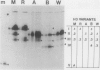
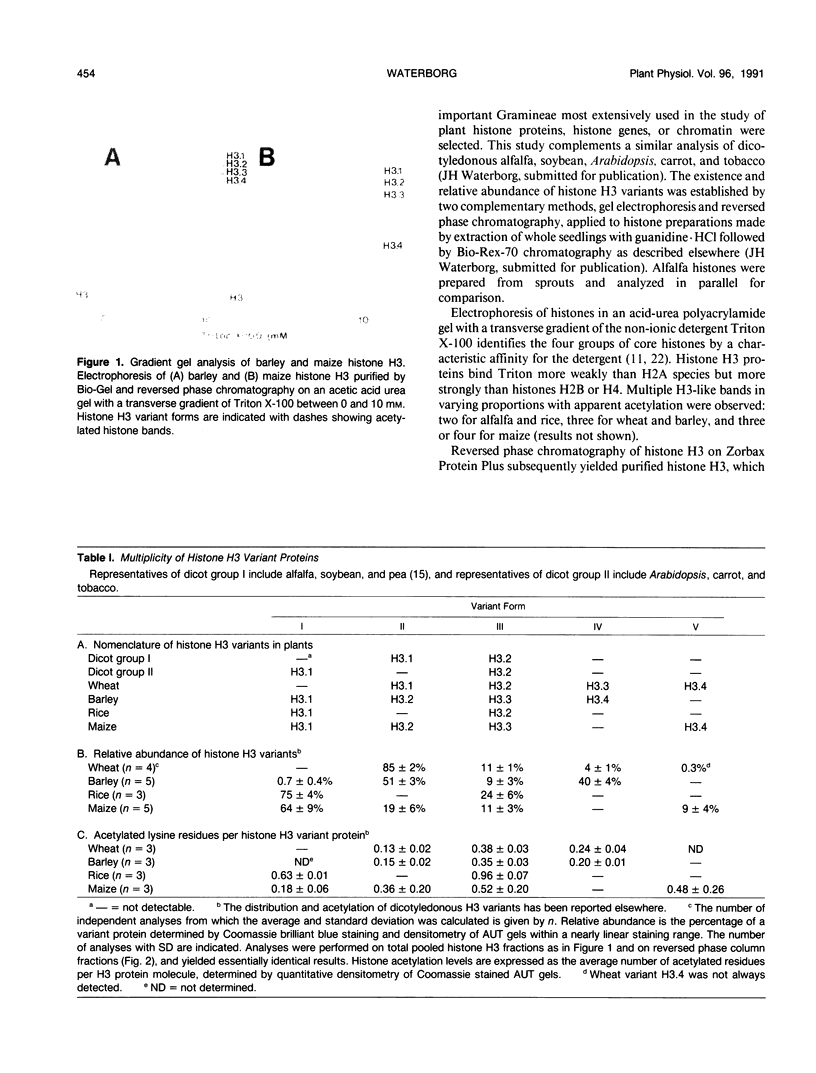
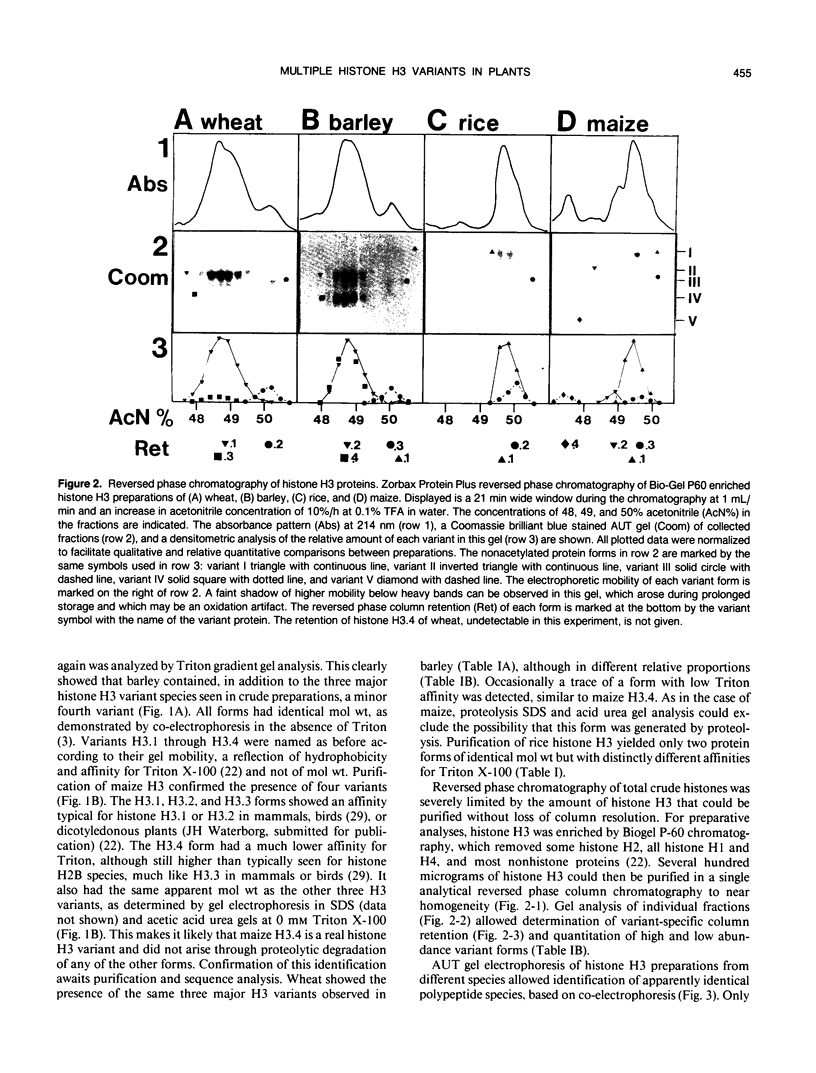
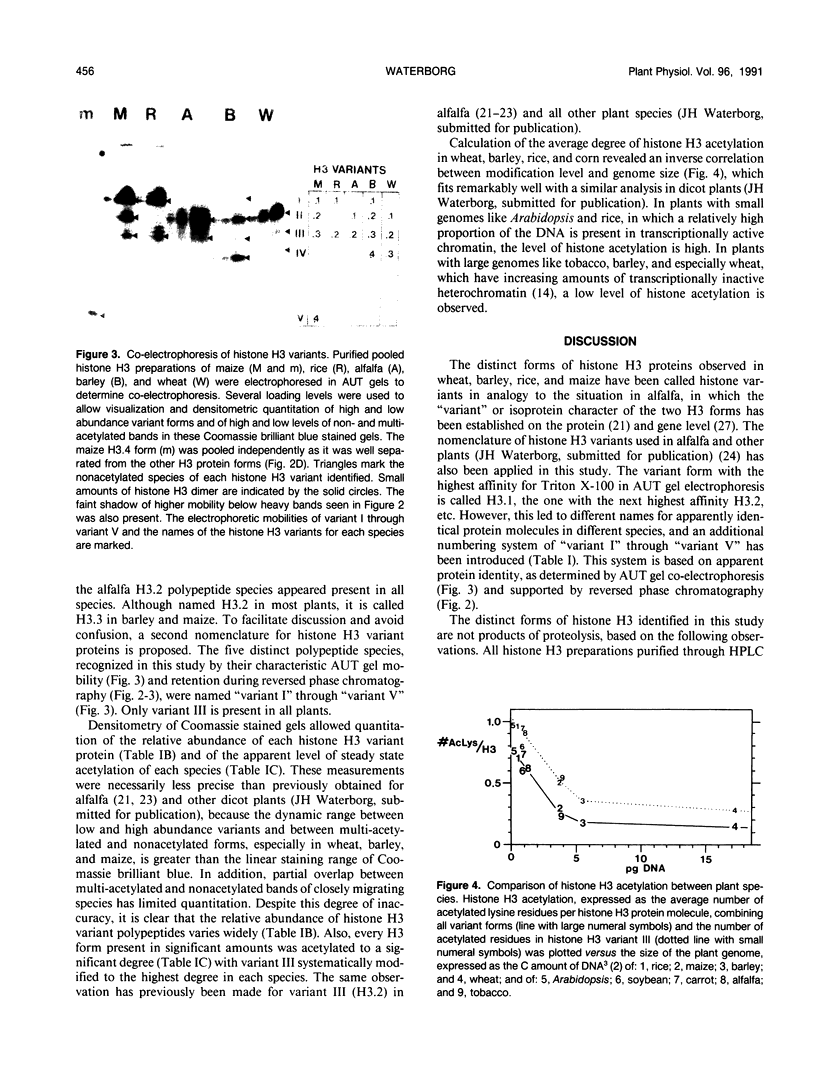
Abstract
Histone H3 proteins were purified to near homogeneity from etiolated seedlings of wheat (Triticum aestivum), barley (Hordeum vulgare), rice (Oryza sativa), maize (Zea mays), and alfalfa (Medicago sativa) to determine the number of histone H3 variants. Five distinct histone H3 variants were identified by gradient gel electrophoresis and reversed phase chromatography. These variants occur in various combinations of two to four forms in each plant species. One minor histone variant form (variant III, named H3.2 in alfalfa) appeared present and identical in all mono- and dicotyledonous plant species tested to date. All H3 proteins were acetylated to multiple levels and in every species the variant III form was acetylated most extensively. The level of histone H3 acetylation showed an inverse correlation with plant genome size. These observations support the idea that acetylated histones and especially variant III proteins are an element of transcriptionally active chromatin.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennett M. D., Smith J. B. Nuclear dna amounts in angiosperms. Philos Trans R Soc Lond B Biol Sci. 1976 May 27;274(933):227–274. doi: 10.1098/rstb.1976.0044. [DOI] [PubMed] [Google Scholar]
- Brandt W. F., Sewell B. T., von Holt C. Mr determination of histones from their electrophoretic mobility in acidic urea gels in the absence of detergents. FEBS Lett. 1986 Jan 6;194(2):273–277. doi: 10.1016/0014-5793(86)80099-0. [DOI] [PubMed] [Google Scholar]
- Cross S. L., Smith M. M. Comparison of the structure and cell cycle expression of mRNAs encoded by two histone H3-H4 loci in Saccharomyces cerevisiae. Mol Cell Biol. 1988 Feb;8(2):945–954. doi: 10.1128/mcb.8.2.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green G. R., Gustavsen L. C., Poccia D. L. Phosphorylation of Plant H2A Histones. Plant Physiol. 1990 Jul;93(3):1241–1245. doi: 10.1104/pp.93.3.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T., Hayashi H., Fusauchi Y., Iwai K. Tetrahymena histone H3. Purification and two variant sequences. J Biochem. 1984 Jun;95(6):1741–1749. doi: 10.1093/oxfordjournals.jbchem.a134788. [DOI] [PubMed] [Google Scholar]
- Patthy L., Smith E. L. Histone 3. V. The amino acid sequence of pea embryo histone 3. J Biol Chem. 1973 Oct 10;248(19):6834–6840. [PubMed] [Google Scholar]
- Vanfleteren J. R., Van Bun S. M., Van Beeumen J. J. The histones of Caenorhabditis elegans: no evidence of stage-specific isoforms. An overview. FEBS Lett. 1989 Nov 6;257(2):233–237. doi: 10.1016/0014-5793(89)81541-8. [DOI] [PubMed] [Google Scholar]
- Waterborg J. H., Harrington R. E., Winicov I. Differential Histone Acetylation in Alfalfa (Medicago sativa) Due to Growth in NaCl : Responses in Salt Stressed and Salt Tolerant Callus Cultures. Plant Physiol. 1989 May;90(1):237–245. doi: 10.1104/pp.90.1.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterborg J. H., Harrington R. E., Winicov I. Dynamic histone acetylation in alfalfa cells. Butyrate interference with acetate labeling. Biochim Biophys Acta. 1990 Jul 30;1049(3):324–330. doi: 10.1016/0167-4781(90)90105-b. [DOI] [PubMed] [Google Scholar]
- Waterborg J. H. Sequence analysis of acetylation and methylation in two histone H3 variants of alfalfa. J Biol Chem. 1990 Oct 5;265(28):17157–17161. [PubMed] [Google Scholar]
- Waterborg J. H., Winicov I., Harrington R. E. Histone variants and acetylated species from the alfalfa plant Medicago sativa. Arch Biochem Biophys. 1987 Jul;256(1):167–178. doi: 10.1016/0003-9861(87)90435-8. [DOI] [PubMed] [Google Scholar]
- Wells D., McBride C. A comprehensive compilation and alignment of histones and histone genes. Nucleic Acids Res. 1989;17 (Suppl):r311–r346. doi: 10.1093/nar/17.suppl.r311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu R. S., Panusz H. T., Hatch C. L., Bonner W. M. Histones and their modifications. CRC Crit Rev Biochem. 1986;20(2):201–263. doi: 10.3109/10409238609083735. [DOI] [PubMed] [Google Scholar]
- Wu S. C., Györgyey J., Dudits D. Polyadenylated H3 histone transcripts and H3 histone variants in alfalfa. Nucleic Acids Res. 1989 Apr 25;17(8):3057–3063. doi: 10.1093/nar/17.8.3057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S. C., Végh Z., Wang X. M., Tan C. C., Dudits D. The nucleotide sequences of two rice histone H3 genes. Nucleic Acids Res. 1989 Apr 25;17(8):3297–3297. doi: 10.1093/nar/17.8.3297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zweidler A. Resolution of histones by polyacrylamide gel electrophoresis in presence of nonionic detergents. Methods Cell Biol. 1978;17:223–233. [PubMed] [Google Scholar]