Abstract
In recent years, the effect of sleep on memory consolidation has received considerable attention. In humans, these studies concentrated mainly on procedural types of memory, which are considered to be hippocampus-independent. Here, we show that sleep also has a persisting effect on hippocampus-dependent declarative memory. In two experiments, we examined high school students’ ability to remember vocabulary. We show that declarative memory is enhanced when sleep follows within a few hours of learning, independent of time of day, and with equal amounts of interference during retention intervals. Sleep deprivation has a detrimental effect on memory, which was significant after a night of recovery sleep. Thus, fatigue accumulating during wake intervals could be ruled out as a confound.
In recent years, the effect of sleep on the consolidation of non-declarative, i.e., motor and visual-procedural, memories has received considerable attention (Maquet et al. 2000; Stickgold et al. 2000). Most recent studies on sleep-associated memory consolidation have focused on non-declarative types of tasks, such as motor sequence learning and perceptual learning (Gais et al. 2000; Walker et al. 2003). However, the first reports of enhanced memory consolidation during sleep came from studies investigating declarative memory for verbal material. Jenkins and Dallenbach (1924) found less forgetting of nonsense syllables after sleep periods than after wakefulness. Fowler et al. (1973) found higher retention of paired-associate words when subjects slept during the first half of the night than when they were awake during daytime. A more recent study confirmed this finding, controlling for circadian rhythm by testing subjects that slept or stayed awake during the first or second half of the night (Plihal and Born 1997). Despite the evidence coming from these studies, several recent reviews came to the conclusion that there is no influence of sleep on declarative memory consolidation or, at least, that such effects are doubtful (Siegel 2001; Smith 2001; Vertes 2004). Several points are raised by these authors. A major criticism of previous studies is that none could show convincingly that lack of sleep has an enduring effect on memory consolidation that persists when retrieval testing is delayed until subjects had recovery sleep to make up for any acute effects of sleep deprivation. Because most recent studies tested memory retention directly after sleep deprivation, i.e., when subjects were under acute fatigue, diminished recall after wakefulness may merely reflect impaired retrieval rather than an enduring effect on memory consolidation (Idzikowski 1984; Plihal and Born 1997). In addition, many of the older studies are confounded with circadian factors, in that they have subjects learn and recall at different times of day for sleep and wake conditions. There are hints that acquisition and retrieval of declarative memories are subject to circadian influences (e.g., Tilley and Warren 1983). Together, previous studies were not able to prove that sleep has an improving effect specifically on the consolidation of declarative memory in humans that is distinct from circadian influences and effects of acute fatigue. One way to show this is to use longer retention intervals containing recovery sleep before retrieval testing, in sleep and sleep deprivation conditions, thus avoiding acute effects of fatigue on recall as well as circadian factors.
The aim of the present experiments was to show in a realistic setting that declarative memory consolidation benefits from sleep beyond effects of acute fatigue or circadian rhythms. In two experiments, we show that high school students’ ability to remember vocabulary, an instance of declarative memory, is improved when sleep follows learning. The experiments were designed to demonstrate that enhancements were persistent after two nights, and were independent of fatigue, time of day, and the amount of interference during retention intervals.
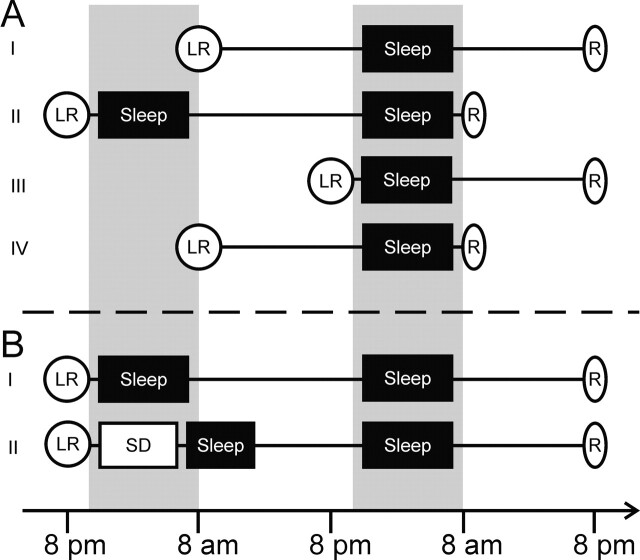
In a first experiment, we investigated whether sleep within a few hours of learning (in the evening) or after >14 h of intervening wakefulness (after learning in the morning) enhances consolidation of declarative memories. To hold constant the amount of interference and to control for the effects of time of day on recall, retention intervals of 24 h and 36 h were used with both morning and evening learning, resulting in four experimental conditions: morning-to-evening, evening-to-morning, evening-to-evening, and morning-to-morning (experimental conditions were performed in the order presented here; see Fig. 1 for an overview of experimental designs). Twelve American high school students (age 17.4 ± 0.2 yr [mean ± SEM], 6 male) participated in all four conditions in a within-subject design. Subjects were native English speakers with no prior knowledge of German.
Figure 1.
Schematic of the experimental design. Timing of the four conditions of the first experiment (A) and the two conditions of the second experiment (B). Subjects participated in all conditions of one experiment according to a within-subject design. (LR) Learning, (R) recall test, (SD) sleep deprivation. Shaded areas represent nighttime intervals.
Subjects learned on each occasion one of four 24-pair English–German vocabulary lists, presented on a sheet of paper with a time limit of 10 min to learn the whole list. Lists consisted of 10 dissimilar and five cognate nouns and five verbs. In addition, two pairs of nouns in the beginning and in the end of each list were excluded from later analysis to rule out recency and primacy effects. Learning took place either at 8 a.m. or at 8 p.m. Immediately after learning, recall was tested to see how many words were initially retained. Recall was tested again after 24 h or 36 h at 8 a.m. or 8 p.m. The recall test was in written format, with the English words given in a different order than during the original presentation. Recall was measured as the number of correctly remembered words; forgetting is given as the average individual percent change in recall performance across the retention interval.
Subjects were required to refrain from drinking alcohol and caffeine during the days of the experiments. They agreed to have regular sleep patterns throughout the experiment and filled out sleep logs to indicate sleep duration and daytime naps. A cash prize was awarded to the subject with the highest average score during recall tests to keep participants motivated. Statistical analysis was based on a repeated-measures GLM and was done in SPSS 12.0.
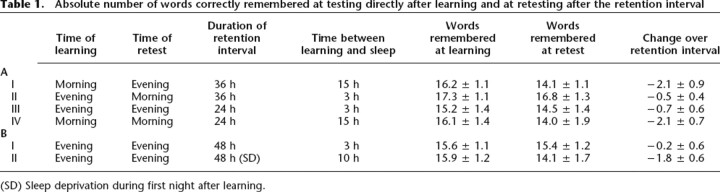
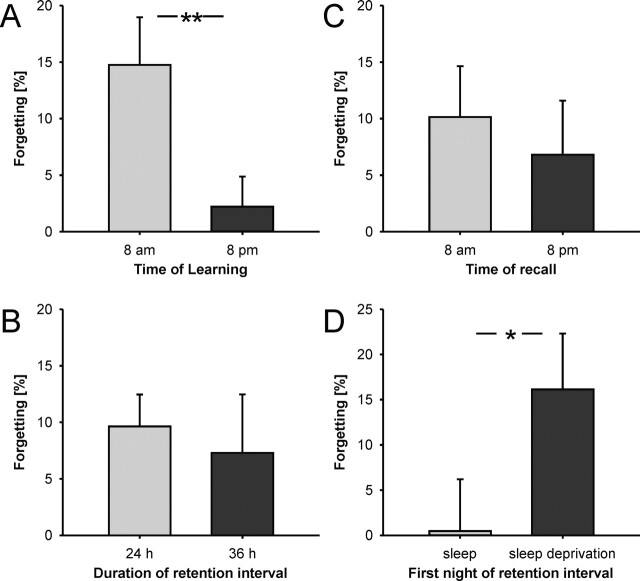
The results of this first experiment are detailed in the upper part of Table 1. The most notable difference between conditions is a much higher rate of forgetting in morning-learning conditions than in evening-learning conditions (F1,11 = 9.8, P < 0.01, Fig. 2A). It is also interesting that, on the other hand, no significant difference was found for retention intervals of 24 h and 36 h (F1,11 = 0.2, P = 0.65, Fig. 2B). The time of the retrieval session did not appear to play a role either: Morning-recall and evening-recall conditions were not significantly different (10.2 ± 4.4% forgetting in the morning recall and 6.9 ± 3.8% in the evening recall, F1,11 = 0.2, P = 0.65, Fig. 2C). With regard to initial learning, all four conditions were comparable and showed no significant difference (see Table 1, F3,33 = 2.5, P > 0.05). Difficulty of all four lists was likewise identical when comparing pre-test performance (F3,92 = 0.82, P = 0.49). Sleep duration, as indicated by the sleep logs, was on average 7.4 h (SD: 0.6 h).
Table 1.
Absolute number of words correctly remembered at testing directly after learning and at retesting after the retention interval
(SD) Sleep deprivation during first night after learning.
Figure 2.
Forgetting indicated as average individual percent change in recall score across periods of sleep and wakefulness. (A,B,C) The first experiment shows that only the time of learning (8 a.m. or 8 p.m.) affects memory retention (A) **P < 0.01; refer to Fig. 1 for experimental design. Retention intervals of 24 h and 36 h (B) and time of recall testing (C) did not significantly change memory performance. Results are averaged across the remaining conditions (time of learning, time of recall, and duration of the retention interval), respectively. See Table 1 for full, non-averaged results. (D) In the second experiment, subjects showed better recall at a retrieval testing 48 h after learning (in the evening) when they had slept within a few hours of learning than when they were sleep-deprived during the first night after learning, *P < 0.05.
In a second experiment, our goal was to test whether the beneficial effect of evening learning found in the first experiment could be attributed to sleep following learning or whether it was related to the time of day. Therefore, subjects learned similar word lists as in the first experiment in the evening before sleeping or before a night of sleep deprivation (Fig. 1). Fourteen male high school students (age 18.1 ± 0.2 yr) were tested twice in randomized, counterbalanced order, with half of the subjects beginning with the sleep condition and the other half beginning with sleep deprivation. Subjects learned at 8 p.m., followed by a first test of initial acquisition performance. Then, subjects either went home to sleep or stayed for a night of sleep deprivation under the observation of an experimenter. During sleep deprivation, subjects were engaged in activities such as watching TV and playing games. Reading was not allowed in order to prevent possible interference in the declarative memory system. At 6 a.m., sleep-deprived subjects were allowed to go home, where they slept on average for 5.6 ± 0.6 h during daytime and for another 7.6 ± 0.6 h during the following night. In the sleep condition, subjects slept on average 7.4 ± 0.3 h and 7.5 ± 0.2 h on the first and second night, respectively. Recall testing took place in both conditions 48 h after initial learning, again at 8 p.m. All procedures for learning and retrieval were otherwise identical to those of the first experiment.
Sleep and sleep deprivation conditions differed significantly with regard to the proportion of words remembered at retesting 48 h after learning. Subjects remembered clearly more of the learned vocabulary when they had slept during the night following learning than when they had stayed awake (F1,13 = 7.1, P < 0.05, Fig. 2D). No difference was observed between conditions at initial learning (F1,13 = 0.2, P > 0.65). Results are detailed in the lower part of Table 1.
To summarize, our results indicate that sleep following learning has a beneficial effect on declarative memory consolidation in humans and that this effect is present when retrieval is postponed until after recovery sleep in the sleep deprivation condition. This finding excludes fatigue as a factor explaining differences in retrieval performance between sleep and sleep deprivation. In addition, our results show that sleep is most effective when it follows within a few hours after learning without longer periods of intervening wakefulness. Interestingly, the influence of sleep on memory seems to be distinctly greater than that of the length of the retention period, at least in the 24–48 h timeframe investigated here. In fact, the data show no relation of forgetting with the time between learning and retrieval over all conditions. Although this might be astonishing, it must be considered that, compared with many other studies measuring forgetting, here only a small number of items had to be encoded, and acquisition performance was very good. In addition, the subjects were relatively young students and used to learning and retaining this kind of material for longer periods.
The lack of interference during sleep has often been discussed as the source of the beneficial effect of sleep on memory (Jenkins and Dallenbach 1924; Grosvenor and Lack 1984; Wixted 2004). Here, both 24-h conditions and both 36-h conditions have identical amounts of waking-associated interference. The amount of interference per se thus cannot be the cause of the observed effects (Benson and Feinberg 1977). It is, however, possible that memories can be consolidated best during a limited period after encoding, which is free of interference. In that case, one role of sleep would be to provide such a period, making memories less sensitive to interference occurring after this period. Still, other recent studies could show that specific physiologic features present during sleep and in different sleep stages—such as the modulation of the neuroendocrine and neurotransmitter milieu and the presence of sleep spindles in the electroencephalogram—play their roles in memory consolidation during sleep as well (Gais and Born 2004).
An issue that is not easily avoided with any kind of long-term memory task is a possible rehearsal of the memory content between learning and retrieval sessions, which might not have been reported by the subjects afterward and which cannot be entirely controlled by the experimenter. In the present experiments, we suppose that such rehearsal, if it occurred, affected all conditions equally. It must be considered, however, that results could be affected systematically if rehearsal happened mainly at a specific time of day (e.g., in the evening before sleep) and concurrently it is more effective after a specific time interval after learning.
The time interval between learning and sleep in the first experiment is confounded with time of day. Therefore, subjects may have been in different states of arousal during morning and evening learning. Because of the known effects of arousal on learning and memory, the results of the first experiment could be confounded by such a circadian effect (Revelle and Loftus 1992). The fact that conditions do not differ significantly in pre-test performance, however, speaks against this interpretation of results. In addition, the second experiment excludes such an explanation, since learning takes place at the same time in both conditions.
Another point to mention is that retention was assessed by percent change between learning and retrieval. Because immediate recall relies partially on working memory, this measure does not necessarily reflect the same type of memory that was assessed at retrieval. The memory trace assessed at retrieval testing is probably different from the one assessed at learning, due to a variety of intervening processes associated with synaptic and systems consolidation of the memory trace. Nevertheless, the immediate recall performance still represents a valid measure of encoding during the preceding learning phase.
The fact that sleep after learning enhances long-term retention in humans has been shown compellingly for procedural memory systems (Stickgold et al. 2000; Walker et al. 2003), but studies have not been able to show convincingly that sleep deprivation has lasting effects also on declarative memory (Smith 2001; Vertes 2004). Many of the earlier studies investigating declarative memory looked at the effects of selective REM sleep deprivation that were overall inconsistent (summarized in Smith 2001). In a study by Ekstrand et al. (1971), neither REM sleep deprivation nor deprivation of stage 4 sleep impaired memory for paired associates. However, this study lacked a control condition of undisturbed sleep. There are only two studies known to the authors that used retention intervals >36 h. These found enhanced memory consolidation for nonsense syllables, but are either single case reports or lack circadian controls (Graves 1936; Richardson and Gough 1963). Another more recent review reports briefly of two studies showing no effect of sleep deprivation on declarative memory tested after 7 d (Smith 2001). The latter are, however, difficult to comment on, because they are not described in detail. It is, nevertheless, possible that ongoing processes of consolidation and forgetting blur the initial effects of sleep over time. Systems consolidation of hippocampus-dependent memories extends over several days and leads to a gradual reorganization of the representation in memory (McClelland et al. 1995; Dudai 2004; Wagner et al. 2004). To grasp such dynamics, different types of retention tests and experimental designs might prove valuable to detect changes at more delayed times.
The material used in the present experiments differed in some respects from that used in previous studies. Most older studies used nonsense syllables (Benson and Feinberg 1975); later studies used mainly paired-associate word lists (Plihal and Born 1997). The advantage of using vocabulary is that this represents a common everyday memory task. On the other hand, it is harder to compare results with other studies using different tasks. It should also be noted in this context that although these tasks represent declarative memory according to the classical definition, they are probably not purely declarative, but may include procedural aspects to varying degrees (Squire 1992; Peigneux et al. 2001). Whether learning vocabulary is dependent on SWS-like previous tasks of declarative memory is a matter of speculation, although this might be assumed (but see also De Koninck et al. 1989).
Our study provides further evidence for the notion that memory consolidation in the declarative memory system benefits from sleep. It extends previous studies in that it shows that consolidation is enhanced when the interval of intervening wakefulness between learning and sleep is short. It also shows that this beneficial effect of sleep is stable over 48 h. These findings are independent of time of day and not due to acute fatigue. Together with previous studies, these data encourage the idea that, for optimal retention, phases of intensive learning, like school, should be followed closely by intervals of sleep.
Footnotes
Article and publication are at http://www.learnmem.org/cgi/doi/10.1101/NA
References
- Benson K., Feinberg I. Sleep and memory: Retention 8 and 24 hours after initial learning. Psychophysiology. 1975;12:192–195. doi: 10.1111/j.1469-8986.1975.tb01275.x. [DOI] [PubMed] [Google Scholar]
- Benson K., Feinberg I. The beneficial effect of sleep in an extended Jenkins and Dallenbach paradigm. Psychophysiology. 1977;14:375–384. doi: 10.1111/j.1469-8986.1977.tb02967.x. [DOI] [PubMed] [Google Scholar]
- De Koninck J., Lorrain D., Christ G., Proulx G., Coulombe D. Intensive language learning and increases in rapid eye movement sleep: Evidence of a performance factor. Int. J. Psychophysiol. 1989;8:43–47. doi: 10.1016/0167-8760(89)90018-4. [DOI] [PubMed] [Google Scholar]
- Dudai Y. The neurobiology of consolidations, or, how stable is the engram? Annu. Rev. Psychol. 2004;55:51–86. doi: 10.1146/annurev.psych.55.090902.142050. [DOI] [PubMed] [Google Scholar]
- Ekstrand B.R., Sullivan M.J., Parker D.F., West J.N. Spontaneous recovery and sleep. J. Exp. Psychol. 1971;88:142–144. doi: 10.1037/h0030642. [DOI] [PubMed] [Google Scholar]
- Fowler M.J., Sullivan M.J., Ekstrand B.R. Sleep and memory. Science. 1973;179:302–304. doi: 10.1126/science.179.4070.302. [DOI] [PubMed] [Google Scholar]
- Gais S., Born J. Declarative memory consolidation: Mechanisms acting during human sleep. Learn. Mem. 2004;11:679–685. doi: 10.1101/lm.80504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gais S., Plihal W., Wagner U., Born J. Early sleep triggers memory for early visual discrimination skills. Nat. Neurosci. 2000;3:1335–1339. doi: 10.1038/81881. [DOI] [PubMed] [Google Scholar]
- Graves E.A. The effect of sleep upon retention. J. Exp. Psychol. 1936;19:316–322. [Google Scholar]
- Grosvenor A., Lack L.C. The effect of sleep before or after learning on memory. Sleep. 1984;7:155–167. doi: 10.1093/sleep/7.2.155. [DOI] [PubMed] [Google Scholar]
- Idzikowski C. Sleep and memory. Br. J. Psychol. 1984;75:439–449. doi: 10.1111/j.2044-8295.1984.tb01914.x. [DOI] [PubMed] [Google Scholar]
- Jenkins J.G., Dallenbach K.M. Obliviscence during sleep and waking. Am. J. Psychol. 1924;35:605–612. [Google Scholar]
- Maquet P., Laureys S., Peigneux P., Fuchs S., Petiau C., Phillips C., Aerts J., Del Fiore G., Degueldre C., Meulemans T., et al. Experience-dependent changes in cerebral activation during human REM sleep. Nat. Neurosci. 2000;3:831–836. doi: 10.1038/77744. [DOI] [PubMed] [Google Scholar]
- McClelland J.L., McNaughton B.L., O’Reilly R.C. Why there are complementary learning systems in the hippocampus and neocortex: Insights from the successes and failures of connectionist models of learning and memory. Psychol. Rev. 1995;102:419–457. doi: 10.1037/0033-295X.102.3.419. [DOI] [PubMed] [Google Scholar]
- Peigneux P., Laureys S., Delbeuck X., Maquet P. Sleeping brain, learning brain. The role of sleep for memory systems. Neuroreport. 2001;12:A111–A124. doi: 10.1097/00001756-200112210-00001. [DOI] [PubMed] [Google Scholar]
- Plihal W., Born J. Effects of early and late nocturnal sleep on declarative and procedural memory. J. Cogn. Neurosci. 1997;9:534–547. doi: 10.1162/jocn.1997.9.4.534. [DOI] [PubMed] [Google Scholar]
- Revelle W., Loftus D.A. 1992. The implications of arousal effects for the study of affect and memory. In Handbook of emotion and memory (ed. S.A. Christianson), pp. 113–150. Lawrence Erlbaum; Hillsdale, NJ [Google Scholar]
- Richardson A., Gough J.E. The long range effect of sleep on retention. Aust. J. Psychol. 1963;15:37–41. [Google Scholar]
- Siegel J.M. The REM sleep-memory consolidation hypothesis. Science. 2001;294:1058–1063. doi: 10.1126/science.1063049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C. Sleep states and memory processes in humans: Procedural versus declarative memory systems. Sleep Med. Rev. 2001;5:491–506. doi: 10.1053/smrv.2001.0164. [DOI] [PubMed] [Google Scholar]
- Squire L.R. Memory and the hippocampus: A synthesis from findings with rats, monkeys, and humans. Psychol. Rev. 1992;99:195–231. doi: 10.1037/0033-295x.99.2.195. [DOI] [PubMed] [Google Scholar]
- Stickgold R., James L., Hobson J.A. Visual discrimination learning requires sleep after training. Nat. Neurosci. 2000;3:1237–1238. doi: 10.1038/81756. [DOI] [PubMed] [Google Scholar]
- Tilley A., Warren P. Retrieval from semantic memory at different times of day. J. Exp. Psychol. Learn. Mem. Cogn. 1983;9:718–724. doi: 10.1037//0278-7393.9.4.718. [DOI] [PubMed] [Google Scholar]
- Vertes R.P. Memory consolidation in sleep: Dream or reality. Neuron. 2004;44:135–148. doi: 10.1016/j.neuron.2004.08.034. [DOI] [PubMed] [Google Scholar]
- Wagner U., Gais S., Haider H., Verleger R., Born J. Sleep inspires insight. Nature. 2004;427:352–355. doi: 10.1038/nature02223. [DOI] [PubMed] [Google Scholar]
- Walker M.P., Brakefield T., Hobson J.A., Stickgold R. Dissociable stages of human memory consolidation and reconsolidation. Nature. 2003;425:616–620. doi: 10.1038/nature01930. [DOI] [PubMed] [Google Scholar]
- Wixted J.T. The psychology and neuroscience of forgetting. Annu. Rev. Psychol. 2004;55:235–269. doi: 10.1146/annurev.psych.55.090902.141555. [DOI] [PubMed] [Google Scholar]