Abstract
Introduction
There are many intra-articular hyaluronic acid (IA-HA) products on the market that have known intrinsic differences in molecular size, source, and structure. The current review summarizes existing evidence describing and assessing these differences, while also identifying whether these differences have an impact on clinical outcomes.
Methods
This systematic review summarized all literature that specifically addresses IA-HA product differences. Included studies summarized basic science and mechanism of action comparisons of IA-HA product differences, or systematic reviews that assess differences in clinical outcomes between IA-HA product differences.
Results
A total of 20 investigations assessed basic science differences between IA-HA products, while 20 investigations provided assessments of the clinical outcome differences between IA-HA product characteristics. The published basic science literature provided a differentiation between low molecular weight (LMW) and high molecular weight (HMW) HA with regard to changes within the synovial fluid, driven by the interactions that these molecules have with receptors in the joint space. These differences in receptor interaction manifest within clinical outcomes, as meta-analyses comparing pain relief after IA-HA suggest that pain reduction is superior in patients who receive HMW HA as opposed to LMW HA.
Conclusion
This review highlights differences between IA-HA characteristics, and how important the molecular weight, derivation of the product, and structure are to variances in reported clinical outcomes to treat osteoarthritis (OA) of the knee. HMW IA-HAs have shown greater efficacy compared to the alternative of LMW products, while avian-derived and cross-linked products have potentially demonstrated an increase in inflammatory events over non-avian-derived, non-cross-linked HAs.
Keywords: knee, joint involved, osteoarthritis, diagnosis, hyaluronic acid
Introduction
Hyaluronic acid (HA) is a main component of the synovial fluid that provides viscosity and elasticity within the joint space. Endogenous HA has rheological properties that allow it to withstand long-term loading while recovering to its original state after the loading force is removed. 1 The progression of knee osteoarthritis (OA) is a result of the reduction of HA molecular weight (MW) distribution, average MW, and concentration of HA within the synovial fluid. 1 This degradation of the synovial fluid results in joint pain, disability, and increased wear on the articulating cartilage of the knee joint. 2 The disability caused by knee OA creates a significant socioeconomic burden by decreasing patient quality of life, causing loss of workability, and further progression of disease that requires surgical management. 3
The large impact of knee OA has resulted in a multitude of non-operative treatment options that aim to manage the symptoms of this disease.4,5 Due to the direct association between HA degradation and knee OA progression, a common treatment option in both clinical practice and the scientific literature is the use of intra-articular injection of HA (IA-HA). IA-HA treatment aims to replenish the synovial joint space with exogenous HA in order to counteract the reduction of HA concentration and distribution that occurs as a result of knee OA disease progression. 2 The use of IA-HA as a treatment for knee OA has demonstrated significant reductions in the symptoms caused by knee OA, as well as a beneficial impact on the time before a patient requires a total knee arthroplasty (TKA).4 -7 As a result of the beneficial clinical outcomes of IA-HA, recent guidelines conditionally recommend the use of this therapy in knee OA patients who have not observed a therapeutic response to conservative treatment options, such as non-steroidal anti-inflammatory drugs (NSAIDs). 8
The HA primary receptor is CD44, while it also interacts with RHAMM, LYVE-1, ICAM-1, and TLRs.2,9 HA-CD44 binding has numerous downstream effects that aim to regulate chondroprotective, anti-inflammatory, subchondral, and proteoglycan synthesis. 2 HA-CD44 binding reduces interleukin (IL)-1B expression within the joint, which decreases matrix metalloproteinase (MMP) development. The effect of this MMP reduction results in less catabolism of cartilage within the knee joint. IA-HA receptor binding also enhances proteoglycan and glycosaminoglycan synthesis within the knee joint, providing protection of the cartilage through proliferation of intrinsic HA development.2,10 Prior publications have provided a comprehensive overview of the signaling pathways and evidence of HA’s mechanism of action,2,11,12 yet a link between this mechanism of action and the clinical outcomes of IA-HA is complicated by numerous intrinsic differences between IA-HA products. 11
Although the beneficial effects of IA-HA have been well documented, evidence has demonstrated that not all IA-HA products elicit the same response. There are a number of important differences in the size, source, and structure of IA-HA products that have implications on their treatment effects. 11 These key differences in IA-HA products include the MW, the presence of cross-linking (CL), and the HA source used to create the IA-HA product.1,6,11 IA-HA products are produced at a wide range of MW, with recent investigations generally separating products into categories of HMW and LMW (LMW).4,6 The process of CL modifies the natural straight-chain structure of the molecule, creating an entangled HA molecule. 11 IA-HA products are manufactured using 1 of 2 processes—using an avian HA source or creating HA through biological fermentation. 11 Despite these differences, guideline developers, formularies, payers, and healthcare professionals continue to assess IA-HA as a class.
The clinical effectiveness of patient-reported outcomes (PROs) is commonly assessed in relation to the minimal clinically important difference (MCID). The MCID is a benchmark for the smallest change in a clinical outcome that would be perceived as beneficial (or harmful) by a patient.13,14 There are many investigations that have determined the MCID for various PROs, including outcomes for patients with knee OA. 14 Understanding the clinical effects of treatments in relation to the MCID is important to better understand if a treatment provides an effect that is not only statistically significant but would actually be important to the patient.13,14 A recent investigation of knee OA injectables determined that HMW HA, but not LMW HA, surpassed the MCID for pain in knee OA patients. 4 This provides an important message—that the differences in size, structure, and source of IA-HA directly contribute to clinically important differences between IA-HA products.
In order to better understand these IA-HA intrinsic product differences and their impacts on clinical outcomes, this study provides a thorough assessment of the literature on these product differences. The current review aimed to summarize all available basic science and mechanism of action evidence assessing the differences that result due to IA-HA product differences, as well as all published evidence synthesis investigations that assessed the efficacy and safety differences caused by these product differences.
Methods
This study conducted a systematic literature review of all evidence that specifically addressed the 3 aforementioned IA-HA product differences. This project followed the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines for systematic reviews, as well as the guidance from the Cochrane handbook for systematic reviews.
Eligibility Criteria
Included studies summarized basic science and mechanism of action comparisons of IA-HA product differences, or systematic reviews that assess differences in clinical outcomes between IA-HA product differences. Studies were excluded if they did not specifically assess intrinsic differences between IA-HA products in a basic science/mechanism of action investigation, or if they were a primary investigation of product differences in clinical outcomes. Only evidence synthesis investigations on product differences were included.
Information Sources and Search Strategy
Relevant literature was retrieved through a systematic search of the Medline, Embase, and Web of Science databases. The search strategy is included in Supplemental Appendix A, which was conducted from database inception to December 10, 2020.
Study Selection
Two independent reviewers assessed the retrieved studies for eligibility in duplicate. Articles were assessed in 2 phases: the title/abstract phase as well as the full-text phase. Any discrepancies were resolved through a consensus meeting. Article screening was conducted using Covidence software (Covidence.org, Melbourne, Australia).
Data Collection Process
All included studies were summarized within a standardized data extraction form. All basic science/mechanism of action study results were summarized within the context of MW, CL, and HA source assessments, while conclusions on clinical outcomes for these product differences were captured and summarized from all included reviews.
Results
Study Selection
The literature search retrieved 1,437 articles. After eligibility review, 379 articles were assessed at the full-text stage. Of these, a total of 20 basic science investigations assessed differences between HA products,10,15 -33 while 20 systematic reviews provided assessments of HA product differences.2,4,6,9,11,12,34 -47 Figure 1 provides an overview of the study screening process. The results of this investigation have summarized the literature pertaining to HA characteristics that differ across available products.
Figure 1.
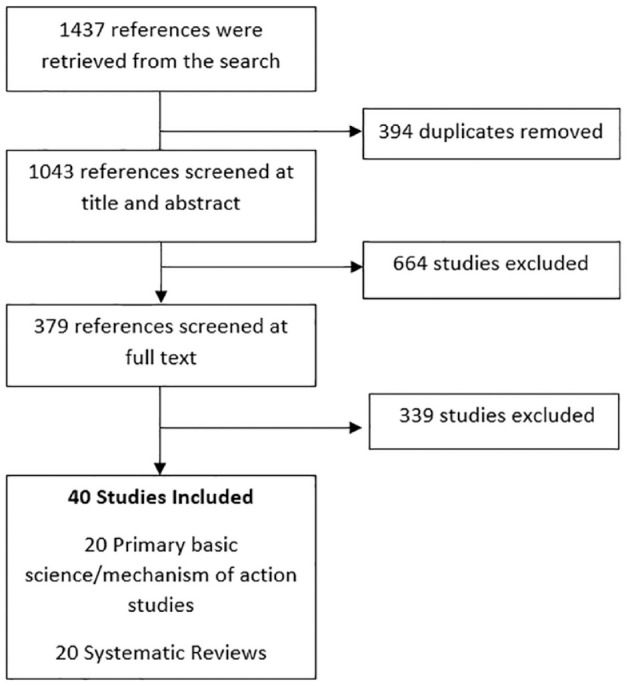
Article screening process.
Overview of Studies
The most researched product difference was MW (30 studies), while CL (9 studies) and HA source (6 studies) investigations were less common and more recent topics of investigation. The concept of IA-HA product differences is a new field of investigation, as 24 (60.0%) of the included studies were published within the last 10 years. Figure 2 shows the yearly trend in publications on IA-HA product differences. The remainder of the results summarize the main findings of the available literature on MW, CL, and manufacturing process differences between IA-HA products.
Figure 2.
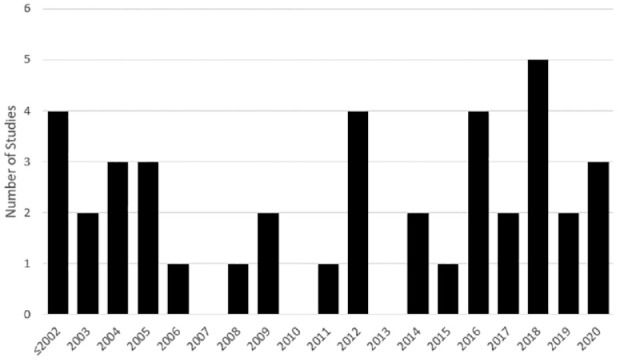
Histogram of IA-HA (intra-articular hyaluronic acid) product difference publications per year.
Molecular Weight
MW basic science evidence
The published basic science literature provided a differentiation between LMW and HMW HA with regard to changes within the synovial fluid, driven by the interactions that these molecules have with receptors in the joint space. 11 Chondroprotective effects have been demonstrated to be MW dependent, with protection of the articular cartilage and chondroregulatory processes benefiting from HMW HA.20,26,29,30 HA molecules interact with RHAMM, LYVE-1, ICAM-1, and TLR receptors, but have a strong affinity for the CD44 receptor. The primary interaction with CD44 receptors elicits the biological mechanisms of action within the synovial fluid, and HMW HA has demonstrated greater affinity to this receptor.2,15 This receptor binding is responsible for numerous downstream effects that combat the symptoms of knee OA. 2 These downstream effects are primarily driven by the reduction of IL-1B and MMPs, while other ILs, such as IL-6 and IL-8, and inflammatory mediators, PGE2 and TNFa, can be suppressed through HA-CD44 binding. 2 The reduction in key mediators, such as IL-1B and MMP, from HA-CD44 binding has been shown to be directly correlated with MW; with HMW HA providing a stronger response. This HA-CD44 MW-dependent affinity has also shown direct implications in the endogenous HA production and cytokine regulation that occurs within the synovium, as LMW HA showed minimal to no stimulation of endogenous HA production, while HMW HA stimulated HA synthesis in a concentration-dependent manner. 10
The HA-CD44 binding also provides an anti-inflammatory response within the joint space through its reduction in IL-1B, MMP, IL-6, IL-8, PGE2, and TNFa expression. 2 HMW HA products have been shown to decrease the pro-inflammatory environment within the synovial fluid to a greater extent than their LMW HA counterparts, as the reductions of these inflammatory mediators appear to be MW-dependent.2,12,16 Binding to HMW HA decreases activation of the CD44 receptor and can lead to inactivation of the inflammatory TLR cascade, causing a reduction of cytokine gene expression, whereas LMW HA can lead to a pro-inflammatory response and greater cytokine expression that contribute to symptoms of knee OA. 12 The inflammatory cascade and presence of inflammatory mediators from smaller HA fragments also increase the inflammatory activity of macrophages within the joint. As larger, HMW HA molecules span across many CD44 receptors, the TLR2 and TLR4 inflammatory cascade is inactivated, while the smaller LMW HA molecules binding to a smaller number of CD44 receptors can create the opposite effect.2,12
The rheologic properties of HMW HA are also more consistent with those of healthy human HA when compared to LMW HA, which better mimics the lubricating environment of the healthy knee joint. 28 The properties that are correlated with the MW of HA include a higher viscosity, a zero sheer rate viscosity, and a characteristic crossover frequency.19,21,23,24,28 LMW HA products have been shown to have similar rheological properties as saline, while HMW HA (non-CL) provides a more robust shear thinning and rapid relaxation time—meaning these products are able to quickly return to their original state after forces from movements like walking are exerted on the molecules.1,28 The greater elastoviscosity of HMW HA products has also been shown to better protect pain-eliciting nerve endings directly. 16
The available basic science evidence on the differences between HMW and LMW HA suggest that the synovial fluid changes caused by HA are not the same for HA products of different MW.20,26,29,30 While HA has an affinity to important inflammatory and chondroprotective mediators, HMW HA molecules appear to provide important and cascading effects to improve the inflammatory and chondroprotective environment within the synovium.2,12 This, however, cannot be said about smaller, LMW HA fragments—as these LMW HA molecules can trigger a further inflammatory response and a lack of important chondroprotective effects.2,12
MW clinical outcome evidence
The aforementioned basic science literature provides a biological basis for differences between HMW and LMW HA products. It is important to consider if these differences translate into realized differences in clinical outcomes. Earlier reviews stated inconsistent conclusions and uncertainty on the differences between MW clinical effectiveness, as there was not a clear difference in pain relief between LMW and HMW HA within published trials.9,35,36,39,40 As the body of evidence on IA-HA treatment for knee OA grew, more recent reviews have demonstrated a clearer distinction between HMW and LMW IA-HA products with regard to pain reduction.4,6,42,44
In an analysis of all knee OA injectable treatments at 3- to 6-month follow-up, HMW HA was the only treatment to surpass the MCID for pain. 4 Not only did HMW HA surpass the MCID while LMW HA did not, no other treatment option for knee OA met this threshold. 4 This result was also seen in an analysis of the clinical importance of knee OA treatment pain relief. 47 A thorough analysis of knee OA treatment effects in relation the MCID yielded results that suggest HMW HA products provide a clinically important improvement in pain, while LMW HA products do not. These effects were further strengthened when the intra-articular placebo effect was accounted for within the analysis. 47
Cross-Linking
Cross-linking basic science evidence
The process of CL changes the structure of the HA chain, which increases the viscosity, but may alter the way in which HA binds to receptors.23,25 CL-HA differs in its HA-CD44 interaction, as only the soluble (untangled) portion of the CL HA products is capable of interacting with the CD44 receptor. 25 Due to this, there would be fewer available HA-CD44 interactions than a comparably sized, straight chain, HMW HA molecule. CL also alters the rheologic properties of HA to a gel-like state, which may increase joint residence time, but differs from healthy human HA.28,38 HMW CL HA has been shown to have superior friction coefficients than LMW non-CL counterparts, although it is uncertain how much of this effect is directly attributable to the CL process, opposed to the HMW of the products tested. 25 While CL HA products demonstrate gel-like rheologic properties and increased joint residence time, the direct implications of this with regard to the chondro-regulatory implications of CD44 binding and the TLR cascade are not documented.
CL HA may increase local inflammation, and also may exhibit a greater percentage of eosinophils within the joint space. 33 These findings were not seen within native, non-CL HA. 33 Another study confirmed the immunostimulatory adverse effects associated with CL, as only the CL product tested exhibited these effects. 32 Within this study, the CL HA tested exhibited a marked inflammatory reaction compared to non-CL HA, causing a significant increase in monocyte and heterophil accumulation within the synovial fluid. This reaction was hypothesized to be a result of a much higher (1 → 3)-β-d-glucan content of the CL HA product, or the unique molecular structure created by the CL process. 32 This study also tested avian-derived non-CL HA and biologically derived non-CL HA, which both did not exhibit these immunostimulatory effects. 32 This suggests that the effects were attributable to the CL nature of the tested IA-HA. The longer residence time of CL products has, however, been suggested to increase the length of nociceptive effects of CL products through a longer residence time within the synovium. 22
Cross-linking clinical outcome evidence
The basic scientific evidence suggests that there is a potential for greater inflammatory response with CL HA products, yet this has not been directly assessed clinically. CL has been associated with a higher incidence of local acute inflammatory reactions, although other reviews have attributed these reactions to the avian source of most CL products.11,37 While there is a basic science rationale for both CL and avian HA to result in increased local inflammatory reactions, no review clearly differentiates which of these characteristics is the primary cause of these reactions. Despite the lack of clinical evidence differentiating the exact cause of these inflammatory events, it is possible, based on the basic science evidence reviewed, that CL contributes to these observed inflammatory events. 32 One review provided a meta-analysis of pain effects between CL versus non-CL HA products. 41 This review concluded that CL products may provide greater pain relief. Despite these findings, the majority of the CL products are HMW, while the non-CL products were LMW. This makes it unclear as to whether the clinical benefits were truly due to the CL process, or if they were a result of the HMW of the HA. 41 There is no evidence that assesses clinical outcomes between CL and non-CL HA products of similar MW, which makes the distinction between CL and non-CL products difficult from a symptom relief perspective.
Manufacturing Source
Manufacturing source basic science evidence
Evidence has highlighted the potential for immunostimulatory activity with the use of avian-derived HA, which was not seen with biologically derived HA. 32 Biologically derived HA has been reported to have an advantageous safety profile as a result of minimal impurities in the product, whereas avian HA may contain such impurities.2,11 The impurities observed within avian-derived HA were primarily (1 → 3)-β-d-glucan, which may be attributable to local flare-up reactions in avian-derived products.11,32 There have also been reports of severe acute inflammatory reactions occurring within avian HA as a result of the reaction to chicken proteins found within the avian HA product. 48 These impurities may contribute to suboptimal, albeit rare, adverse events that are a result of the manufacturing process of the HA product. 48
Manufacturing source clinical outcome evidence
Avian HA studies report a greater number of adverse events, including effusions and acute inflammatory reactions, than biologically derived HA products. 11 One investigation noted that avian HA products that were not CL had similar safety profiles as biologically derived HA, suggesting that the presence of CL may be a larger contributor to the inflammatory events seen after IA-HA treatment with certain products. 32 These results do not provide a clear differentiation between CL and avian characteristics as the driving factor behind the observed inflammatory and adverse reactions seen in CL, avian-derived products. Regardless, it is of note that products with both of these characteristics have demonstrated an increase in adverse effects.11,32
Discussion
Implications of Results
It is clear that there are key differences between IA-HA products that warrant consideration when deciding the best treatment for each individualized patient. MW, CL structure, and HA source are 3 key factors that can impact outcomes. This thorough investigation assessing the available literature on IA-HA product differences provides a number of key considerations for clinicians, as well as policymakers. First, the presence of numerous factors that create differential treatment effects between IA-HA products makes it apparent that not all IA-HA products work in the same manner, both biologically and clinically. MW has been shown across numerous studies to have a direct impact on the cellular response through CD44 binding and toll-like receptor cascade inhibition within the knee joint, causing different biological responses with regard to the anti-inflammatory, chondroprotective, proteoglycan synthesis, analgesic, and subchondral effects.2,28 These effects are derived from the regulation of a number of key mediators, primarily driven by IL-1B and MMPs.
This evidence suggests that HMW HA elicits a more favorable response from a biological and rheological perspective, which is confirmed by the multitude of meta-analyses that have demonstrated superior clinical outcome results from HMW HA.4,6 It is important to note that, while some earlier reviews found no difference between HMW and LMW HA products, the difference has become more apparent in recent meta-analyses.4,43,44 In addition, time points earlier than 3 months after injection did not always demonstrate a clear distinction between MW, while time points of 3 to 6 months typically demonstrated a distinction between LMW and HMW HA products.4,42,43,49 This is an important difference between meta-analyses of HA treatments, as earlier time points tend to favor shorter-acting treatments like corticosteroid injection, while relatively longer follow-ups tend to favor IA-HA treatment.4,5,43,50
The use of CL in HA products also demonstrated differentiation between products. While the evidence of biological and rheological differences due to CL was well reported, there is limited evidence addressing the specific clinical outcomes associated with CL versus non-CL IA-HA products. This is largely due to confounding with MW, as many HMW HA investigations in the literature are also CL products. Little evidence exists comparing CL and non-CL products of the same MW, which makes it difficult to ascertain the clinical relevance of CL specifically. 41 Some reviews noted the potential for an increase in local reactions with the use of CL products, which was also a concern with avian-derived products.37,45
Cost-effectiveness Considerations
Evidence also exists for differences in cost-effectiveness between IA-HA products, as well as differences in the cost-effectiveness of IA-HA use in early/moderate knee OA versus later OA stages.51,52 This evidence provides a holistic overview of the differences that exist across IA-HA products, as the basic biological effects, clinical outcomes, and cost-effectiveness are not equivalent across the IA-HA class. Understanding the mechanisms of action, the timeframe of clinical effects, and differences between IA-HA products may also help clinicians develop robust multimodal care models for individual patients, as knee OA treatments can work together in a synergistic manner. One such synergy is the balance of short-term effects that IA corticosteroids provide, along with the longer-term effects that HMW HA provide.49,53
While differences in clinical outcomes were addressed within this article, the broader implications of these product differences are important to consider. With better symptom management, delaying the need for surgery, reduction in medication use, earlier return to work, and improved quality of life, the impacts of selecting the optimal IA-HA can expand to provide beneficial health and socioeconomic implications.3,7,51,54,55 Recent data from a large administrative database suggested that prior to TKA for knee OA, patients who were treated with IA-HA had drastically reduced medical costs than individuals who were not treated with IA-HA. 55 The potentially widespread implications from IA-HA use, particularly with a product that has beneficial product characteristics, stem far beyond pain relief, thus suggesting that future clinical decision-making and clinical practice guidelines should consider these product differences when evaluating the appropriate use of IA-HA for the treatment of knee OA.
Appropriate Patient Selection
This review highlighted the importance of appropriate HA selection, yet an equally important consideration is the appropriate patient for IA-HA treatment. Numerous studies have investigated the importance of patient characteristics in their prognosis following IA-HA treatment for knee OA, with consistent conclusions suggesting that IA-HA provides greater benefit to individuals who are earlier in their disease progression.52,56,57 These findings, in conjunction with optimal HA product selection, can maximize the likelihood of providing optimal outcomes for patients suffering from knee OA.
Future Directions
The evidence of IA-HA product differences has become apparent within the literature; however, key stakeholders and clinical practice guideline groups have yet to consistently account for these differences within their recommendations. The AAOS (American Academy of Orthopaedic Surgeons) knee OA guideline published in 2013 was one of the most impactful guidelines for knee OA treatment and insurance coverage decisions, yet differences such as MW were not considered in the final recommendation. 58 At that time, product differences in clinical outcomes were not as adequately reported within the literature—prompting the AAOS guideline to simply state that differences, particularly due to MW, may exist. 58 Since 2013, the wealth of information on these differences calls for future guideline developers and other key stakeholders to consider the differential effects that products have instead of assessing IA-HA treatments as a class.4,6,42,44
Strengths and Limitations
This study is strengthened by its comprehensive and systematic search for available literature. A key limitation in understanding these product differences arises due to the potential confounding of multiple characteristics. For example, HMW products have demonstrated better outcomes, but many studies assessing HMW HA were also cross-linked, so it is unclear as to what CL contributes to these effects. Similarly, CL has been proposed to be associated with increased adverse event rates; however, it is unclear whether these are more likely a result of the avian-derived HA that is also cross-linked. There is an importance for considering product differentiating characteristics instead of assessing HA as a class in future research investigations, as the available literature demonstrates clear differences between available IA-HA products.
Conclusion
Many differences exist across IA-HA products. This review summarizes the mechanism of action of IA-HA—specifically highlighting differences between IA-HA characteristics, and how important the MW, derivation of the product, and structure are to variances in reported clinical outcomes to treat OA of the knee. Specifically, HMW IA-HAs have shown greater efficacy compared to the alternative of lower MW products, while avian-derived and cross-linked products have potentially demonstrated an increase in inflammatory adverse events over non-avian-derived, non-cross-linked counterparts. Given the differences in properties of HAs and their mechanism of action, not all HAs have the same clinical effect; thus, these properties should be considered by physicians, providers, and stakeholders prior to choosing an HA.
Supplemental Material
Supplemental material, sj-docx-1-car-10.1177_19476035231154530 for Intra-articular Hyaluronic Acid Treatments for Knee Osteoarthritis: A Systematic Review of Product Properties by Eric Ferkel, Ajay Manjoo, Damion Martins, Mohit Bhandari, Paul Sethi and Mathew Nicholls in CARTILAGE
Footnotes
Acknowledgments and Funding: The authors would like to thank Mark Phillips of Global Research Solutions for his medical writing assistance. The author(s) have received no financial support for the research, authorship, and/or publication of this article. The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Ferring Pharmaceuticals Inc. All authors had final review and complete control over the publication and writing of this manuscript.
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: EF serves as a consultant for Arthrex and Ferring. MN, AM, and DM have nothing to disclose. MB serves as a consultant for Sanofi Aventis, Smith and Nephew, and AgNovos Healthcare. PS serves as a consultant for Arthrex and Pacira.
Ethical Approval: Ethical approval was not sought for the present study because all data was obtained from a previously conducted trial. No patients were directly enrolled within this investigation.
Supplementary material for this article is available on the Cartilage website at http://cart.sagepub.com/supplemental.
References
- 1. Nicholls M, Manjoo A, Shaw P, Niazi F, Rosen J. Rheological properties of commercially available hyaluronic acid products in the United States for the treatment of osteoarthritis knee pain. Clin Med Insights Arthritis Musculoskelet Disord. 2018;11:1179544117751622. doi: 10.1177/1179544117751622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Altman RD, Manjoo A, Fierlinger A, Niazi F, Nicholls M. The mechanism of action for hyaluronic acid treatment in the osteoarthritic knee: a systematic review. BMC Musculoskelet Disord. 2015;16:321. doi: 10.1186/s12891-015-0775-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rosen J, Sancheti P, Fierlinger A, Niazi F, Johal H, Bedi A. Potential impact of biologically derived hyaluronic acid on quality of life in patients with knee osteoarthritis in the United States. Adv Ther. 2016;33(12):2200-10. doi: 10.1007/s12325-016-0433-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Phillips M, Vannabouathong C, Devji T, Patel R, Gomes Z, Patel A, et al. Differentiating factors of intra-articular injectables have a meaningful impact on knee osteoarthritis outcomes: a network meta-analysis. Knee Surg Sports Traumatol Arthrosc. 2020;28(9):3031-9. doi: 10.1007/s00167-019-05763-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bannuru RR, Schmid CH, Kent DM, Vaysbrot EE, Wong JB, McAlindon TE. Comparative effectiveness of pharmacologic interventions for knee osteoarthritis: a systematic review and network meta-analysis. Ann Intern Med. 2015;162(1):46-54. doi: 10.7326/M14-1231. [DOI] [PubMed] [Google Scholar]
- 6. Hummer CD, Angst F, Ngai W, Whittington C, Yoon SS, Duarte L, et al. High molecular weight intraarticular hyaluronic acid for the treatment of knee osteoarthritis: a network meta-analysis. BMC Musculoskelet Disord. 2020;21:702. doi: 10.1186/s12891-020-03729-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Altman R, Lim S, Steen RG, Dasa V. Hyaluronic acid injections are associated with delay of total knee replacement surgery in patients with knee osteoarthritis: evidence from a large U.S. health claims database. PLoS ONE. 2015;10(12):e0145776. doi: 10.1371/journal.pone.0145776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bannuru RR, Osani MC, Vaysbrot EE, Arden NK, Bennell K, Bierma-Zeinstra SMA, et al. OARSI guidelines for the non-surgical management of knee, hip, and polyarticular osteoarthritis. Osteoarthritis Cartilage. 2019;27(11):1578-89. doi: 10.1016/j.joca.2019.06.011. [DOI] [PubMed] [Google Scholar]
- 9. Moreland LW. Intra-articular hyaluronan (hyaluronic acid) and hylans for the treatment of osteoarthritis: mechanisms of action. Arthritis Res Ther. 2003;5(2):54-67. doi: 10.1186/ar623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Smith MM, Ghosh P. The synthesis of hyaluronic acid by human synovial fibroblasts is influenced by the nature of the hyaluronate in the extracellular environment. Rheumatol Int. 1987;7(3):113-22. doi: 10.1007/BF00270463. [DOI] [PubMed] [Google Scholar]
- 11. Altman RD, Bedi A, Karlsson J, Sancheti P, Schemitsch E. Product differences in intra-articular hyaluronic acids for osteoarthritis of the knee. Am J Sports Med. 2016;44(8):2158-65. doi: 10.1177/0363546515609599. [DOI] [PubMed] [Google Scholar]
- 12. Altman R, Bedi A, Manjoo A, Niazi F, Shaw P, Mease P. Anti-inflammatory effects of intra-articular hyaluronic acid: a systematic review. Cartilage. 2019;10(1):43-52. doi: 10.1177/1947603517749919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Devji T, Carrasco-Labra A, Qasim A, Phillips M, Johnston BC, Devasenapathy N, et al. Evaluating the credibility of anchor based estimates of minimal important differences for patient reported outcomes: instrument development and reliability study. BMJ. 2020;369:m1714. doi: 10.1136/bmj.m1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Carrasco-Labra A, Devji T, Qasim A, Phillips MR, Wang Y, Johnston BC, et al. Minimal important difference estimates for patient-reported outcomes: a systematic survey. J Clin Epidemiol. 2021;133:61-71. doi: 10.1016/j.jclinepi.2020.11.024. [DOI] [PubMed] [Google Scholar]
- 15. Gotoh S, Onaya J, Abe M, Miyazaki K, Hamai A, Horie K, et al. Effects of the molecular weight of hyaluronic acid and its action mechanisms on experimental joint pain in rats. Ann Rheum Dis. 1993;52(11):817-22. doi: 10.1136/ard.52.11.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gomis A, Pawlak M, Balazs EA, Schmidt RF, Belmonte C. Effects of different molecular weight elastoviscous hyaluronan solutions on articular nociceptive afferents. Arthritis Rheum. 2004;50(1):314-26. doi: 10.1002/art.11421. [DOI] [PubMed] [Google Scholar]
- 17. Karatay S, Kiziltunc A, Yildirim K, Karanfil RC, Senel K. Effects of different hyaluronic acid products on synovial fluid NO levels in knee osteoarthritis. Clin Rheumatol. 2005;24(5):497-501. doi: 10.1007/s10067-004-1077-2. [DOI] [PubMed] [Google Scholar]
- 18. Nunoda D, Yamamoto K, Katori Y, Watanabe Y. Effects of hyaluronic acid on extracellular matrix degradation in a rabbit osteoarthritis model. Proceedings of the 5th Combined Meeting of the Orthopaedic Research Societies of Canada, USA, Japan and Europe, Banff, AB, 2005, Poster No. 272. [Google Scholar]
- 19. Falcone SJ, Palmeri DM, Berg RA. Rheological and cohesive properties of hyaluronic acid. J Biomed Mater Res A. 2006;76(4):721-8. doi: 10.1002/jbm.a.30623. [DOI] [PubMed] [Google Scholar]
- 20. Hsieh YS, Yang SF, Lue KH, Chu SC, Lu KH. Effects of different molecular weight hyaluronan products on the expression of urokinase plasminogen activator and inhibitor and gelatinases during the early stage of osteoarthritis. J Orthop Res. 2008;26(4):475-84. doi: 10.1002/jor.20524. [DOI] [PubMed] [Google Scholar]
- 21. Fam H, Kontopoulou M, Bryant JT. Effect of concentration and molecular weight on the rheology of hyaluronic acid/bovine calf serum solutions. Biorheology. 2009;46(1):31-43. doi: 10.3233/BIR-2009-0521. [DOI] [PubMed] [Google Scholar]
- 22. Boettger MK, Kümmel D, Harrison A, Schaible HG. Evaluation of long-term antinociceptive properties of stabilized hyaluronic acid preparation (NASHA) in an animal model of repetitive joint pain. Arthritis Res Ther. 2011;13(4):R110. doi: 10.1186/ar3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bhuanantanondh P, Grecov D, Kwok E. Rheological study of viscosupplements and synovial fluid in patients with osteoarthritis. J Med Biol Eng. 2012;32(1):12-6. [Google Scholar]
- 24. Abd. Rahim NEH, Zulkifly AH, Mohamed F, Ku Zaifah N. Viscosity and viscoelasticity of osteoarthritic synovial fluids in comparison to intraarticular injection of hyaluronic acid (Synvisc and Suplasyn). Malays Orthop J. 2012;6(2, Suppl A):75. [Google Scholar]
- 25. Elmorsy S, Funakoshi T, Sasazawa F, Todoh M, Tadano S, Iwasaki N. Chondroprotective effects of high-molecular-weight cross-linked hyaluronic acid in a rabbit knee osteoarthritis model. Osteoarthritis Cartilage. 2014;22(1):121-7. doi: 10.1016/j.joca.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 26. Sato E, Ando T, Ichikawa J, Okita G, Sato N, Wako M, et al. High molecular weight hyaluronic acid increases the differentiation potential of the murine chondrocytic ATDC5 cell line. J Orthop Res. 2014;32(12):1619-27. doi: 10.1002/jor.22691. [DOI] [PubMed] [Google Scholar]
- 27. Gómez-Aristizábal A, Kim KP, Viswanathan S. A systematic study of the effect of different molecular weights of hyaluronic acid on mesenchymal stromal cell-mediated immunomodulation. PLoS ONE. 2016;11(1):e0147868. doi: 10.1371/journal.pone.0147868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nicholls M, Manjoo A, Shaw P, Niazi F, Rosen J. A comparison between rheological properties of intra-articular hyaluronic acid preparations and reported human synovial fluid. Adv Ther. 2018;35(4):523-30. doi: 10.1007/s12325-018-0688-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Oliveira MZ, Albano MB, Stirma GA, Namba MM, Vidigal L, Cunha LAMD. Intra-articular viscosupplementation of hyaluronic acids in an experimental osteoarthritis model. Rev Bras Ortop. 2018;53(3):293-9. doi: 10.1016/j.rboe.2018.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sirin DY, Kaplan N, Yilmaz I, Karaarslan N, Ozbek H, Akyuva Y, et al. The association between different molecular weights of hyaluronic acid and CHAD, HIF-1α, COL2A1 expression in chondrocyte cultures. Exp Ther Med. 2018;15(5):4205-12. doi: 10.3892/etm.2018.5943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lokhnauth J, Driscoll KE, Bendele A, Niazi F, Liang A, Larsen CC. Viscosupplementation may preserve tibial cartilage and collagen in osteoarthritis: findings from a preclinical model of osteoarthritis. J Exp Orthop. 2020;7(1):39. doi: 10.1186/s40634-020-00256-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yoshioka K, Katayama M, Nishiyama T, Harada K, Takeshita S, Kawamata Y. Biocompatibility study of different hyaluronan products for intra-articular treatment of knee osteoarthritis. BMC Musculoskelet Disord. 2019;20(1):424. doi: 10.1186/s12891-019-2815-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Schiavinato A, Finesso M, Cortivo R, Abatangelo G. Comparison of the effects of intra-articular injections of Hyaluronan and its chemically cross-linked derivative (Hylan G-F20) in normal rabbit knee joints. Clin Exp Rheumatol. 2002;20(4):445-54. [PubMed] [Google Scholar]
- 34. Ghosh P, Guidolin D. Potential mechanism of action of intra-articular hyaluronan therapy in osteoarthritis: are the effects molecular weight dependent? Semin Arthritis Rheum. 2002;32(1):10-37. doi: 10.1053/sarh.2002.33720. [DOI] [PubMed] [Google Scholar]
- 35. Lo GH, LaValley M, McAlindon T, Felson DT. Intra-articular hyaluronic acid in treatment of knee osteoarthritis: a meta-analysis. JAMA. 2003;290(23):3115-21. doi: 10.1001/jama.290.23.3115. [DOI] [PubMed] [Google Scholar]
- 36. Aggarwal A, Sempowski IP. Hyaluronic acid injections for knee osteoarthritis. Systematic review of the literature. Can Fam Physician. 2004;50:249-56. [PMC free article] [PubMed] [Google Scholar]
- 37. Goldberg VM, Coutts RD. Pseudoseptic reactions to hylan viscosupplementation: diagnosis and treatment. Clin Orthop. 2004;419:130-7. doi: 10.1097/00003086-200402000-00021. [DOI] [PubMed] [Google Scholar]
- 38. Agerup B, Berg P, Akermark C. Non-animal stabilized hyaluronic acid: a new formulation for the treatment of osteoarthritis. BioDrugs. 2005;19(1):23-30. doi: 10.2165/00063030-200519010-00003. [DOI] [PubMed] [Google Scholar]
- 39. Benke M, Shaffer B. Viscosupplementation treatment of arthritis pain. Curr Pain Headache Rep. 2009;13(6):440-6. doi: 10.1007/s11916-009-0072-3. [DOI] [PubMed] [Google Scholar]
- 40. Colen S, van den Bekerom MPJ, Mulier M, Haverkamp D. Hyaluronic acid in the treatment of knee osteoarthritis: a systematic review and meta-analysis with emphasis on the efficacy of different products. BioDrugs. 2012;26(4):257-68. doi: 10.2165/11632580-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 41. Rutjes AWS, Jüni P, da Costa BR, Trelle S, Nüesch E, Reichenbach S. Viscosupplementation for osteoarthritis of the knee: a systematic review and meta-analysis. Ann Intern Med. 2012;157(3):180-91. doi: 10.7326/0003-4819-157-3-201208070-00473. [DOI] [PubMed] [Google Scholar]
- 42. Johal H, Devji T, Schemitsch EH, Bhandari M. Viscosupplementation in knee osteoarthritis: evidence revisited. JBJS Rev. 2016;4(4):e11-111. doi: 10.2106/JBJS.RVW.15.00098. [DOI] [PubMed] [Google Scholar]
- 43. Zhao H, Liu H, Liang X, Li Y, Wang J, Liu C. Hylan G-F 20 versus low molecular weight hyaluronic acids for knee osteoarthritis: a meta-analysis. BioDrugs. 2016;30(5):387-96. doi: 10.1007/s40259-016-0186-1. [DOI] [PubMed] [Google Scholar]
- 44. Bhandari M, Bannuru RR, Babins EM, Martel-Pelletier J, Khan M, Raynauld JP. et al. Intra-articular hyaluronic acid in the treatment of knee osteoarthritis: a Canadian evidence-based perspective. Ther Adv Musculoskelet Dis. 2017;9(9):231-46. doi: 10.1177/1759720X17729641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Cooper C, Rannou F, Richette P, Bruyère O, Al-Daghri N, Altman RD. et al. Use of intraarticular hyaluronic acid in the management of knee osteoarthritis in clinical practice. Arthritis Care Res (Hoboken). 2017;69(9):1287-96. doi: 10.1002/acr.23204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Caramona L, De La Mata J. Viscosupplementation in non-severe knee osteoarthritis: a network meta-analysis of high quality randomized clinical trials. Clin Exp Rheumatol. 2018;36(Suppl 109):S-23. [Google Scholar]
- 47. Vannabouathong C, Bhandari M, Bedi A, Khanna V, Yung P, Shetty V, et al. Nonoperative treatments for knee osteoarthritis: an evaluation of treatment characteristics and the intra-articular placebo effect: a systematic review. JBJS Rev. 2018;6(7):e5. doi: 10.2106/JBJS.RVW.17.00167. [DOI] [PubMed] [Google Scholar]
- 48. Hamburger MI, Lakhanpal S, Mooar PA, Oster D. Intra-articular hyaluronans: a review of product-specific safety profiles. Semin Arthritis Rheum. 2003;32(5):296-309. doi: 10.1053/sarh.2002.50008. [DOI] [PubMed] [Google Scholar]
- 49. Bannuru RR, Natov NS, Dasi UR, Schmid CH, McAlindon TE. Therapeutic trajectory following intra-articular hyaluronic acid injection in knee osteoarthritis–meta-analysis. Osteoarthritis Cartilage. 2011;19(6):611-9. doi: 10.1016/j.joca.2010.09.014. [DOI] [PubMed] [Google Scholar]
- 50. Jevsevar DS, Shores PB, Mullen K, Schulte DM, Brown GA, Cummins DS. Mixed treatment comparisons for nonsurgical treatment of knee osteoarthritis: a network meta-analysis. J Am Acad Orthop Surg. 2018;26(9):325-36. doi: 10.5435/JAAOS-D-17-00318. [DOI] [PubMed] [Google Scholar]
- 51. Rosen J, Sancheti P, Fierlinger A, Niazi F, Johal H, Bedi A. Cost-effectiveness of different forms of intra-articular injections for the treatment of osteoarthritis of the knee. Adv Ther. 2016;33(6):998-1011. doi: 10.1007/s12325-016-0331-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Rosen J, Niazi F, Dysart S. Cost-effectiveness of treating early to moderate stage knee osteoarthritis with intra-articular hyaluronic acid compared to conservative interventions. Adv Ther. 2020;37(1):344-52. doi: 10.1007/s12325-019-01142-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Bannuru RR, Natov NS, Obadan IE, Price LL, Schmid CH, McAlindon TE. Therapeutic trajectory of hyaluronic acid versus corticosteroids in the treatment of knee osteoarthritis: a systematic review and meta-analysis. Arthritis Rheum. 2009;61(12):1704-11. doi: 10.1002/art.24925. [DOI] [PubMed] [Google Scholar]
- 54. Delbarre A, Amor B, Bardoulat I, Tetafort A, Pelletier-Fleury N. Do intra-articular hyaluronic acid injections delay total knee replacement in patients with osteoarthritis—a Cox model analysis. PLoS ONE. 2017;12(11):e0187227. doi: 10.1371/journal.pone.0187227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Concoff A, Niazi F, Farrokhyar F, Alyass A, Rosen J, Nicholls M. Delay to TKA and costs associated with knee osteoarthritis care using intra-articular hyaluronic acid: analysis of an administrative database. Clin Med Insights Arthritis Musculoskelet Disord. 2021;14:1179544121994092. doi: 10.1177/1179544121994092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Altman RD, Farrokhyar F, Fierlinger A, Niazi F, Rosen J. Analysis for prognostic factors from a database for the intra-articular hyaluronic acid (Euflexxa) treatment for osteoarthritis of the knee. Cartilage. 2016;7(3):229-37. doi: 10.1177/1947603515620890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Nicholls M, Shaw P, Niazi F, Bhandari M, Bedi A. The impact of excluding patients with end-stage knee disease in intra-articular hyaluronic acid trials: a systematic review and meta-analysis. Adv Ther. 2019;36(1):147-61. doi: 10.1007/s12325-018-0847-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Jevsevar DS. Treatment of osteoarthritis of the knee: evidence-based guideline, 2nd edition. J Am Acad Orthop Surg. 2013;21(9):571-6. doi: 10.5435/JAAOS-21-09-571. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-car-10.1177_19476035231154530 for Intra-articular Hyaluronic Acid Treatments for Knee Osteoarthritis: A Systematic Review of Product Properties by Eric Ferkel, Ajay Manjoo, Damion Martins, Mohit Bhandari, Paul Sethi and Mathew Nicholls in CARTILAGE


