Abstract
Aim:
To determine whether continuous glucose monitoring (CGM) can reduce hypoglycaemia in patients with post-bariatric hypoglycaemia (PBH).
Materials and Methods:
In an open-label, nonrandomized, pre-post design with sequential assignment, CGM data were collected in 22 individuals with PBH in two sequential phases: (i) masked (no access to sensor glucose or alarms); and (ii) unmasked (access to sensor glucose and alarms for low or rapidly declining sensor glucose). Twelve participants wore the Dexcom G4 device for a total of 28 days, while 10 wore the Dexcom G6 device for a total of 20 days.
Results:
Participants with PBH spent a lower percentage of time in hypoglycaemia over 24 hours with unmasked versus masked CGM (<3.3 mM/L, or <60 mg/dL: median [median absolute deviation {MAD}] 0.7 [0.8]% vs. 1.4 [1.7]%, P = 0.03; <3.9 mM/L, or <70 mg/dL: median [MAD] 2.9 [2.5]% vs. 4.7 [4.8]%; P = 0.04), with similar trends overnight. Sensor glucose data from the unmasked phase showed a greater percentage of time spent between 3.9 and 10 mM/L (70–180 mg/dL) (median [MAD] 94.8 [3.9]% vs. 90.8 [5.2]%; P = 0.004) and lower glycaemic variability over 24 hours (median [MAD] mean amplitude of glycaemic excursion 4.1 [0.98] vs. 4.4 [0.99] mM/L; P = 0.04). During the day, participants also spent a greater percentage of time in normoglycaemia with unmasked CGM (median [MAD] 94.2 [4.8]% vs. 90.9 [6.2]%; P = 0.005), largely due to a reduction in hyperglycaemia (>10 mM/L, or 180 mg/dL: median [MAD] 1.9 [2.2]% vs. 3.9 [3.6]%; P = 0.02).
Conclusions:
Real-time CGM data and alarms are associated with reductions in low sensor glucose, elevated sensor glucose, and glycaemic variability. This suggests CGM allows patients to detect hyperglycaemic peaks and imminent hypoglycaemia, allowing dietary modification and self-treatment to reduce hypoglycaemia. The use of CGM devices may improve safety in PBH, particularly for patients with hypoglycaemia unawareness.
Keywords: bariatric surgery, continuous glucose monitoring, glycaemic control, hypoglycaemia
1 |. INTRODUCTION
Bariatric surgery is an effective approach to weight loss and obesity-associated comorbidities. For example, surgery is associated with improved glycaemic control, remission of type 2 diabetes, reduction in cardiovascular disease, reduced mortality and reduction in diabetes-related complications, in comparison to medical management.1–7 However, improvements in overall glucose metabolism are associated with an increased risk of hypoglycaemia in some individuals, a condition termed post-bariatric hypoglycaemia (PBH).
Post-bariatric hypoglycaemia can occur after either Roux-en-Y gastric bypass (RYGB) or sleeve gastrectomy. Hypoglycaemia can also develop after other upper gastrointestinal surgeries, including fundoplication in adults8,9 and children.10,11 Prevalence estimates depend on the methods used, ranging from <1% requiring hospitalization12,13 to 36% of individuals with symptoms of hypoglycaemia reported on surveys.14 Onset of this condition is typically observed at least 1 year postoperatively, but occasionally many years later.
Patients with PBH can experience both autonomic and neuroglycopenic symptoms of hypoglycaemia, typically 1 to 3 hours after meals, and especially after consumption of high glycaemic index carbohydrates. Recurrent hypoglycaemia can contribute to a high prevalence of hypoglycaemia unawareness. One study using continuous glucose monitoring (CGM) found that 75% of asymptomatic post-RYGB patients had sensor glucose levels below 3.1 mM/L (55 mg/dL).15 Such hypoglycaemia unawareness can lead to impaired quality of life, job loss and disability, and reduced safety due to loss of consciousness, falls, and seizures.
The pathophysiology of PBH is complex and incompletely understood. Glycaemic patterns are characterized by a precipitous rise in plasma glucose following a meal, followed by a rapid fall to hypoglycaemic levels.16 Early post-meal rises in glucose are typically associated with rapid gastric emptying of ingested nutrients into the proximal intestine; this can trigger symptoms of dumping syndrome, with lightheadedness, palpitations, fatigue, and abdominal cramping.17,18 Postprandial rises in glucose and exaggerated secretion of the incretin hormone glucagon-like peptide-1 (GLP-1) together trigger excessive secretion of insulin and subsequent rapid drops in glucose levels.19,20 Additional contributors to PBH may include reduction in counterregulatory hormone response,21,22 including blunting of hepatic glucose production during hypoglycaemia,23 shifts in the gut microbiome24 and alterations in the bile acid-FXR-FGF19 axis.25–27
Unfortunately, PBH is a chronic condition and long-term treatment options are often incompletely effective but include dietary modification to reduce high-glycaemic-index carbohydrate intake and to ensure adequate protein and micronutrient intake,28 acarbose or miglitol to slow carbohydrate absorption,29 and somatostatin analogues (eg, octreotide or pasireotide)30 and diazoxide31,32 to reduce insulin secretion.
Given that hypoglycaemia unawareness can threaten safety, and current therapies are unable to fully eliminate hypoglycaemia,33 additional approaches are needed. We hypothesized that use of a real-time CGM device with alarms would allow patients with PBH to detect imminent, precipitous decreases in sensor glucose and/or low sensor glucose values, allowing them to self-treat before severe hypoglycaemia develops. We tested this hypothesis by analysing glycaemic patterns in patients with PBH during sequential periods of wearing masked and unmasked CGM devices in a real-world setting to determine whether availability of sensor glucose information would allow patients to decrease glycaemic variability and reduce hypoglycaemia frequency and severity.
2 |. METHODS
The Joslin Diabetes Center Committee on Human Studies approved the study. Written informed consent was obtained from all participants. Participants aged 18 to 75 years, with a history of RYGB and clinical diagnosis of PBH, were recruited from the Joslin Hypoglycemia Clinic (Figure S1).
Diagnosis of PBH required fulfilment of Whipple’s triad (symptoms of hypoglycaemia, low plasma or capillary glucose [<3 mM/L], and relief of symptoms with treatment to increase glucose), with or without neuroglycopenia (defined by prior episodes of documented hypoglycaemia associated with altered mental status or consciousness requiring assistance of others, with or without seizure). CGM was not utilized to diagnose PBH. Exclusion criteria were current diagnosis of diabetes or current use of diabetes medications (except acarbose), major systemic illness, cardiac arrhythmia, hypertension, active coronary artery disease, fasting hypoglycaemia, insulinoma, pregnancy, active alcohol or substance abuse, and recent steroid or investigational drug exposure. Participants were investigated as indicated during clinical care to rule out other contributors to hypoglycaemia, including adrenal insufficiency and autonomous insulin secretion, which was excluded by assessment of glucose and insulin levels after an overnight or longer fast.
The study used an open-label, nonrandomized, pre-post design with sequential assignment to evaluate the efficacy of unmasked CGM in reducing the frequency of hypoglycaemia and glycaemic variability in the real-world setting, in the presence of participants’ existing treatment plan (including both meal plan and medications). Masked monitoring was performed first in all participants, as providing unmasked data to participants first could have altered their dietary or other patterns and reduced the ability of the study to independently assess any effect of the CGM intervention. Participants were not permitted to wear personal CGM devices during study participation. At study initiation, the Dexcom G4 Professional CGM device was available for blinded use. Participants wearing the G4 device (n = 13) attended five study visits, each 7 days apart (Figure S2A). During the course of the study, the Dexcom G6 was approved by the US Food and Drug Administration, and the study protocol was modified to reflect the longer duration of sensor wear (10 days for the G6 versus 7 days for the G4 device); participants on the G6 system (n = 10) attended three visits, each 10 days apart (Figure S2B).
Visit 1 included consent, review of inclusion and exclusion criteria, medical history, physical examination, and laboratory testing (glycated haemoglobin, complete blood count, comprehensive chemistry, urine analysis, and pregnancy test if applicable). Participants completed the Hypoglycaemia Fear Survey version II (HFS-II)34 to assess fear of hypoglycaemia and its impact on their daily routine, and two surveys to assess hypoglycaemia awareness: the Gold Score35 and a modified Clarke Hypoglycemia Awareness Questionnaire.36 Participants using the G6 system also completed the Edinburgh Hypoglycaemia Symptom Scale (EHSS)37 and the Dumping Symptom Rating Scale (DSRS).18 Surveys were repeated at subsequent visits, but the Hypoglycaemia Fear Survey was inadvertently omitted for Visits 2/3 for participants in the Dexcom G6 phase. Participants were instructed in completion of a detailed log to record symptoms, capillary glucose, any action taken to address hypoglycaemia (eg, food, glucose type and quantity, effectiveness), activity, oral intake (food and drink), and alarms (for the unmasked phase) to complete during both phases. Participants were asked to continue their current medical nutrition therapy-based meal plans during the study.
The Dexcom CGM sensor and transmitter were inserted on the anterior abdomen and set to masked mode. Participants were provided a capillary glucose monitor and instructed in its use. Those who wore the G4 system were specifically taught how to calibrate the CGM with the capillary glucose monitor 2 hours after sensor insertion and then once every 12 hours during the period of wear. The G6 system did not require calibration. For participants using the G4 system, a new CGM sensor was placed and again set to masked mode during Visit 2 (7 days after Visit 1). During the masked mode, no alarms or other information were received by the participant.
Unmasked monitoring (in which participants were aware of sensor glucose data and received alarms) was initiated during Visits 3 and 4 (7 days apart) and completed at Visit 5 for those wearing a G4 system. For those wearing the G6 system, the unmasked mode was initiated during Visit 2 and completed at Visit 3. Participants were provided a handheld receiver to display glucose values and sound alarms. Alarms were set for (i) sensor glucose <3.9 mM/L; (ii) sensor glucose 3.1 mM/L; and (iii) trend alarm for glucose decrease of ≥0.17 mM/L/min. Participants were instructed in the meaning of the alarms and the suggested responses (Figure S3).
At the final study visit, patterns of hypoglycaemia and any recommendations for dietary or medication adjustments were reviewed with the participant by the study clinician.
2.1 |. Statistical analysis
Demographic data are reported as mean ± standard deviation. Each sensor glucose reading (every 5 minutes) counted as a datapoint for a participant. Median and median absolute deviation (MAD) values for CGM variables are reported; paired differences were analysed with the Wilcoxon signed-rank tests, as noted. Percent distribution was calculated by dividing the number of sensor glucose values above or below a given threshold by the total number of values within that timeframe. Hypoglycaemic events were defined as having glucose levels lower than or equal to the threshold for at least 15 minutes, ending when glucose exceeds the threshold. Hypoglycaemic event values were normalized by dividing the number of events by the number of days of data. Daytime was defined as 6:00 AM to 11:59 PM and nighttime was defined as 12:00 AM to 5:59 AM. Glucose metrics were calculated using the R package iglu,38 while rGV was used for calculation of hypoglycaemia episodes.39 All analyses were performed using R.
Surveys were administered to assess hypoglycaemia unawareness and hypoglycaemia-related behaviours and fears. For both Gold and Clarke scores, scores ≤2 indicate normal hypoglycaemia awareness, scores of 3 indicate undetermined awareness status, and scores ≥4 indicate impaired awareness of hypoglycaemia.35,36 The HFS-II survey includes a behaviour subsection (HFS-B), which addresses behaviours performed to avoid hypoglycaemia and yields scores from 0 to 60, and a worry subsection (HFS-W), which addresses concerns about hypoglycaemia and yields scores from 0 to 72.34 For both subsections, a higher score indicates a greater impact of hypoglycaemia. Hypoglycaemia fear questionnaires were administered to participants using the G6 device only at their first visit due to study staff oversight. Gold and Clarke raw scores were analysed as both continuous variables and as categorical values using the categories described above. Differences in raw Gold and Clarke scores between study visits were analysed using repeated-measures ANOVA, followed by post-hoc Tukey honestly significant difference test. Categorical Gold and Clarke scores were analysed via a Cochran-Mantel-Haenszel test and a Kruskal-Wallis test. HFS-B and HFS-W scores were analysed as continuous variables via repeated-measures analysis of variance.
3 |. RESULTS
The study enrolled 25 participants, of whom 13 wore a G4 device and 10 wore a G6 device; two participants were lost to follow-up before CGM download, and one was excluded due to corrupted data, leaving a final study cohort of 22 participants with analysable CGM datasets (Table 1). Ninety percent of participants were female, consistent with the higher prevalence of PBH in women.40,41 The mean body mass index was 30.6 ± 6.0 kg/m2, with a postoperative duration of 9.6 ± 8.0 years (range 1.1 to 30.6 years). Eighty-two percent of participants had experienced level 3 hypoglycaemia (severely altered cognition requiring the assistance of others42) and all participants had experienced neuroglycopenia. Hypoglycaemia (<3 mM/L) was confirmed by capillary (n = 22) and/or venous (n = 3) glucose values. Ninety-five percent of participants had received PBH-specific medical nutrition therapy in the past, while medication use had included acarbose (95%), octreotide (5%), and/or diazoxide (5%). Changes in therapy during the study included clinician initiation of acarbose due to concern for safety due to recurrent severe hypoglycaemia and syncope (n = 1, masked phase), and participant self-modification during the unmasked phase, including acarbose dose reduction (n = 2), discontinuation (n = 1) and cornstarch initiation (n = 1).
TABLE 1.
Participant characteristics
| Characteristic | |
|---|---|
| Age, mean ± SD years | 51.1 ± 13.0 |
| Sex: female/male | 20/2 |
| Race (White/black/not reported), n/n/n | 19/1/2 |
| Ethnicity (Hispanic or Latino/Not Hispanic or Latino), n/n | 4/18 |
| HbA1c mean ± SD, % | 5.4 ± 0.4 |
| Preoperative BMI, mean ± SD kg/m2 | 47.6 ± 6.8 |
| Current BMI, mean ± SD kg/m2 | 30.6 ± 6.2 |
| Years postoperatively, mean ± SD | 9.6 ± 8.0 |
| Years between surgery and hypoglycaemia onset, mean ± SD | 4.7 ± 5.7 |
| Level 3 hypoglycaemia, % | 82 |
| Neuroglycopenia, % | 100 |
| Visit 1 EHSS score | 51.3 ±20.4 |
| Visit 1 DSRS score | 24.3 ± 6.6 |
| Pharmacotherapy for PBH during participation, n (%) | |
| Acarbosea | 10 (45) |
| Diazoxideb | 1 (5) |
| Octreotideb | 1 (5) |
Note: Data obtained from the 22 participants with analysable CGM data.
Abbreviations: BMI, body mass index; DSRS, Dumping Symptom Rating Scale; EHSS, Edinburgh Hypoglycaemia Symptom Scale; HbA1c, glycated haemoglobin; PBH, post-bariatric hypoglycaemia.
Acarbose (25 mg pre-meals) was prescribed clinically for one participant 4 days after the start of the unmasked phase, due to concerns for safety. Two participants self-adjusted doses of acarbose (one decreased the dose, one stopped it) during the unmasked phase of the study.
For the participants on diazoxide and octreotide at study onset (n = 2), doses were not adjusted during participation.
There were no changes in octreotide or diazoxide therapy. Thirty-six percent of participants reported some alcohol intake during screening history query. The EHSS, which surveys hypoglycaemia symptoms with a possible range of scores from 18 to 126, yielded a mean score of 51.3 ± 20.4. The DSRS, which surveys dumping syndrome symptoms with a possible range from 9 to 63, yielded a mean score of 24.3 ± 6.6.
The CGM devices were active for a median (MAD) 96.8 (3.6)% of masked and 98.1 (1.7)% of unmasked phase wear. Masked data analysis revealed patterns consistent with prior studies of masked data for this population.43
The CGM results over 24 hours in the masked versus unmasked phases are displayed in Table 2. No significant differences between the masked and unmasked phase were found for mean, median, peak, nadir, or range over 24 hours (Figure 1A). However, distribution of glucose values differed between masked and unmasked wear. As seen in the ambulatory glucose profile in Figure 1B and in the bar graph in Figure 1C, the percentage of time below 3.9 mM/L was significantly lower for the unmasked phase (median [MAD] 2.9 [2.5]% unmasked vs. 4.7 [4.8]% masked; P = 0.04)—a mean reduction of 26 minutes per day. Fifteen of the 22 participants spent less time below 3.9 mM/L when unmasked (Figure 1D). Similar reductions in time below 3.3 mM/L were observed (0.7 [0.8]% unmasked vs. 1.4 [1.7]% masked; P = 0.03). We also observed a reduction in the number of events (normalized per day of CGM device wear) in which sensor glucose level was 3.3 mM/L for at least 15 minutes (median [MAD] unmasked 0.2 [0.3] vs. masked 0.3 [0.4]; P = 0.04), with similar numerical reductions for events <3 mM/L (Table 2 and Figure 2A).
TABLE 2.
24-hour glucose metrics
| Masked | Unmasked | P value | |
|---|---|---|---|
| Sensor glucose metrics | |||
| Median (quartiles), mM/L | 5.4 (4.8, 6.3) | 5.4 (4.8, 6.3) | 0.45 |
| Mean, mM/L | 5.8 (0.7) | 5.8 (0.4) | 0.35 |
| Peak, mM/L | 14.6 (2.9) | 14.2 (2.4) | 0.53 |
| Nadir, mM/L | 2.2 (0.0) | 2.3 (0.2) | 0.65 |
| Range, mM/L | 12.3 (2.9) | 11.6 (2.8) | 0.47 |
| Percent distribution | |||
| Above 13.9 mM/L | 0.1 (0.2) | 0.1 (0.1) | 0.22 |
| Above 10 mM/L | 3.1 (2.8) | 1.7 (1.6) | 0.05 |
| 3.9 to 10 mM/L | 90.8 (5.2) | 94.8 (3.9) | 0.004 |
| Below 3.9 mM/L | 4.7 (4.8) | 2.9 (2.5) | 0.04 |
| Below 3.3 mM/L | 1.4 (1.7) | 0.7 (0.8) | 0.03 |
| Below 3.0 mM/L | 0.6 (0.8) | 0.2 (0.3) | 0.14 |
| Glycaemic variability | |||
| Standard deviation, mM/L | 1.7 (0.4) | 1.5 (0.3) | 0.09 |
| Mean coefficient of variation, % | 23.6 (5.1) | 23.0 (5.0) | 0.25 |
| MAGE, mM/L | 4.4 (1.0) | 4.1 (1.0) | 0.04 |
| CONGA-1 hour, mM/L | 2.0 (0.5) | 1.8 (0.4) | 0.34 |
| CONGA-2 hours, mM/L | 2.0 (0.6) | 2.1 (0.4) | 0.54 |
| CONGA-4 hours, mM/L | 2.2 (0.6) | 2.1 (0.4) | 0.35 |
| CONGA-24 hours, mM/L | 2.0 (0.6) | 1.8 (0.4) | 0.15 |
| Risk metrics | |||
| Average daily risk range | 27.1 (7.0) | 23.8 (6.9) | 0.15 |
| Low blood glucose index | 2.0 (1.6) | 1.75 (1.0) | 0.28 |
| High blood glucose index | 0.8 (0.6) | 0.6 (0.4) | 0.07 |
| Normalized hypoglycaemic events | |||
| Below 3.9 mM/L | 1.6 (1.5) | 1.8 (1.3) | 0.41 |
| Below 3.3 mM/L | 0.7 (0.6) | 0.5 (0.6) | 0.04 |
| Below 3.0 mM/L | 0.3 (0.4) | 0.2 (0.3) | 0.08 |
Note: Data shown are median (median absolute deviations), unless otherwise indicated (n = 22). P values were generated using Wilcoxon’s signed-rank test.
Abbreviations: CONGA, continuous overall net glycaemic action; MAGE, mean amplitude of glycaemic excursion.
FIGURE 1.
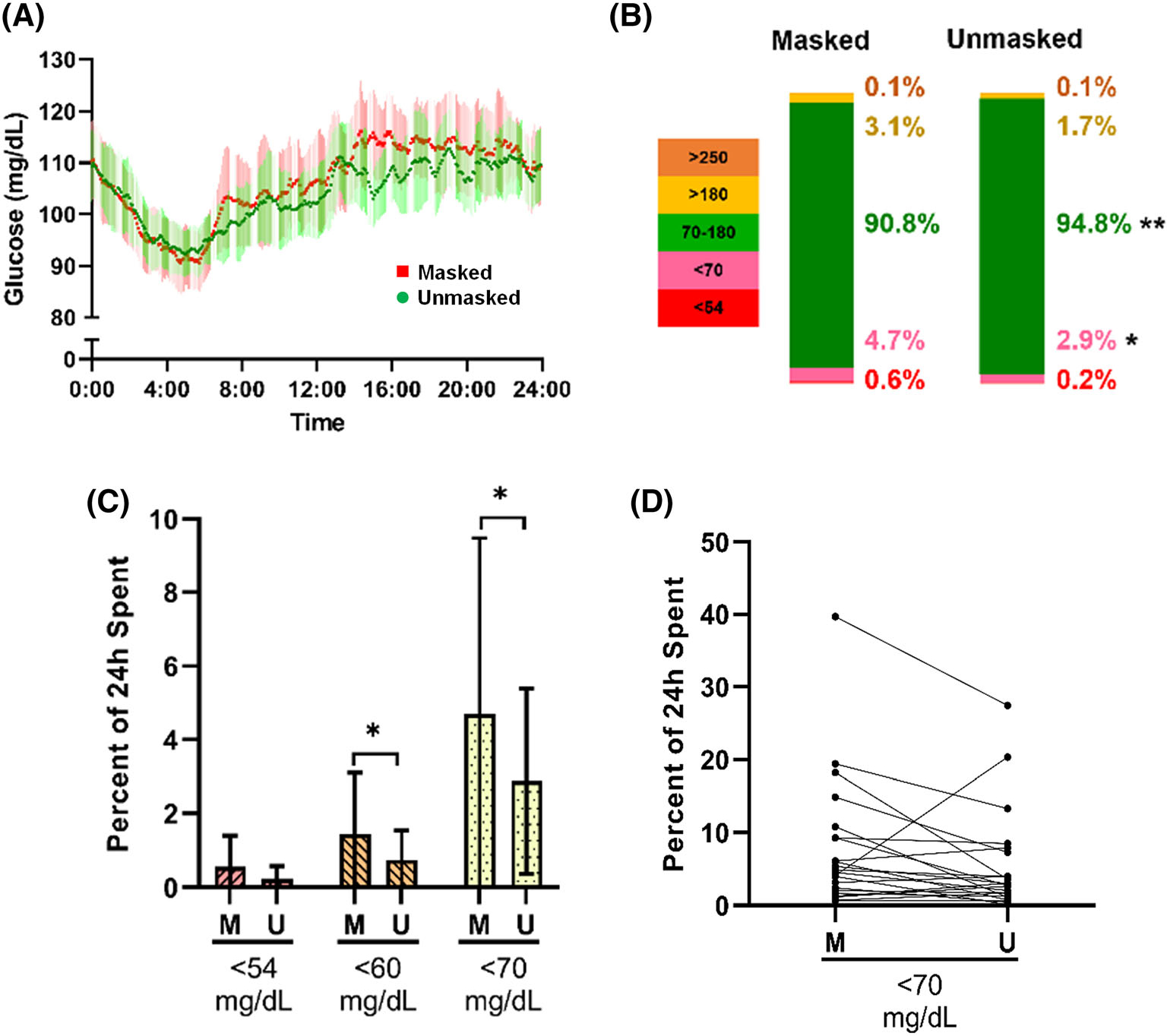
Effect of unmasking on continuous glucose monitoring-derived sensor glucose patterns and time spent in low glucose ranges. (A) Paired comparison for the entire cohort in masked (red) versus unmasked (green) phase, according to time of day (mean, 95% confidence intervals). (B) Ambulatory glucose profile, comparing median percent distributions between phases over 24 hours. (C) The median percent distributions ± median absolute deviations (MAD) in each phase for the 24-hour data. (D) Line segments representing each participant’s time spent below 3.9 mM/L when masked (M) and unmasked (U) (n = 22). *P < 0.05
FIGURE 2.
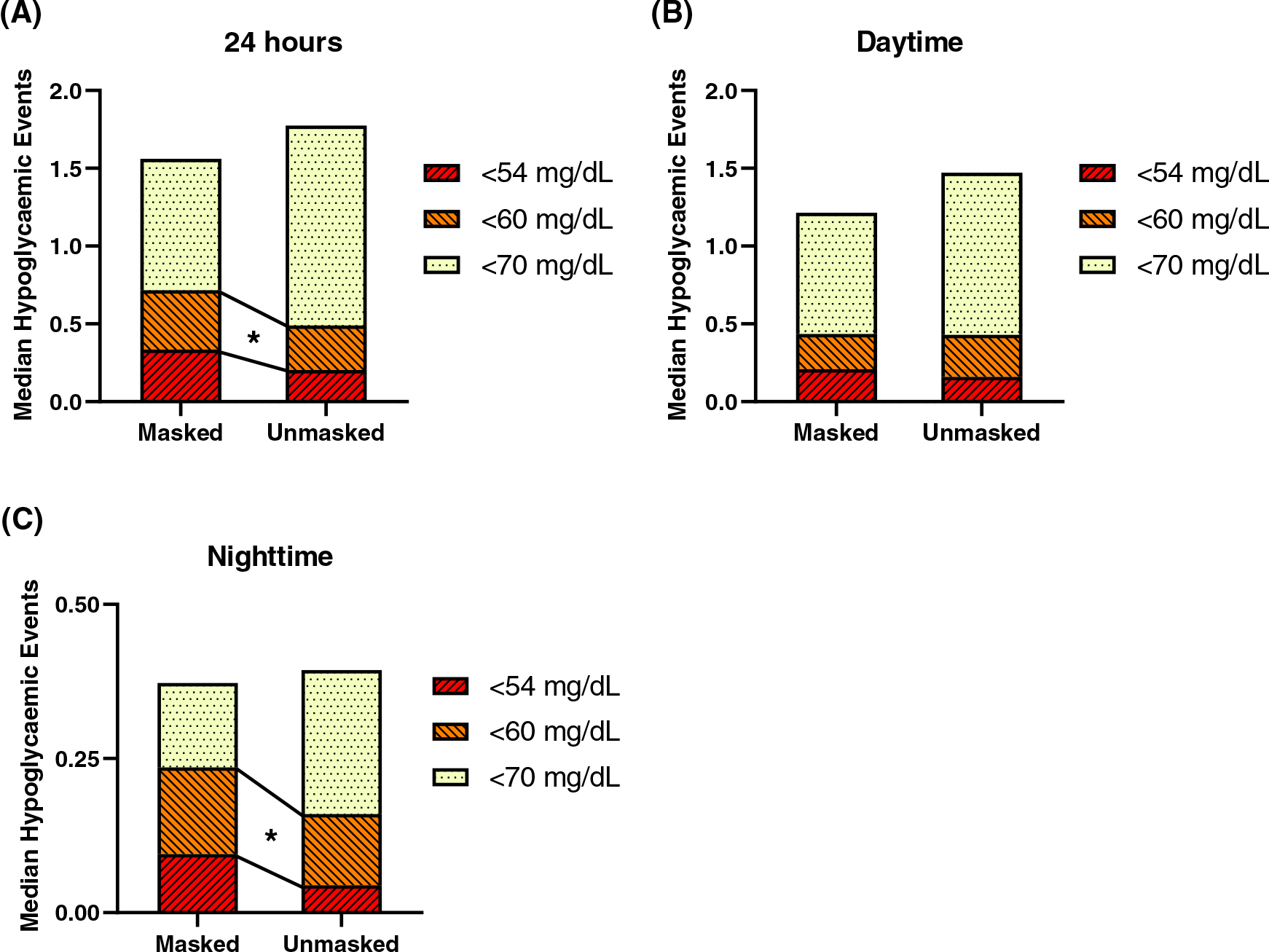
Effect of unmasking on normalized hypoglycaemic events. Normalized hypoglycaemic events over (A) 24 hours, (B) daytime hours, and (C) nighttime hours. Hypoglycaemic events are defined as having glucose lower than or equal to the threshold for at least 15 minutes, ending when glucose levels exceeded the threshold. Hypoglycaemic event values were normalized by dividing the number of events by the number of days of data
Likewise, percentage of time in range (between 3.9 and 10 mM/L) was greater for the unmasked phase (median [MAD] 94.8 [3.9]%) than the masked phase (median [MAD] 90.8 [5.2]%; P = 0.004 [Figures 1B and S4A]). Seventeen of the 22 participants spent more time between 3.9 and 10 mM/L when unmasked (Figure S4B). Glycaemic variability, as calculated by mean amplitude of glycaemic excursion (MAGE), was significantly lower for the unmasked (median [MAD] 4.1 [1.0] mM/L) as compared with the masked phase (median [MAD] 4.4 [1.0] mM/L; P = 0.04). Standard deviation tended to be lower in the unmasked phase, but other measures of glycaemic variability did not differ.
We next analysed CGM data during daytime and nighttime periods independently (Tables 3 and 4, respectively). There were no significant differences in median, mean, peak, nadir, or range during either daytime or nighttime. However, the distribution of sensor glucose values differed in masked and unmasked phases. During the daytime, the percent of sensor glucose values between 3.9 and 10 mM/L was higher in the unmasked versus masked phase (median [MAD] 94.2 [4.8]% vs. 90.9 [6.2]%; P = 0.005) while the percent above 10 mM/L was lower in the unmasked phase (median [MAD] 1.9 [2.2]% vs. 3.9 [3.6]%; P = 0.02 [Figure 3A]). Percent values below 3.9 mM/L were also numerically lower in the unmasked phase, but differences did not reach statistical significance. Glycaemic variability was significantly lower in the unmasked phase during the day, with significant reductions in daytime standard deviation (median [MAD] 1.5 [0.4] mM/L unmasked vs. 1.7 [0.4] mg/dL masked; P = 0.03) and continuous overall net glycaemic action (CONGA)-24 hours (median [MAD] 1.9 [0.5] mM/L unmasked vs. 2.2 [0.7] mg/dL masked; P = 0.03). While the percent time spent in hypoglycaemic ranges tended to be lower in the unmasked phase during the night (Figure 3B), the number of events with sensor glucose 3.3 mM/L was significantly lower during the unmasked phase (P = 0.02; Table 4 and Figure 2C). Interestingly, several measures of glycaemic variability were higher at night, reaching significance for CONGA-1 hour and CONGA-2 hours (P < 0.05 for both). There were no significant differences between percent distribution data derived from Dexcom G4 as compared with G6 systems (Tables S2–S4).
TABLE 3.
Daytime glucose metrics
| Masked | Unmasked | P value | |
|---|---|---|---|
| Daytime sensor glucose metrics | |||
| Median (quartiles), mM/L | 5.5 (4.8, 6.4) | 5.4 (4.9, 6.5) | 0.15 |
| Mean, mM/L | 5.9 (0.7) | 6.0 (0.4) | 0.13 |
| Peak, mM/L | 14.6 (2.9) | 13.5 (2.8) | 0.31 |
| Nadir, mM/L | 2.2 (0.0) | 2.4 (0.3) | 0.88 |
| Range, mM/L | 11.6 (3.3) | 11.2 (3.0) | 0.46 |
| Percent distribution | |||
| Above 13.9 mM/L | 0.2 (0.3) | 0.02 (0.0) | 0.10 |
| Above 10 mM/L | 3.9 (3.6) | 1.9 (2.2) | 0.02 |
| 3.9–10 mM/L | 90.9 (6.2) | 94.2 (4.8) | 0.005 |
| Below 3.9 mM/L | 4.6 (5.0) | 2.9 (3.0) | 0.07 |
| Below 3.3 mM/L | 1.4 (1.6) | 0.8 (0.9) | 0.20 |
| Below 3.0 mM/L | 0.7 (1.0) | 0.3 (0.4) | 0.22 |
| Glycaemic variability | |||
| Standard deviation, mM/L | 1.7 (0.4) | 1.5 (0.4) | 0.03 |
| Mean coefficient of variation, % | 23 (5.1) | 22.4 (5.0) | 0.21 |
| MAGE, mM/L | 4.6 (1.1) | 4.1 (1.1) | 0.08 |
| CONGA-1 hour, mM/L | 2.1 (0.6) | 1.9 (0.5) | 0.10 |
| CONGA-2 hours, mM/L | 2.2 (0.7) | 2.0 (0.6) | 0.10 |
| CONGA-4 hours, mM/L | 2.2 (0.5) | 2.0 (0.5) | 0.06 |
| CONGA-24 hours, mM/L | 2.2 (0.7) | 1.9 (0.5) | 0.03 |
| Risk metrics | |||
| Average daily risk range | 24.5 (7.4) | 22.2 (7.7) | 0.03 |
| Low blood glucose index | 1.9 (1.6) | 1.7 (0.9) | 0.48 |
| High blood glucose index | 1.0 (0.8) | 0.7 (0.6) | 0.03 |
| Normalized hypoglycaemic events | |||
| Below 3.9 mM/L | 1.2 (1.4) | 1.5 (1.1) | 0.75 |
| Below 3.3 mM/L | 0.4 (0.5) | 0.4 (0.5) | 0.14 |
| Below 3 mM/L | 0.2 (0.3) | 0.2 (0.2) | 0.18 |
Note: Daytime refers to 6:00 am to 23:59 pm. Data shown are median (median absolute deviations), unless otherwise indicated. P values were generated using Wilcoxon’s signed-rank tests.
Abbreviations: CONGA, continuous overall net glycaemic action; MAGE, mean amplitude of glycaemic excursion.
TABLE 4.
Nighttime glucose metrics
| Masked | Unmasked | P value | |
|---|---|---|---|
| Nighttime sensor glucose metrics | |||
| Median (quartiles), mM/L | 5.0 (4.8, 5.7) | 5.2 (4.8, 5.9) | 0.68 |
| Mean, mM/L | 5.3 (0.9) | 5.5 (0.5) | 0.31 |
| Peak, mM/L | 10.1 (2.2) | 10.8 (3.4) | 0.50 |
| Nadir, mM/L | 2.5 (0.4) | 3.0 (1.1) | 0.35 |
| Range, mM/L | 7.6 (2.8) | 7.8 (3.3) | 0.86 |
| Percent distribution | |||
| Above 13.9 mM/L | 0 (0) | 0 (0) | 0.40 |
| Above 10 mM/L | 0.2 (0.3) | 0.5 (0.8) | 0.40 |
| 3.9–10 mM/L | 93.3 (5.8) | 96.5 (4.7) | 0.08 |
| Below 3.9 mM/L | 5.5 (5.7) | 2.8 (2.7) | 0.07 |
| Below 3.3 mM/L | 1.1 (1.6) | 0.8 (1.1) | 0.06 |
| Below 3 mM/L | 0.7 (1.0) | 0 (0) | 0.06 |
| Glycaemic variability | |||
| Standard deviation, mM/L | 1.1 (0.4) | 1.2 (0.4) | 0.77 |
| Mean coefficient of variation, % | 13.9 (6.7) | 15.2 (8.0) | 0.17 |
| MAGE, mM/L | 3.3 (1.4) | 3.2 (1.2) | 0.10 |
| CONGA-1 hour, mM/L | 1.1 (0.7) | 1.2 (0.7) | 0.03 |
| CONGA-2 hours, mM/L | 1.4 (0.9) | 1.5 (0.7) | 0.04 |
| CONGA-4 hours, mM/L | 1.4 (0.7) | 1.4 (0.7) | 0.32 |
| CONGA-24 hours, mM/L | 1.4 (0.7) | 1.5 (0.5) | 0.31 |
| Risk metrics | |||
| Average daily risk range | 9.1 (3.2) | 10.0 (5.6) | 0.92 |
| Low blood glucose index | 2.3 (2.3) | 1.8 (1.0) | 0.16 |
| High blood glucose index | 0.2 (0.63) | 0.3 (0.4) | 0.44 |
| Normalized hypoglycaemic events | |||
| Below 3.9 mM/L | 0.4 (0.3) | 0.4 (0.4) | 0.28 |
| Below 3.3 mM/L | 0.2 (0.3) | 0.2 (0.2) | 0.02 |
| Below 3.0 mM/L | 0.1 (0.1) | 0.04 (0.1) | 0.08 |
Note: Nighttime refers to 12:00 am to 5:59 am. Data shown are median (median absolute deviations), unless otherwise indicated. P values were generated using Wilcoxon signed-rank tests.
Abbreviations: CONGA, continuous overall net glycaemic action; MAGE, mean amplitude of glycaemic excursion.
FIGURE 3.
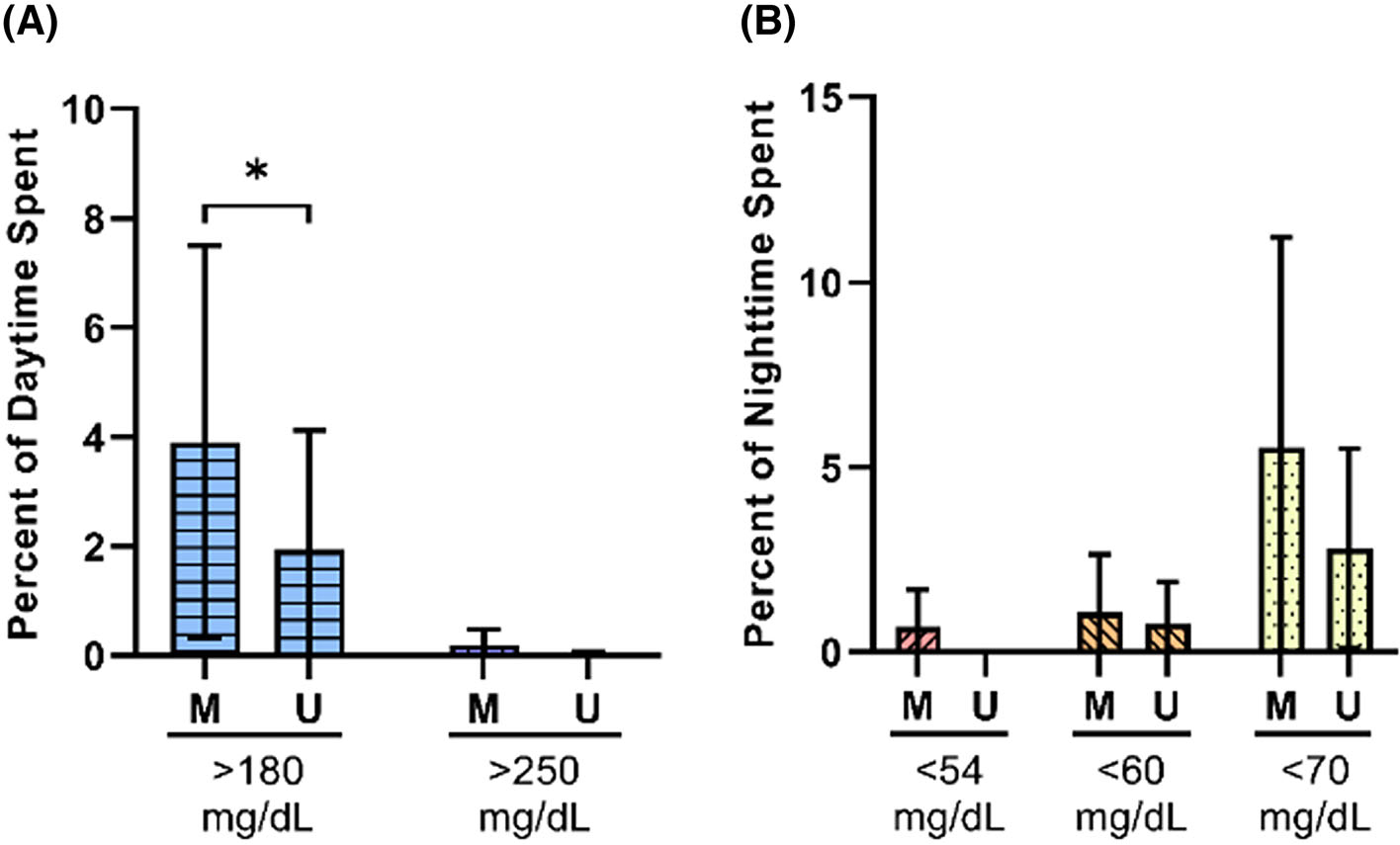
Effect of unmasking on percent distribution for daytime and nighttime data. The median percent distributions ± median absolute deviations in each phase for the (A) daytime and (B) nighttime data (n = 22). *P < 0.05. M, masked; U, unmasked
We performed a sensitivity analysis which excluded those five study participants who had adjustment of hypoglycaemia therapies of any type during the course of the study (described above). Analysis of this subcohort (n = 17) demonstrated similar patterns to those of the entire cohort. For all comparisons that were significant in the entire cohort, directionality and magnitude of change was similar in the subcohort analysis, with significance remaining for time above 10 mM/L during the day (reduced in unmasked phase), daytime CONGA-24 hours (reduced in masked phase), and nighttime CONGA-1 hour and CONGA-2 hours (increased in masked phase).
During the unmasked phase, alarms were triggered by glycaemic excursions (at least one value <3.9 or >10 mM/L); for the 10 participants wearing the G6 device, alarm information was available from the device download. Surprisingly, there were only six alarms in two participants wearing the G6 device in the unmasked phase (two alarms <3.9 mM/L and two >10 mM/L in one participant, two alarms <3.9 mM/L in another participant). By comparison, there were 64 episodes in the masked phase (which would have triggered alarms if not masked) in these same participants.
We analysed hypoglycaemia awareness and fear based on surveys completed by participants at each study visit. The Gold score, reflecting self-reported unawareness, was significantly higher after completion of the unmasked phase, with the percentage of participants reporting impaired awareness increasing from 50% after completion of the masked phase, to 67% after completion of the unmasked phase (Figure S5A/B). Similarly, there was a significant increase in raw Clarke score during the course of the study, with a significant increase in the percentage reporting impaired awareness (Figure S5C/D). Fear of hypoglycaemia was assessed using the HFS-II survey. There were no significant differences between visits, but scores for both domains (behaviour and worry) trended higher after completion of the unmasked phase (Figure S5E/F).
There were no direct adverse effects of sensor wear, including infection, bleeding, or severe cutaneous irritation. Two participants reported mild erythema at the site of a single Dexcom G4 sensor insertion. One participant reported transient discomfort at the site of the sensor after a single insertion.
4 |. DISCUSSION
Similarly to patients with diabetes, patients with PBH can experience severe hypoglycaemia with neuroglycopenia, and recurrent hypoglycaemia can be associated with hypoglycaemia unawareness, further compromising patient safety. We hypothesized that the availability of sensor glucose data from CGM could help reduce hypoglycaemia and mitigate glycaemic variability in patients with PBH in a real-world setting. To test this hypothesis, we compared glycaemic patterns during masked (no alarms or sensor glucose data available to participants) and unmasked (full data and alarms available) CGM in a real-world setting.
Our study cohort displayed glycaemic patterns in general agreement with those of previous analyses of patients with PBH.43,44 Relative to healthy individuals,45 they spent more time in hypoglycaemia and had increased measures of glycaemic variability, consistent with PBH.
Paired analysis of unmasked data as compared with masked data for individual participants revealed that provision of real-time sensor glucose data and alarms informing patients of impending or current low sensor glucose can significantly: (i) increase time in range; (ii) shorten the percent time spent in hypoglycaemic range by up to 50%; (iii) reduce the number of episodes with sensor glucose <3.3 mM/L; and (iv) reduce glycaemic variability. The observed 4% improvement in time in range represents a meaningful change, as defined by the recent international consensus statement for CGM metrics for clinical trials.46 The reduction in sensor glucose values below 3.9 mM/L translates to a 26-minute reduction over 24 hours, a substantial and likely clinically meaningful improvement.
We next evaluated whether the significant reductions in low sensor glucose over 24 hours were preferentially observed during daytime or nocturnal hours. While the number of data points was reduced with the time-stratified analysis, the improvement in sensor glucose values associated with unmasked CGM wear was greater during daytime periods. Interestingly, unmasked CGM had a greater impact on reduction of time spent in hyperglycaemia and increased time in range during the day, with a trend for reduction in sensor glucose values of <3.9 mM/L. Likewise, measures of glycaemic variability were numerically lower, reaching significance for standard deviation and CONGA-24 hours during daytime hours. These patterns were also observed in the sensitivity analysis of those participants without medication adjustment. Collectively, these findings suggest that patients’ increased awareness of post-meal spikes may be a significant contributor to improvement in glycaemic patterns in the unmasked phase, potentially leading to improved avoidance of foods which provoke glycaemic spikes and reduction in variability from day to day.
During nocturnal hours, there was a trend for reduced sensor glucose levels at several thresholds (<3.9, 3.3, and 3.0 mM/L) and a reduction in events <3.3 mM/L lasting ≥15 minutes. This is especially important given that symptom awareness is reduced during nocturnal hours,47,48 and that recurrent nocturnal hypoglycaemia may contribute particularly to hypoglycaemia unawareness.49 Interestingly, both CONGA-1 hour and CONGA-2 hours were higher in the unmasked phase at night, with similar trends for other markers of glycaemic variability, for unclear reasons. It is important to note that one participant spent more time in hypoglycaemia and less time in range during the unmasked phase (Figures 1D and S4B). Inspection of this individual’s unmasked CGM data and food diary revealed several days of little to no food intake, underscoring the importance of maintaining adequate nutrition while avoiding high glycaemic index carbohydrates.
We hypothesized that the reduction in low sensor glucose values and events with unmasked CGM device wear would probably be related to changes in behaviour linked to either awareness of glucose levels or receipt of alarms, prompting treatment with glucose tablets/gel, and/or a snack to achieve euglycaemia. Additionally, early treatment of hypoglycaemia and prevention of severe hypoglycaemia may increase awareness,50 further reducing subsequent hypoglycaemia. As described above, we were surprised to find only six alarms in two participants wearing the G6 device in the 10-day unmasked phase. By comparison, there were 64 episodes in the masked phase (which would have triggered alarms if not masked) in these same participants. This again demonstrates a marked improvement in low sensor glucose values. Moreover, the markedly reduced number of alarm-triggering low sensor glucose levels in the unmasked phase suggests that awareness of low glucose was likely to be the major contributor to change in glycaemia, rather than the alarms themselves.
Interestingly, participants reported greater impairment of awareness after the unmasked phase (Figure S5A–D). This could reflect participants’ new realization—based on CGM data—that hypoglycaemia does not always present with symptoms, leading them to report greater unawareness of hypoglycaemia. This also underscores that short-term reduction in hypoglycaemia does not always restore awareness, as demonstrated in individuals with diabetes.51,52 Whether prolonged CGM use could improve awareness in PBH will be an important question to address in future studies. Until then, these results demonstrate that continued use of CGM to improve safety in those with chronic hypoglycaemia unawareness is clinically impactful and appropriate.
Participants also reported increased fear of hypoglycaemia with unmasked CGM (Figure S5F). Thus, although unmasked CGM may mitigate the risks associated with hypoglycaemia unawareness, our study highlights the potential psychosocial impacts of CGM wear. Fear of hypoglycaemia could be related to alarms or just newly appreciated awareness of sensor glucose data, specifically that glucose levels were lower than previously recognized. As noted above, there were few alarms provided in the unmasked phase. This information, together with the finding that the reduction in glycaemic variability was greater for daytime hours, suggests to us that awareness of sensor glucose values was probably a greater factor than alarm number in both the benefits of CGM as well as a potential contributor to fear.
One study comparing CGM wear to self-monitoring of blood glucose in individuals with T1D (assessed at baseline and 6 months), similarly found that CGM wear does not decrease worry related to hypoglycaemia; however, there are significant improvements in measures of hypoglycaemia confidence, specifically in staying safe while sleeping and when driving, as well as improvements in disease-related and interpersonal distress.53 In this same study, satisfaction with CGM use correlated with disease-specific quality of life. Given that longer-term studies show improvements in many psychosocial variables in those with diabetes,54,55 longer-duration studies will be required to determine whether similar beneficial impact of CGM wear—beyond glycaemic variables—can also be observed in patients with PBH. Studies of longer-term wear would also allow more individualized implementation of CGM technology, including adjustment of alarm thresholds, with the goal of balancing improvements in safety versus the potential burden of constant glucose information and/or alarms.
The strengths of our study include the paired design for individual participants, allowing assessment of the impact of awareness of CGM sensor glucose data. We acknowledge several limitations of our study. CGM technology detects interstitial glucose, and is less accurate in hypoglycaemic ranges,56,57 as recently demonstrated for patients with congenital hyperinsulinism.58 CGM has been shown to be less sensitive and specific than meal provocation testing for detection of chemical hypoglycaemia.16,44 CGM readings can be affected by non-glycaemic factors, such as changes in blood flow and compression at sensor sites,59 and interfering medications such as acetaminophen (for the Dexcom G4 device).60 We did not require verification of sensor glucose values via capillary glucose monitoring, so it is possible that some sensor glucose values did not reflect true reductions in plasma glucose levels. While each participant used a single CGM system for the duration of the study, allowing paired comparison, the required change in sensor system from G4 to G6 during the course of the study may have introduced additional variables (although we found no significant difference in glycaemic patterns between the two systems’ data). Our study duration was relatively short, preventing us from assessing potentially progressive longer-term therapeutic impact of CGM-derived glucose awareness. With longer-term wear, as would be expected in the clinical setting, CGM data review would likely lead to further individualization of recommendations for meal plan and medication use. While the R package iglu and rGV were used to analyse the CGM data, other analysis tools such as easyGV61 are available. A majority of our participants (12 of 22) were taking medication(s) for PBH, which may have reduced the sensitivity to detect the impact of CGM on overall glycaemic patterns as compared with untreated individuals due to relatively low rates of low sensor glucose at baseline. Acarbose or cornstarch therapies were adjusted (either increased or decreased) by the participant (n = 4) or their clinician (n = 1) during the study in five individuals. Moreover, despite our instructions to continue current meal plans, participation in the study may have modified behaviour during the course of the study due to interactions with study personnel, increased focus on self-care, and provision of capillary blood glucose monitors and supplies, independent of CGM device wear, as would be expected in the “real-world” clinical environment. Likewise, increased self-awareness about food choices could have contributed to observed differences—an indirect effect of CGM data awareness. Unfortunately, food and activity logs provided to participants were incomplete for many, preventing us from using them to compare dietary behaviour with glycaemic outcomes. The surveys used to evaluate hypoglycaemia awareness and fear were designed for those with diabetes-related hypoglycaemia and have not been validated for patients with PBH.
Our study provides evidence for CGM as a therapeutic tool for PBH to reduce hypoglycaemia and potentially improve safety. Future studies could investigate if early and more prolonged use of personal CGM devices, implemented at time of diagnosis, decreases the frequency and severity of hypoglycaemia, and reduces the development of hypoglycaemia unawareness.62 Also, it would be important to investigate the possible synergy between dietary and lifestyle modifications and CGM use in decreasing the risk of hypoglycaemia. Future studies may consider including individuals with a history of hypoglycaemia due to other upper gastrointestinal surgeries, who could also potentially benefit from CGM use, as recently shown in infants with a history of fundoplication.63 Further studies of the impact of CGM on quality of life, mood, psychosocial functioning, and safety in PBH will be important, particularly in individuals who may be intolerant of medications used to treat PBH.
In summary, we report the efficacy of CGM device wear to address hypoglycaemia and glycaemic variability in patients with PBH in a real-world setting. These findings provide evidence supporting the implementation of CGM use in clinical care for patients with PBH, especially those with hypoglycaemia unawareness who are at greatest risk for hypoglycaemia-related injury.
Supplementary Material
ACKNOWLEDGMENTS
We gratefully acknowledge the time and effort of study participants and the assistance of Maya Abdalla and the Clinical Research Unit nursing staff.
Funding information
Dexcom
Footnotes
DISCLOSURE OF INTERESTS
Funding for this study was received via an investigator-initiated research grant to Mary Elizabeth Patti from Dexcom. Data analysis and manuscript preparation were performed by the study team without input from Dexcom. We also acknowledge support from the Diabetes Research Center grant P30 DK 036836. Mary Elizabeth Patti reports personal consulting fees from Astra Zeneca, Fractyl, Hanmi Pharmaceutical, MBX Biosciences, Recordati, Poxel, and Eiger Pharmaceuticals and grants from the Chan-Zuckerberg Initiative and Helmsley Trust, outside the submitted work. Donald C. Simonson is a stockholder/shareholder of GI Windows and his spouse is owner of Phase V Technologies (neither related to the current study).
SUPPORTING INFORMATION
Additional supporting information can be found online in the Supporting Information section at the end of this article.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1.Kirwan JP, Courcoulas AP, Cummings DE, et al. Diabetes remission in the alliance of randomized trials of medicine versus metabolic surgery in type 2 diabetes (ARMMS-T2D). Diabetes Care. 2022;45(7):1574–1583. doi: 10.2337/dc21-2441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Purnell JQ, Dewey EN, Laferrère B, et al. Diabetes remission status during seven-year follow-up of the longitudinal assessment of bariatric surgery study. J Clin Endocrinol Metab. 2021;106(3):774–788. doi: 10.1210/clinem/dgaa849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roth AE, Thornley CJ, Blackstone RP. Outcomes in bariatric and metabolic surgery: an updated 5-year review. Curr Obes Rep. 2020;9(3):380–389. doi: 10.1007/s13679-020-00389-8 [DOI] [PubMed] [Google Scholar]
- 4.Mingrone G, Panunzi S, De Gaetano A, et al. Metabolic surgery versus conventional medical therapy in patients with type 2 diabetes: 10-year follow-up of an open-label, single-Centre, randomised controlled trial. Lancet. 2021;397(10271):293–304. doi: 10.1016/s01406736(20)32649–0 [DOI] [PubMed] [Google Scholar]
- 5.Doumouras AG, Wong JA, Paterson JM, et al. Bariatric surgery and cardiovascular outcomes in patients with obesity and cardiovascular disease:: a population-based retrospective cohort study. Circulation. 2021;143(15):1468–1480. doi: 10.1161/circulationaha.120.052386 [DOI] [PubMed] [Google Scholar]
- 6.Adams TD, Gress RE, Smith SC, et al. Long-term mortality after gastric bypass surgery. N Engl J Med. 2007;357(8):753–761. doi: 10.1056/NEJMoa066603 [DOI] [PubMed] [Google Scholar]
- 7.Fisher DP, Johnson E, Haneuse S, et al. Association between bariatric surgery and macrovascular disease outcomes in patients with type 2 diabetes and severe obesity. Jama. 2018;320(15):1570–1582. doi: 10.1001/jama.2018.14619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zaloga GP, Chernow B. Postprandial hypoglycemia after Nissen fundoplication for reflux esophagitis. Gastroenterology. 1983;84(4):840–842. [PubMed] [Google Scholar]
- 9.Kataria R, Linn S, Malik Z, Abbas AE, Parkman H, Schey R. Post-fundoplication dumping syndrome: a frequent ”rare“ complication. ACG Case Rep J. 2018;5:e1. doi: 10.14309/crj.2018.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cournot M, Marquie JC, Ansiau D, et al. Relation between body mass index and cognitive function in healthy middle-aged men and women. Neurology. 2006;67(7):1208–1214. doi: 10.1212/01.wnl.0000238082.13860.50 [DOI] [PubMed] [Google Scholar]
- 11.Calabria AC, Gallagher PR, Simmons R, Blinman T, De Leon DD. Post-operative surveillance and detection of postprandial hypoglycemia after fundoplasty in children. J Pediatr. 2011;159(4):597–601.e1. doi: 10.1016/j.jpeds.2011.03.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marsk R, Jonas E, Rasmussen F, Näslund E. Nationwide cohort study of post-gastric bypass hypoglycaemia including 5,040 patients undergoing surgery for obesity in 1986–2006 in Sweden. Diabetologia. 2010;53(11):2307–2311. doi: 10.1007/s00125-010-1798-5 [DOI] [PubMed] [Google Scholar]
- 13.Sarwar H, Chapman WH 3rd, Pender JR, et al. Hypoglycemia after Roux-en-Y gastric bypass: the BOLD experience. Obes Surg. 2014; 24(7):1120–1124. doi: 10.1007/s11695-014-1260-8 [DOI] [PubMed] [Google Scholar]
- 14.Fischer LE, Wolfe BM, Fino N, et al. Postbariatric hypoglycemia: symptom patterns and associated risk factors in the longitudinal assessment of bariatric surgery study. Surg Obes Relat Dis. 2021;17(10):1787–1798. doi: 10.1016/j.soard.2021.04.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kefurt R, Langer FB, Schindler K, Shakeri-Leidenmühler S, Ludvik B, Prager G. Hypoglycemia after Roux-En-Y gastric bypass: detection rates of continuous glucose monitoring (CGM) versus mixed meal test. Surg Obes Relat Dis. 2015;11(3):564–569. doi: 10.1016/j.soard.2014.11.003 [DOI] [PubMed] [Google Scholar]
- 16.Salehi M, Vella A, McLaughlin T, Patti ME. Hypoglycemia after gastric bypass surgery: current concepts and controversies. J Clin Endocrinol Metab. 2018;103(8):2815–2826. doi: 10.1210/jc.2018-00528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nguyen NQ, Debreceni TL, Bambrick JE, et al. Rapid gastric and intestinal transit is a major determinant of changes in blood glucose, intestinal hormones, glucose absorption and postprandial symptoms after gastric bypass. Obesity (Silver Spring). 2014;22(9):2003–2009. doi: 10.1002/oby.20791 [DOI] [PubMed] [Google Scholar]
- 18.Laurenius A, Olbers T, Näslund I, Karlsson J. Dumping syndrome following gastric bypass: validation of the dumping symptom rating scale. Obes Surg. 2013;23(6):740–755. doi: 10.1007/s11695-012-0856-0 [DOI] [PubMed] [Google Scholar]
- 19.Goldfine AB, Mun EC, Devine E, et al. Patients with neuroglycopenia after gastric bypass surgery have exaggerated incretin and insulin secretory responses to a mixed meal. J Clin Endocrinol Metab. 2007;92(12):4678–4685. doi: 10.1210/jc.2007-0918 [DOI] [PubMed] [Google Scholar]
- 20.Salehi M, Prigeon RL, D’Alessio DA. Gastric bypass surgery enhances glucagon-like peptide 1-stimulated postprandial insulin secretion in humans. Diabetes. 2011;60(9):2308–2314. doi: 10.2337/db11-0203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abrahamsson N, Börjesson JL, Sundbom M, Wiklund U, Karlsson FA, Eriksson JW. Gastric bypass reduces symptoms and hormonal responses in hypoglycemia. Diabetes. 2016;65(9):2667–2675. doi: 10.2337/db16-0341 [DOI] [PubMed] [Google Scholar]
- 22.Øhrstrøm CC, Hansen DL, Kielgast UL, et al. Counterregulatory responses to postprandial hypoglycemia after roux-en-Y gastric bypass. Surg Obes Relat Dis. 2021;17(1):55–63. doi: 10.1016/j.soard.2020.08.037 [DOI] [PubMed] [Google Scholar]
- 23.Salehi M, Gastaldelli A, DeFronzo R. Prandial hepatic glucose production during hypoglycemia is altered after gastric bypass surgery and sleeve gastrectomy. Metabolism. 2022;131:155199. doi: 10.1016/j.metabol.2022.155199 [DOI] [PubMed] [Google Scholar]
- 24.Zhou LY, Deng MQ, Xiao XH. Potential contribution of the gut microbiota to hypoglycemia after gastric bypass surgery. Chin Med J. 2020;133(15):1834–1843. doi: 10.1097/cm9.0000000000000932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mulla CM, Goldfine AB, Dreyfuss JM, et al. Plasma FGF-19 levels are increased in patients with post-bariatric hypoglycemia. Obes Surg. 2019;29(7):2092–2099. doi: 10.1007/s11695-019-03845-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van den Broek M, de Heide LJM, Sips FLP, et al. Altered bile acid kinetics contribute to postprandial hypoglycaemia after roux-en-Y gastric bypass surgery. Int J Obes. 2021;45(3):619–630. doi: 10.1038/s41366-020-00726-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van Furth AM, van den Broek M, Emous M, de Heide LJM, Kuipers F, van Beek AP. Cholecystectomy increases the risk of dumping syndrome and postbariatric hypoglycemia after bariatric surgery. Surg Obes Relat Dis. 2020;16(12):1939–1947. doi: 10.1016/j.soard.2020.08.009 [DOI] [PubMed] [Google Scholar]
- 28.Suhl E, Anderson-Haynes SE, Mulla C, Patti ME. Medical nutrition therapy for post-bariatric hypoglycemia: practical insights. Surg Obes Relat Dis. 2017;13(5):888–896. doi: 10.1016/j.soard.2017.01.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Øhrstrøm CC, Worm D, Højager A, et al. Postprandial hypoglycaemia after roux-en-Y gastric bypass and the effects of acarbose, sitagliptin, verapamil, liraglutide and pasireotide. Diabetes Obes Metab. 2019;21(9):2142–2151. doi: 10.1111/dom.13796 [DOI] [PubMed] [Google Scholar]
- 30.Scarpellini E, Arts J, Karamanolis G, et al. International consensus on the diagnosis and management of dumping syndrome. Nat Rev Endocrinol. 2020;16(8):448–466. doi: 10.1038/s41574-020-0357-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thondamm SK, Nair S, Wile D, Gill GV. Diazoxide for the treatment of hypoglycaemic dumping syndrome. QJM. 2013;106(9):855–858. doi: 10.1093/qjmed/hcr234 [DOI] [PubMed] [Google Scholar]
- 32.Gonzalez-Gonzalez A, Delgado M, Fraga-Fuentes MD. Use of diazoxide in management of severe postprandial hypoglycemia in patient after Roux-en-Y gastric bypass. Surg Obes Relat Dis. 2013;9(1):e18–e19. doi: 10.1016/j.soard.2011.05.010 [DOI] [PubMed] [Google Scholar]
- 33.Michaels AD, Hunter Mehaffey J, Brenton French W, Schirmer BD, Kirby JL, Hallowell PT. Hypoglycemia following bariatric surgery: our 31-year experience. Obes Surg. 2017;27(12):3118–3123. doi: 10.1007/s11695-017-2734-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cox DJ, Irvine A, Gonder-Frederick L, Nowacek G, Butterfield J. Fear of hypoglycemia: quantification, validation, and utilization. Diabetes Care. 1987;10(5):617–621. doi: 10.2337/diacare.10.5.617 [DOI] [PubMed] [Google Scholar]
- 35.Gold AE, Macleod KM, Frier BM. Frequency of severe hypoglycemia in patients with type I diabetes with impaired awareness of hypoglycemia. Diabetes Care. 1994;17(7):697–703. doi: 10.2337/diacare.17.7.697 [DOI] [PubMed] [Google Scholar]
- 36.Clarke WL, Cox DJ, Gonder-Frederick LA, Julian D, Schlundt D, Polonsky W. Reduced awareness of hypoglycemia in adults with IDDM. A prospective study of hypoglycemic frequency and associated symptoms. Diabetes Care. 1995;18(4):517–522. doi: 10.2337/diacare.18.4.517 [DOI] [PubMed] [Google Scholar]
- 37.Deary IJ, Hepburn DA, MacLeod KM, Frier BM. Partitioning the symptoms of hypoglycaemia using multi-sample confirmatory factor analysis. Diabetologia. 1993;36(8):771–777. doi: 10.1007/BF00401150 [DOI] [PubMed] [Google Scholar]
- 38.Broll S, Urbanek J, Buchanan D, et al. Interpreting blood GLUcose data with R package iglu. PLoS One. 2021;16(4):e0248560. doi: 10.1371/journal.pone.0248560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Olawsky E, Zhang Y, Eberly LE, Helgeson ES, Chow LS. A new analysis tool for continuous glucose monitor data. J Diabetes Sci Technol. 2022;16(6):1496–1504. doi: 10.1177/19322968211028909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee CJ, Clark JM, Schweitzer M, et al. Prevalence of and risk factors for hypoglycemic symptoms after gastric bypass and sleeve gastrectomy. Obesity. 2015;23(5):1079–1084. doi: 10.1002/oby.21042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee CJ, Brown TT, Schweitzer M, Magnuson T, Clark JM. The incidence and risk factors associated with developing symptoms of hypoglycemia after bariatric surgery. Surg Obes Relat Dis. 2018;14(6):797–802. doi: 10.1016/j.soard.2018.01.028 [DOI] [PubMed] [Google Scholar]
- 42.Ratner RE. Hypoglycemia: new definitions and regulatory implications. Diabetes Technol Ther. 2018;20(S2):S250–S253. doi: 10.1089/dia.2018.0113 [DOI] [PubMed] [Google Scholar]
- 43.Lee D, Dreyfuss JM, Sheehan A, Puleio A, Mulla CM, Patti ME. Glycemic patterns are distinct in post-bariatric hypoglycemia after gastric bypass (PBH-RYGB). J Clin Endocrinol Metab. 2021;106(8):2291–2303. doi: 10.1210/clinem/dgab323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Honka H, Chuang J, D’Alessio D, Salehi M. Utility of continuous glucose monitoring vs meal study in detecting hypoglycemia after gastric bypass. J Clin Endocrinol Metab. 2022;107(5):e2095–e2102. doi: 10.1210/clinem/dgab913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shah VN, DuBose SN, Li Z, et al. Continuous glucose monitoring profiles in healthy nondiabetic participants: a multicenter prospective study. J Clin Endocrinol Metab. 2019;104(10):4356–4364. doi: 10.1210/jc.2018-02763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Battelino T, Alexander CM, Amiel SA, et al. Continuous glucose monitoring and metrics for clinical trials: an international consensus statement. Lancet Diabetes Endocrinol. 2023;11(1):42–57. doi: 10.1016/s2213-8587(22)00319-9 [DOI] [PubMed] [Google Scholar]
- 47.Banarer S, Cryer PE. Sleep-related hypoglycemia-associated autonomic failure in type 1 diabetes: reduced awakening from sleep during hypoglycemia. Diabetes. 2003;52(5):1195–1203. doi: 10.2337/diabetes.52.5.1195 [DOI] [PubMed] [Google Scholar]
- 48.Lin YK, Groat D, Chan O, et al. Associations between the time in hypoglycemia and hypoglycemia awareness status in type 1 diabetes patients using continuous glucose monitoring systems. Diabetes Technol Ther. 2020;22(11):787–793. doi: 10.1089/dia.2020.0016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Veneman T, Mitrakou A, Mokan M, Cryer P, Gerich J. Induction of hypoglycemia unawareness by asymptomatic nocturnal hypoglycemia. Diabetes. 1993;42(9):1233–1237. doi: 10.2337/diab.42.9.1233 [DOI] [PubMed] [Google Scholar]
- 50.Hendrieckx C, Hagger V, Jenkins A, Skinner TC, Pouwer F, Speight J. Severe hypoglycemia, impaired awareness of hypoglycemia, and self-monitoring in adults with type 1 diabetes: results from diabetes MILES-Australia. J Diabetes Complications. 2017;31(3):577–582. doi: 10.1016/j.jdiacomp.2016.11.013 [DOI] [PubMed] [Google Scholar]
- 51.Lin YK, Hung M, Sharma A, et al. Impaired awareness of hypoglycemia continues to BE a risk factor for severe hypoglycemia despite the use of continuous glucose monitoring system IN type 1 diabetes. Endocr Pract. 2019;25(6):517–525. doi: 10.4158/ep-2018-0527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zekarias K, Moheet A, Kumar A, Seaquist ER. Does continuous glucose monitoring (CGM) restore hypoglycemia awareness in patients with type 1 diabetes (T1D) who previously had impaired awareness of hypoglycemia (IAH)?jdiabetesjAmerican Diabetes Association. Diabetes. 2022;67(1):2175. doi: 10.2337/db18-2175-PUB [DOI] [Google Scholar]
- 53.Polonsky WH, Hessler D, Ruedy KJ, Beck RW. The impact of continuous glucose monitoring on markers of quality of life in adults with type 1 diabetes: further findings from the DIAMOND randomized clinical trial. Diabetes Care. 2017;40(6):736–741. doi: 10.2337/dc17-0133 [DOI] [PubMed] [Google Scholar]
- 54.Gilbert TR, Noar A, Blalock O, Polonsky WH. Change in hemoglobin A1c and quality of life with real-time continuous glucose monitoring use by people with insulin-treated diabetes in the landmark study. Diabetes Technol Ther. 2021;23(S1):S35–s39. doi: 10.1089/dia.2020.0666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nana M, Moore SL, Ang E, Lee ZX, Bondugulapati LNR. Flash glucose monitoring: impact on markers of glycaemic control and patient-reported outcomes in individuals with type 1 diabetes mellitus in the real-world setting. Diabetes Res Clin Pract. 2019;157:107893. doi: 10.1016/j.diabres.2019.107893 [DOI] [PubMed] [Google Scholar]
- 56.Shah VN, Laffel LM, Wadwa RP, Garg SK. Performance of a factory-calibrated real-time continuous glucose monitoring system utilizing an automated sensor applicator. Diabetes Technol Ther. 2018;20(6): 428–433. doi: 10.1089/dia.2018.0143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wadwa RP, Laffel LM, Shah VN, Garg SK. Accuracy of a factory-calibrated, real-time continuous glucose monitoring system during 10 days of use in youth and adults with diabetes. Diabetes Technol Ther. 2018;20(6):395–402. doi: 10.1089/dia.2018.0150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Worth C, Dunne MJ, Salomon-Estebanez M, et al. The hypoglycaemia error grid: a UK-wide consensus on CGM accuracy assessment in hyperinsulinism. Front Endocrinol. 2022;13:1016072. doi: 10.3389/fendo.2022.1016072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mensh BD, Wisniewski NA, Neil BM, Burnett DR. Susceptibility of interstitial continuous glucose monitor performance to sleeping position. J Diabetes Sci Technol. 2013;7(4):863–870. doi: 10.1177/193229681300700408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Denham D Effect of repeated doses of acetaminophen on a continuous glucose monitoring system with Permselective membrane. J Diabetes Sci Technol. 2021;15(2):517–518. doi: 10.1177/1932296820948544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Moscardo V, Giménez M, Oliver N, Hill NR. Updated software for automated assessment of glucose variability and quality of glycemic control in diabetes. Diabetes Technol Ther. 2020;22(10):701–708. doi: 10.1089/dia.2019.0416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hermanns N, Heinemann L, Freckmann G, Waldenmaier D, Ehrmann D. Impact of CGM on the management of hypoglycemia problems: overview and secondary analysis of the HypoDE study. J Diabetes Sci Technol. 2019;13(4):636–644. doi: 10.1177/1932296819831695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sato D, Morino K, Ohashi N, et al. Octreotide improves early dumping syndrome potentially through incretins: a case report. Endocr J. 2013; 60(7):847–853. doi: 10.1507/endocrj.ej12-0288 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


