Abstract
Objective
Osteochondral allograft (OCA) transplantation is a restorative surgical option for large, full-thickness chondral or osteochondral defects in the knee. Variability in outcomes reporting has led to a broad range of graft survival rates. Using rate of salvage surgery following OCA as a failure metric, the purpose of this study was to analyze the incidence and risk factors for failure in a nationwide cohort.
Design
The M151Ortho PearlDiver database was queried for patients aged 20 to 59 who underwent primary OCA between 2010 and 2020. Patients with prior cartilage procedures or arthroplasty were excluded. Kaplan-Meier survival analysis was performed to characterize cumulative rate of salvage surgery, defined as any patient subsequently undergoing revision OCA, autologous chondrocyte implantation (ACI), osteochondral autograft transfer system (OATS), unicompartmental knee arthroplasty (UKA), or total knee arthroplasty (TKA). Multivariable logistic regression was used to determine the effect of several variables on odds of salvage surgery.
Results
Around 6,391 patients met inclusion criteria. Cumulative 5-year salvage rate was 1.71%, with 68.8% in the first 2 years. Age 20 to 29 and concomitant or prior bony realignment procedures were associated with significantly decreased rate of salvage surgery (age—adjusted odds ratio [aOR] = 0.49, 95% confidence interval [CI], 0.24-0.99, P = 0.046; realignment—aOR = 0.24, 95% CI, 0.04-0.75, P = 0.046).
Conclusions
In the largest OCA cohort studied to date, less than 2% of patients required salvage surgery. Young age and bony realignment were protective. These findings suggest that OCA in the knee is a durable cartilage-restoration procedure, especially in young patients with corrected alignment.
Keywords: osteochondral allograft transplantation, OCA, 5-year survivorship, cartilage restoration
Introduction
Articular cartilage injuries of the knee are highly prevalent, with chondral or osteochondral pathology observed in 60% to 66% of knee arthroscopy patients.1 -4 Often painful and debilitating, chondral lesions have limited capacity for intrinsic healing secondary to the avascular, hypocellular, and aneural nature of cartilage. 5 Self-repair of cartilaginous lesions depends on factors such as patient age, size or thickness of the defect, and location.6,7 Small defects may adapt with the formation of near-normal hyaline cartilage, whereas larger chondral or osteochondral defects will instead incorporate fibrocartilage.5,6 The predominance of type 1 cartilage in maladaptive fibrocartilage may be transiently effective but ultimately inadequate, without the friction-reducing and load-bearing characteristics of native hyaline tissue. 6 This process does not ensure symptom resolution and may predispose the subchondral bone to further degenerative damage and osteoarthritis (OA).6,8 Several surgical techniques have been developed to address symptomatic cartilage defects, especially in young, active patients; although indications vary based on pathology and patient demand, controversy remains in distinguishing a clearly superior technique.9,10 Surgical treatment options for focal chondral defects can be broadly categorized as palliative (chondroplasty), repair (marrow stimulation with microfracture or drilling), or restoration.9 -11 Restorative procedures include cell-based methods such as autologous chondrocyte implantation (ACI), in addition to whole-tissue reconstructive techniques such as osteochondral autograft transfer system (OATS) and osteochondral allograft (OCA) transplantation.10,12 -14
Modern OCA is a single-stage procedure involving transfer of a size-matched cartilage and subchondral bone allograft to treat large (>2 cm2), full-thickness chondral or osteochondral defects.14,15 It may also be used for multifocal or multicompartmental defects. 16 In comparison to other cartilage procedures, OCA is efficient and uniquely restorative of both cartilage and underlying bone. The procedure has also demonstrated significant improvement in patient-reported outcomes (PROs) and good mid- to long-term survivorship.16 -18
Although OCA use is common, there is no consistently used method to evaluate failure. Metrics used to assess outcomes are variable, including PROs on postoperative symptoms and function, incorporation on imaging, graft removal/revision, arthroplasty, or in many cases, a combination of these factors. This variability in reporting lacks clinical utility and prevents analysis of graft durability. Failure rates using these methods range widely from approximately 8% to 43%.12,16,18 -29 A salvage surgery addressing the same articular surface—defined in this study as revision OCA, other cartilage-restoration procedure, or knee arthroplasty—is the clearest indication of failure. A similar study from Gillinov et al. evaluated incidence of salvage surgery following ACI. 30 Previous studies using secondary surgeries as a failure metric for OCA have been limited by small sample sizes and inconsistent follow-up.19,21,23,24,27,29
The primary purpose of our study is to use a nationwide administrative claims patient registry to investigate the incidence and timing of salvage cartilage surgery within 5 years of index OCA. Secondary goals include identification of pertinent patient factors that influence risk of OCA failure. Using the incidence of salvage procedures as a utilitarian failure metric, we aim to provide large-scale data to guide clinical decision-making regarding OCA transplant in the knee.
Methods
Data Source
This retrospective epidemiological analysis was completed using the de-identified data from the M151Ortho data set within the larger PearlDiver (PearlDiver Technologies, Colorado Springs, CO) Mariner database from January 1, 2010, through October 31, 2020. PearlDiver is an insurance claims database compiled from Humana Incorporated insurance claims, contains records of more than 150 million patients, and provides researchers the ability to longitudinally track patients and characterize short-, medium-, and long-term disease progression and/or postoperative complications As these data are de-identified, this analysis did not require Institutional Review Board (IRB) approval at our institution.
Patient Selection
The M151Ortho data set was queried for all patients who underwent an OCA (CPT-27415, CPT-29867) between the ages of 20 and 59 years. Those patients who previously received an OCA, OAT (CPT-27416, CPT-29866), unilateral knee arthroplasty (CPT-27446), chondroplasty (CPT-29877), or microfracture (CPT-29879) before their index OCA were excluded from the study as previous arthroplasty or cartilage repair has been previously associated with increased rates of chondrocyte implantation failure. These age and exclusion criteria were used to remain consistent with previously published literature.12,30 -32
Potential risk factors of interest were extracted from the aforementioned database; these included sex, age, body mass index (BMI), Elixhauser Comorbidity Index (ECI), bony realignment procedures with index OCA or within 1 year prior (femoral shaft osteotomy CPT-27450, tibial tubercle osteotomy CPT-27418, proximal tibial osteotomy CPT-27455 or CPT-27457), and comorbidities including vitamin D deficiency, tobacco use, diabetes, hypertension, and obstructive pulmonary disease. Of note, ECI was used in this study as a cumulative comorbidity index, functioning to practically quantify severity of comorbid conditions, rather than as a mortality predictor. Age was divided into four 10-year brackets: 20 to 29, 30 to 39, 40 to 49, or 50 to 59 years; patients age 20 to 59 were specifically analyzed as this represents a range that captures a population most likely to undergo restorative cartilage procedures, specifically OCA.11,26 BMI was divided into <25, 25 to 40 (overweight or obese), and >40 (Class III obesity), and ECI, a combined comorbidity index used to predict inpatient mortality, was stratified into 0, 1 to 3, or >3. These variables were selected for the model to be consistent with models from previously published literature. 30
Outcomes
Rates of salvage surgery were calculated over a 5-year period for each patient and was defined as any patient subsequently undergoing a revision OCA (CPT-27415, CPT-29867), ACI (CPT-27412), OATS (CPT-27416, CPT-29866), unicompartmental knee arthroplasty (UKA, CPT-27446), or total knee arthroplasty (TKA, CPT-27447). These procedures were chosen to reflect clinical recommendations for appropriate revision procedures following failure of index OCA transplant in the knee.33,34
Statistical Analysis
Kaplan-Meier survival analysis was performed to characterize the cumulative rate of salvage surgery over a 5-year period following an index OCA. All patients in the cohort were included in this analysis. However, patients who lacked sufficient follow-up for reasons such as change in insurance, no further follow-up with the physician, or death were censored at the time point when data were no longer available. Multivariable logistic regression was used to determine how patient demographics, comorbidities, arthroscopic versus open operation, and concomitant bony realignment changed the odds of undergoing a secondary surgery within 5 years of the index OCA. A P value of <0.05 was determined to represent statistical significance a priori. All statistical analyses were performed using the R Statistical Package (v4.2.1; R Core Team 2022, Vienna, Austria) embedded within PearlDiver. Mean and standard deviation (SD) were calculated for all quantitative variables.
Results
Study Population
A total of 6,391 patients met the inclusion criteria; see Table 1 for demographic breakdown and surgical differences. The number of patients available for follow-up in years 1, 2, 3, 4, and 5 were 5,650; 5,015; 4,400; 3,868; and 3,331 patients, respectively. Bony realignment procedures were performed in 477 patients (7.5%); 466 of those were performed at the time of OCA and 11 occurred within 1 year prior to the index OCA procedure.
Table 1.
Demographics of Patients Undergoing Osteochondral Allograft.
| Sample Size | Total | |
|---|---|---|
| Value | Percentage | |
| Age, years, mean (SD) | 37.7 | (10.3) |
| 20-29 | 1,598 | 25.0 |
| 30-39 | 1,991 | 31.2 |
| 40-49 | 1,849 | 28.9 |
| 50-59 | 953 | 14.9 |
| Surgical approach | ||
| Open | 3,950 | 60.4 |
| Arthroscopic | 2,589 | 39.6 |
| Sex | ||
| Male | 3,212 | 50.3 |
| Female | 3,174 | 49.7 |
| BMI | ||
| >30 | 778 | 12.2 |
| >40 | 288 | 4.5 |
| ECI score, mean (SD) | 2 | (2.3) |
| 0 | 1,948 | 30.5 |
| 1-3 | 3,261 | 51.0 |
| >3 | 1,182 | 18.5 |
| Bony realignment | ||
| Yes | 477 | 7.5 |
| No | 5,914 | 92.5 |
| Comorbidities | ||
| Tobacco use | 907 | 14.2 |
| Diabetes | 410 | 6.4 |
| Hypertension | 1,123 | 17.6 |
| Lung disease | 505 | 7.9 |
| Hypovitaminosis D | 456 | 7.1 |
BMI = body mass index; ECI = Elixhauser Comorbidity Index.
Subsequent Surgery and Timing
Salvage surgery over time is depicted as a cumulative incidence by Kaplan-Meier failure analysis in Figure 1 . Over 5 years, a total of 109 patients required secondary procedures. The cumulative rate was 1.71%. Most of these procedures occurred in the first (n = 43, 39.4%) and second year (n = 32, 29.4%), with the first 2 years accounting for 68.8% of total surgeries over the 5 years assessed. The percentage of salvage surgeries in the following years was 19.3% (year 3), 9.1% (year 4), and 2.8% (year 5).
Figure 1.
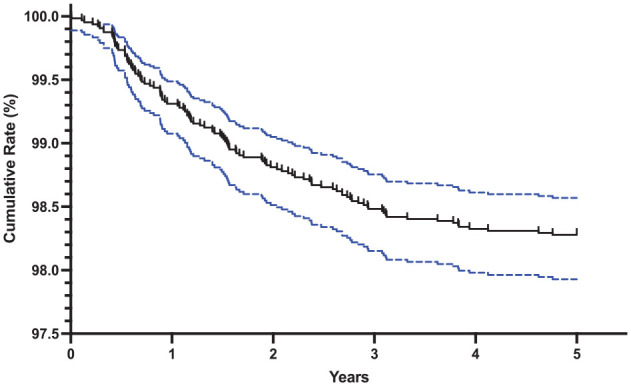
Kaplan-Meier failure analysis depicting the cumulative rate of subsequent surgery following an osteochondral allograft. The blue dotted lines depict the 95% confidence interval. Cumulative rates at 1 (43, 0.67%), 2 (32, 1.17%), 3 (21, 1.50%), 4 (10, 1.66%), 5 (3, 1.71%) years.
Risk Factors for OC Allograft Surgical Salvage
The results of the logistic regression analysis performed to identify risk factors for salvage surgery are presented in Table 2 . Younger age was associated with a significantly decreased risk of subsequent surgery (aOR = 0.49, 95% CI, 0.24-0.99; P = 0.046). Previous or concomitant bony realignment was also associated with a significant decrease in the rate of salvage surgery following OCA transplantation (aOR = 0.24, 95% CI, 0.04-0.75; P = 0.046). There was no relationship between sex, BMI, ECI score, or specific diagnoses like tobacco use, diabetes, hypertension, lung disease, hypovitaminosis D (P > 0.05 for each).
Table 2.
Risk Factors for Undergoing Subsequent Surgery Following an Osteochondral Allograft.
| Risk Factor | N, Total | N, Subsequent (%) | aOR (95% CI) | P Value |
|---|---|---|---|---|
| Age, years, mean (SD) | ||||
| 20-29 | 1,598 | 15 (0.94) | 0.49 (0.24-0.99) | 0.046 |
| 30-39 | 1,991 | 29 (1.46) | 0.74 (0.41-1.37) | 0.330 |
| 40-49 | 1,849 | 46 (2.49) | 1.25 (0.74-2.19) | 0.426 |
| 50-59 | 953 | 20 (2.10) | Reference | N/A |
| Surgical Approach | ||||
| Open | 3,950 | 72 (1.82) | 2.00 (0.59-5.10) | 0.197 |
| Arthroscopic | 2,589 | 42 (1.62) | Reference | N/A |
| Sex | ||||
| Male | 3,212 | 54 (1.68) | 0.99 (0.67-1.46) | 0.949 |
| Female | 3,174 | 56 (1.76) | Reference | N/A |
| BMI | ||||
| ≥25 | 778 | 13 (1.67) | 0.65 (0.26-1.26) | 0.215 |
| ≥40 | 288 | <11 (N/A) | 1.73 (0.60-4.79) | 0.293 |
| ECI score, mean (SD) | ||||
| 0 | 1,948 | 30 (1.54) | 0.77 (0.40-1.51) | 0.437 |
| 1-3 | 3,261 | 52 (1.59) | 0.73 (0.43-1.28) | 0.262 |
| >3 | 1,182 | 28 (2.37) | Reference | N/A |
| Bony Realignment | ||||
| Yes | 477 | <11 (N/A) | 0.24 (0.04-0.76) | 0.046 |
| No | 5,914 | N/A | Reference | N/A |
| Comorbidities | ||||
| Tobacco use | 907 | 16 (1.76) | 0.82 (0.45-1.42) | 0.508 |
| Diabetes | 410 | <11 (N/A) | 1.05 (0.47-2.08) | 0.927 |
| Lung disease | 1,123 | 15 (1.34) | 1.71 (0.91-3.01) | 0.076 |
| Hypertension | 505 | 26 (5.25) | 1.10 (0.63-1.85) | 0.735 |
| Hypovitaminosis D | 456 | <11 (N/A) | 1.00 (0.46-1.42) | 0.995 |
aOR = adjusted odds ratio; CI = confidence interval; BMI = body mass index; ECI = Elixhauser Comorbidity Index.
Bold font indicates statistical significance (P value of <0.05).
Discussion
In this analysis of the largest known OCA transplant cohort, 5-year rate of salvage surgery was 1.71%. Young age and bony realignment surgery were also identified as protective against risk of requiring salvage surgery. This rate of failure is much lower than previously reported in the literature, with the nearest being 8% as reported by Cook et al. and Sadr et al.18,20 These rates may be larger secondary to a limited inter-provider variability—the cases from Cook et al. were all performed by a single surgeon, and the cohort from Sadr et al. was gathered from a single clinic. In a recent database study, Burroughs et al. captured 1,631 patients that underwent OCA between 2010 to 2018 and determined the cumulative rate of secondary surgery to be 23.9%. 32 Our present analysis captured a larger sample of patients compared to this study, likely secondary to utilization of the larger M151Ortho data set which is the most comprehensive data set provided by PearlDiver. Sampling error is a function of sample size; therefore, the large nationwide scope of our results may be more representative of the population as a whole and suggests a promisingly lower rate of failure of the OCA transplant. Furthermore, we excluded patients who had previous underwent cartilage-related surgery, in an attempt to represent the risk of OCA failure for all-comers. Prior research has determined a significant relationship between reoperation after OCA and number of prior ipsilateral knee surgeries. 12 This may explain the discrepancy between the low rate of reoperation, or failure, in this study as compared to the existing literature. It also suggests that OCA may be a better option for patients without surgical history in the operative knee.
Previous studies on the topic have reported outcomes of OCA transplant in the knee using an assortment of metrics including PROMs, graft incorporation via imaging evaluation, gross appearance of failure with second-look arthroscopy, or removal/revision of the allograft. This lack of standardization in outcomes reporting is not clinically useful, as it prevents effective comparison between patient cohorts and clear communication of risk to patients; evidence of this issue is the wide range of reported OCA graft “failure” rates, from 8% to 43%.12,16,18 -29 As posited by Gillinov et al. in their analysis of ACI, another cartilage restoration procedure, the use of salvage surgery as a failure metric can provide immense clarity regarding graft durability. 30 Other studies have reported rates of reoperation following OCA, but are inadequate due to small cohort size, single surgeon series, or noncomprehensive inclusion of secondary surgery.12,16,35 The lack of standardized language to describe OCA outcomes is problematic, and possibly detrimental to patient-centered communication; patients may identify any reoperation as a relative failure, due to the large burden associated with orthopedic surgery and recovery.
This study reports a 5-year operation-free survival of 98.3%. This 5-year graft survival rate is higher than others reported in the literature; Burroughs et al. report a 88% 5-year survival, and a systematic review including 1,036 patients from Familiari et al. report 86.7%.20,35 These short- to mid-term outcomes are very promising; however, it should be noted that due to the database nature of this study, we were not able to differentiate between initial osteochondral defect pathologies (i.e., location, size, focality) and treatment characteristics (i.e. freshness, storage, technique), which have been shown to adversely affect survival rates. 20 In addition, graft durability in this study should be understood merely as a proxy for important variables such as functionality and patient satisfaction, which were unable to be assessed. When we broke down the results by postoperative year, the first 2 years accounted for 68.8% of all salvage surgeries in the 5-year period assessed. Rate of salvage then decreased stepwise according to postoperative year. A similar trend was reported by Burroughs et al. and suggests that failure of OCA transplants is identified early. 35 Reported failure mechanisms specific to OCA are commonly related to transplant integrity or bony incorporation and include graft fracture, subchondral bone collapse, subsidence, and cartilaginous delamination.36,37 Bone healing, and thus incorporation of an OCA, occurs in three phases—inflammation, repair, and remodeling—triggered by an initial immune response; successful transplant requires the coordination of a multifaceted healing immune response, prolonged to approximately 9 to 12 months due to the introduction of foreign material.27,36 OCAs are incorporated through “creeping substitution,” through which allograft is gradually repopulated by viable recipient cells, with an appropriate balance of osteoclastic and osteoblastic activity. 36 Stannard et al. similarly reported that a majority of failures occurred in postoperative year 1; interestingly, there was a large overlap (46%) in their study between failures and patient noncompliance, resulting in mechanical failure. 27 The authors suggest that the greatest risk for noncompliance occurs in conjunction with the reported period of initial pain relief from the procedure itself, between 6 weeks and 6 months; this is far before the gradual process of graft integration is complete, suggesting that pre-emptive return to activity results in the high failure rates of OCA in the early postoperative years.
The effect of age on OCA transplants is controversial, with literature reporting both older or younger age as risk factors.12,17,23,24,28 This study identified that age between 20 and 29 was associated with decreased odds of salvage surgery. Although the mechanism remains unclear, the known relationship between increasing age and loss of healing capacity, cellular degeneration, and immunoprotection intuitively supports the notion that there is increased risk with increased age. 24 Wang et al.’s study demonstrates a marked decrease in allograft survivorship in patients aged 40 or older. 23 Kunze et al. also conclude that age is an additional important risk factor for failure after OCA, in addition to higher BMI. 28 Although the aforementioned studies report a similar trend, an important distinction must be made between their results and that of this study—young age was associated with significantly decreased odds of requiring salvage procedures, but no significant odds were identified for older age cohorts. Based on our results, patients in the third decade of life may be good candidates for OCA transplantation in the knee, but more research must be done to further characterize risk profile for older patients. Overall, our study adds evidence to the discussion on best indications for OCA per age, but due to the current heterogeneity of data, clinical decision-making must take into account patient-specific goals and weigh the risk of subsequent surgery with the potential for functional improvement.
Prior or concomitant bony realignment was also identified as a protective factor against the risk of salvage surgery following OCA transplantation. Realignment osteotomy is often indicated as a concomitant or staged procedure with OCA transplantation, in patients in which the weight-bearing axis falls through the cartilage defect. 38 High tibial osteotomy or lateral distal femoral osteotomy, for varus and valgus malalignment, respectively, act to decrease the load on the graft and effectively increase survival. 39 Tibial tubercle osteotomy similarly can transfer the load from the damaged patellofemoral chondral surface to a healthier area, in patients with patellar maltracking. 38 A recent systematic review from Kunze et al. reported that concomitant procedures—such as realignment osteotomy, or meniscus transplant—at the time of OCA were not associated with graft failure. 28 Our results take this one step further, suggesting that patients having undergone bony realignment prior or with current indications for realignment may be better candidates for OCA with longer graft durability. Of note, we have no information on the coronal plane alignment of the patients in our study, limiting the impact of our findings related to associated osteotomies.
As with all retrospective analyses of insurance claims data sets, this study is not without its limitations. First and foremost, the interpretation of the results relies on accurate coding and is subject to potential sampling bias. Fortunately, this study primarily relies on CPT codes to identify the sample population and outcome of interest, as opposed to International Classification of Diseases (ICD) codes, which provides more certainty in their accuracy. However, due to reliance on CPT codes, we were unable to confirm or report patient or procedure-specific details such as injury severity, activity demand, laterality, graft site, surgical technique, size/preparation of the allograft, and perioperative or intraoperative complications. There is a small chance that some salvage procedures were not captured by the included set of CPT codes; however, our best attempt was made to gather all salvage procedures based on current clinic recommendations for revision after failed cartilage restoration. Second, given the use of billing codes, there is no way to determine postoperative course via adherence to postoperative recommendations or PROMs, both of which likely influence the rates of allograft failure and salvage surgery. Similarly, we are unable to elucidate patient-specific indications for subsequent surgeries. Similar to the limitations described by Gillinov et al., there was a large portion of patients without the full 5-year follow-up, which may change the true rate of allograft failure. 30 However, the used Kaplan-Meier function censors those patients without the full 5-year follow-up which provides a more accurate estimate of the true rate of allograft failure over the initial 5-year postoperative period. Finally, further research is needed to address the reoperation risk in other populations considering OCA for cartilage defects, namely those with more extensive knee surgical history.
Conclusion
In an analysis of 6,391 patients who underwent OCA transplant, less than 2% of patients required a failure-defining articular cartilage salvage surgery. Most subsequent surgeries occurred in the first two postoperative years. Young age and concomitant or prior bony realignment procedures were protective factors against graft failure. These findings suggest that OCA in the knee is a durable cartilage replacement procedure, especially in young patients with corrected bony malalignment. The use of salvage surgery as a utilitarian failure metric can clarify success of cartilage procedures and should be interpreted alongside other outcome metrics for optimal clinical decision-making.
Footnotes
Acknowledgment and Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: COI/disclosure forms previously submitted. Author Brett Owens discloses the following potential COI: consulting for Conmed, Mitek, Vericel Corp, Miach, Linvatec, DePuy Synthes Products, Musculoskeletal Transplant Foundation, Medical Device Business Services; royalties Conmed, Linvatec; stock options Vivorte; honoraria Vericel.
Ethical Approval: Not applicable.
ORCID iD: Rory A. Byrne  https://orcid.org/0000-0002-0099-9016
https://orcid.org/0000-0002-0099-9016
References
- 1. Hjelle K, Solheim E, Strand T, Muri R, Brittberg M. Articular cartilage defects in 1,000 knee arthroscopies. Arthroscopy. 2002;18(7):730-4. doi: 10.1053/jars.2002.32839 [DOI] [PubMed] [Google Scholar]
- 2. Arøen A, Løken S, Heir S, Alvik E, Ekeland A, Granlund OG, et al. Articular cartilage lesions in 993 consecutive knee arthroscopies. Am J Sports Med. 2004;32(1):211-5. doi: 10.1177/0363546503259345 [DOI] [PubMed] [Google Scholar]
- 3. Curl WW, Krome J, Gordon ES, Rushing J, Smith BP, Poehling GG. Cartilage injuries: a review of 31,516 knee arthroscopies. Arthroscopy. 1997;13(4):456-60. doi: 10.1016/S0749-8063(97)90124-9 [DOI] [PubMed] [Google Scholar]
- 4. Widuchowski W, Widuchowski J, Trzaska T. Articular cartilage defects: study of 25,124 knee arthroscopies. Knee. 2007;14(3):177-82. doi: 10.1016/j.knee.2007.02.001 [DOI] [PubMed] [Google Scholar]
- 5. Buckwalter JA. Articular cartilage: injuries and potential for healing. J Orthop Sports Phys Ther. 1998;28(4):192-202. doi: 10.2519/jospt.1998.28.4.192 [DOI] [PubMed] [Google Scholar]
- 6. Roseti L, Grigolo B. Current concepts and perspectives for articular cartilage regeneration. J Exp Orthop. 2022;9:61. doi: 10.1186/s40634-022-00498-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. McCormick F, Harris JD, Abrams GD, Frank R, Gupta A, Hussey K, et al. Trends in the surgical treatment of articular cartilage lesions in the United States: an analysis of a large private-payer database over a period of 8 years. Arthroscopy. 2014;30(2):222-6. doi: 10.1016/j.arthro.2013.11.001 [DOI] [PubMed] [Google Scholar]
- 8. Mundi R, Bedi A, Chow L, Crouch S, Simunovic N, Sibilsky Enselman E, et al. Cartilage restoration of the knee: a systematic review and meta-analysis of level 1 studies. Am J Sports Med. 2016;44(7):1888-95. doi: 10.1177/0363546515589167 [DOI] [PubMed] [Google Scholar]
- 9. DeFroda SF, Bokshan SL, Yang DS, Daniels AH, Owens BD. Trends in the surgical treatment of articular cartilage lesions in the United States from 2007 to 2016. J Knee Surg. 2021;34(14):1609-16. doi: 10.1055/s-0040-1712946 [DOI] [PubMed] [Google Scholar]
- 10. Frank RM, Lee S, Levy D, Poland S, Smith M, Scalise N, et al. Osteochondral allograft transplantation of the knee: analysis of failures at 5 years. Am J Sports Med. 2017;45(4):864-74. doi: 10.1177/0363546516676072 [DOI] [PubMed] [Google Scholar]
- 11. Richter DL, Schenck RC, Jr, Wascher DC, Treme G. Knee articular cartilage repair and restoration techniques. Sports Health. 2016;8(2):153-60. doi: 10.1177/1941738115611350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chahla J, Stone J, Mandelbaum BR. How to manage cartilage injuries? Arthroscopy. 2019;35(10):2771-3. doi: 10.1016/j.arthro.2019.08.021 [DOI] [PubMed] [Google Scholar]
- 13. Nikolaou VS, Giannoudis PV. History of osteochondral allograft transplantation. Injury. 2017;48(7):1283-6. doi: 10.1016/j.injury.2017.05.005 [DOI] [PubMed] [Google Scholar]
- 14. Chui K, Jeys L, Snow M. Knee salvage procedures: the indications, techniques and outcomes of large osteochondral allografts. World J Orthop. 2015;6(3):340-50. doi: 10.5312/wjo.v6.i3.340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gilat R, Haunschild ED, Huddleston HP, Tauro TM, Patel S, Wolfson TS, et al. Osteochondral allograft transplant for focal cartilage defects of the femoral condyles: clinically significant outcomes, failures, and survival at a minimum 5-year follow-up. Am J Sports Med. 2021;49(2):467-75. doi: 10.1177/0363546520980087 [DOI] [PubMed] [Google Scholar]
- 16. Anderson DE, Robinson KS, Wiedrick J, Crawford DC. Efficacy of fresh osteochondral allograft transplantation in the knee for adults 40 years and older. Orthop J Sports Med. 2018;6(11). doi: 10.1177/2325967118805441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Assenmacher AT, Pareek A, Reardon PJ, Macalena JA, Stuart MJ, Krych AJ. Long-term outcomes after osteochondral allograft: a systematic review at long-term follow-up of 12.3 years. Arthroscopy. 2016;32(10):2160-8. doi: 10.1016/j.arthro.2016.04.020 [DOI] [PubMed] [Google Scholar]
- 18. Cook JL, Rucinski K, Crecelius C, Fenkell B, Stannard JP. Assessment of outcomes after multisurface osteochondral allograft transplantations in the knee. Orthop J Sports Med. 2022;10(6). doi: 10.1177/23259671221102452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Familiari F, Cinque ME, Chahla J, Godin JA, Olesen ML, Moatshe G, et al. Clinical outcomes and failure rates of osteochondral allograft transplantation in the knee: a systematic review. Am J Sports Med. 2018;46(14):3541-9. doi: 10.1177/0363546517732531 [DOI] [PubMed] [Google Scholar]
- 20. Sadr KN, Pulido PA, McCauley JC, Bugbee WD. Osteochondral allograft transplantation in patients with osteochondritis dissecans of the knee. Am J Sports Med. 2016;44(11):2870-5. doi: 10.1177/0363546516657526 [DOI] [PubMed] [Google Scholar]
- 21. Chahla J, Sweet MC, Okoroha KR, Nwachukwu BU, Hinckel B, Farr J, et al. Osteochondral allograft transplantation in the patellofemoral joint: a systematic review. Am J Sports Med. 2019;47(12):3009-18. doi: 10.1177/0363546518814236 [DOI] [PubMed] [Google Scholar]
- 22. Wang D, Kalia V, Eliasberg CD, Wang T, Coxe FR, Pais MD, et al. Osteochondral allograft transplantation of the knee in patients aged 40 years and older. Am J Sports Med. 2018;46(3):581-9. doi: 10.1177/0363546517741465 [DOI] [PubMed] [Google Scholar]
- 23. Wang D, Rebolledo BJ, Dare DM, Pais MD, Cohn MR, Jones KJ, et al. Osteochondral allograft transplantation of the knee in patients with an elevated body mass index. Cartilage. 2019;10(2):214-21. doi: 10.1177/1947603518754630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Thomas D, Shaw KA, Waterman BR. Outcomes after fresh osteochondral allograft transplantation for medium to large chondral defects of the knee. Orthop J Sports Med. 2019;7(3). doi: 10.1177/2325967119832299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chahal J, Gross AE, Gross C, Mall N, Dwyer T, Chahal A, et al. Outcomes of osteochondral allograft transplantation in the knee. Arthrosc J Arthrosc Relat Surg. 2013;29(3):575-88. doi: 10.1016/j.arthro.2012.12.002 [DOI] [PubMed] [Google Scholar]
- 26. Stannard JP, Cook JL. Prospective assessment of outcomes after primary unipolar, multisurface, and bipolar osteochondral allograft transplantations in the knee: a comparison of 2 preservation methods. Am J Sports Med. 2020;48(6):1356-64. doi: 10.1177/0363546520907101 [DOI] [PubMed] [Google Scholar]
- 27. Kunze KN, Ramkumar PN, Manzi JE, Wright-Chisem J, Nwachukwu BU, Williams RJ. Risk factors for failure after osteochondral allograft transplantation of the knee: a systematic review and exploratory meta-analysis. Am J Sports Med. 2023;51:1356-67. doi: 10.1177/03635465211063901 [DOI] [PubMed] [Google Scholar]
- 28. Briggs DT, Sadr KN, Pulido PA, Bugbee WD. The use of osteochondral allograft transplantation for primary treatment of cartilage lesions in the knee. Cartilage. 2015;6(4):203-7. doi: 10.1177/1947603515595072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gillinov SM, Fosam A, Burroughs PJ, Schneble CA, McLaughlin WM, Moran J, et al. Incidence, timing, and risk factors for 5-year revision surgery after autologous chondrocyte implantation in 533 patients. Am J Sports Med. 2022;50(11):2893-9. doi: 10.1177/03635465221111115 [DOI] [PubMed] [Google Scholar]
- 30. Nawaz SZ, Bentley G, Briggs TWR, Carrington RWJ, Skinner JA, Gallagher KR, et al. Autologous chondrocyte implantation in the knee: mid-term to long-term results. J Bone Jt Surg. 2014;96(10):824-30. doi: 10.2106/JBJS.L.01695 [DOI] [PubMed] [Google Scholar]
- 31. Krishnan SP, Skinner JA, Bartlett W, Carrington RW, Flanagan AM, Briggs TW, et al. Who is the ideal candidate for autologous chondrocyte implantation? J Bone Joint Surg Br. 2006;88(1):61-4. doi: 10.1302/0301-620X.88B1.16796 [DOI] [PubMed] [Google Scholar]
- 32. Burroughs PJ, Kahan JB, Moran J, Gillinov SM, Joo PY, Schneble CA, et al. Subsequent surgery up to 10 years after osteochondral allograft and osteochondral autograft: an analysis of more than 2000 patients. Orthop J Sports Med. 2022;10(12). doi: 10.1177/23259671221139127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Luk J, Stoker AM, Teixeiro E, Kuroki K, Schreiner AJ, Stannard JP, et al. Systematic review of osteochondral allograft transplant immunology: how we can further optimize outcomes. J Knee Surg. 2021;34(1):30-8. doi: 10.1055/s-0040-1721670 [DOI] [PubMed] [Google Scholar]
- 34. Tschon M, Veronesi F, Giannini S, Fini M. Fresh osteochondral allotransplants: outcomes, failures and future developments. Injury. 2017;48(7):1287-95. doi: 10.1016/j.injury.2017.05.006 [DOI] [PubMed] [Google Scholar]
- 35. Frank RM, Cotter EJ, Lee S, Poland S, Cole BJ. Do outcomes of osteochondral allograft transplantation differ based on age and sex? a comparative matched group analysis. Am J Sports Med. 2018;46(1):181-91. doi: 10.1177/0363546517739625 [DOI] [PubMed] [Google Scholar]
- 36. Zarkadis NJ, Kusnezov NA, Garcia EJ, Pallis MP, Waterman BR. Return to preoperative function after autologous cartilage implantation of the knee in active military servicemembers. Orthop J Sports Med. 2017;5(5). doi: 10.1177/2325967117706057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sherman SL, Garrity J, Bauer K, Cook J, Stannard J, Bugbee W. Fresh osteochondral allograft transplantation for the knee: current concepts. J Am Acad Orthop Surg. 2014;22(2):121-33. doi: 10.5435/JAAOS-22-03-199 [DOI] [PubMed] [Google Scholar]
- 38. Gross AE, Shasha N, Aubin P. Long-term followup of the use of fresh osteochondral allografts for posttraumatic knee defects. Clin Orthop. 2005;435:79-87. doi: 10.1097/01.blo.0000165845.21735.05 [DOI] [PubMed] [Google Scholar]
- 39. Patel RM, Wright-Chisem J, Williams RJ. Anteriorizing tibial tubercle osteotomy for patellofemoral cartilage lesions. Arthrosc Tech. 2021;10(9):e2181-7. doi: 10.1016/j.eats.2021.05.021 [DOI] [PMC free article] [PubMed] [Google Scholar]


