Abstract
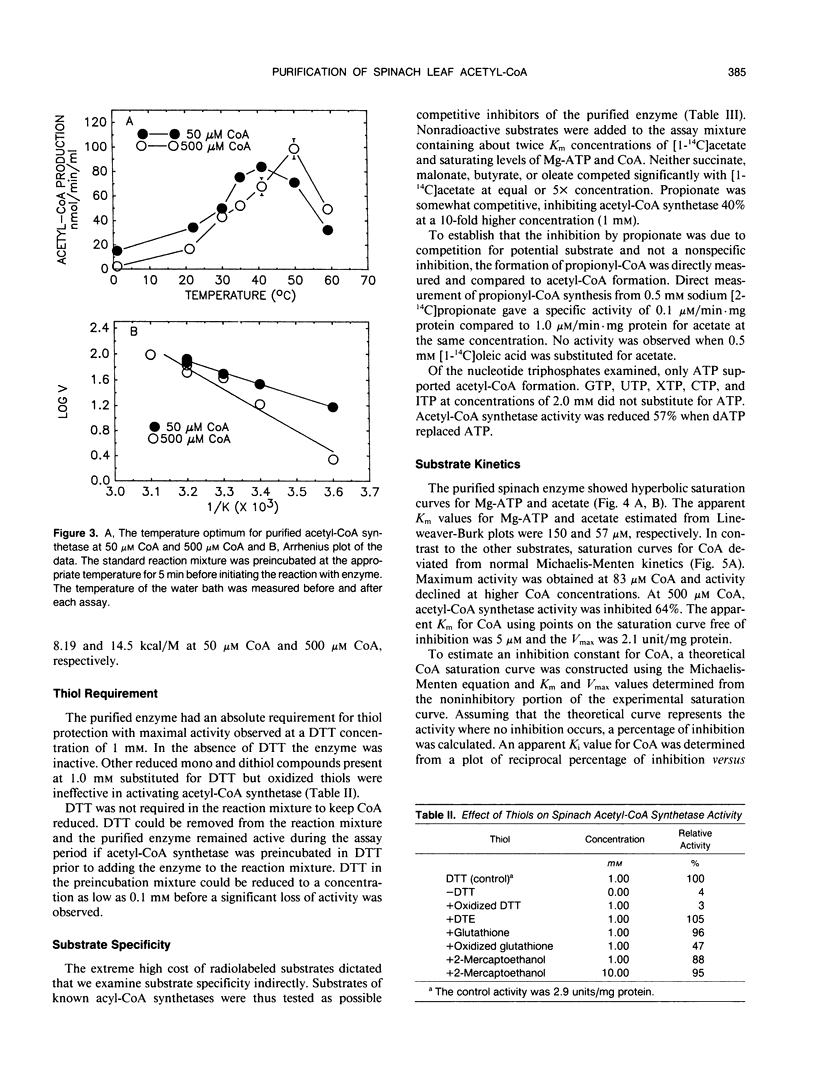
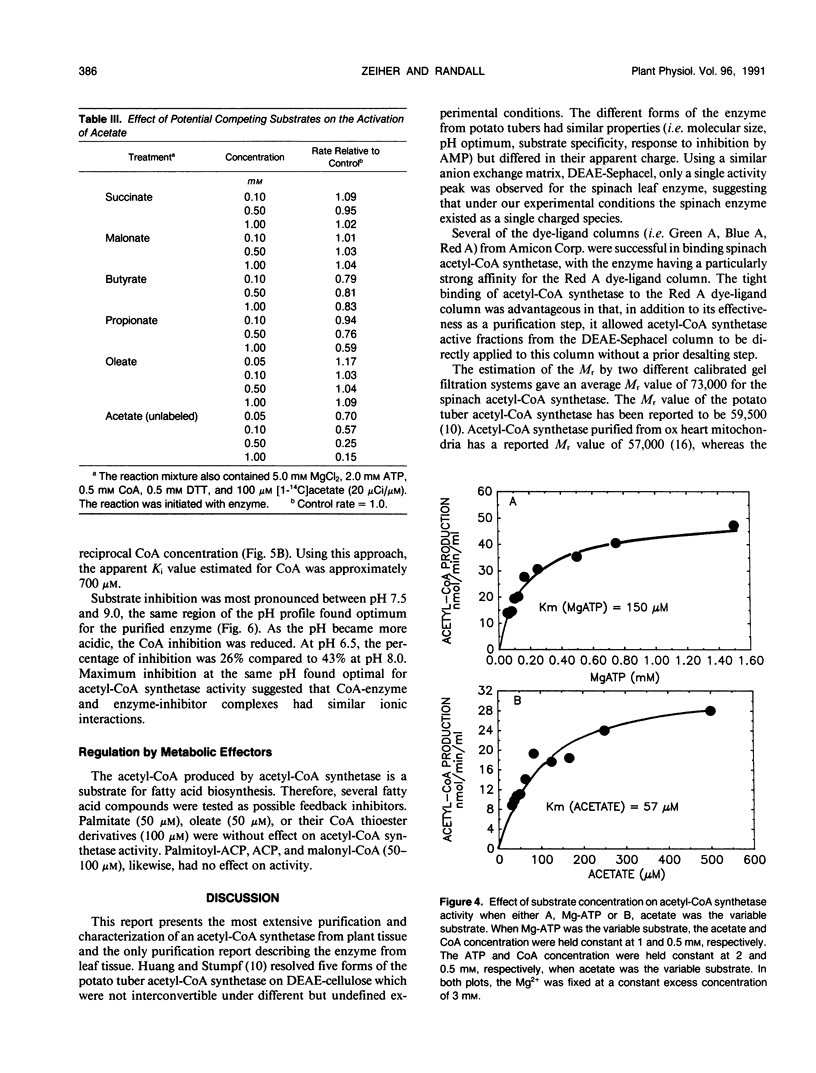
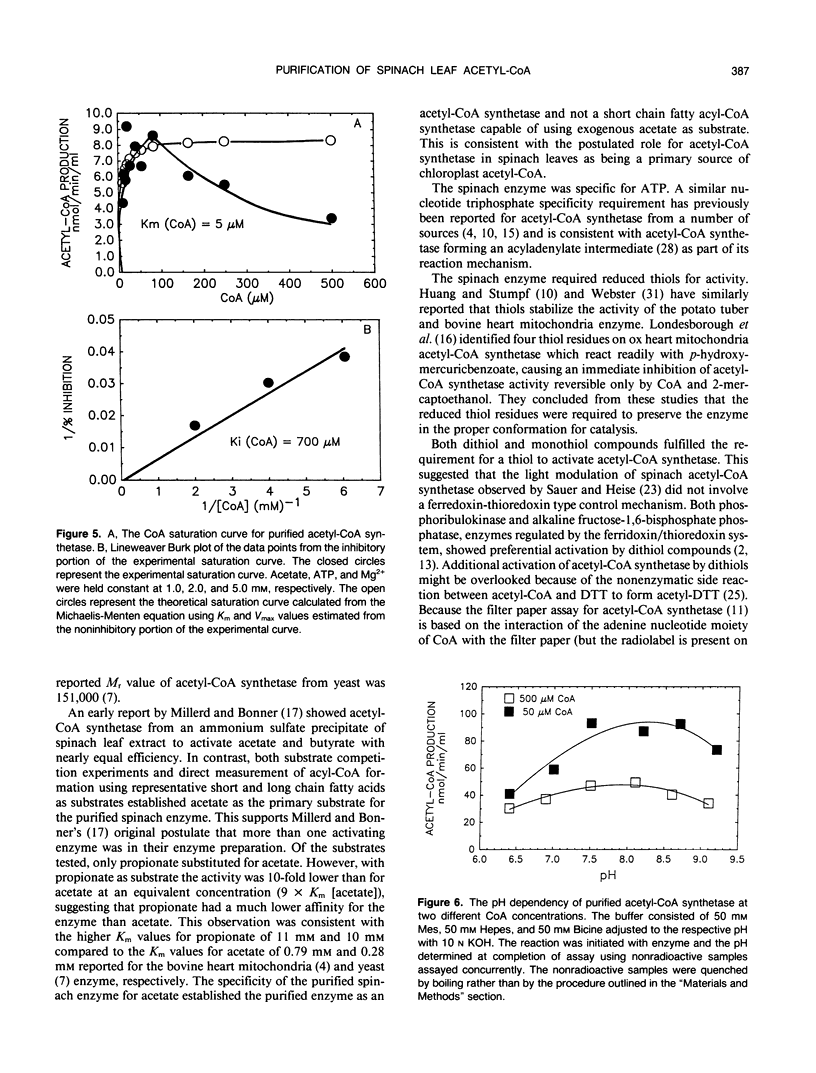
Acetyl-coenzyme A (CoA) synthetase was purified 364-fold from leaves of spinach (Spinacia oleracea L.) using ammonium sulfate fractionation followed by ion exchange, dye-ligand, and gel permeation chromatography. The final specific activity was 2.77 units per milligram protein. The average Mr value of the native enzyme was about 73,000. The Michaelis constants determined for Mg-ATP, acetate, and coenzyme A were 150, 57, and 5 micromolar, respectively. The purified enzyme was sensitive to substrate inhibition by CoA with an apparent Ki for CoA of 700 micromolar. The enzyme was specific for acetate; other short and long chain fatty acids were ineffective as substrates. Several intermediates and end products of fatty acid synthesis were examined as potential inhibitors of acetyl-CoA synthetase activity, but none of the compounds tested significantly inhibited acetyl-CoA synthetase activity in vitro. The properties of the purified enzyme support the postulated role of acetyl-CoA synthetase as a primary source of chloroplast acetyl-CoA.
Full text
PDF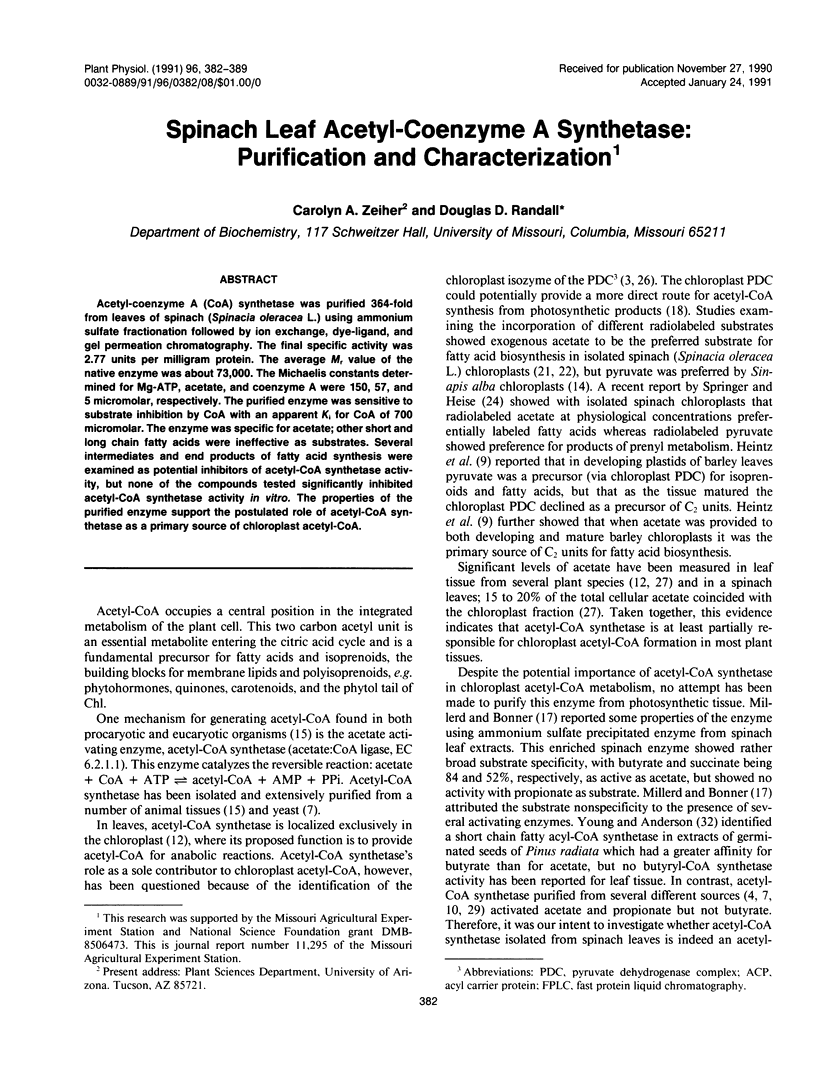




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CAMPAGNARI F., WEBSTER L. T., Jr Purification and properties of acetyl coenzyme A synthetase from bovine heart mitochondria. J Biol Chem. 1963 May;238:1628–1633. [PubMed] [Google Scholar]
- Cleland W. W. Statistical analysis of enzyme kinetic data. Methods Enzymol. 1979;63:103–138. doi: 10.1016/0076-6879(79)63008-2. [DOI] [PubMed] [Google Scholar]
- Frenkel E. P., Kitchens R. L. Purification and properties of acetyl coenzyme A synthetase from bakers' yeast. J Biol Chem. 1977 Jan 25;252(2):504–507. [PubMed] [Google Scholar]
- Heintze A., Görlach J., Leuschner C., Hoppe P., Hagelstein P., Schulze-Siebert D., Schultz G. Plastidic Isoprenoid Synthesis during Chloroplast Development : Change from Metabolic Autonomy to a Division-of-Labor Stage. Plant Physiol. 1990 Jul;93(3):1121–1127. doi: 10.1104/pp.93.3.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang K. P. A sensitive assay method of acetyl CoA synthetase. Anal Biochem. 1970 Sep;37(1):98–104. doi: 10.1016/0003-2697(70)90263-0. [DOI] [PubMed] [Google Scholar]
- Huang K. P., Stumpf P. K. Fat metabolism in higher plants. XLI. Properties of potato acetyl coenzyme A synthetase. Arch Biochem Biophys. 1970 Sep;140(1):158–173. doi: 10.1016/0003-9861(70)90019-6. [DOI] [PubMed] [Google Scholar]
- Kuhn D. N., Knauf M., Stumpf P. K. Subcellular localization of acetyl-CoA synthetase in leaf protoplasts of Spinacia oleracea. Arch Biochem Biophys. 1981 Jul;209(2):441–450. doi: 10.1016/0003-9861(81)90301-5. [DOI] [PubMed] [Google Scholar]
- Londensborough J. C., Yuan S. L., Webster L. T., Jr The molecular weight and thiol residues of acetyl-coenzyme A synthetase from ox heart mitochondria. Biochem J. 1973 May;133(1):23–36. doi: 10.1042/bj1330023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MILLERD A., BONNER J. Acetate activation and acetoacetate formation in plant systems. Arch Biochem Biophys. 1954 Apr;49(2):343–355. doi: 10.1016/0003-9861(54)90204-0. [DOI] [PubMed] [Google Scholar]
- Murthy V. K., Steiner G. Hepatic acetic thiokinase: possible regulatory step in lipogenesis. Metabolism. 1972 Mar;21(3):213–221. doi: 10.1016/0026-0495(72)90043-1. [DOI] [PubMed] [Google Scholar]
- Ohlrogge J. B., Kuhn D. N., Stumpf P. K. Subcellular localization of acyl carrier protein in leaf protoplasts of Spinacia oleracea. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1194–1198. doi: 10.1073/pnas.76.3.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roughan P. G., Holland R., Slack C. R. Acetate is the preferred substrate for long-chain fatty acid synthesis in isolated spinach chloroplasts. Biochem J. 1979 Dec 15;184(3):565–569. doi: 10.1042/bj1840565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roughan P. G., Holland R., Slack C. R. On the control of long-chain-fatty acid synthesis in isolated intact spinach (Spinacia oleracea) chloroplasts. Biochem J. 1979 Nov 15;184(2):193–202. doi: 10.1042/bj1840193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokes G. B., Stumpf P. K. Fat metabolism in higher plants. The nonenzymatic acylation of dithiothreitol by acyl coenzyme A. Arch Biochem Biophys. 1974 Jun;162(2):638–648. doi: 10.1016/0003-9861(74)90226-4. [DOI] [PubMed] [Google Scholar]
- WEBSTER L. T., Jr STUDIES OF THE ACETYL COENZYME A SYNTHETASE REACTION. I. ISOLATION AND CHARACTERIZATION OF ENZYME-BOUND ACETYL ADENYLATE. J Biol Chem. 1963 Dec;238:4010–4015. [PubMed] [Google Scholar]
- Webster L. T., Jr Studies of the acetyl coenzyme A synthetase reaction. 3. Evidence of a double requirement for divalent cations. J Biol Chem. 1965 Nov;240(11):4164–4169. [PubMed] [Google Scholar]
- Webster L. T., Jr Studies of the acetyl coenzyme A synthetase reaction. II. Crystalline acetyl coenzyme A synthetase. J Biol Chem. 1965 Nov;240(11):4158–4163. [PubMed] [Google Scholar]
- Young O. A., Anderson J. W. Properties and substrate specificity of some reactions catalysed by a short-chain fatty acyl-coenzyme A synthetase from seeds of Pinus radiata. Biochem J. 1974 Mar;137(3):423–433. doi: 10.1042/bj1370423. [DOI] [PMC free article] [PubMed] [Google Scholar]


