Abstract
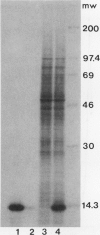
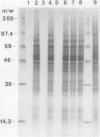
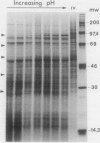
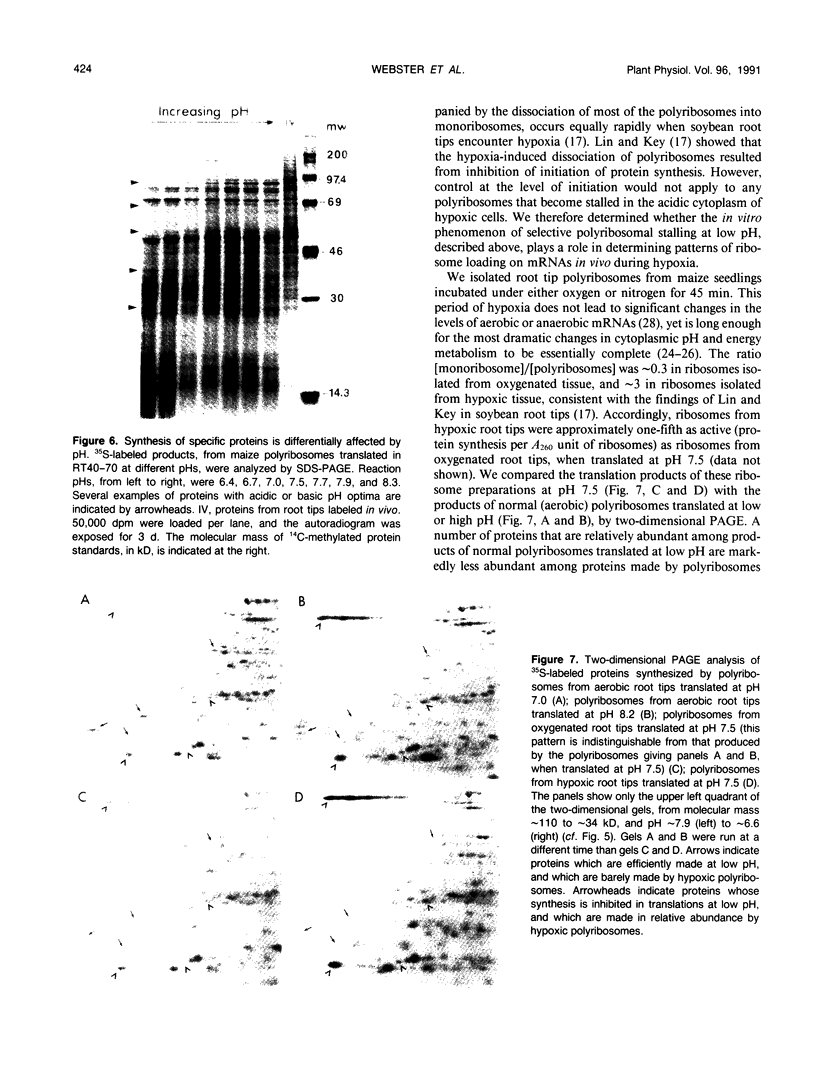
We show that the control of gene expression at the level of elongation and termination of protein synthesis can be observed in vitro. Free cytoplasmic polyribosomes were isolated from maize (Zea mays) root tips, and translated in root tip extracts that had been fractionated with ammonium sulfate to contain elongation factors, and be depleted in initiation factors. The root tip extract performs elongation and termination reactions as efficiently as wheat germ extracts. The translation products of the maize system are the same as made in vivo. The dependence of these in vitro elongation and termination reactions on pH was determined. Total protein synthesis in this system exhibits an optimum at pH ∼7.5. However, the pH dependence of rates of synthesis of individual proteins is not at all uniform; many polyribosomes become stalled when translated at low pH. These data were compared with the elongation and termination capacity of polyribosomes isolated from oxygenated and hypoxic root tips (tissue having, respectively, high and low cytoplasmic pH values). We observed an inverse relationship between the relative abundance of many specific translatable mRNAs in polyribosomes of hypoxic root tips, and the relative rates of elongation and termination reactions on the different mRNAs at low pH in vitro. These results suggest that changes in intracellular pH in hypoxic root tips can be sensed directly by the translational machinery and thereby selectively modulate gene expression.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beachy R. N., Thompson J. F., Madison J. T. Isolation of polyribosomes and messenger RNA active in in vitro synthesis of soybean seed proteins. Plant Physiol. 1978 Feb;61(2):139–144. doi: 10.1104/pp.61.2.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry J. O., Carr J. P., Klessig D. F. mRNAs encoding ribulose-1,5-bisphosphate carboxylase remain bound to polysomes but are not translated in amaranth seedlings transferred to darkness. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4190–4194. doi: 10.1073/pnas.85.12.4190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehling E., Weidner M. Temperature characteristics and adaptive potential of wheat ribosomes. Plant Physiol. 1986 Jan;80(1):181–186. doi: 10.1104/pp.80.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim N. G., Burke J. P., Beattie D. S. The sensitivity of rat liver and yeast mitochondrial ribosomes to inhibitors of protein synthesis. J Biol Chem. 1974 Nov 10;249(21):6806–6811. [PubMed] [Google Scholar]
- Johnson J., Cobb B. G., Drew M. C. Hypoxic Induction of Anoxia Tolerance in Root Tips of Zea mays. Plant Physiol. 1989 Nov;91(3):837–841. doi: 10.1104/pp.91.3.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Key J. L., Lin C. Y., Chen Y. M. Heat shock proteins of higher plants. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3526–3530. doi: 10.1073/pnas.78.6.3526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk M. M., Kirk D. L. Translational regulation of protein synthesis, in response to light, at a critical stage of Volvox development. Cell. 1985 Jun;41(2):419–428. doi: 10.1016/s0092-8674(85)80015-5. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laroche A., Hopkins W. G. Isolation and in vitro translation of polysomes from mature rye leaves. Plant Physiol. 1987 Feb;83(2):371–376. doi: 10.1104/pp.83.2.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lax S. R., Lauer S. J., Browning K. S., Ravel J. M. Purification and properties of protein synthesis initiation and elongation factors from wheat germ. Methods Enzymol. 1986;118:109–128. doi: 10.1016/0076-6879(86)18068-2. [DOI] [PubMed] [Google Scholar]
- Lin C. Y., Key J. L. Dissocation and reassembly of polyribosomes in relation to protein synthesis in the soybean root. J Mol Biol. 1967 Jun 14;26(2):237–247. doi: 10.1016/0022-2836(67)90294-x. [DOI] [PubMed] [Google Scholar]
- Lopo A. C., MacMillan S., Hershey J. W. Translational control in early sea urchin embryogenesis: initiation factor eIF4F stimulates protein synthesis in lysates from unfertilized eggs of Strongylocentrotus purpuratus. Biochemistry. 1988 Jan 12;27(1):351–357. doi: 10.1021/bi00401a053. [DOI] [PubMed] [Google Scholar]
- Morrissey J. H. Silver stain for proteins in polyacrylamide gels: a modified procedure with enhanced uniform sensitivity. Anal Biochem. 1981 Nov 1;117(2):307–310. doi: 10.1016/0003-2697(81)90783-1. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Reger B. J., Smillie R. M., Fuller R. C. Protein synthesis by isolated etioplasts and chloroplasts from pea and wheat and the effects of chloramphenicol and cycloheximide. Plant Physiol. 1972 Jul;50(1):19–23. doi: 10.1104/pp.50.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts J. K., Callis J., Jardetzky O., Walbot V., Freeling M. Cytoplasmic acidosis as a determinant of flooding intolerance in plants. Proc Natl Acad Sci U S A. 1984 Oct;81(19):6029–6033. doi: 10.1073/pnas.81.19.6029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts J. K., Callis J., Wemmer D., Walbot V., Jardetzky O. Mechanisms of cytoplasmic pH regulation in hypoxic maize root tips and its role in survival under hypoxia. Proc Natl Acad Sci U S A. 1984 Jun;81(11):3379–3383. doi: 10.1073/pnas.81.11.3379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts J. K., Lane A. N., Clark R. A., Nieman R. H. Relationships between the rate of synthesis of ATP and the concentrations of reactants and products of ATP hydrolysis in maize root tips, determined by 31P nuclear magnetic resonance. Arch Biochem Biophys. 1985 Aug 1;240(2):712–722. doi: 10.1016/0003-9861(85)90080-3. [DOI] [PubMed] [Google Scholar]
- Rowlands A. G., Montine K. S., Henshaw E. C., Panniers R. Physiological stresses inhibit guanine-nucleotide-exchange factor in Ehrlich cells. Eur J Biochem. 1988 Jul 15;175(1):93–99. doi: 10.1111/j.1432-1033.1988.tb14170.x. [DOI] [PubMed] [Google Scholar]
- Sachs M. M., Freeling M., Okimoto R. The anaerobic proteins of maize. Cell. 1980 Jul;20(3):761–767. doi: 10.1016/0092-8674(80)90322-0. [DOI] [PubMed] [Google Scholar]
- Sun S. M., Buchbinder B. U., Hall T. C. Cell-free Synthesis of the Major Storage Protein of the Bean, Phaseolus vulgaris L. Plant Physiol. 1975 Dec;56(6):780–785. doi: 10.1104/pp.56.6.780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taliercio E. W., Chourey P. S. Post-transcriptional control of sucrose synthase expression in anaerobic seedlings of maize. Plant Physiol. 1989 Aug;90(4):1359–1364. doi: 10.1104/pp.90.4.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]