the morphology, proliferation, and viability, and levels of Fe3+, glutathione (GSH), reactive oxygen species (ROS), malondialdehyde (MDA), and 5 proteins related to ferroptosis with or without intervention of ferrostatin-1 (Fer-1). In addition, we compared the effect of Fer-1 and liproxstatin-1 (Lip-1) on ferroptosis and the protection of chondrocytes by detecting several markers of both ferroptosis and OA.
Results
After stimulation of IL-1β, there were significant changes on the shape of chondrocyte, with lower viability and proliferation. There was accumulation of intracellular Fe3+, GSH, ROS, and MDA, with the changes of expression of 5 ferroptosis-related proteins. With the contribution of Fer-1, results above were reversed. Moreover, there was no significant difference in GPX4 and ACSL4 between Fer-1 and Lip-1 group. However, the expression of COLX, ADAMTS5, and MMP-13 are lower after the treatment of Fer-1 compared with Lip-1.
Conclusions
Ferroptosis plays an important role in human OA chondrocytes, which can be reversed by Fer-1, illustrating that inhibitor of ferroptosis may be a potential treatment of OA. Moreover, Lip-1 and Fer-1 can both alleviate the level of ferroptosis in OA chondrocytes, but Fer-1 had a more protective effect.
Keywords: osteoarthritis, chondrocyte, ferroptosis, ferrostatin-1
Introduction
Osteoarthritis (OA) is a common chronic degenerative and progressive disease in the middle-aged and elder people, especially in obese population. 1 According to the data of the World Health Organization in 2019, about 250 million people around the world are suffering from OA. 2 The pathology of OA is complex and not completely studied, resulting in the lack of targeted treatment. 3
As the only kind of cells in cartilage, chondrocyte plays a decisive role in maintaining the stability and reconstruction of cartilage. In OA environment, abnormal death of chondrocytes usually causes matrix degradation, resulting in the imbalance of the cartilage homeostasis. 4 Traditional cell death modes such as apoptosis, necrosis, and autophagy have been previously shown to contribute to the occurrence and development of OA.5 -7 In recent years, new programmed cell death modes have been found, such as ferroptosis, necroptosis, pyroptosis, and chondroptosis.8 -11
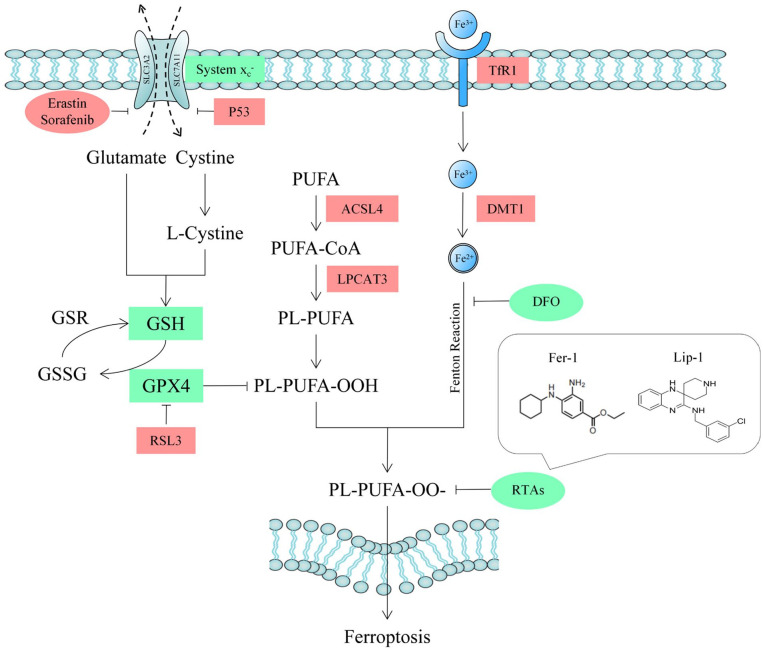
As one of them, ferroptosis was first found in 2012, which has unique characteristics in morphology and biochemistry. In 2018, Nomenclature Committee on Cell Death (NCCD) formally defined ferroptosis as a programmed cell death mode caused by abnormal oxidation regulated by glutathione peroxidase 4 (GPX4). 12 Morphologically, ferroptosis is characterized by smaller cell volume and mitochondria as well as increased density of mitochondrial membrane and cristae. 13 At present, it is found that the major factors mediating ferroptosis are the disorder of iron metabolism and glutathione (GSH)-related substances as well as the accumulation of lipid peroxide. 14 Fe3+ enters the cell through membrane proteins transferrin receptor 1 (TfR1), then stored as unstable Fe2+. Reduced utilization of Fe3+ or abnormal function of TfR1 results in the accumulation of Fe2+, which can generate a large number of reactive oxygen species (ROS) through Fenton reaction. 15 GSH is a protective substance in cells and the main substrate of GPX4, which can combine with lipid peroxide to reduce ROS, so as to play an important role in antioxidant. As a subunit of a cystine/glutamate antiporter, solute carrier family 7 member 11 (SLC7A11) can promote the expression of intracellular GSH, and is downregulated by Protein 53 (P53). 16 Long-chain acyl-CoA synthetase 4 (ACSL4) is a key enzyme regulating lipid composition as well as a biomarker of ferroptosis, accelerating the process of lipid peroxidation, leading to the production of malondialdehyde (MDA). 17 In addition, chondrocyte also secretes inflammatory molecules such as matrix metallopeptidase 13 (MMP-13) and a disintegrin and metalloproteinase with thrombospondinmotif-5 (ADAMTS5),18,19 which aggravate the destruction of cartilage. Based on the mechanism of ferroptosis, people have found several types of inducers and inhibitors. 20 Ferrostatin-1 (Fer-1) is considered to be a specific inhibitor as well as a probe for studying ferroptosis, which can reduce ROS generating from lipid peroxidation as a kind of radiotrapping antioxidants (RTAs), which is showed in Fig.1 . 21
Figure 1.
The schematic drawing of GPX4-dependent ferroptosis and target of RTAs. GPX4 = glutathione peroxidase 4; RTA = radiotrapping antioxidant; SLC3A2 = solute carrier family 3 membrane 2; SLC7A11 = solute carrier family 7 membrane 11; P53 = protein 53; GSH = glutathione; GSR = glutathione reductase; GSSG = oxidized glutathione; PUFA = polyunsaturated fatty acid; ACSL4 = acyl-CoA synthetase long-chain family member 4; LPCAT3 = lysophophatidylcholne acyltransferase 3; PUFA-CoA = polyunsaturated fatty acid coenzyme A; PL = phospholipid; TfR1 = transferrin receptor 1; Fe2+ = ferrous iron; Fe3+ = ferric iron; DMT1 = divalent metal transporter 1; DFO = deferoxamine.
In this study, we used interleukin-1β (IL-1β) to stimulate chondrocytes extracted from cartilage of patients with OA in vitro. We detected cell viability, level of proteins, and series of markers to investigate the effect of Fer-1 on chondrocytes treated with IL-1β. Subsequently, we compared the effects of Fer-1 and another inhibitor of ferroptosis (Liproxstatin-1, Lip-1) on chondrocytes in the aspect of ferroptosis and OA inflammatory environment. We aim to give a proof that ferroptosis plays a role in human chondrocytes of OA so as to provide a theoretical basis for the development of new anti-OA drugs in the future.
Materials and Methods
Isolation and Culture of Human Chondrocytes
The study was designed according to the Declaration of Helsinki and was approved by Ethic Committee of the Second Affiliated Hospital of Harbin Medical University (Ref: KY 2021-256). Written informed consent was obtained for 5 patients aging from 60 to 65 years old with OA, without rheumatoid arthritis, acute trauma, tumor, or infection of knee joint. The diagnosis of OA was based on clinical and radiological evidence of degenerative changes during surgery. The discarded specimens after total knee arthroplasty (TKA) were transferred into the biosafety cabinet within half an hour and washed twice with phosphate-buffered saline (PBS) solution to remove the blood, fat, and synovial fluid. The exposed cartilage tissue was cut into the final size about 0.5 mm × 0.5 mm, and put into 0.2% type II collagenase (Biosharp, China, BS163). After 4 hours, α-MEM medium (Cytiva, USA, SH30265.01) was added, with 10% fetal bovine serum (ExCell Bio, China, FSD500), 1% penicillin, and 1% streptomycin (Beyotime, China, C0222). Primary chondrocytes could be seen climbing out after about 7 days under optical microscope (ZEISS Axio Vert.A1, Germany). The medium was changed every 2 days and digested when the chondrocytes climbed to about 60% of the bottom. Chondrocytes used in this study were from passage 2 to 3 (P2-3).
Identification of Chondrocytes
A chondrocyte suspension of 10 μl was taken to cell counting. According to the results, an appropriate density of chondrocytes was seeded into 6-well plates, with about 104 cells per well. After 24 hours, chondrocytes were washed twice with PBS, treated with 40 g/L of paraformaldehyde (LEAGENE, China, DF0135) for 30 minutes; 1% toluidine blue solution (Solarbio, China, G3660) was added in the volume fraction, staying for 30 minutes at room temperature and discarded. Chondrocytes were then decolorized slowly by absolute ethanol, and observed under optical microscope.
Cell Viability Assay
Chondrocytes were seeded in 96-well plates at a density at about 2,000 cells per well. After adhesion, cells were treated with IL-1β (PEPROTECH, USA, 200-01B) or IL-1β + Fer-1 (MedChemExpress, China, HY-100579), respectively. After adding 10 ul of CCK-8 reagent (Beyotime, China, C0037) at 24 and 48 hours, respectively, chondrocytes were put into the incubator for 30 minutes away from light. The absorbance of each well was measured at 450 nm by microplate reader (BIO-RAD iMark, USA). Then the cell viability of chondrocyte of each group was calculated according to the instruction.
Cell Proliferation Test
Chondrocytes of each group were seeded in a 24-well plate at a density of about 105 cells/ml; 200 μl of 0.1% EdU solution (Beyotime, China, C0078S) was added in each well. After incubated for 4 hours at 37°C, chondrocytes were fixed with 4% paraformaldehyde for 15 minutes, and then infiltrated by 0.3% Triton X-100 (Biosharp, China, BS084) for 15 minutes. Then cells were incubated with Click solution for 30 minutes at 37°C in the dark. After being washed with PBS solution for 3 times, cells were incubated with 0.1% Hoechst 33342 for 10 minutes. Images were obtained under a fluorescence microscope (Leica DM 4000 B, Germany).
Detection of Intracellular Fe3+, GSH, and Lipid Peroxidation
Chondrocytes of each group were broken by Ultrasonic Cell Crusher (SONICS vibra cell, USA). After centrifuged at 3,500 × g for 10 minutes at 4°C (Heal Force Neofuge 13R, China), the mixture of dd-H2O, iron standard solution (Jiancheng, China, A039-2-1), GSH standard solution (Solarbio, China, BC1170), MDA standard solution (Solarbio, China, BC0025), and the supernatant to be tested with chromogenic agent were boiled, centrifuged, and added to a 96-well plate, respectively. The absorbance was measured at 520 nm for Fe3+ detection; 412 nm for GSH detection; and 450, 520, and 600 nm for MDA detection by microplate reader. Then, the concentration of each marker was calculated according to the instruction.
Detection of Intracellular ROS
Chondrocytes of each group were seeded in 96-well plates at a density at about 3,000 cells per well. After treatment, the medium was replaced by 30 μl of H2DCFDA (Biosharp, China, BL714A) working solution with the concentration of 10 μM to fully cover the cells. Chondrocytes were then incubated at 37°C for 2 hours in the dark and washed twice with serum-free medium to fully remove the probes that did not enter the cells. Cells were then observed under the fluorescence microscope with fluorescein isothiocyante (FITC) filter, according to the instructions.
Western Blot Analysis
Chondrocytes of each group were washed twice with PBS, lysed in radio immunoprecipitation assay (RIPA) with 1% phenylmethanesulfonyl fluoride (PMSF) for 10 minutes, and centrifuged at 15,000 × g for 10 minutes at 4°C. Protein concentrations were determined through the bicinchoninic acid (BCA) protein assay (Beyotime, China, P0012S). Then, proteins were separated by 12.5% SDS-PAGE and subsequently transferred to polyvinylidene fluoride (PVDF) membranes (0.2 μm; Bio-Rad, USA). After blocking with 5% skim milk (Beyotime, China, P0216-300g) for 90 minutes at room temperature, the membranes were incubated overnight at 4°C with primary antibodies below: GPX4 (Affinity, USA, DF6701), SLC7A11 (Affinity, USA, DF12509), P53 (Wanleibio, China, WL01919), ACSL4 (Affinity, USA, DF12141), TfR1 (Wanleibio, China, WL03500), ADAMTS5 (Abcam, UK ab41037), MMP-13 (Wanleibio, China, WL04694), and β-actin (Wanleibio, China, WL01372). Then, the membranes were incubated with secondary antibodies (Wanleibio, China, WLA023a) for 1 hour at room temperature. The targeted proteins were detected according to the previous protocol. Blots were visualized using enhanced chemiluminescence reagents (Beyotime, China, P0018S). The density of each band was quantified by image J version 1.51.
Immunofluorescence Staining
Chondrocytes of each group were seeded into 24-well plates. After treated respectively, chondrocytes were fixed with 4% paraformaldehyde at room temperature for 15 minutes. After washed by PBS solution for 3 times, cells were infiltrated by 0.1% Triton X-100 for 30 minutes and blocked by goat serum (Boster, China, AR0009) for 1 hour at room temperature. Chondrocytes were then incubated with GPX4 and SLC7A11 antibody at 4°C overnight. The next day, cells were incubated with Cy3-conjugated goat anti-rabbit secondary antibody (Beyotime, China, BL033A) for 2 hour away from light. After washed 3 times with PBS solution, chondrocytes were incubated with 4′,6-diamidino-2-phenylindole (DAPI; Beyotime, China, C1005) for 10 minutes and washed twice. Images were obtained under a fluorescence microscope.
ELISA Analysis
Chondrocyte supernatant was collected and centrifuged for 20 minutes at 1,000 × g at 2°C to 8°C, then was added to a 96-well plate covered by antibody of ADAMTS5 (Elabscience, USA, E-EL-H5590c) and MMP-13 (Elabscience, USA, E-EL-H6023), 100 μl for each well. After incubated for 90 minutes at 37°C, Biotinylated Detection Ab was added to each well. After incubated for 60 minutes at 37°C, chondrocytes were washed with wash buffer for 3 times; 100 μl of HRP (horseradish peroxidase) conjugate was then added to each well and incubated for 30 minutes at 37°C away from light; 90 μl of substrate reagent and 50 μl stop solution were then added. The optical density (OD) value of each well was determined at once by microplate reader at 450 nm.
Quantitative Real-Time Polymerase Chain Reaction
Total RNA of chondrocytes was extracted with Trizol (Beyotime, China, R0016) buffer according to the protocol. After determining the concentration, 2 µg of total RNA were used to synthesize cDNA by using a cDNA synthesis kit. SYBR Green (ES Science, China, QP002) was applied to perform the quantitative real-time polymerase chain reaction (qRT-PCR). GAPDH served as internal reference to normalize the expression level by calculating with the 2-∆∆CT method. The sequences of primers are listed in Table 1 .
Table 1.
Primers Used for RT-qPCR Amplification.
| Gene | Primers | Sequence |
|---|---|---|
| ACSL4 | Forward | CATCCCTGGAGCAGATACTCT |
| Reverse | TCACTTAGGATTTCCCTGGTCC | |
| GPX4 | Forward | GAGGCAAGACCGAAGTAAACTAC |
| Reverse | CCGAACTGGTTACACGGGAA | |
| COLX | Forward | CACTACCCAACACCAAGACA |
| Reverse | CTGGTTTCCCTACAGCTGAT | |
| SOX9 | Forward | TGCTCGGGCACTTATTGG |
| Reverse | TCCTCAGGCTTTGCGATTT | |
| ADAMTS5 | Forward | GAACATCGACCAACTCTACTCCG |
| Reverse | CAATGCCCACCGAACCATCT | |
| MMP-13 | Forward | ACTGAGAGGCTCCGAGAAATG |
| Reverse | GAACCCCGCATCTTGGCTT | |
| GAPDH | Forward | CACTCAGACCCCCACCACAC |
| Reverse | GATACATGACAAGGTGCGGCT |
RT-qPCR = quantitative real-time polymerase chain reaction; ACSL4 = Acyl-CoA synthetase long-chain family member 4; GPX4 = glutathione peroxidase 4; COLX = collagenase X; SOX9 = sex determing region Y-box9; ADAMTS5 = a disintegrin and metalloproteinase with thrombospondinmotif-5; MMP-13 = matrix metallopeptidase 13. GAPDH = glyceraldehyde-3-phosphate dehydrogenase.
Statistical Analysis
All the data were presented as the means ± standard deviation (SD). Statistical analyses were performed using GraphPad Prism version 6.02. Differences in numerical data between 3 groups were determined by one-way analysis of variance (ANOVA) followed by a Bonferroni post hoc test. P < 0.05 was defined as statistically significant.
Results
Fer-1 Increased the Cytoactive of OA Chondrocytes After the Stimulation of IL-1β
The morphology of chondrocytes of P2 or P3 in each group was shown under optical microscope. The result of identification by toluidine blue is shown in the Supplemental Material (S1). Chondrocytes in the control group were triangular and closely arranged, which became significantly longer and formed branches in IL-1β stimulated group. The longer the treatment, the more obvious the deformation and sparse cell arrangement. When treated with IL-1β + Fer-1, the deformation amplitude of chondrocytes decreased and the branches formed became less ( Fig. 2A ). In the images of EdU assay, red highlights represented chondrocytes in proliferation period ( Fig. 2B ). The results showed that within 4 hours of EdU solution treatment, the proportion of cells in proliferation period in IL-1β treated group was significantly less than that in IL-1β + Fer-1 treated group and control group (P = 0.0006, P < 0.0001, respectively) ( Fig. 2C ). The results of CCK-8 assay showed that the viability of chondrocytes in IL-1β treated group at 24 and 48 hours was significantly lower than that in IL-1β + Fer-1 treated group and control group (P < 0.0001) ( Fig. 2D-E ), demonstrating that ferroptosis stimulated by IL-1β significantly affects the cytoactive of OA chondrocytes, which can be reversed by Fer-1.
Figure 2.
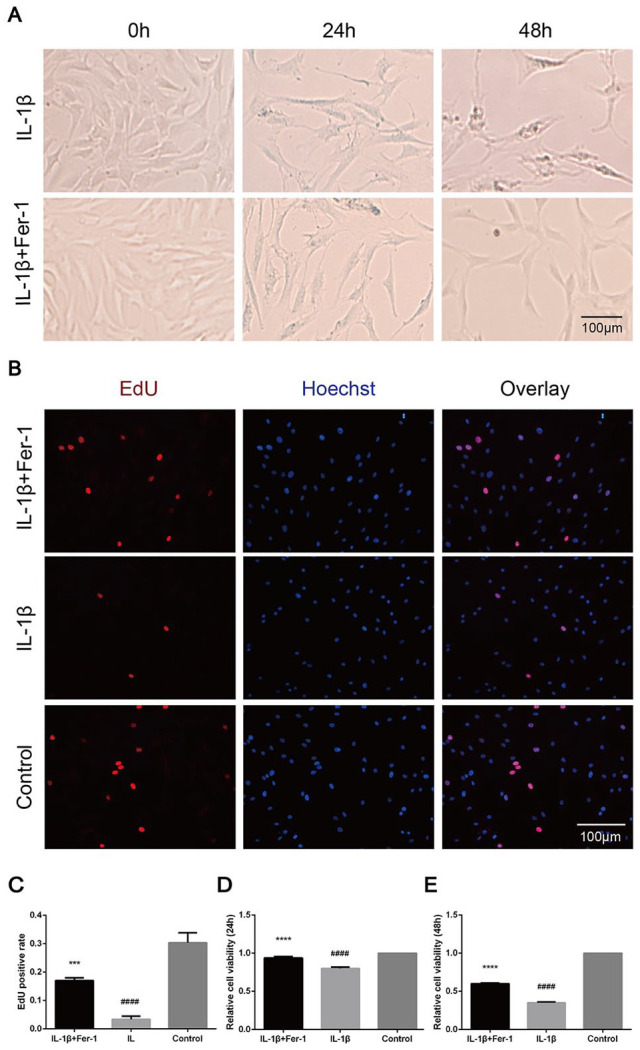
Fer-1 increased the cytoactive of OA chondrocytes after the stimulation of IL-1β. (A) Shape and state of chondrocytes of each group. (B) The proliferation of chondrocytes detected through EdU. The nuclei were stained with Hoechst. (C) EdU-positive cell rate of each group. (D-E) The viability of chondrocytes detected through CCK-8 within 24 and 48 hours. Error bars represent SD. OA = osteoarthritis; EdU = 5-Ethynyl-2’-deoxyuridine; CCK-8 = cell count kit 8; IL-1β = interleukin-1β; SD = standard deviation. ***P < 0.001, ****P < 0.0001 versus IL-1β treated group, ####P < 0.0001 versus control group.
Fer-1 Reduced Iron Overload Caused by IL-1β in OA Chondrocytes
The concentration of Fe3+ of chondrocytes in each group was measured by microplate reader. It showed an increase of Fe3+ in IL-1β treated group, which was decreased in IL-1β + Fer-1 treated group and control group (P < 0.0001), indicating a possible accumulation of Fe3+ in OA chondrocytes ( Fig. 3A ). Furthermore, we detected the expression of TfR1 of chondrocytes in each group. Similarly, we found an increase in IL-1β treated group, demonstrating an overstorage of Fe3+ indirectly (P < 0.0001). However, there was no decrease of TfR1 level in IL-1β + Fer-1 treated group, suggesting that Fer-1 did not lighten the accumulation of intracellular Fe3+ by reducing TfR1 expression (P = 0.83) ( Fig. 3B-C ). The similar conclusion was also observed in the detection of immunofluorescence staining ( Fig. 3D ).
Figure 3.
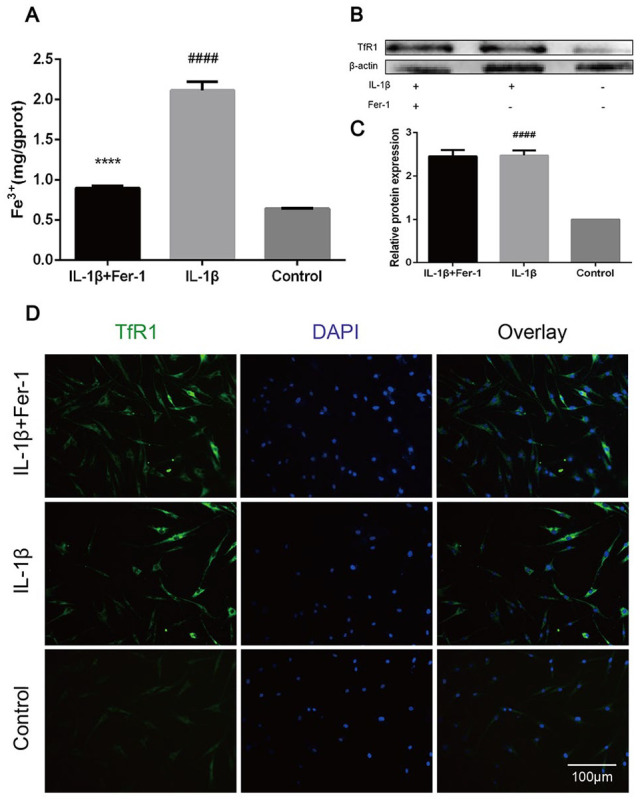
Fer-1 reduced iron overload caused by IL-1β in OA chondrocytes. (A) The level of intracellular Fe3+ after treatment of IL-1β or IL-1β + Fer-1. (B) The protein expression level of TfR1 of each group detected through Western blot analysis. (C) The band density ratio of TfR1 to β-actin in the Western blot quantified by densitometry. (D) The total protein level of TfR1 in the chondrocytes of each group evaluated by immunofluorescence staining. The nuclei were stained with DAPI. ****P < 0.0001 versus IL-1β treated group, ####P < 0.0001 versus control group. Error bars represent SD. IL-1β = interleukin-1β; OA = osteoarthritis; TfR1 = transferrin receptor 1; DAPI = 4′,6-diamidino-2-phenylindole; SD = standard deviation.
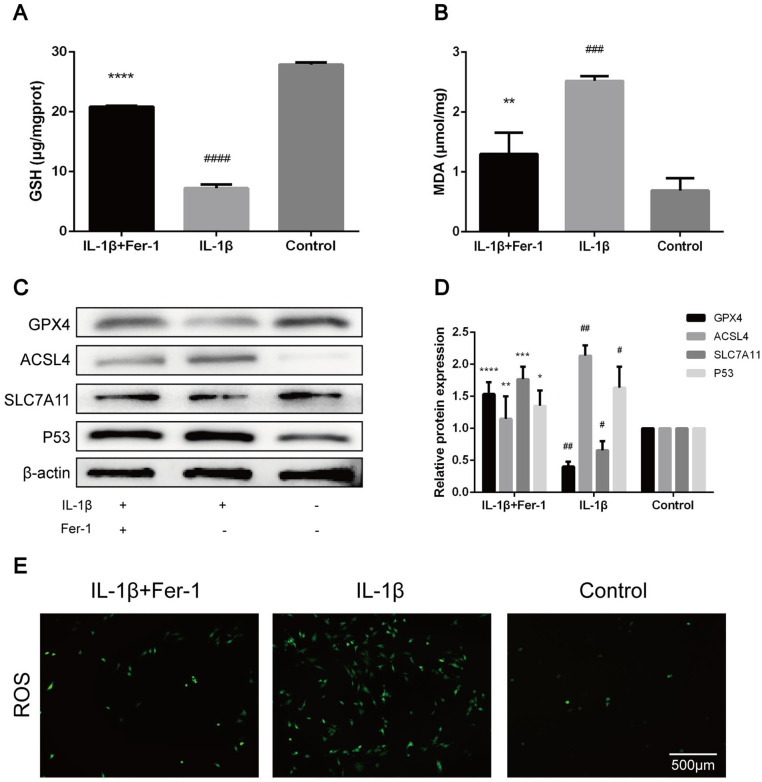
Fer-1 Reversed the Changes of OA-Related Oxidation Levels in Chondrocyte Caused by IL-1β
The GSH level of chondrocytes in IL-1β treated group was significantly lower than that in control group (P < 0.0001), which was significantly higher than that in chondrocytes in IL-1β + Fer-1 treated group (P < 0.0001) ( Fig. 4A ). On the contrary, the level of MDA in chondrocytes of IL-1β treated group was significantly higher than that of control group (P = 0.002), which was significantly lower than that in chondrocytes in IL-1β + Fer-1 treated group (P = 0.0016) ( Fig. 4B ). Among the marker proteins of ferroptosis, we selected to detect the expression levels of GPX4, ACSL4, SLC7A11, and P53. It showed that IL-1β increased the level of ACSL4 and P53 in chondrocytes while inhibited the expression of GPX4 and SLC7A11, which were reversed by Fer-1 ( Fig. 4C ). The band density ratio of 4 proteins was quantified by densitometry, which was significantly different between IL-1β treated group and control group as well as IL-1β treated group and IL-1β + Fer-1 treated group (P < 0.05) ( Fig. 4D ). The level of ROS was accumulated in IL-1β treated group while got opposite outcome in IL-1β + FER-1 treated group ( Fig. 4E ). Also, through immunofluorescence staining, we detected the level of total GPX4 and SLC7A11 of each group, which is highlighted by green fluorescence. In IL-1β treated group, the number of green highlighted spots dispersed in the cytoplasm decreased significantly with lower fluorescence intensity. When treated with IL-1β + Fer-1 at the same time, the number and intensity of green highlighted spots increased (Fig. 5A and B). The result demonstrated that Fer-1 reversed the changes of OA-related oxidation levels in chondrocyte caused by IL-1β, and further proved that ferroptosis takes part in the progress of OA chondrocytes.
Figure 4.
Fer-1 reversed the changes of OA-related oxidation levels in chondrocyte caused by IL-1β. (A) The level of GSH in chondrocytes after treatment of IL-1β or IL-1β + Fer-1. (B) The level of MDA in chondrocytes after treatment of IL-1β or IL-1β + Fer-1. (C) The protein expression level of GPX4, ACSL4, SLC7A11, and P53 of each group detected through Western blot analysis. (D) The band density ratio of GPX4, ACSL4, SLC7A11, and P53 to β-actin in the Western blot quantified by densitometry. (E) Intracellular ROS of chondrocytes detected by H2DCFDAfluorescent probe. Error bars represent SD. OA = osteoarthritis; IL-1β = interleukin-1β; GSH = glutathione; MDA = malondialdehyde; GPX4 = glutathione peroxidase; ACSL4 = acyl-CoA synthetase long-chain family member 4; SLC7A11 = solute carrier family 7 membrane 11; P53 = protein 53; ROS = reactive oxygen species; H2DCFDA = 2’,7’,-Dichlorodihydrofluorescein diacetate; SD = standard deviation. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 versus IL-1β treated group, #P < 0.05, ##P < 0.01, ###P < 0.001, ####P < 0.0001 versus control group.
Figure 5.
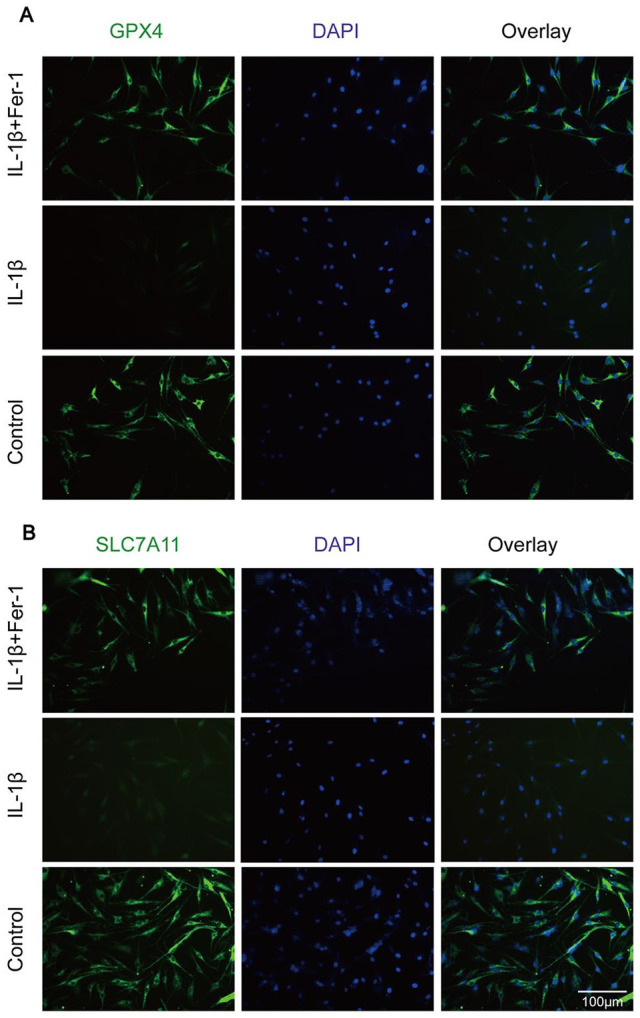
(A) The total protein level of GPX4 in the chondrocytes of each group evaluated by immunofluorescence staining. The nuclei were stained with DAPI. (B) The total protein level of SLC7A11 in the chondrocytes of each group was evaluated by immunofluorescence staining. The nuclei were stained with DAPI. GPX4 = glutathione peroxidase; SLC7A11 = solute carrier family 7 membrane 11; DAPI = 4′,6-diamidino-2-phenylindole.
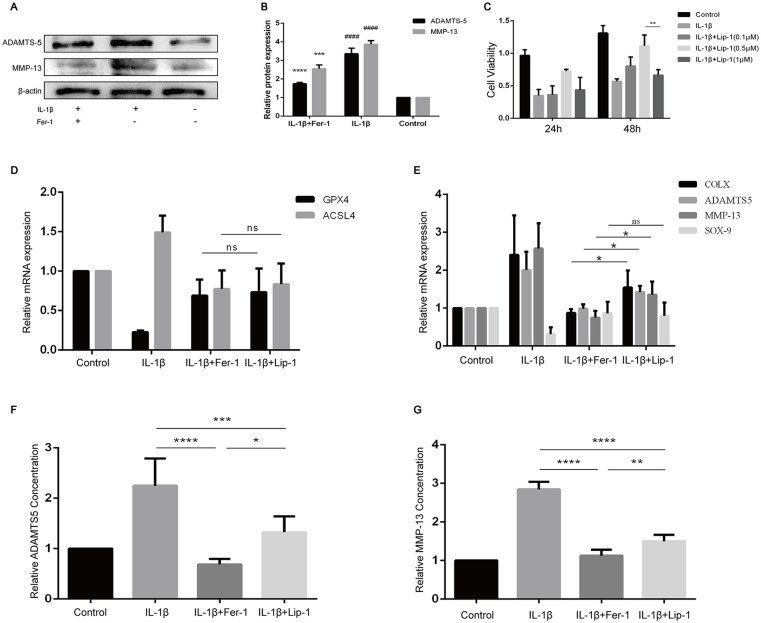
Fer-1 Protected Chondrocyte from OA Inflammatory Environment Better than Lip-1
With the finding of the increase of ferroptosis-related oxidation level in OA chondrocytes, we sought to determine whether Fer-1 can reduce the expression of OA-related markers and protect chondrocytes in OA extracellular environment. The results showed that the protein expression levels of ADAMTS5 and MMP-13 in IL-1β treated group were significantly higher than those in the control group (P < 0.0001) and in IL-1β + Fer-1 treated group (P < 0.0001, Fig. 6A-B ). After confirming the existence of ferroptosis in OA chondrocytes, we tend to compare the effect of Fer-1 with another RTA named Lip-1. CCK-8 results showed that the viability of chondrocytes was reduced when treated with Lip-1 under the concentration of 1 μM (P < 0.01, Fig. 6C ). Therefore, we considered 0.5 μM as the optimal concentration of Lip-1, which was used in the subsequent experiments. PCR results showed that there was no significant difference in GPX4 and ACSL4 expression between chondrocytes treated by Fer-1 and Lip-1 ( Fig. 6D ). However, the expressions of COLX, ADAMTS5, and MMP-13 in chondrocytes are lower after the treatment of Fer-1 compared with Lip-1 (P < 0.05), while the expression of SOX9 seemed to be not significantly different ( Fig. 6E ). Through enzyme-linked immuno-sorbent assay (ELISA) analysis, we found that both Fer-1 and Lip-1 had the ability to reduce the level of ADAMTS5 and MMP-13 in the cell supernatant (P < 0.0001), which of Fer-1 is more significant (P < 0.05, P < 0.01, respectively) ( Fig. 6F-G ).
Figure 6.
Fer-1 reduced the secretion of OA markers of chondrocyte. (A) The protein expression level of ADAMTS5 and MMP-13 of each group detected through Western blot analysis. (B) The band density ratio of ADAMTS5 and MMP-13 to β-actin in the Western blot quantified by densitometry. (C) Viability of chondrocytes treated with different concentrations of Lip-1. (D-E) relative expression of mRNAs related to ferroptosis and OA inflammation in different groups. (F) The level of ADAMTS5 concentration of different groups in the cell supernatant. (G) The level of MMP-13 concentration of different groups in the cell supernatant. OA = osteoarthritis; ADAMTS5 = a disintegrin and metalloproteinase with thrombospondinmotif-5; MMP-13 = matrix metallopeptidase 13; IL-1β = interleukin-1β; GPX4 = glutathione peroxidase; ACSL4 = acyl-CoA synthetase long-chain family member 4. *P < 0.05, ***P < 0.001, ****P < 0.0001. ns = no significance.
Discussion
As a progressive disease with high disability, OA is mainly characterized by disintegration of matrix and reduction of chondrocytes, especially knee osteoarthritis (KOA). The conservative treatment of KOA includes oral analgesic drugs and intra-articular injection. 22 The operation of KOA mainly includes arthroscopic debridement, high tibial osteotomy (HTO), unicondylar knee arthroplasty (UKA), and TKA.23 -25 But in fact, these are only remedial methods after the occurrence of OA, which cause considerable economic burden to the patients, mainly because of its unclear pathogenesis. 26 As an independent cell death mode, ferroptosis has become a research hotspot. Studies have shown that ferroptosis plays a role in diseases such as cancer, neurodegenerative diseases, intracerebral hemorrhage and ischemia-reperfusion injury.27 -30 Recently, Yao et al. 31 found that the expression of ROS, lipid peroxides, and related proteins showed a characteristic change of ferroptosis in mouse chondrocytes with OA. But at present, no such study is available in human chondrocytes. The aim of our study is to confirm that ferroptosis of chondrocytes takes part in the progress of human OA.
We extracted human chondrocytes from specimens after TKA to maximize the reset of the environment of OA in vitro, and treated chondrocytes with IL-1β or IL-1β + Fer-1. We found the morphology of chondrocytes changed significantly, with decreased cell viability under the effect of IL-1β. However, after adding Fer-1, the extent of such changes was reduced, indicating that ferroptosis maybe one of the mechanism which can influence the growth of chondrocytes.
Intracellular iron homeostasis depends on the balance between iron absorption, secretion, and storage. 32 When there is too much intracellular Fe3+ that cannot be used in time, it will cause iron overload and affect cell function. Jing et al. 33 observed a significant trabecular bone loss in mice with iron overload. A clinical cohort study of elderly OA patients found that the serum Fe3+ level in OA patients increased and was positively correlated with the severity of OA. 34 Our study demonstrated that there is an accumulation of Fe3+ in chondrocytes of OA, with higher expression of TfR1. In addition, level of Fe3+ decreased after the treatment of Fer-1, giving a proof on the protection of Fer-1 from iron overload. However, overexpression of TfR1 maintained, probably because of the targeting effect of Fer-1 on the lipid peroxidation, not on TfR1, same to the conclusion of Miotto et al. 21
Oxidative stress is another major component of mechanism of ferroptosis. Reduced GSH is an essential intracellular antioxidant synthesized from glutamate, glycine, and cysteine, which is transported by cystine/glutamate antiporter system Xc– (SLC7A11 and SLC3A2). 35 GSH is a necessary cofactor of GPX4 for eliminating lipid peroxides. 36 Lipid peroxidation refers to the oxidative deterioration of polyunsaturated fatty acids (PUFAs) and lipids in cells, which can destroy the membrane of mitochondrion and cells, leading to cell dysfunction. Studies have shown that there are symbols of ferroptosis in neurodegenerative diseases, that is, the expression of oxidative stress protective proteins such as GSH and GPX4 decreases, while the level of lipid peroxidation increases. 37 Our study showed the same character in OA chondrocytes, proving that ferroptosis contributes to human OA. Moreover, we found that Fer-1 can reverse the changes of levels of GSH, MDA, ROS, as well as protein markers of ferroptosis (GPX4, SLC7A11, P53, ACSL4) caused by IL-1β, resulting in the reduction of oxidative stress response in chondrocytes.
ADAMTS5 and MMP-13 are the main mediators of OA cartilage destruction, with low expression in normal cartilage and significant increase in OA cartilage, leading to matrix degradation and cartilage destruction. 38 ADAMTS5 and MMP-13 are considered as molecules to predict the progress and treatment value of OA. 39 To confirm the protection of Fer-1 on chondrocytes and cartilage matrix in inflammatory environment of OA, we subsequently detected the expression of related factors in cells or cell supernatant. Through PCR and ELISA analysis, it was suggested that Fer-1 had a protective effect on chondrocytes, which was better than Lip-1.
At present, studies have put eyes on the treatment by promoting ferroptosis of tumor cells or inhibiting ferroptosis of normal cells, especially RTAs (such as Fer-1, Lip-1, Vitamin E). Vitamin E (Vit E) is a naturally occurring fat-soluble antioxidant. Tantavisut et al. 40 have found that Vit E is reduced in the serum of OA patients. From the aspect of ferroptosis, Vit E can reduce intracellular lipid peroxidation by inhibiting 15-lipoxygenase-1 (15-LOX-1), and can also cooperatively maintain lipid redox balance with GPX4 to prevent ferroptosis in hematopoietic stem and progenitor cells (HSPCs).41,42 However, the evidence suggests that α-tocopherol (α-TOH), the most biologically active form of Vit E and Nature’s premier lipid-soluble RTA, is a relatively poor inhibitor of ferroptosis compared with either Fer-1 or Lip-1. 43 Several clinical trials also showed that the application of Vit E will not reduce the clinical symptoms and the cartilage loss of OA patients. Moreover, different isomers of Vit E might possess distinct anti-osteoarthritic effects. 44
Fer-1 and Lip-1 were firstly discovered by high-throughput screening of small molecule libraries using cell assays. Some studies have shown that Lip-1 and Fer-1 can also significantly inhibit intracellular 15-LOX-1, thereby reducing lipid hydroxides (LOOH), except from working as RTA. 43 In this study, we found that Lip-1 showed significant toxicity to chondrocytes at a lower concentration than Fer-1. In addition, at the optimal concentration, Fer-1 showed a similar effect on the inhibition of ferroptosis, while a stronger effect on the protection of chondrocytes by reducing the expression of inflammatory factors of OA. In the part in vivo of previous studies, intraperitoneal injection and focal lesion injection of Fer-1 were the two most common treating methods. In view of the few studies on the toxicity and the potential protective effect on tumor cells of oral Fer-1, it seems that intra-articular injection of Fer-1, which is also closest to the simulation in vitro, may be an effective and safe method to alleviate OA.
As far as we know, our study is the first one which proved that there is ferroptosis mechanism in OA chondrocytes of human. At the same time, the application of inhibitor of ferroptosis in vitro can significantly reverse the characteristic changes of ferroptosis, and reduce the secretion of inflammatory molecules and cell dysfunction, so as to protect chondrocytes from death. Moreover, through the comparison, it was found that Lip-1 and Fer-1 can both alleviate the level of ferroptosis in OA chondrocytes, but Fer-1 had more protective effect on chondrocytes.
There are still some limitations in this study. Our study explored the effect of ferroptosis in chondrocytes of OA in vitro, and confirmed the involvement of ferroptosis mechanism in chondrocytes in terms of cell morphology and markers related to ferroptosis. However, animal models have not been carried out, so that experiment in vivo has not been considered in our study. At the same time, the development of human OA usually takes decades, which is mediated by stress, inflammatory environment, and several other factors. We used IL-1β to stimulate chondrocytes for 48 hours to induce OA, which is slightly different from the real situation. In addition, we confirmed the role of ferroptosis in chondrocytes of OA, but the related pathways and key molecules have not been discussed, which are needed to be studied in the future.
In conclusion, our study observed the changes of markers related to ferroptosis in human chondrocytes of OA, which can be prevented by Fer-1, proving that ferroptosis plays an important role in human chondrocytes of OA. At present, the conservative treatment of OA is still anti-inflammatory and analgesic, which is a lack of targeting drugs. This study provided a theoretical basis for the development of therapeutic drugs for early OA, that is, ferroptosis inhibitors, especially Fer-1, may be one of the potential options.
Supplemental Material
Supplemental material, sj-tif-1-car-10.1177_19476035221142011 for Ferroptosis Plays a Role in Human Chondrocyte of Osteoarthritis Induced by IL-1β In Vitro by Wenbo Xu, Bowen Zhang, Chunyang Xi, Yong Qin, Xin Lin, Bo Wang, Pengyu Kong and Jinglong Yan in CARTILAGE
Footnotes
Author Contributions: W.X., P.K., X.L., and J.Y. contributed to the collection of sample and manuscript writing. P.K., B.Z., and C.X. performed data analysis and generated figures. Y.Q. and B.W. consulted on the interpretation of results. Every author read and approved the final manuscript.
Acknowledgments and Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The study was supported by the National Natural Science Foundation of China (No. 82072472).
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical Approval: The study was designed according to the Declaration of Helsinki, and was approved by Ethic Committee of the Second Affiliated Hospital of Harbin Medical University (Ref: KY 2021-256).
ORCID iDs: Yong Qin  https://orcid.org/0000-0003-0541-8054
https://orcid.org/0000-0003-0541-8054
Jinglong Yan  https://orcid.org/0000-0003-0622-6249
https://orcid.org/0000-0003-0622-6249
Supplementary material for this article is available on the Cartilage website at http://cart.sagepub.com/supplemental.
References
- 1. Loeser RF, Collins JA, Diekman BO. Ageing and the pathogenesis of osteoarthritis. Nat Rev Rheumatol. 2016;12(7):412-20. doi: 10.1038/nrrheum.2016.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hunter DJ, Bierma-Zeinstra S. Osteoarthritis. Lancet. 2019;393(10182):1745-59. doi: 10.1016/S0140-6736(19)30417-9. [DOI] [PubMed] [Google Scholar]
- 3. Zhang W, Ouyang H, Dass CR, Xu J. Current research on pharmacologic and regenerative therapies for osteoarthritis. Bone Res. 2016;4:15040. doi: 10.1038/boneres.2015.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Komori T. Cell death in chondrocytes, osteoblasts, and osteocytes. Int J Mol Sci. 2016;17(12):2045. doi: 10.3390/ijms17122045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hwang HS, Kim HA. Chondrocyte apoptosis in the pathogenesis of osteoarthritis. Int J Mol Sci. 2015;16(11):26035-54. doi: 10.3390/ijms161125943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Riegger J, Brenner RE. Evidence of necroptosis in osteoarthritic disease: investigation of blunt mechanical impact as possible trigger in regulated necrosis. Cell Death Dis. 2019;10(10):683. doi: 10.1038/s41419-019-1930-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sasaki H, Takayama K, Matsushita T, Ishida K, Kubo S, Matsumoto T, et al. Autophagy modulates osteoarthritis-related gene expression in human chondrocytes. Arthritis Rheum. 2012;64(6):1920-8. doi: 10.1002/art.34323. [DOI] [PubMed] [Google Scholar]
- 8. Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM, Gleason CE, et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149(5):1060-72. doi: 10.1016/j.cell.2012.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Galluzzi L, Kroemer G. Necroptosis: a specialized pathway of programmed necrosis. Cell. 2008;135(7):1161-3. doi: 10.1016/j.cell.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 10. An S, Hu H, Li Y, Hu Y. Pyroptosis plays a role in osteoarthritis. Aging Dis. 2020;11(5):1146-57. doi: 10.14336/AD.2019.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pérez H E, Luna M J, Rojas M L, Kouri JB. Chondroptosis: an immunohistochemical study of apoptosis and Golgi complex in chondrocytes from human osteoarthritic cartilage. Apoptosis. 2005;10(5):1105-10. doi: 10.1007/s10495-005-0649-1. [DOI] [PubMed] [Google Scholar]
- 12. Galluzzi L, Vitale I, Aaronson SA, Abrams JM, Adam D, Agostinis P, et al. Molecular mechanisms of cell death: recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 2018;25(3):486-541. doi: 10.1038/s41418-017-0012-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cao JY, Dixon SJ. Mechanisms of ferroptosis. Cell Mol Life Sci. 2016;73(11-12):2195-209. doi: 10.1007/s00018-016-2194-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chen X, Kang R, Kroemer G, Tang D. Broadening horizons: the role of ferroptosis in cancer. Nat Rev Clin Oncol. 2021;18(5):280-96. doi: 10.1038/s41571-020-00462-0. [DOI] [PubMed] [Google Scholar]
- 15. Hirayama T, Miki A, Nagasawa H. Organelle-specific analysis of labile Fe(ii) during ferroptosis by using a cocktail of various colour organelle-targeted fluorescent probes. Metallomics. 2019;11(1):111-7. doi: 10.1039/c8mt00212f. [DOI] [PubMed] [Google Scholar]
- 16. Jiang L, Kon N, Li T, Wang S, Su T, Hibshoosh H, et al. Ferroptosis as a p53-mediated activity during tumour suppression. Nature. 2015;520(7545):57-62. doi: 10.1038/nature14344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wang W, Li X, Ding N, Teng J, Zhang S, Zhang Q, et al. MiR-34a regulates adipogenesis in porcine intramuscular adipocytes by targeting ACSL4. BMC Genet. 2020;21(1):33. doi: 10.1186/s12863-020-0836-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hu Q, Ecker M. Overview of MMP-13 as a promising target for the treatment of osteoarthritis. Int J Mol Sci. 2021;22(4):1742. doi: 10.3390/ijms22041742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Karsenty G. An aggrecanase and osteoarthritis. N Engl J Med. 2005;353(5):522-3. doi: 10.1056/NEJMcibr051399. [DOI] [PubMed] [Google Scholar]
- 20. Jiang X, Stockwell BR, Conrad M. Ferroptosis: mechanisms, biology and role in disease. Nat Rev Mol Cell Biol. 2021;22(4):266-82. doi: 10.1038/s41580-020-00324-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Miotto G, Rossetto M, Di Paolo ML, Orian L, Venerando R, Roveri A, et al. Insight into the mechanism of ferroptosis inhibition by ferrostatin-1. Redox Biol. 2020;28:101328. doi: 10.1016/j.redox.2019.101328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Deyle GD, Allen CS, Allison SC, Gill NW, Hando BR, Petersen EJ, et al. Physical therapy versus glucocorticoid injection for osteoarthritis of the knee. N Engl J Med. 2020;382(15):1420-9. doi: 10.1056/NEJMoa1905877. [DOI] [PubMed] [Google Scholar]
- 23. Thorlund JB, Juhl CB, Roos EM, Lohmander LS. Arthroscopic surgery for degenerative knee: systematic review and meta-analysis of benefits and harms. BMJ. 2015;350:h2747. doi: 10.1136/bmj.h2747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Primeau CA, Birmingham T, Leitch KM, Willits KR, Litchfield RB, Fowler PJ, et al. Total knee replacement after high tibial osteotomy: time-to-event analysis and predictors. CMAJ. 2021;193(5):E158-E166. doi: 10.1503/cmaj.200934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Evans JT, Whitehouse MR. Partial versus total knee replacement for knee osteoarthritis. Lancet. 2019;394(10200):712-3. doi: 10.1016/S0140-6736(19)31612-5. [DOI] [PubMed] [Google Scholar]
- 26. Kennish L, Attur M, Oh C, Krasnokutsky S, Samuels J, Greenberg J, et al. Age-dependent ferritin elevations and HFE C282Y mutation as risk factors for symptomatic knee osteoarthritis in males: a longitudinal cohort study. BMC Musculoskelet Disord. 2014;15:8. doi: 10.1186/1471-2474-15-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Liang C, Zhang X, Yang M, Dong X. Recent progress in ferroptosis inducers for cancer therapy. Adv Mater. 2019;31(51):e1904197. doi: 10.1002/adma.201904197. [DOI] [PubMed] [Google Scholar]
- 28. La Rosa P, Petrillo S, Turchi R, Berardinelli F, Schirinzi T, Vasco G, et al. The Nrf2 induction prevents ferroptosis in Friedreich’s Ataxia. Redox Biol. 2021;38:101791. doi: 10.1016/j.redox.2020.101791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kenny EM, Fidan E, Yang Q, Anthonymuthu TS, New LA, Meyer EA, et al. Ferroptosis contributes to neuronal death and functional outcome after traumatic brain injury. Crit Care Med. 2019;47(3):410-8. doi: 10.1097/CCM.0000000000003555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Liu P, Feng Y, Li H, Chen X, Wang G, Xu S, et al. Ferrostatin-1 alleviates lipopolysaccharide-induced acute lung injury via inhibiting ferroptosis. Cell Mol Biol Lett. 2020;25:10. doi: 10.1186/s11658-020-00205-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yao X, Sun K, Yu S, Luo J, Guo J, Lin J, et al. Chondrocyte ferroptosis contribute to the progression of osteoarthritis. J Orthop Translat. 2021;27:33-43. doi: 10.1016/j.jot.2020.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Galaris D, Barbouti A, Pantopoulos K. Iron homeostasis and oxidative stress: an intimate relationship. Biochim Biophys Acta Mol Cell Res. 2019;1866(12):118535. doi: 10.1016/j.bbamcr.2019.118535. [DOI] [PubMed] [Google Scholar]
- 33. Jing X, Du T, Chen K, Guo J, Xiang W, Yao X, et al. Icariin protects against iron overload-induced bone loss via suppressing oxidative stress. J Cell Physiol. 2019;234(7):10123-37. doi: 10.1002/jcp.27678. [DOI] [PubMed] [Google Scholar]
- 34. GBD 2017 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392(10159):1789-858. doi: 10.1016/S0140-6736(18)32279-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Stockwell BR, Friedmann Angeli JP, Bayir H, Bush A, Conrad M, Dixon S, et al. Ferroptosis: a regulated cell death nexus linking metabolism, redox biology, and disease. Cell. 2017;171(2):273-85. doi: 10.1016/j.cell.2017.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Muri J, Kopf M. Redox regulation of immunometabolism. Nat Rev Immunol. 2021;21(6):363-81. doi: 10.1038/s41577-020-00478-8. [DOI] [PubMed] [Google Scholar]
- 37. Vitalakumar D, Sharma A, Flora SJS. Ferroptosis: a potential therapeutic target for neurodegenerative diseases. J Biochem Mol Toxicol. 2021;35(8):e22830. doi: 10.1002/jbt.22830. [DOI] [PubMed] [Google Scholar]
- 38. Latourte A, Cherifi C, Maillet J, Ea HK, Bouaziz W, Funck-Brentano T, et al. Systemic inhibition of IL-6/Stat3 signalling protects against experimental osteoarthritis. Ann Rheum Dis. 2017;76(4):748-55. doi: 10.1136/annrheumdis-2016-209757. [DOI] [PubMed] [Google Scholar]
- 39. Saberi H, Bierma-Zeinstra SM, Bay-Jensen AC. Osteoarthritis year in review 2018: biomarkers (biochemical markers). Osteoarthritis Cartilage. 2019;27(3):412-23. doi: 10.1016/j.joca.2018.12.002. [DOI] [PubMed] [Google Scholar]
- 40. Tantavisut S, Tanavalee A, Honsawek S, Suantawee T, Ngarmukos S, Adisakwatana S, et al. Effect of Vitamin E on oxidative stress level in blood, synovial fluid, and synovial tissue in severe knee osteoarthritis: a randomized controlled study. BMC Musculoskelet Disord. 2017;18(1):281. doi: 10.1186/s12891-017-1637-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zhang X, Wu S, Guo C, Guo K, Hu Z, Peng J, et al. Vitamin E exerts neuroprotective effects in pentylenetetrazole kindling epilepsy via suppression of ferroptosis. Neurochem Res. 2022;47(3):739-47. doi: 10.1007/s11064-021-03483-y. [DOI] [PubMed] [Google Scholar]
- 42. Hu Q, Zhang Y, Lou H, Ou Z, Liu J, Duan W, et al. GPX4 and Vitamin E cooperatively protect hematopoietic stem and progenitor cells from lipid peroxidation and ferroptosis. Cell Death Dis. 2021;12(7):706. doi: 10.1038/s41419-021-04008-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zilka O, Shah R, Li B, Friedmann Angeli JP, Griesser M, Conrad M, et al. On the mechanism of cytoprotection by ferrostatin-1 and liproxstatin-1 and the role of lipid peroxidation in ferroptotic cell death. ACS Cent Sci. 2017;3(3):232-43. doi: 10.1021/acscentsci.7b00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Brand C, Snaddon J, Bailey M, Cicuttini F. Vitamin E is ineffective for symptomatic relief of knee osteoarthritis: a six month double blind, randomised, placebo controlled study. Ann Rheum Dis. 2001;60(10):946-9. doi: 10.1136/ard.60.10.946. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-tif-1-car-10.1177_19476035221142011 for Ferroptosis Plays a Role in Human Chondrocyte of Osteoarthritis Induced by IL-1β In Vitro by Wenbo Xu, Bowen Zhang, Chunyang Xi, Yong Qin, Xin Lin, Bo Wang, Pengyu Kong and Jinglong Yan in CARTILAGE