Abstract
We investigated the role of the ventromedial prefrontal cortex (vmPFC) in extinction of conditioned taste aversion (CTA) by microinfusing a protein synthesis inhibitor or N-methyl-d-asparate (NMDA) receptors antagonist into the vmPFC immediately following a non-reinforced extinction session. We found that the protein synthesis blocker anisomycin, but not the NMDA receptors antagonist D,L-2-amino-5-phosphonovaleric acid, impaired CTA extinction in the vmPFC. Anisomycin microinfusion into vmPFC had no effect on CTA acquisition and by itself did not induce CTA. These findings show the necessary role functional protein synthesis is playing in the vmPFC during the learning of CTA extinction.
Experimental extinction is the decline in the frequency or intensity of the conditioned response following the withdrawal of reinforcement and assumed to reflect relearning rather than unlearning (Bouton 1994; Rescorla 1996; Berman and Dudai 2001; Myers and Davis 2002).
The ventromedial prefrontal cortex (vmPFC) has been suggested to play a significant role in the extinction of conditioned fear responses (Milad and Quirk 2002; Akirav et al. 2006). For example, lesions or inhibition of protein synthesis in the vmPFC impair extinction of conditioned fear (Morgan et al. 1993; Quirk et al. 2000; Santini et al. 2004). Furthermore, vmPFC electrical stimulation that mimics extinction-induced conditioned stimulus firing responses reduces conditioned fear (Milad and Quirk 2002; Milad et al. 2004), and stimulating the mediodorsal thalamic inputs to mPFC was associated with the maintenance of extinction of learned fear (Herry and Garcia 2002, 2003).
Here, we aimed to generalize the involvement of the vmPFC in extinction learning to another aversive memory not related to fear response. Toward that end, we used conditioned taste aversion (CTA), in which rats learn to avoid a taste if the first encounter with that taste is followed by malaise (Garcia et al. 1955). CTA acquisition and the resulting aversion memory is robust and long lasting. Nevertheless, CTA memory can be extinguished following retrieval in the absence of reinforcer. Both acquisition and extinction of CTA are subserved by the insular cortex (Rosenblum et al. 1993; Berman et al. 1998; Eisenberg et al. 2003; Bermudez-Rattoni 2004; Belelovsky et al. 2005) and the amygdala (Yasoshima et al. 2000; Bahar et al. 2003; Akirav 2006). A recent study reported that c-Fos protein expression increases dramatically in both the infralimbic and prelimbic areas (IL and PL; together they compose the vmPFC) after rats have extinguished CTA (Mickley et al. 2005). This suggests that the vmPFC has a role in the maintenance of CTA extinction. Moreover, anatomical data show connections between the vmPFC and the insular cortex that could be important for CTA extinction (Gabbott et al. 2003).
Here we examined whether a blockade of protein synthesis or N-methyl-d-asparate (NMDA) receptor in the vmPFC would interfere with CTA extinction learning. The requirement for protein synthesis is a universal of long-term memory consolidation (Dudai 1996), and NMDA receptor activation in the vmPFC is known to be involved in learning in a variety of paradigms (Tronel and Sara 2003; Takehara-Nishiuchi et al. 2005; Akirav and Maroun 2006). We hypothesized that CTA extinction is dependent on the vmPFC, and thus should be blocked by inhibition of protein synthesis and/or blockade of NMDA receptor in the vmPFC following a non-reinforced session.
The subjects were male Wistar rats (∼60 d old, 250–300 g), caged individually at 22 ± 2°C under 12-h light/12-h dark cycles. All tests were performed between 10 a.m. and 4 p.m. The experiments were approved by the University of Haifa Ethics and Animal Care Committee, and adequate measures were taken to minimize pain or discomfort in accordance with the guidelines laid down by the NIH in the US regarding the care and use of animals for experimental procedures. Saccharin (0.1% w/v) was used as the conditioned stimulus (CS), and lithium chloride (LiCl, 0.15 M, 2% body weight, intraperitoneally [i.p.]) as the malaise-inducing agent (unconditioned stimulus [US]). Rats were pre-trained to get their water ration once a day for 10 min from two pipettes each containing 10 mL of tap water. On the conditioning day, they were allowed to drink the saccharin solution instead of water for 10 min, and 40 min after the offset of the drinking period, they were injected with LiCl i.p. For the next two days, the rats were presented daily for 10 min with two pipettes each containing 10 mL of tap water. Three days after training, a 10-min multiple-choice test (consisting of three water pipettes and three saccharin pipettes, each containing 5 mL) was performed to determine the acquired aversion. The conditioned rats prefer water to saccharin at a ratio of 9:1. This test session is defined as extinction training since animals are exposed to non-reinforced presentations of a CS (saccharin) that had previously been paired with an US (LiCl). To measure CTA extinction, the test was repeated for six successive days. The conditioned aversion is presented as an aversion index, defined as mL of water/(mL of water + mL of saccharin) × 100, consumed in the test; hence, 50 indicates equal preference.
Microinfusion into the vmPFC was performed via chronically implanted cannulae. Rats were implanted bilaterally with a guide stainless cannula (23 gauge) aimed at the IL and PL areas (anteroposterior, +3 mm relative to bregma; lateral, ±0.5 mm; ventral, −5 mm; Paxinos and Watson 1998). The cannulae were positioned in place with acrylic dental cement and secured by two skull screws. A stylus was placed in the guide cannula to prevent clogging. Animals were allowed 1 wk to recuperate. For microinfusion, the stylus was removed from the guide cannula, and a 28 gauge injection cannula, extending 1.0 mm from the tip of the guide cannula, was inserted. The injection cannula was connected via PE20 tubing to a Hamilton microsyringe driven by a microinfusion pump (CMA/100; Carnegie Medicin). Microinfusion was performed bilaterally in a 0.5-μL volume per hemisphere delivered over 1 min. The injection cannula was left in position before withdrawal for an additional 1 min to minimize dragging of the injected liquid along the injection tract.
We microinfused the protein synthesis inhibitor anisomycin (100 μg/0.5 μL), the NMDA receptor antagonist D,L-2-amino-5-phosphonovaleric acid (APV; 2.5 μg/0.5 μL or 5 μg/0.5 μL) (Sigma) or vehicle (saline 0.9%) into the vmPFC. The doses are based on previous work by Akirav and Maroun (2006).
At the completion of the behavioral experiments animals were deeply anesthetized and microinfused into the vmPFC with 0.5 μL of India ink. Frozen brain slices (60 μm) were examined under a light microscope following Nissl staining to verify the cannula location. Placements of the cannulae were found to be correct in all injected rats (Fig. 1).
Figure 1.
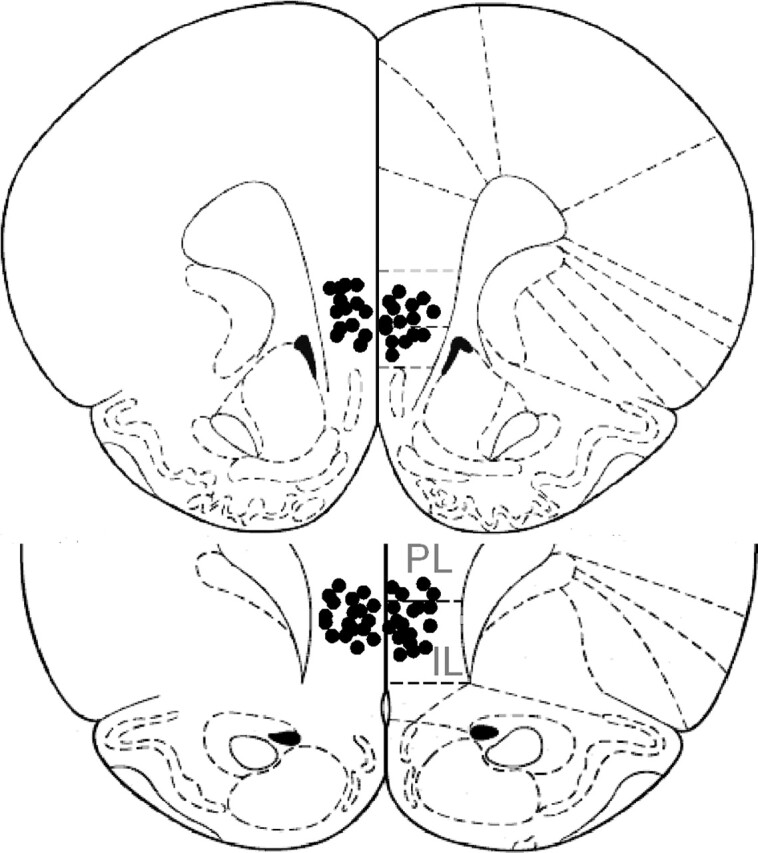
Schematic drawing of IL (infralimbic cortex) and PL (prelimbic cortex) injector tip positions (together they compose the vmPFC). Shown is a coronal view at position 3.20 and 2.70 mm anterior to bregma. Adapted with permission from Elsevier 1986, Paxinos and Watson 1986.
During the first retrieval test, i.e., before drug infusions, both groups showed a similar and marked aversion to saccharin. Anisomycin infusion into the vmPFC immediately following this test (i.e., 2 min following the offset of drinking; Aniso, n = 10) blocked extinction of CTA, compared with the vehicle animals (Vehicle, n = 10; Fig. 2A). ANOVA conducted on aversion index throughout the six test days revealed significant effects of the treatment (F(1,18) = 5.398, P < 0.05) and test day (F(1,18) = 16.215, P < 0.001), but not the interaction between treatment and day (F(1,18) = 1.556, NS). Post hoc comparison unveiled a significant difference between the vehicle and anisomycin groups throughout the experiment (P < 0.05), except for the first test day (before drug infusion) and the last test day (day 6). Moreover, both anisomycin and vehicle groups showed a significant decrease in aversion levels throughout the experiment, indicating that both groups extinguish. Thus, anisomycin microinfused following the test on day 1 shifted the aversion curve up compared with the vehicle group, but by the last day of testing there was no significant difference between the groups.
Figure 2.
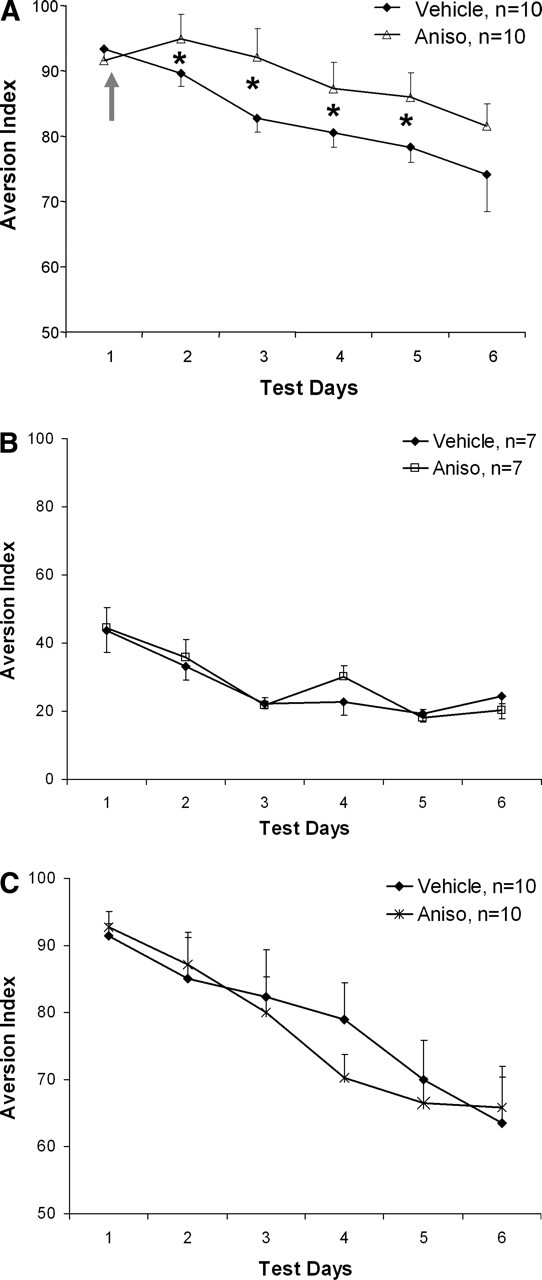
CTA extinction learning is dependent on protein synthesis in the vmPFC. (A) Animals microinfused with anisomycin (100 μg/0.5 μL) into the vmPFC immediately following test day 1 showed marked aversion to saccharin compared with the vehicle animals throughout the experiment (*P < 0.05), except for the first test day (before drug infusion) and the last test day (day 6). Arrow denotes time of drug microinfusion. (B) Animals microinfused with anisomycin into the vmPFC immediately following the first exposure to saccharin and that received an i.p. injection of saline (instead of LiCl) were not significantly different from vehicle animals in their aversion to saccharin. (C) Animals microinfused with anisomycin into the vmPFC following exposure to saccharin on conditioning day were not significantly different from vehicle animals in their aversion to saccharin.
In order to verify that microinfusion of anisomycin had no aversive value by itself and in consequence did not induce CTA, animals were microinfused with anisomycin (or saline) into the vmPFC immediately after the first exposure to saccharin (i.e., 2 min following the offset of drinking) and received i.p. injection of saline instead of LiCl (n = 7 each). Both groups did not show any aversion to saccharin as indicated by an aversion ratio <50 throughout the six test days (Fig. 2B). ANOVA did not reveal a significant effect of the treatment (F(1,12) < 1, NS), the test day (F(1,12) < 1, NS), or the interaction between treatment and day (F(1,12) < 1, NS).
We also investigated whether protein synthesis in vmPFC is specifically required for CTA extinction learning. For this purpose we evaluated the effect of anisomycin infusion in vmPFC on CTA acquisition. Animals were microinfused with anisomycin or vehicle on conditioning day (n = 10 each) shortly after the first exposure to saccharin and ∼30 min before LiCl treatment. Both groups showed marked aversion to saccharin as indicated by an aversion ratio >90 on the first test day (Fig. 2C). There was a significant effect of the test day for both groups (F(1,12) = 55.211, P < 0.001), but not of the treatment (F(1,12) < 1, NS) or the interaction between treatment and day (F(1,12) < 1, NS). Thus, protein synthesis in the vmPFC is not required for CTA acquisition.
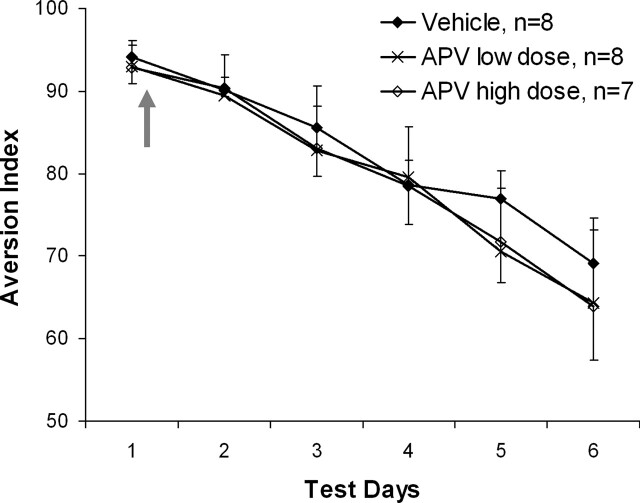
Next we examined whether CTA extinction in the vmPFC is dependent on NMDA receptor function. Hence, animals were microinfused with vehicle (n = 8), low (APV low dose, n = 8) or high (APV high dose, n = 7) doses of APV into the vmPFC immediately following the first retrieval test (i.e., 2 min following the offset of drinking; Fig. 3). ANOVA conducted on aversion index throughout the six test days revealed a significant effect of the test day (F(1,20) = 57.559, P < 0.001), but not of the treatment (F(2,20) < 1, NS) or the interaction between treatment and day (F(2,20) < 1, NS). Hence, NMDA function in the vmPFC is not required for CTA extinction learning.
Figure 3.
CTA extinction learning in the vmPFC is not dependent on NMDA receptor functions. Animals microinfused with APV into the vmPFC (low dose: 2.5 μg/0.5 μL; high dose: 5 μg/0.5 μL) immediately following test day 1 were not significantly different from vehicle animals in their aversion to saccharin. Arrow denotes time of drug microinfusion.
In this study we contribute to the generalization of the role of the vmPFC in extinction learning to another learning paradigm than fear conditioning, CTA. We show that protein synthesis in the vmPFC is required for long-term CTA extinction but not for CTA acquisition. We further suggest that CTA extinction in the vmPFC is not dependent on NMDA receptor function. This corroborates findings of Berman and Dudai (2001), showing that CTA extinction is dependent on protein synthesis, but not on the function of the NMDA receptors in the insular cortex. Yet, Fresquet et al. (2003) showed that the transaction of the frontal portions of the brain fails to affect CTA extinction. However, our experiment specifically targeted the PL and IL cortices, whereas in the lesion experiment the frontal transactions disconnected much more than just the PL and IL from the rest of the brain. Furthermore, the frontal transaction was made before CTA acquisition whereas in our study the drugs were infused following a retention test. Recent studies indicate that, as opposed to post-training lesion, pre-training lesion does not affect extinction of aversive conditioning (Anglada-Figueroa and Quirk 2005; Simon et al. 2005). This suggests compensation or partial recovery of function in animals lesioned before conditioning.
It could be argued that anisomycin did not attenuate extinction but rather reinstated the original aversion by functioning as a negative reinforcer in association with the taste (i.e., anisomycin serves as an US). However, microinfusing anisomycin into the vmPFC after the first saccharin exposure without LiCl injection did not result in aversion to saccharin. Thus, the resistance to extinction detected in the anisomycin group does not result from enhanced conditioning.
Inhibition of protein synthesis in either the vmPFC (present study), the insular cortex (Berman and Dudai 2001; Eisenberg et al. 2003), or the basolateral amygdala (Bahar et al. 2003) impaired CTA extinction. There are close anatomical and functional interactions between the IL and PL of the vmPFC, the insular cortex, and the amygdala (Krettek and Price 1977; Sesack et al. 1989; Hurley et al. 1991; Gabbott et al. 2003, 2005) that may support an important interplay between these areas during CTA extinction. Moreover, the mPFC (Santini et al. 2004) and the basolateral amygdala (Lin et al. 2003) are also involved in fear extinction whereas the insular cortex is not (Santini et al. 2004). This suggests that the neural pathway that learns CTA extinction is partly different from the one that learns fear extinction.
Inhibition of protein synthesis in vmPFC did not interfere with CTA acquisition. This is congruent with others showing that the ablation of the entire PFC failed to impair CTA acquisition (Divac et al. 1975; Isaac et al. 1989; Mogensen and Divac 1993). However, Hernadi et al. (2000) showed that lesions of the mediodorsal division of the PFC impaired CTA acquisition. The anatomical difference between our study (ventromedial division) and Hernadi et al. (2000) should be considered according to the differential contribution of dorsal and vmPFC to the acquisition of conditioned fear (Morgan and LeDoux 1995).
The intact CTA acquisition following protein synthesis inhibition in the vmPFC suggests that the effects of anisomycin on CTA extinction cannot be attributed to tissue damage or to a strong effect on basal neural activity. In support of that, it has been demonstrated that anisomycin infused to the insular cortex had no effect on CTA retrieval (Rosenblum et al. 1993) and we have recently shown that anisomycin had no effect on short-term recognition memory when infused into the vmPFC (Akirav and Maroun 2006). Additionally, anisomycin was found to impair performance in a retention test, when it was infused to the vmPFC immediately after the acquisition of a recognition task, but the deficit was no longer evident when anisomycin was infused 3 h after acquisition (Akirav and Maroun 2006).
We also show that CTA extinction in the vmPFC is not dependent on NMDA receptor activation, as previously shown in the insular cortex (Berman and Dudai 2001). However, NMDA receptor antagonist infusion in the basolateral amygdala impaired conditioned fear extinction (Falls et al. 1992; Lin et al. 2003) whereas NMDA receptors partial agonist D-cycloserine (Walker et al. 2002) facilitated fear extinction. It remains to be demonstrated if NMDA-dependent processes in the basolateral amygdala underlie CTA extinction. We suggest that the lack of effect of APV on CTA extinction is not due to low dose infusion, since this dose of APV (2.5 μg/0.5 μL) was shown to be optimal in inducing learning impairment in other paradigms such as fear conditioning and object recognition (Maren et al. 2003; Akirav and Maroun 2006). We microinfused another set of animals with a higher dose of APV (5 μg/0.5 μL) in order to verify that NMDA receptors in the vmPFC are not necessary for CTA extinction. We did not continue to examine the role of NMDA receptors in CTA acquisition since the purpose of this study was to investigate the mechanisms involved in CTA extinction.
When an animal consumes a new taste, it reduces its ingestion and, if there are no negative consequences afterward, it becomes recognized as a safe signal, leading to a gradual increase of its consumption. However, if the gustatory stimulus is paired with post-digestive malaise, the animal will recognize the taste as an aversive cue, thus developing CTA (Bermudez-Rattoni 2004; Ramirez-Lugo et al. 2006). It has been suggested that NMDA receptors are only involved in the aversive taste memory trace formation and not in an association of the taste with the absence of a negative reinforcer (for example in extinction or attenuation of neophobia) in the insular cortex and the nucleus accumbens (Berman and Dudai 2001; Ramirez-Lugo et al. 2006). Anisomycin, on the other hand, was found to be involved in both aversive and safe taste memory trace formation in the insular cortex and the amygdala (Rosenblum et al. 1993; Berman and Dudai 2001; Bahar et al. 2003; Rodriguez-Ortiz et al. 2005). This may explain the dissociation between the effects of anisomycin and APV in CTA extinction in vmPFC. However, Rosenblum et al. (1997) showed that NMDA receptors are involved in the formation of another safe taste memory trace in the insular cortex (i.e., latent inhibition). Thus, NMDA receptors may have different effects on learning, depending on the time of administration of the drug.
In conclusion, our data is consistent with the idea that extinction is an active learning process that is distinct from the acquisition of the original association and requires additional training to develop (Davis and Myers 2002). Further, the response to a CS may be orchestrated by different molecular mechanisms (protein synthesis, NMDA receptor function) in different brain regions (insular cortex, amygdala, and prefrontal cortex) at different time windows. The present study is also consistent with the general role of the PFC in the process of inhibiting CS-evoked conditioned response that takes place in extinction.
Acknowledgments
Supported by The Ebelin and Gerd Bucerius ZEIT Foundation to M.M. We thank Noam Hikind for technical assistance.
Footnotes
Article and publication are at http://www.learnmem.org/cgi/doi/10.1101/lm.191706
References
- Akirav I. 2006. NMDA partial agonist reverses blocking of extinction of aversive memory by GABAA agonist in the amygdala. Neuropsychopharmacology (in press). [DOI] [PubMed] [Google Scholar]
- Akirav I., Maroun M. 2006. Ventromedial prefrontal cortex is obligatory for consolidation and reconsolidation of object recognition memory. Cereb. Cortex (in press). [DOI] [PubMed] [Google Scholar]
- Akirav I., Raizel H., Maroun M. Enhancement of conditioned fear extinction by infusion of the GABAA agonist muscimol into the rat prefrontal cortex and amygdala. Eur. J. Neurosci. 2006;23:758–764. doi: 10.1111/j.1460-9568.2006.04603.x. [DOI] [PubMed] [Google Scholar]
- Anglada-Figueroa D., Quirk G.J. Lesions of the basal amygdala block expression of conditioned fear but not extinction. J. Neurosci. 2005;25:9680–9685. doi: 10.1523/JNEUROSCI.2600-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahar A., Samuel A., Hazvi S., Dudai Y. The amygdalar circuit that acquires taste aversion memory differs from the circuit that extinguishes it. Eur. J. Neurosci. 2003;17:1527–1530. doi: 10.1046/j.1460-9568.2003.02551.x. [DOI] [PubMed] [Google Scholar]
- Belelovsky K., Elkobi A., Kaphzan H., Nairn A.C., Rosenblum K.A. A molecular switch for translational control in taste memory consolidation. Eur. J. Neurosci. 2005;22:2560–2568. doi: 10.1111/j.1460-9568.2005.04428.x. [DOI] [PubMed] [Google Scholar]
- Berman D.E., Dudai Y. Memory extinction, learning anew, and learning the new: Dissociations in the molecular machinery of learning in cortex. Science. 2001;23:2417–2419. doi: 10.1126/science.1058165. [DOI] [PubMed] [Google Scholar]
- Berman D.E., Hazvi S., Rosenblum K., Seger R., Dudai Y. Specific and differential activation of mitogen-activated protein kinase cascades by unfamiliar taste in the insular cortex of the behaving rat. J. Neurosci. 1998;1:10037–10044. doi: 10.1523/JNEUROSCI.18-23-10037.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermudez-Rattoni F. Molecular mechanisms of taste-recognition memory. Nat. Rev. Neurosci. 2004;5:209–217. doi: 10.1038/nrn1344. [DOI] [PubMed] [Google Scholar]
- Bouton M.E. Context-specificity of target versus feature inhibition in a feature-negative discrimination. J. Exp. Psychol. Anim. Behav. Process. 1994;20:51–65. [PubMed] [Google Scholar]
- Davis M., Myers K.M. The role of glutamate and γ-aminobutyric acid in fear extinction: Clinical implications for exposure therapy. Biol. Psychiatry. 2002;15:998–1007. doi: 10.1016/s0006-3223(02)01507-x. [DOI] [PubMed] [Google Scholar]
- Divac I., Gade A., Wikmark R.G. Taste aversion in rats with lesions in the frontal lobes: No evidence for interoceptive agnosia. J. Comp. Physiol. Psychol. 1975;3:43–45. [Google Scholar]
- Dudai Y. Consolidation: Fragility on the road to the engram. Neuron. 1996;17:367–370. doi: 10.1016/s0896-6273(00)80168-3. [DOI] [PubMed] [Google Scholar]
- Eisenberg M., Kobilo T., Berman D.E., Dudai Y. Stability of retrieved memory: Inverse correlation with trace dominance. Science. 2003;22:1102–1104. doi: 10.1126/science.1086881. [DOI] [PubMed] [Google Scholar]
- Falls W.A., Miserendino M.J., Davis M. Extinction of fear-potentiated startle: Blockade by infusion of an NMDA antagonist into the amygdala. J. Neurosci. 1992;12:854–863. doi: 10.1523/JNEUROSCI.12-03-00854.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fresquet N., Yamamoto J., Sandner G. Frontal lesions do not alter the differential extinction of taste aversion conditioning in rats, when using two methods of sucrose delivery. Behav. Brain Res. 2003;17:25–34. doi: 10.1016/s0166-4328(02)00317-0. [DOI] [PubMed] [Google Scholar]
- Gabbott P.L., Warner T.A., Jays P.R., Bacon S.J. Areal and synaptic interconnectivity of prelimbic (area 32), infralimbic (area 25) and insular cortices in the rat. Brain Res. 2003;12:59–71. doi: 10.1016/j.brainres.2003.08.056. [DOI] [PubMed] [Google Scholar]
- Gabbott P.L., Warner T.A., Jays P.R., Salway P., Busby S.J. Prefrontal cortex in the rat: Projections to subcortical autonomic, motor, and limbic centers. J. Comp. Neurol. 2005;14:145–177. doi: 10.1002/cne.20738. [DOI] [PubMed] [Google Scholar]
- Garcia J., Kimmeldorf D.J., Koelling R.A. Conditioned aversion to saccharin resulting from exposure to γ radiation. Science. 1955;122:157–158. [PubMed] [Google Scholar]
- Hernadi I., Karadi Z., Vigh J., Petyko Z., Egyed R., Berta B., Lenard L. Alterations of conditioned taste aversion after microiontophoretically applied neurotoxins in the medial prefrontal cortex of the rat. Brain Res. Bull. 2000;53:751–758. doi: 10.1016/s0361-9230(00)00361-0. [DOI] [PubMed] [Google Scholar]
- Herry C., Garcia R. Prefrontal cortex long-term potentiation, but not long-term depression, is associated with the maintenance of extinction of learned fear in mice. J. Neurosci. 2002;15:577–583. doi: 10.1523/JNEUROSCI.22-02-00577.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herry C., Garcia R. Behavioral and paired-pulse facilitation analyses of long-lasting depression at excitatory synapses in the medial prefrontal cortex in mice. Behav. Brain Res. 2003;30:89–96. doi: 10.1016/j.bbr.2003.09.017. [DOI] [PubMed] [Google Scholar]
- Hurley K.M., Herbert H., Moga M.M., Saper C.B. Efferent projections of the infralimbic cortex of the rat. J. Comp. Neurol. 1991;8:249–276. doi: 10.1002/cne.903080210. [DOI] [PubMed] [Google Scholar]
- Isaac W.L., Nonneman A.J., Neisewander J., Landers T., Brado M.T. Prefrontal cortex lesions differentially disrupt cocaine-reinforced conditioned place preference but not conditioned taste aversion. Behav. Neurosci. 1989;103:345–355. doi: 10.1037//0735-7044.103.2.345. [DOI] [PubMed] [Google Scholar]
- Krettek J.E., Price J.L. The cortical projections of the mediodorsal nucleus and adjacent thalamic nuclei in the rat. J. Comp. Neurol. 1977;171:157–191. doi: 10.1002/cne.901710204. [DOI] [PubMed] [Google Scholar]
- Lin C.H., Yeh S.H., Lu H.Y., Gean P.W. The similarities and diversities of signal pathways leading to consolidation of conditioning and consolidation of extinction of fear memory. J. Neurosci. 2003;10:8310–8317. doi: 10.1523/JNEUROSCI.23-23-08310.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S., Ferrario C.R., Corcoran K.A., Desmond T.J., Frey K.A. Protein synthesis in the amygdala, but not the auditory thalamus, is required for consolidation of Pavlovian fear conditioning in rats. Eur. J. Neurosci. 2003;18:3080–3088. doi: 10.1111/j.1460-9568.2003.03063.x. [DOI] [PubMed] [Google Scholar]
- Mickley G.A., Kenmuir C.L., Yocom A.M., Wellman J.A., Biada J.M. A role for prefrontal cortex in the extinction of a conditioned taste aversion. Brain Res. 2005;27:176–182. doi: 10.1016/j.brainres.2005.05.033. [DOI] [PubMed] [Google Scholar]
- Milad M.R., Quirk G.J. Neurons in medial prefrontal cortex signal memory for fear extinction. Nature. 2002;7:70–74. doi: 10.1038/nature01138. [DOI] [PubMed] [Google Scholar]
- Milad M.R., Vidal-Gonzalez I., Quirk G.J. Electrical stimulation of medial prefrontal cortex reduces conditioned fear in a temporally specific manner. Behav. Neurosci. 2004;118:389–394. doi: 10.1037/0735-7044.118.2.389. [DOI] [PubMed] [Google Scholar]
- Mogensen J., Divac I. Behavioural changes after ablation of subdivisions of the rat prefrontal cortex. Acta Neurobiol. Exp. (Wars.) 1993;53:439–449. [PubMed] [Google Scholar]
- Morgan M.A., LeDoux J.E. Differential contribution of dorsal and ventral medial prefrontal cortex to the acquisition and extinction of conditioned fear in rats. Behav. Neurosci. 1995;109:681–688. doi: 10.1037//0735-7044.109.4.681. [DOI] [PubMed] [Google Scholar]
- Morgan M.A., Romanski L.M., LeDoux J.E. Extinction of emotional learning: Contribution of medial prefrontal cortex. Neurosci. Lett. 1993;26:109–113. doi: 10.1016/0304-3940(93)90241-c. [DOI] [PubMed] [Google Scholar]
- Myers K.M., Davis M. Behavioral and neural analysis of extinction. Neuron. 2002;14:567–584. doi: 10.1016/s0896-6273(02)01064-4. [DOI] [PubMed] [Google Scholar]
- Paxinos G., Watson C. 1986. The rat brain in stereotaxic coordinates, 3rd ed. Academic Press; San Diego, CA. [Google Scholar]
- Quirk G.J., Russo G.K., Barron J.L., Lebron K. The role of ventromedial prefrontal cortex in the recovery of extinguished fear. J. Neurosci. 2000;15:6225–6231. doi: 10.1523/JNEUROSCI.20-16-06225.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez-Lugo L., Zavala-Vega S., Bermudez-Rattoni F. NMDA and muscarinic receptors of the nucleus accumbens have differential effects on taste memory formation. Learn. Mem. 2006;13:45–51. doi: 10.1101/lm.103206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rescorla R.A. Preservation of pavlovian associations through extinction. Q. J. Exp. Psychol. 1996;49B:245–258. [Google Scholar]
- Rodriguez-Ortiz C.J., De la Cruz V., Gutierrez R., Bermudez-Rattoni F. Protein synthesis underlies post-retrieval memory consolidation to a restricted degree only when updated information is obtained. Learn. Mem. 2005;12:533–537. doi: 10.1101/lm.94505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenblum K., Meiri N., Dudai Y. Taste memory: The role of protein synthesis in gustatory cortex. Behav. Neural Biol. 1993;59:49–56. doi: 10.1016/0163-1047(93)91145-d. [DOI] [PubMed] [Google Scholar]
- Rosenblum K., Berman D.E., Hazvi S., Lamprecht R., Dudai Y. NMDA receptor and the tyrosine phosphorylation of its 2B subunit in taste learning in the rat insular cortex. J. Neurosci. 1997;1:5129–5135. doi: 10.1523/JNEUROSCI.17-13-05129.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santini E., Ge H., Ren K., de Pena Ortiz S., Quirk G.J. Consolidation of fear extinction requires protein synthesis in the medial prefrontal cortex. J. Neurosci. 2004;23:5704–5710. doi: 10.1523/JNEUROSCI.0786-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sesack S.R., Deutch A.Y., Roth R.H., Bunney B.S. Topographical organization of the efferent projections of the medial prefrontal cortex in the rat: An anterograde tract-tracing study with Phaseolus vulgaris leucoagglutinin. J. Comp. Neurol. 1989;8:213–242. doi: 10.1002/cne.902900205. [DOI] [PubMed] [Google Scholar]
- Simon B., Knuckley B., Churchwell J., Powell D.A. Post-training lesions of the medial prefrontal cortex interfere with subsequent performance of trace eyeblink conditioning. J. Neurosci. 2005;25:10740–10746. doi: 10.1523/JNEUROSCI.3003-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takehara-Nishiuchi K., Kawahara S., Kirino Y. NMDA receptor-dependent processes in the medial prefrontal cortex are important for acquisition and the early stage of consolidation during trace, but not delay eyeblink conditioning. Learn. Mem. 2005;12:606–614. doi: 10.1101/lm.5905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tronel S., Sara S.J. Blockade of NMDA receptors in prelimbic cortex induces an enduring amnesia for odor-reward associative learning. J. Neurosci. 2003;23:5472–5476. doi: 10.1523/JNEUROSCI.23-13-05472.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker D.L., Ressler K.J., Lu K.T., Davis M. Facilitation of conditioned fear extinction by systemic administration or intra-amygdala infusions of D-cycloserine as assessed with fear-potentiated startle in rats. J. Neurosci. 2002;15:2343–2351. doi: 10.1523/JNEUROSCI.22-06-02343.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasoshima Y., Morimoto T., Yamamoto T. Different disruptive effects on the acquisition and expression of conditioned taste aversion by blockades of amygdalar ionotropic and metabotropic glutamatergic receptor subtypes in rats. Brain Res. 2000;869:15–24. doi: 10.1016/s0006-8993(00)02397-0. [DOI] [PubMed] [Google Scholar]