ABSTRACT
Background
Post-operative acute kidney injury (PO-AKI) is a common surgical complication consistently associated with subsequent morbidity and mortality. Prior kidney dysfunction is a major risk factor for PO-AKI, however it is unclear whether serum creatinine, the conventional kidney function marker, is optimal in this population. Serum cystatin C is a kidney function marker less affected by body composition and might provide better prognostic information in surgical patients.
Methods
This was a pre-defined, secondary analysis of a multi-centre prospective cohort study of pre-operative functional capacity. Participants were aged ≥40 years, undergoing non-cardiac surgery. We assessed the association of pre-operative estimated glomerular filtration rate (eGFR) calculated using both serum creatinine and serum cystatin C with PO-AKI within 3 days after surgery, defined by KDIGO creatinine changes. The adjusted analysis accounted for established AKI risk factors.
Results
A total of 1347 participants were included (median age 65 years, interquartile range 56–71), of whom 775 (58%) were male. A total of 82/1347 (6%) patients developed PO-AKI. These patients were older, had higher prevalence of cardiovascular disease and related medication, were more likely to have intra-abdominal procedures, had more intraoperative transfusion, and were more likely to be dead at 1 year after surgery 6/82 (7.3%) vs 33/1265 (2.7%) (P = .038). Pre-operative eGFR was lower in AKI than non-AKI patients using both creatinine and cystatin C. When both measurements were considered in a single age- and sex-adjusted model, eGFR-Cysc was strongly associated with PO-AKI, with increasing risk of AKI as eGFR-Cysc decreased below 90, while eGFR-Cr was no longer significantly associated.
Conclusions
Data from over 1000 prospectively recruited surgical patients confirms pre-operative kidney function as major risk factor for PO-AKI. Of the kidney function markers available, compared with creatinine, cystatin C had greater strength of association with PO-AKI and merits further assessment in pre-operative assessment of surgical risk.
Keywords: AKI, creatinine, cystatin C, prognosis, surgery
KEY LEARNING POINTS.
What was known:
Post-operative acute kidney injury (PO-AKI) is a common and serious complication of major surgery.
Estimates of kidney function using creatinine may be confounded in several disease states associated with low muscle mass, where cystatin C may perform better.
Less was known about use of cystatin C to stratify kidney risk before major surgery.
This study adds:
Compared with creatinine, cystatin C was more strongly associated with risk of PO-AKI.
Lower creatinine relative to cystatin C was associated with worse physical function after surgery.
Assessment of kidney function by creatinine may be confounded by lower muscle mass, and thus creatinine production in some surgical patients.
Potential impact:
Cystatin C should be considered for the assessment of kidney function in the pre-operative work-up of high-risk patient groups at risk of sarcopenia.
Creatinine and cystatin C might provide in complementary information in pre-operative assessment of high-risk candidates for major surgery, including patients at risk of physical function outcomes.
INTRODUCTION
Currently, an estimated 300 million surgical patients undergo non-cardiac procedures each year with a steady year-on-year increase particularly in older, higher risk patients [1–3]. Around 10% of them sustain post-operative acute kidney injury (PO-AKI) [4]. While many cases of PO-AKI are asymptomatic, even self-limiting occurrence of PO-AKI has been associated with increased risk of hospital readmission, development or progression of chronic kidney disease (CKD), and both short-term and late mortality [5–7]. Importantly, development of CKD is strongly associated with accelerated cardiovascular disease and premature cardiovascular death [8–10]. Accordingly, PO-AKI may represent a short-term injury with significant potential for longer term harm, mediated by the development or worsening of CKD [11]. Furthermore, pre-existing CKD is well recognized as one of the strongest risk factors for development of PO-AKI, allowing for a vicious cycle of CKD progression [11]. However, the association between estimated glomerular filtration rate based on creatinine (eGFR-Cr) and risk of adverse events after surgery may be confounded by decreased muscle mass in certain disease states. Decreased muscle mass tends to lower creatinine production, lowering serum creatinine and leading to an overestimate of kidney function [12]. As a result, after age adjustment, pre-operative eGFR-Cr has been associated with a U-shaped relationship with risk of death [13].
In contrast to serum creatinine, cystatin C is a protein released at constant rate from nucleated cells and is independent of muscle mass, age, sex and race, and excreted exclusively by glomerular filtration [14]. Equations estimating GFR based on cystatin C (eGFR-Cysc) have been developed from large diverse populations and validated against measured GFR [15]. Cystatin C has been shown to predict all-cause mortality and cardiovascular morbidity in a range of clinical settings including the general population [16], the elderly [17], and individuals with acute and chronic cardiovascular disease [18–20]. After critical illness which is near universally accompanied by profound muscle wasting, cystatin C provides a more accurate measure of kidney function [21] and is more strongly associated with ongoing risk of death [22, 23]. However, cystatin C has not been extensively explored as a pre-operative predictor of AKI and other adverse outcomes after major surgery, despite many major-surgical candidates having low muscle mass. In addition, kidney function and injury are also highly related to pre-existing cardiovascular disease and risk, however it is unclear how markers of cardiac risk (biomarkers and functional capacity) predict AKI relative to markers of pre-operative kidney function.
In this secondary analysis of the Measurement of Exercise Tolerance before Surgery (METS) study [24], we sought to examine the relative strengths of pre-operative serum cystatin C versus creatinine as predictors of PO-AKI. We examined the relationship between proposed pre-operative markers of cardiac risk and results of cardiopulmonary exercise testing on risk of PO-AKI. We hypothesized that creatinine might underperform cystatin C as a measure of baseline kidney function in patients with low muscle mass. To explore lower creatinine as a potential biomarker of low pre-operative muscle mass, we examined the relationship between creatinine:cystatin C ratio, as a potential index of muscle mass and creatinine production [25], and mobility status 1 year after surgery.
MATERIALS AND METHODS
Study design and setting
This is a secondary analysis of the METS international prospective observational cohort study of pre-operative assessment before non-cardiac surgery at 25 hospitals in Canada, the UK, Australia and New Zealand [24]. The study protocol and methods have been published previously [26]. The study received prior research ethics approvals and was conducted in accordance with the principles of the Declaration of Helsinki and the Research Governance Framework; all participants gave written informed consent to take part before surgery.
Participants
Participants were aged 40 years or older, undergoing elective non-cardiac surgery under general anaesthesia and/or regional anaesthesia with a planned overnight stay in hospital, and with at least one of the following peri-operative risk factors: intermediate or high-risk surgery [intraperitoneal, intrathoracic or major vascular (suprainguinal or lower extremity vascular procedures)], coronary artery disease, heart failure, cerebrovascular disease, diabetes mellitus, pre-operative kidney insufficiency (defined as eGFR-Cr ≤60 mL/min/1.73 m2 (since this is relevant to the focus of this present study), peripheral arterial disease, hypertension, a history of tobacco smoking within the previous year or age 70 years or more. The exclusion criteria were: planned procedure using only endovascular technique, use of cardiopulmonary exercise testing for risk stratification as part of routine care, insufficient time for cardiopulmonary exercise testing before surgery, presence of an implantable cardioverter-defibrillator, known or suspected pregnancy, previous enrolment in the study, severe hypertension (>180/100 mmHg), active cardiac conditions or other contraindications precluding cardiopulmonary exercise testing [26, 27]. In this AKI-focused analysis, we additionally excluded patients with prior end-stage kidney disease on maintenance dialysis pre-operatively (where AKI diagnosis does not apply) and those undergoing major urological procedures (cystectomy and nephrectomy) where post-operative change in kidney function might be expected as an accompaniment of the procedure.
Study conduct and data collection
A detailed and standardized dataset was collected before surgery, during the hospital stay, and at 30 days and 1 year after surgery. Researchers collected data directly from participants and their medical records. Each participant underwent pre-operative cardiopulmonary exercise testing. Blood was sampled before surgery and on the first, second and third days after surgery, as long as the participant remained in hospital. Quality of life was assessed using the EuroQol-5 Dimension (EQ-5D) pre-operatively, at 30 days and 1 year. Blood samples were drawn at any point between study recruitment and surgery to measure serum creatinine, cystatin C, Troponin isoforms, highly sensitive C-reactive protein (hs-CRP) and N-terminal pro-hormone of brain natriuretic peptide (NT-proBNP). These samples were stored at −70°C to −80°C at each study site, then analysed at the Aberdeen Royal Infirmary (Aberdeen, UK) using the Siemens Vista immunoassay analyser (Siemens Healthcare Diagnostics, Frimley, UK) creatinine assay used an IDMS enzymatic method while cystatin C particle-enhanced nephelometric immunoassay was traceable to the ERM-DA471/IFCC standard. Finally, as part of the METS protocol participants underwent pre-operative symptom limited cycle ergometer CPET as described and published previously [24, 26].
Exposures and outcomes
Our primary exposures of interest were pre-operative kidney function as assessed by eGFR-Cr and eGFR-Cysc based on simultaneous sampling in preassessment visit, centrally assayed and calculated using the Chronic Kidney Disease Epidemiology Collaboration formulas relevant at the time of the study [15, 28]. Our primary outcome was PO-AKI based on the 3-day observations available from the METS study data. Based on the 2012 KDIGO creatinine criteria for AKI [29], this was defined as a 1.5-fold increase in serum creatinine from pre-operative baseline within the first 3 days post-operatively or a >26 µmol/L creatinine increase within a 2-day interval (pre-operative compared with Day 1 or 2, Day 1 compared with Day 2 or 3, or Day 2 compared with Day 3). Hourly urine output data was not available for AKI diagnosis. For AKI diagnosis pre-operative creatinine values hospital measurements were used as baseline to ensure consistency with post-operative samples also measured locally.
Statistical analysis
We used the R Version 4.1.1 [30] and the rms package [31] to analyse the data. Data are presented as median with interquartile range and between group comparison were undertaken using Wilcoxon rank sum or Chi-squared tests for continuous and categorical data respectively. Paired data were analysed using Wilcoxon signed rank sum or McNemar's tests for continuous and categorical data respectively. In univariate analysis, we considered the association between PO-AKI and the following additional exposures including age, sex, ASA-score, Furosemide use, ACE/A2RB use, NSAID use, diabetes, hypertension, coronary artery disease, congestive cardiac failure, surgical procedure category, pre-assessment NT-proBNP and hs-CRP, as well as measurement of pre-operative exercise capacity [CPET anaerobic threshold, peak oxygen consumption and the Duke Activity Status Index (DASI)]. As a prospective study including only patients with pre-operative biomarker assessment, missing data was infrequent and handled in multi-variable analysis by case-wise deletion.
We explored the relationship between eGFR-Cr and eGFR-Cysc (exposures) and AKI (outcome) in multivariable logistic regression analysis adjusting for age and sex; measurements of eGFR were modelled as a non-linear predictors using a restricted cubic splines with 5 knots following the method of Harrell [31]. We assessed multi-collinearity between eGFR by calculation of the variance inflation factor with a threshold of >5. To assess the most informative variables associated with AKI, we undertook backward selection based on minimization of the AIC from a model containing all the following variables associated with AKI: age, sex, hypertension, coronary artery disease, pre-operative NT-proBNP and eGFR.
Finally, in an exploratory analysis, we considered the creatinine:cystatin C ratio as an index of pre-operative muscle mass. We hypothesized that low pre-operative muscle mass as measured by creatinine:cystatin C ratio might correlate with ability to physically rehabilite after surgery; hence, this ratio might predict impaired mobility status at 1 year after surgery. Self-reported mobility status at 1 year was assessed the mobility domain of the EQ-5D-3L (response levels: no difficulty walking around, some difficulty and confined to bed). As a competing endpoint, death before 1 year was considered equivalent to ‘confined to bed’. Modelling for the relationship between creatinine:cystatin C ratio and impaired mobility at 1 year was conducted similarly using logistic regression accounting for sex, age, body mass index (BMI) and pre-operative mobility status.
Sample size calculation
This was a planned secondary analysis of a prospectively collected data. The sample size was determined based on the comparisons being made in the principal analysis, which has been published previously [24]. For this sub-study, we included all available cases with the sample size determined by this availability.
RESULTS
Study sample
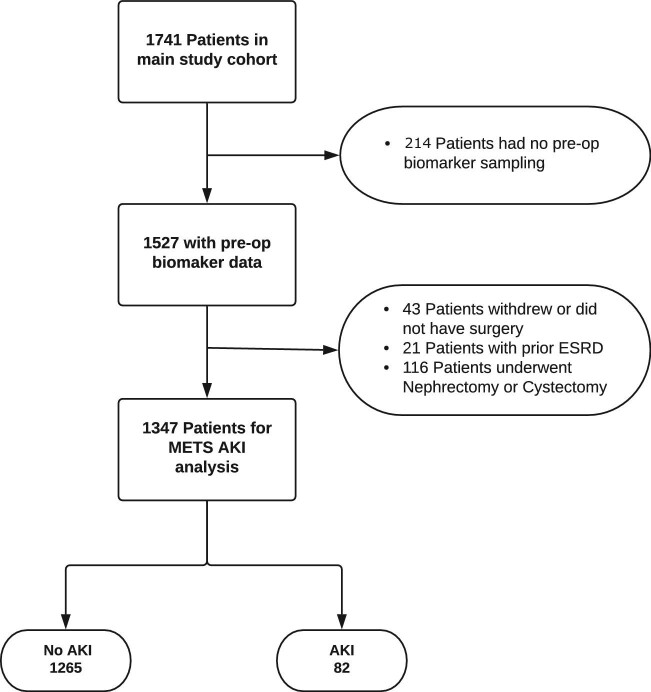
A total of 1741 patients were recruited into the METS study between March 2013 and March 2016. After predefined exclusion of patients that did not have pre-assessment biomarker measures (n = 214), patients who did not have surgery (n = 43), patients with prior end-stage kidney disease (n = 21) and patients who underwent nephrectomy and/or cystectomy (n = 116), we analysed data obtained from 1347 participants (Fig. 1). Median age was 65 (interquartile range 57–72) years, and 785 (58.3%) were male. A total of 1246 (93%) patients were classified as American Society of Anesthesiologists Physical Status (ASA-PS) class 2 or higher.
Figure 1:
Patient flow diagram showing the number of cases included in the analysis.
Primary outcome
A total of 82 patients (6.1%) developed PO-AKI, with 70 in Stage 1, 12 in Stage 2–3 and 4 patients requiring kidney replacement therapy, 80 patients sustained a >26 µmol/dL rise and 41 experienced a >1.5-fold increase in creatinine with respect to baseline. Baseline characteristics of the cohort by AKI status are summarized in Table 1. Overall, patients developing PO-AKI were older, had higher prevalence of hypertension, coronary artery disease or heart failure, but not diabetes, and were more likely to take angiotensin-converting enzyme inhibitors, angiotensin-2 blockers or loop diuretics. They also had higher ASA score, were more likely to have intra-abdominal procedures and had more blood transfusion intra-operatively (Table 1). Patients experiencing AKI were more likely to have died by 1 year after surgery [6/82 (7.3%) vs 33/1265 (2.7%), P = .038].
Table 1:
Demographic, surgical parameters and biomarkers by AKI outcome.
| No AKI | AKI | P | Missing data (%) | |
|---|---|---|---|---|
| Number of cases | 1265 | 82 | ||
| Age, years | 65.00 (56.00, 71.00) | 67.50 (60.00, 75.00) | .009 | 0 |
| Male sex (%) | 729 (57.6) | 56 (68.3) | .075 | 0 |
| Height (cm) | 168 (162, 175) | 170 (166, 175) | .051 | 8.9 |
| Weight (kg) | 80.0 (69.0, 92.0) | 82.5 (69.9, 96.9) | .444 | 8.9 |
| BMI (kg/m2) | 28.0 (24.7, 31.8) | 27.4 (24.7, 31.9) | .996 | 8.9 |
| Pre-existing conditions | ||||
| Coronary artery disease | 146 (11.5) | 19 (23.2) | .003 | 0 |
| Diabetes | 248 (19.6) | 17 (20.7) | .916 | 0 |
| Congestive cardiac failure | 20 (1.6) | 4 (4.9) | .079 | 0 |
| Hypertension | 697 (55.1) | 58 (70.7) | .008 | 0 |
| ASA-PS grade | .014 | 0.2 | ||
| Class 1 | 96 (7.6) | 2 (2.4) | ||
| Class 2 | 739 (58.6) | 39 (47.6) | ||
| Class 3 | 405 (32.1) | 38 (46.3) | ||
| Class 4 | 22 (1.7) | 3 (3.7) | ||
| Pre-operative medication | ||||
| ACE inhibitor/ARB | 471 (37.2) | 43 (52.4) | .009 | 0 |
| Frusemide | 46 (3.6) | 8 (9.8) | .014 | 0 |
| NSAIDs | 91 (7.2) | 6 (7.3) | 1 | 0 |
| Insulin | 51 (4.0) | 7 (8.5) | .082 | 0 |
| Pre-operative CPET | ||||
| Anaerobic threshold (mL/kg/min) | 12.00 (10.00, 14.70) | 11.50 (10.00, 13.07) | .377 | 17.6 |
| Peak oxygen consumption (mL/kg/min) | 18.50 (15.00, 22.00) | 18.10 (14.40, 21.80) | .46 | 12 |
| DASI | 42.70 (26.95, 52.95) | 38.20 (23.45, 50.70) | .25 | 0.5 |
| Surgical procedure | .037 | 0 | ||
| Vascular | 22 (1.7) | 5 (6.1) | ||
| Intra-thoracic | 31 (2.5) | 3 (3.7) | ||
| Intra-peritoneal or retro-peritoneal | 425 (33.6) | 36 (43.9) | ||
| Orthopaedic | 351 (27.7) | 16 (19.5) | ||
| Urologic or gynaecologic surgerya | 298 (23.6) | 18 (22.0) | ||
| Head and neck | 103 (8.1) | 3 (3.7) | ||
| Neurosurgery | 7 (0.6) | 0 (0.0) | ||
| Other | 28 (2.2) | 1 (1.2) | ||
| Minimally invasive surgery | 424 (33.5) | 26 (31.7) | .829 | 0 |
| General anaesthesia | 1036 (81.9) | 75 (91.5) | .040 | 0 |
| Any intra-op transfusion, % | 2.2 | 7.3 | .0167 | 0.2 |
| Post-op care | .002 | 0 | ||
| Intensive care unit | 107 (8.5) | 13 (15.9) | ||
| High-dependency unit | 152 (12.0) | 18 (22.0) | ||
| Surgical ward | 977 (77.2) | 51 (62.2) | ||
| Day surgery | 29 (2.3) | 0 (0.0) | ||
| Discharge status | <.001 | 0 | ||
| Home | 1228 (97.1) | 76 (92.7) | ||
| Rehabilitation facility | 36 (2.8) | 3 (3.7) | ||
| Died | 1 (0.1) | 3 (3.7) | ||
| One year mortality | 33 (2.7) | 6 (7.3) | .038 | 1.8 |
| Peri-operative creatinine, µmol/L | ||||
| Hospital pre-op | 74 (65, 87) | 82 (66, 99) | .004 | 0 |
| Day 1 | 73 (62, 85) | 117 (96, 146) | <.001 | 18 |
| Day 2 | 69 (59, 82) | 121 (94, 148) | <.001 | 37.2 |
| Day 3 | 66 (55, 78) | 93 (78, 133) | <.001 | 47.7 |
| Pre-operative study bloods | ||||
| Creatinine, µmol/L | 73 (63, 85) | 83 (65, 97) | .001 | 0 |
| eGFR-Cr, mL/min/1.73 m2 | 87.5 (75.9, 96.9) | 77.5 (62.1, 95.2) | <.001 | 0 |
| Cystatin C, mg/dL | 0.85 (0.74, 0.97) | 1.02 (0.79, 1.19) | <.001 | 0 |
| eGFR-Cysc, mL/min/1.73 m2 | 92.4 (75.8, 104.4) | 72.6 (57.8, 103.8) | <.001 | 0 |
| NT-proBNP, pg/mL | 82 (40, 160) | 129 (48, 426) | .001 | 0 |
| hs-CRP, mg/dL | 1.92 (0.85, 4.76) | 3.15 (1.26, 6.38) | .003 | 0 |
| Troponin-I, ng/mL | 0.00 (0.00, 0.00) | 0.00 (0.00, 0.00) | .307 | 0 |
Except where indicated, data are presented as n (%) or median (IQR).
aExcludes cystectomy and nephrectomy.
ACE: angiotensin-converting enzyme; ARB: angiotensin-receptor blocker; NSAID: non-steroidal anti-inflammatory drug.
Primary analysis: pre-operative kidney function and risk of PO-AKI
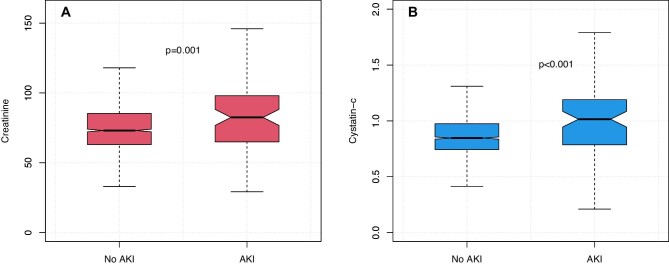
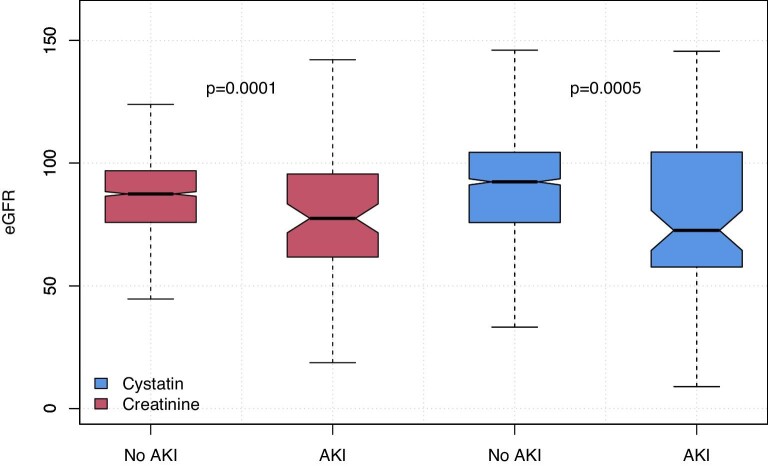
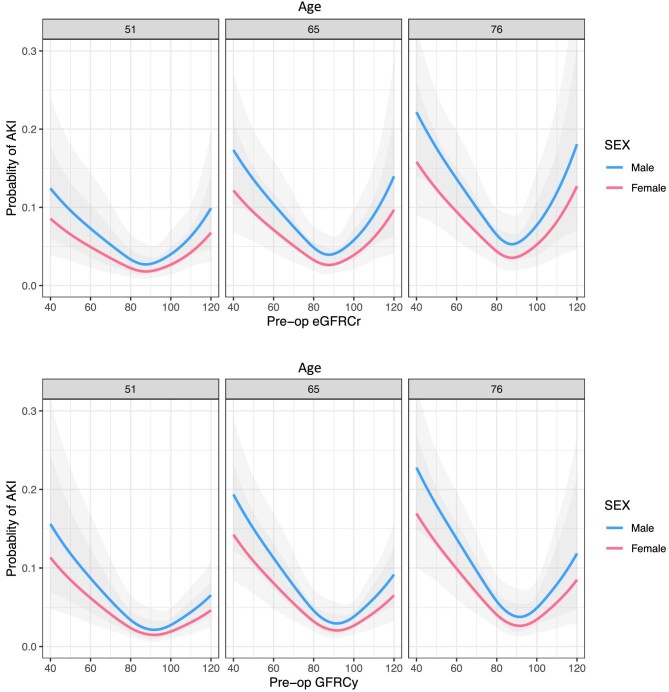
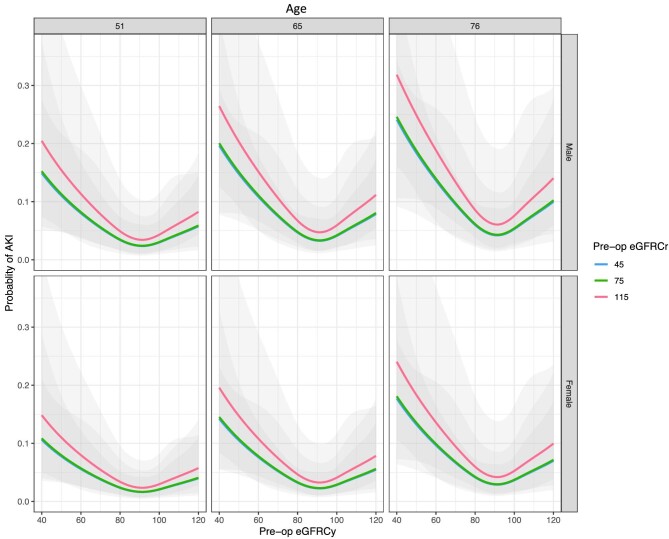
Both pre-operative creatinine and cystatin C were significantly higher in patients developing AKI, creatinine 83 vs 73 µmol/L (P = .001) and cystatin C 1.02 vs 0.85 mg/dL (Wilcoxon rank sum test, P < .001) (Fig. 2). In the entire cohort, 9.7% of patients had an eGFR <60 mL/min/1.73 m2 based on creatinine and 10.8% when calculated using cystatin C (McNemar's test P < .001). eGFR was lower in AKI patients using both measurements (Fig. 3), creatinine and cystatin C were highly correlated (Pearson R2 0.60, Supplementary data, Fig. S1). There was no significant difference between creatinine and cystatin C–based eGFR measurements in patients developing AKI (78 vs 73, respectively, P = .554, Wilcoxon signed rank test); however, in those without AKI, creatinine eGFR was slightly lower (87 vs 92, P < .001). In multivariable logistic regression accounting for age and sex, modelling eGFR using a restricted cubic spline, both eGFR-Cr and eGFR-Cysc showed a significant non-linear relationship with risk of developing PO-AKI with nadir of risk around an eGFR of 90 and with risk of AKI increasing with lower eGFR (Fig. 4, Supplementary data, Tables S1 and S2). When both measurements were considered in a single model eGFR-Cr was no longer a significant predictor of AKI while eGFR-Cysc remained strongly associated with increasing risk of AKI as eGFR-Cysc decreased below 90 (Fig. 5, Supplementary data, Table S3).
Figure 2:
Pre-operative creatinine (A) and cystatin C (B) by AKI outcome. Wilcoxon Rank Sum Test.
Figure 3:
Pre-operative eGFR-Cr and eGFR-Cysc by AKI outcome. Wilcoxon Rank Sum Test.
Figure 4:
Age- and sex-adjusted probability of AKI by baseline creatinine (upper panels) or cystatin C (lower panels) estimates of GFR (in mL/min/1.73 m2) in logistic regression modelling treating eGFR as a non-linear covariate fitted to a restricted cubic spline. Three side-by-side panels show modelling of AKI for the 25th, 50th and 75th centiles of age (51, 65 and 76 years, respectively) For eGFR-Cr a U-shaped relationship with increasing risk at low and high eGFR is apparent, for eGFR-Cysc the relationship is J-shaped with highest risk with lower eGFR below 90.
Figure 5:
Age- and sex-adjusted probability of AKI by baseline creatinine and cystatin C estimates of GFR in logistic regression modelling including both eGFR measures (in mL/min/1.73 m2) as non-linear covariates fitted to restricted cubic splines. Three side-by-side panels show modelling of AKI for the 25th, 50th and 75th centiles of age (51, 65 and 76 years, respectively). In this model eGFR-Cr was not significantly associated with risk of AKI while eGFR-Cysc remained strongly associated. Note lines for eGFR creatinine of 45 and 75 in blue and green are closely overlapped.
Secondary analyses
Neither pre-operative CPET anaerobic threshold, peak oxygen consumption nor the DASI differed significantly between patients developing PO-AKI and those who did not (Table 1). NT-proBNP and hs-CRP were significantly higher in those developing AKI (Table 1).
Multivariable analysis
To address the pre-operative factors most strongly associated with AKI development we developed a multivariable model containing age, sex, hypertension, coronary artery disease, and pre-assessment NTProBNP, eGFR-Cr and eGFR-Cysc. The eGFR measurements were modelled as non-linear effects. After backward selection, only sex, NT-proBNP, hypertension diagnosis and eGFR-Cysc (non-linear) were retained in the final model, with eGFR-Cysc being the most significant predictor, accounting for 84% of the total Wald statistic for the model (Supplementary data, Table S4).
Exploratory analysis of mobility status
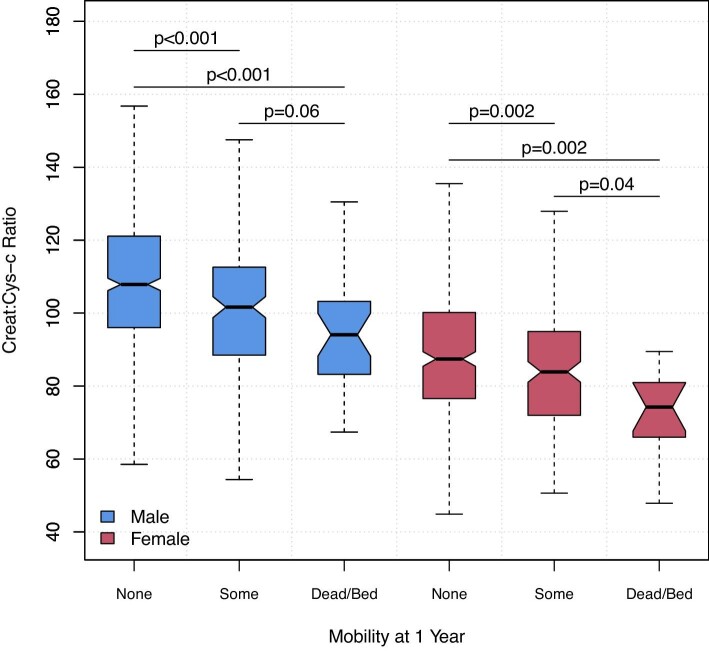
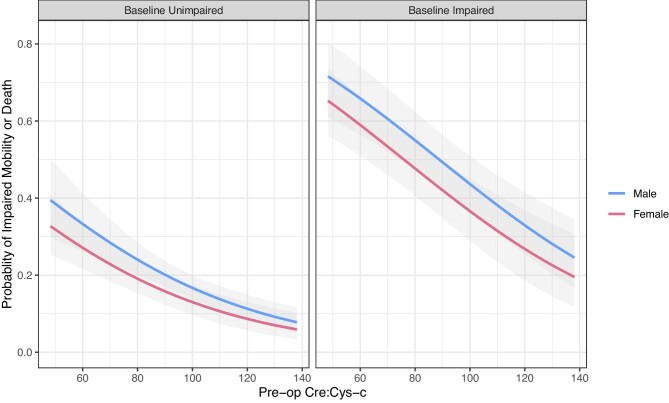
To explore the relationship between kidney function tests and skeletal muscle functional capacity, we examined the relationship between creatinine, cystatin C and creatinine:cystatin C ratio, and EQ-5D-SL mobility status at 1 year. Outcome data were not available in 78/1347 cases, leaving 1269 patients included in this analysis; only 3 respondents were bedbound at 1 year and this group was merged with deaths before 1 year to provide three levels. Increasing cystatin C was significantly associated with worse mobility/survival status at 1 year (Table 2); conversely, creatinine did not significantly differ across outcomes with rather a trend to a lower value with worse mobility/survival status. Creatinine:cystatin C ratio, a putative measure of muscle mass, showed stepwise decline with worse mobility and survival outcome in males and females (Fig. 6). In multivariable modelling, accounting for sex, age, BMI and baseline mobility status, creatinine:cystatin C ratio was an independent predictor of any impaired mobility (some mobility problems, bedbound or dead) at 1 year (P < .001) (Fig. 7).
Table 2:
Frequency and characteristics of patients by mobility status at 1 year post-operatively based on self-reported mobility or death by 1 year (n = 39).
| Mobility status at 1 year | Unimpaired | Some problems | Bed-bound or deada | P |
|---|---|---|---|---|
| Number | 891 | 336 | 42 | |
| Male sex, n (%) | 544 (61) | 173 (51) | 29 (69) | .004 |
| Age | 65 (57, 72) | 66 (59, 73) | 67.5 (62, 77) | .004 |
| Pre-operative: some or greater mobility problems, n (%) | 196 (22) | 192 (57) | 16 (38) | <.001 |
| Pre-op creatinine | 75 (65, 86) | 72 (62, 87) | 67 (57, 88) | .228 |
| Pre-op cystatin C | 0.84 (0.74, 0.95) | 0.89 (0.77, 1.07) | 0.96 (0.79, 1.08) | <.001 |
| Creatinine:cystatin C ratio | 89 (76, 101) | 81 (69, 95) | 79 (67, 87) | <.001 |
Data are presented as n (%) or median (IQR).
aThree bed-bound and 39 dead.
Figure 6:
Pre-operative creatinine:cystatin C ratio and mobility and survival status at 1 year after surgery. Within each gender worse, functional outcome is associated with progressively lower creatinine:cystatin C ratio, a putative index of skeletal muscle mass. Notches approximate the 95% confidence interval for that median. Wilcoxon Rank Sum Test.
Figure 7:
Logistic regression for multivariable logistic regression for the presence of impaired mobility (death or bedbound, some impairment of mobility) at 1 year. Creatinine:cystatin C ratio pre-operatively remained a very significant association of the adverse mobility outcome even after accounting for gender, age, BMI and baseline mobility status. Predictions are shown for median age of 65 years and BMI of 25 kg/m2. Creatinine:cystatin C ratio is based on creatinine in µmol/L and cystatin C in mg/dL.
DISCUSSION
The main finding of this analysis was that pre-operative kidney function remains one of the principal risk factors for the subsequent development of PO-AKI. However, when compared side by side, cystatin C was a stronger predictor of PO-AKI than creatinine, which provided no additional value to a statistical model that included cystatin C. This is important because many conditions that require major surgery may be associated with reduced muscle mass (e.g. cancer, heart failure, etc.), where creatinine may provide an inaccurate overestimate of underlying kidney function. Our data support the need for further research on the accuracy and clinical utility of cystatin C in assessing risk of PO-AKI.
Previous studies in cardiac surgery have suggested that in comparison with serum creatinine or eGFR-Cr pre-operative, cystatin C provided better graduated estimated risks of PO-AKI [32], post-operative complications and long-term mortality [33, 34]. Our current analysis extends these findings to the general non-cardiac surgical population, which is a setting were cystatin C has not been extensively evaluated. In this study we have not assessed pre-operative cystatin C as a diagnostic biomarker for AKI (an event that had not yet occurred), but instead we consider it as potentially better index of baseline kidney function as a risk factor for AKI. Actual occurrence of AKI will be determined by combination of many exposures and risk factors. Hence, in this analysis, we consider the strength of association of pre-operative eGFR-Cysc and PO-AKI in logistic regression rather than assessing ability to ‘discriminate PO-AKI cases’ as a diagnostic test.
Of the other factors significantly associated with PO-AKI in this study, heart disease, hypertension and elevated NT Pro-BNP all suggest a role for cardiovascular disease in PO-AKI pathophysiology. However, neither CPET parameters nor the self-reported functional capacity DASI assessment of cardiopulmonary exercise capacity was associated with risk of AKI.
As well as the association of low eGFR with AKI, values of eGFR-Cr above 90 were also associated with increasing risk of AKI—potentially such patients may actually have lower muscle mass, and this might account for increased risk of complications including AKI. However, given the small numbers of AKI cases overall, and the fact that eGFR equations are not well validated for eGFR >90, we would only suggest that clinicians should not be reassured of a low risk of AKI based on an apparently high eGFR-Cr more than that physiologically plausible for age.
Finally, in an exploratory analysis we found that the creatinine:cystatin C ratio, a potential proxy of muscle mass, was associated with poor mobility or death at 1 year, even after accounting for baseline mobility status. This suggests the potential for utilization of creatinine and cystatin C in tandem in a dual assessment of both baseline kidney function and muscle mass before surgery, which is increasingly recognized as a risk factor for many adverse postoperative outcomes [35].
This study has several strengths. Patients were prospectively recruited from multiple centres across three continents, and an analysis of creatinine, cystatin C and AKI outcomes was a pre-specified secondary analysis. Cystatin C and creatinine values for eGFR calculations were measured in a central laboratory, avoiding any inconsistencies in measurement, while AKI diagnosis was based on local creatinine blood tests for internal consistency. The prospective study protocols [26] also ensured high quality data collection and limited missing data enabling a number of sub-studies such as this one [36–39]. The are some limitations, however. The overall AKI event rate was low at 6%, limiting the power of the statistical analysis and suggesting the results may not be directly generalizable to higher risk populations. However, this AKI rate is representative of that seen in other mixed major surgery populations [40]. In addition, while representation of women was 42% in the study cohort only 26 women had AKI (32% of AKI group), given that eGFR equations are sex specific caution should be taken in the interpretation of these results for women. Similarly, only 7% of the population, and five patients with AKI, were of Black ethnicity and other non-white ethnicity was not recorded. Other eGFR formula exist for cystatin C might provide differing results—our choice was pre-specified and represents the most widely endorsed formula and other formulas generally have similar coefficients and diverge mostly at higher eGFR [41]. Only 3 days of bloods were available for AKI assessment. While the majority of PO-AKI is evident within the first 3 days, strictly PO-AKI would be defined as occurring up to 7 days after surgery and some late cases might have been missed. In addition, lack of sequential data to determine peak creatinine after Day 3 precluded analysis based on the maximum stage of AKI achieved, as the full time-course of AKI was not captured and AKI severity might thereby be underestimated. It must be noted that AKI as an outcome itself is defined by a change in creatinine concentration, while absolute value of baseline creatinine defines one of our measures of pre-operative AKI risk. While detection of a fold change, or small rapid increase in a marker, will be relatively insensitive to the calibration of the test or the absolute baseline value, low baseline or acutely falling creatinine generation might slow or attenuate rise in creatinine in AKI, potentially masking its detection in some patients with low muscle mass. Similarly, hourly urine output data was not available for AKI diagnosis; however, this definition is rarely employed clinically, and the pathophysiological significance of shorter periods post-operative oliguria has been challenged [42]. It should be acknowledged that PO-AKI diagnosis itself is not necessarily patient-centred and long-term data on CKD outcomes were not available. The METS study was conducted before the COVID-19 pandemic, which may limit the external validity to contemporary surgical patients [43]. Finally, in our analysis of creatinine:cystatin C ratio, its interpretation as a potential marker of creatinine production and muscle mass could be confounded by several factors, including inaccuracy of eGFR-Cysc as an estimate of true GFR in several disease states, influence of dietary creatinine intake on creatinine production and variation in tubular clearance of creatinine, and should thus be regarded as hypothesis generating only.
CONCLUSIONS
Data from a large cohort of prospectively recruited non-cardiac surgical patients suggests that pre-operative kidney dysfunction is a strong risk-factor for PO-AKI, and compared with creatinine, cystatin C was more strongly associated with this risk. It is likely that in some patients, assessment of kidney function by creatinine may be confounded by lower muscle mass, and thus creatinine production. In support of sarcopenia as a potential explanation for relatively low pre-operative creatinine values, we found that that a lower creatinine relative to cystatin C was associated with worse physical function after surgery. Cystatin C should therefore be considered for the assessment of kidney function in the pre-operative work-up of high-risk patient groups at risk of sarcopenia. Future investigations could focus on the complementary information that Creatinine and cystatin C might provide in pre-operative assessment of high-risk candidates for major surgery including patients at risk of poor physical function outcomes.
Supplementary Material
Contributor Information
John R Prowle, Critical Care and Peri-operative Medicine Research Group, William Harvey Research Institute, Faculty of Medicine, Queen Mary University of London, London, UK; Adult Critical Care Unit, Barts Health NHS Trust, London, UK.
Bernard Croal, NHS Grampian-Clinical Biochemistry, Aberdeen Royal Infirmary, Foresterhill, Aberdeen, UK.
Thomas E F Abbott, Critical Care and Peri-operative Medicine Research Group, William Harvey Research Institute, Faculty of Medicine, Queen Mary University of London, London, UK; Adult Critical Care Unit, Barts Health NHS Trust, London, UK.
Brian H Cuthbertson, Department of Anesthesiology and Pain Medicine, University of Toronto, Toronto, ON, Canada; Interdepartmental Division of Critical Care Medicine, University of Toronto, Toronto, ON, Canada; Department of Critical Care Medicine, Sunnybrook Health Sciences Centre, Toronto, ON, Canada.
Duminda N Wijeysundera, Department of Anesthesiology and Pain Medicine, University of Toronto, Toronto, ON, Canada; Department of Anesthesia, St Michael's Hospital, Toronto, ON, Canada; Institute of Health Policy, Management and Evaluation, Dalla Lana School of Public Health, University of Toronto, Toronto, ON, Canada.
for the METS study investigators:
P S Myles, M A Shulman, S Wallace, C Farrington, B Thompson, M Ellis, B Borg, R K Kerridge, J Douglas, J Brannan, J Pretto, M G Godsall, N Beauchamp, S Allen, A Kennedy, E Wright, J Malherbe, H Ismail, B Riedel, A Melville, H Sivakumar, A Murmane, K Kenchington, Y Kirabiyik, U Gurunathan, C Stonell, K Brunello, K Steele, O Tronstad, P Masel, A Dent, E Smith, A Bodger, M Abolfathi, P Sivalingam, A Hall, T W Painter, S Macklin, A Elliott, A M Carrera, N C S Terblanche, S Pitt, J Samuels, C Wilde, K Leslie, A MacCormick, D Bramley, A M Southcott, J Grant, H Taylor, S Bates, M Towns, A Tippett, F Marshall, C D Mazer, J Kunasingam, A Yagnik, C Crescini, S Yagnik, C J L McCartney, S Choi, P Somascanthan, K Flores, D N Wijeysundera, W S Beattie, K Karkouti, H A Clarke, A Jerath, S A McCluskey, M Wasowicz, J T Granton, L Day, J Pazmino-Canizares, P Oh, R Belliard, L Lee, K Dobson, V Chan, R Brull, N Ami, M Stanbrook, K Hagen, D Campbell, T Short, J Van Der Westhuizen, K Higgie, H Lindsay, R Jang, C Wong, D Mcallister, M Ali, J Kumar, E Waymouth, C Kim, J Dimech, M Lorimer, J Tai, R Miller, R Sara, A Collingwood, S Olliff, S Gabriel, H Houston, P Dalley, S Hurford, A Hunt, L Andrews, L Navarra, A Jason-Smith, H Thompson, N McMillan, G Back, B L Croal, M Lum, D Martin, S James, H Filipe, M Pinto, S Kynaston, R M Pearse, T E F Abbott, M Phull, C Beilstein, P Bodger, K Everingham, Y Hu, E Niebrzegowska, C Corriea, T Creary, M Januszewska, T Ahmad, J Whalley, R Haslop, J McNeil, A Brown, N MacDonald, M Pakats, K Greaves, S Jhanji, R Raobaikady, E Black, M Rooms, H Lawrence, M Koutra, K Pirie, M Gertsman, S Jack, M Celinski, D Levett, M Edwards, K Salmon, C Bolger, L Loughney, L Seaward, H Collins, B Tyrell, N Tantony, K Golder, G L Ackland, R C M Stephens, L Gallego-Paredes, A Reyes, A Gutierrez del Arroyo, A Raj, and R Lifford
FUNDING
The METS Study was funded by peer-reviewed grants from the Canadian Institutes of Health Research, Heart and Stroke Foundation of Canada, Ontario Ministry of Health and Long-Term Care, Ontario Ministry of Research and Innovation, National Institute of Academic Anaesthesia, UK Clinical Research Network, Australian and New Zealand College of Anaesthetists, and Monash University. T.E.F.A. was funded by a Medical Research Council, UK National Institute of Health and Care Research UK, and British Journal of Anaesthesia clinical research training fellowship (grant reference MR/M017974/1) and an NIHR Clinical Lectureship.
DATA AVAILABILITY STATEMENT
The data underlying this article will be shared on reasonable request to the corresponding author.
CONFLICT OF INTEREST STATEMENT
J.R.P. has received honoraria from Paion Ltd, Baxter Inc., Biomerieux SA and Nikkiso Europe GmbH; he is a scientific advisor for Mission Therapeutics Ltd, Jafron Biomedical Co. Ltd and Nephrolyx GmbH, and serves on a Data Monitoring and Safety Board for Novartis Inc.; he is a specialty editor for the Clinical Kidney Journal and Blood Purification. B.H.C. and D.N.W. are supported in part by Merit Awards from the Department of Anesthesiology and Pain Medicine at the University of Toronto. D.N.W. is supported in part by the Endowed Chair in Translational Anesthesiology Research at St Michael's Hospital and the University of Toronto. D.N.W. has received honoraria from Edwards Lifesciences within the last 5 years and is a member of the Scientific Advisory Board for Surgical Safety Technologies Inc. T.E.F.A. is an NIHR Clinical Lecturer, an editor of the British Journal of Anaesthesia, has received research funding from the Medical Research Council, the National Institute of Academic Anaesthesia and Barts Charity, and has received consultancy fees from MSD and Edwards Life Sciences unrelated to this work. All other authors report no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years, and no other relationships or activities that could appear to have influenced the submitted work.
REFERENCES
- 1. Weiser TG, Haynes AB, Molina Get al. Estimate of the global volume of surgery in 2012: an assessment supporting improved health outcomes. Lancet 2015;385:S11. http://www.ncbi.nlm.nih.gov/pubmed/26313057 [DOI] [PubMed] [Google Scholar]
- 2. Fowler AJ, Abbott TEF, Prowle Jet al. Age of patients undergoing surgery. Br J Surg 2019;106:1012–8. https://www.ncbi.nlm.nih.gov/pubmed/31115918 [DOI] [PubMed] [Google Scholar]
- 3. Abbott TEF, Fowler AJ, Dobbs TDet al. Frequency of surgical treatment and related hospital procedures in the UK: a national ecological study using hospital episode statistics. Br J Anaesth 2017;119:249–57. https://www.ncbi.nlm.nih.gov/pubmed/28854546 [DOI] [PubMed] [Google Scholar]
- 4. Lei VJ, Luong T, Shan Eet al. Risk stratification for postoperative acute kidney injury in major noncardiac surgery using preoperative and intraoperative data. JAMA Netw Open 2019;2:e1916921. https://www.ncbi.nlm.nih.gov/pubmed/31808922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Grams ME, Sang Y, Coresh Jet al. Acute kidney injury after major surgery: a retrospective analysis of Veterans Health Administration data. Am J Kidney Dis 2016;67:872–80. http://www.ncbi.nlm.nih.gov/pubmed/26337133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Grams ME, Sang Y, Coresh Jet al. Candidate surrogate end points for ESRD after AKI. J Am Soc Nephrol 2016;27:2851–9. https://www.ncbi.nlm.nih.gov/pubmed/26857682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bihorac A, Yavas S, Subbiah Set al. Long-term risk of mortality and acute kidney injury during hospitalization after major surgery. Ann Surg 2009;249:851–8. 10.1097/SLA.0b013e3181a40a0b [DOI] [PubMed] [Google Scholar]
- 8. Wu VC, Wu CH, Huang TMet al. Long-term risk of coronary events after AKI. J Am Soc Nephrol 2014;25:595–605. https://www.ncbi.nlm.nih.gov/pubmed/24503241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gansevoort RT, Correa-Rotter R, Hemmelgarn BRet al. Chronic kidney disease and cardiovascular risk: epidemiology, mechanisms, and prevention. Lancet 2013;382:339–52. https://www.ncbi.nlm.nih.gov/pubmed/23727170 [DOI] [PubMed] [Google Scholar]
- 10. Jankowski J, Floege J, Fliser Det al. Cardiovascular disease in chronic kidney disease: pathophysiological insights and therapeutic options. Circulation 2021;143:1157–72. https://www.ncbi.nlm.nih.gov/pubmed/33720773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Prowle JR, Forni LG, Bell Met al. Postoperative acute kidney injury in adult non-cardiac surgery: joint consensus report of the Acute Disease Quality Initiative and PeriOperative Quality Initiative. Nat Rev Nephrol 2021;17:605–18. https://www.ncbi.nlm.nih.gov/pubmed/33976395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Prowle JR, Kolic I, Purdell-Lewis Jet al. Serum creatinine changes associated with critical illness and detection of persistent renal dysfunction after AKI. Clin J Am Soc Nephrol 2014;9:1015–23. http://www.ncbi.nlm.nih.gov/pubmed/24742481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chaudery H, MacDonald N, Ahmad Tet al. International Surgical Outcomes Study (ISOS) Group . Acute kidney injury and risk of death after elective surgery: prospective analysis of data from an international cohort study. Anesth Analg 2019;128:1022–9. https://www.ncbi.nlm.nih.gov/pubmed/30418232 [DOI] [PubMed] [Google Scholar]
- 14. Bagshaw SM, Bellomo R.. Cystatin C in acute kidney injury. Curr Opin Crit Care 2010;16:533–9. http://www.ncbi.nlm.nih.gov/pubmed/20736828 [DOI] [PubMed] [Google Scholar]
- 15. Inker LA, Schmid CH, Tighiouart Het al. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med 2012;367:20–9. https://www.ncbi.nlm.nih.gov/pubmed/22762315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Shlipak MG, Coresh J, Gansevoort RT.. Cystatin C versus creatinine for kidney function-based risk. N Engl J Med 2013;369:2459. https://www.ncbi.nlm.nih.gov/pubmed/24350959 [DOI] [PubMed] [Google Scholar]
- 17. Shlipak MG, Sarnak MJ, Katz Ret al. Cystatin C and the risk of death and cardiovascular events among elderly persons. N Engl J Med 2005;352:2049–60. https://www.ncbi.nlm.nih.gov/pubmed/15901858 [DOI] [PubMed] [Google Scholar]
- 18. Ix JH, Shlipak MG, Chertow GMet al. Association of cystatin C with mortality, cardiovascular events, and incident heart failure among persons with coronary heart disease: data from the Heart and Soul Study. Circulation 2007;115:173–9. https://www.ncbi.nlm.nih.gov/pubmed/17190862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jernberg T, Lindahl B, James Set al. Cystatin C: a novel predictor of outcome in suspected or confirmed non-ST-elevation acute coronary syndrome. Circulation 2004;110:2342–8. https://www.ncbi.nlm.nih.gov/pubmed/15477399 [DOI] [PubMed] [Google Scholar]
- 20. Sarnak MJ, Katz R, Stehman-Breen COet al. Cystatin C concentration as a risk factor for heart failure in older adults. Ann Intern Med 2005;142:497–505. https://www.ncbi.nlm.nih.gov/pubmed/15809461 [DOI] [PubMed] [Google Scholar]
- 21. Haines RW, Fowler AJ, Liang Ket al. Comparison of cystatin C and creatinine in the assessment of measured kidney function during critical illness. Clin J Am Soc Nephrol 2023;18:997–1005. https://www.ncbi.nlm.nih.gov/pubmed/37256861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ravn B, Prowle JR, Mårtensson Jet al. Superiority of serum cystatin C over creatinine in prediction of long-term prognosis at discharge from ICU. Crit Care Med 2017;45:e932–40. https://www.ncbi.nlm.nih.gov/pubmed/28614196 [DOI] [PubMed] [Google Scholar]
- 23. Nateghi Haredasht F, Viaene L, Vens Cet al. Comparison between cystatin C- and creatinine-based estimated glomerular filtration rate in the follow-up of patients recovering from a stage-3 AKI in ICU. J Clin Med 2022;11:7264. https://www.ncbi.nlm.nih.gov/pubmed/36555881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wijeysundera DN, Pearse RM, Shulman MAet al. METS study investigators . Assessment of functional capacity before major non-cardiac surgery: an international, prospective cohort study. Lancet 2018;391:2631–40. [DOI] [PubMed] [Google Scholar]
- 25. Kashani KB, Frazee EN, Kukralova Let al. Evaluating muscle mass by using markers of kidney function: development of the sarcopenia index. Crit Care Med 2017;45:e23–9. https://www.ncbi.nlm.nih.gov/pubmed/27611976 [DOI] [PubMed] [Google Scholar]
- 26. Wijeysundera DN, Pearse RM, Shulman MAet al. Measurement of Exercise Tolerance before Surgery (METS) study: a protocol for an international multicentre prospective cohort study of cardiopulmonary exercise testing prior to major non-cardiac surgery. BMJ Open 2016;6:e010359. http://www.ncbi.nlm.nih.gov/pubmed/26969643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. American Thoracic Society, American College of Chest Physicians . ATS/ACCP statement on cardiopulmonary exercise testing. Am J Respir Crit Care Med 2003;167:211–77. http://www.ncbi.nlm.nih.gov/pubmed/12524257 [DOI] [PubMed] [Google Scholar]
- 28. Levey AS, Stevens LA, Schmid CHet al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009;150:604–12. http://www.ncbi.nlm.nih.gov/pubmed/19414839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kidney Disease: Improving Global Outcomes . KDIGO Clinical Practice Guideline for Acute Kidney Injury; section 2: AKI definition. Kidney Int Suppl (2011) 2012;2:19–36.25018918 [Google Scholar]
- 30. R Core Team . R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria, 2022 [Google Scholar]
- 31. Harrell FE. rms: Regression Modeling Strategies. R package version 6.7-1 Vanderbilt University School of Medicine Nashville TN USA. https://CRAN.R-project.org/package=rms [Google Scholar]
- 32. Shlipak MG, Coca SG, Wang Zet al. TRIBE-AKI Consortium . Presurgical serum cystatin C and risk of acute kidney injury after cardiac surgery. Am J Kidney Dis 2011;58:366–73. https://www.ncbi.nlm.nih.gov/pubmed/21601336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mooney JF, Croal BL, Cassidy Set al. Relative value of cystatin C and creatinine-based estimates of glomerular filtration rate in predicting long-term mortality after cardiac surgery: a cohort study. BMJ Open 2019;9:e029379. https://www.ncbi.nlm.nih.gov/pubmed/31530601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Dardashti A, Nozohoor S, Algotsson Let al. The predictive value of s-cystatin C for mortality after coronary artery bypass surgery. J Thorac Cardiovasc Surg 2016;152:139–46. https://www.ncbi.nlm.nih.gov/pubmed/27056756 [DOI] [PubMed] [Google Scholar]
- 35. Pipek LZ, Baptista CG, Nascimento RFVet al. The impact of properly diagnosed sarcopenia on postoperative outcomes after gastrointestinal surgery: a systematic review and meta-analysis. PLoS One 2020;15:e0237740. https://www.ncbi.nlm.nih.gov/pubmed/32822372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ladha KS, Cuthbertson BH, Abbott TEFet al. Functional decline after major elective non-cardiac surgery: a multicentre prospective cohort study. Anaesthesia 2021;76:1593–9. https://www.ncbi.nlm.nih.gov/pubmed/34254670 [DOI] [PubMed] [Google Scholar]
- 37. Wijeysundera DN, Beattie WS, Hillis GSet al. International and National Coordinators; Central Project Office Operations Committee; CPET Methods Committee; Outcome Adjudication Committee; International Steering Committee . Integration of the Duke Activity Status Index into preoperative risk evaluation: a multicentre prospective cohort study. Br J Anaesth 2020;124:261–70. https://www.ncbi.nlm.nih.gov/pubmed/3186471931864719 [Google Scholar]
- 38. Abbott TEF, Pearse RM, Beattie WSet al. Chronotropic incompetence and myocardial injury after noncardiac surgery: planned secondary analysis of a prospective observational international cohort study. Br J Anaesth 2019;123:17–26. https://www.ncbi.nlm.nih.gov/pubmed/31029407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Abbott TEF, Pearse RM, Cuthbertson BHet al. METS study investigators . Cardiac vagal dysfunction and myocardial injury after non-cardiac surgery: a planned secondary analysis of the measurement of exercise tolerance before surgery study. Br J Anaesth 2019;122:188–97. https://www.ncbi.nlm.nih.gov/pubmed/30686304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. O'Connor ME, Hewson RW, Kirwan CJet al. Acute kidney injury and mortality 1 year after major non-cardiac surgery. Br J Surg 2017;104:868–76. https://www.ncbi.nlm.nih.gov/pubmed/28218392 [DOI] [PubMed] [Google Scholar]
- 41. Inker LA, Tighiouart H, Adingwupu OMet al. CKD-EPI and EKFC GFR estimating equations: performance and other considerations for selecting equations for implementation in adults. J Am Soc Nephrol 2023;34:1953–64. https://www.ncbi.nlm.nih.gov/pubmed/37796982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Puckett JR, Pickering JW, Palmer SCet al. Low versus standard urine output targets in patients undergoing major abdominal surgery: a randomized noninferiority trial. Ann Surg 2017;265:874–81. https://www.ncbi.nlm.nih.gov/pubmed/27763895 [DOI] [PubMed] [Google Scholar]
- 43. Abbott TEF, Fowler AJ, Dobbs TDet al. Mortality after surgery with SARS-CoV-2 infection in England: a population-wide epidemiological study. Br J Anaesth 2021;127:205–14. https://www.ncbi.nlm.nih.gov/pubmed/34148733 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.