Abstract
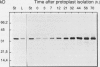
When auxin was omitted during either the preparation or the culture of tobacco mesophyll protoplasts, as well as during both periods, synthesis of β-glucanase was spontaneously induced. In contrast, when protoplasts were prepared and cultured in the presence of 16 micromolar 1-naphthaleneacetic acid (optimal concentration for protoplast division), the expression of β-glucanase was maintained close to the minimal level observed in tobacco leaves. This inhibitory effect was only promoted by active auxins (1-naphthaleneacetic acid, 2,4-dichlorophenoxyacetic acid, 2,4,5-trichlorophenoxyacetic acid, and 3-indoleacetic acid) but not by inactive auxin analogs. Tobacco protoplasts responded to exogenous elicitors from the cell wall of Phytophthora megasperma glycinea (Pmg) by accumulating β-glucanase in the presence of 16 micromolar 1-naphthaleneacetic acid. At higher auxin concentrations, the elicitor-induced β-glucanase synthesis was inhibited. Naphthaleneacetic acid concentration (3 × 10−5 molar) required to inhibit by 50% the expression of this defense reaction triggered by a near-optimal elicitor concentration was about 100 times higher than that sufficient to inhibit by 50% the spontaneous expression in nonelicited protoplasts. This is the first demonstration of an auxin-fungal elicitor interaction in the control of a defined defense reaction. The above observations were extended to soybean cell protoplasts. The Pmg elicitor-induced stimulation of the synthesis of pathogenesis related P17 polypeptides and of a 39-kilodalton peptide immunologically related to tobacco β-glucanase was only observed when the spontaneous accumulation of these proteins was inhibited in auxin-treated protoplasts.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ayers A. R., Ebel J., Valent B., Albersheim P. Host-Pathogen Interactions: X. Fractionation and Biological Activity of an Elicitor Isolated from the Mycelial Walls of Phytophthora megasperma var. sojae. Plant Physiol. 1976 May;57(5):760–765. doi: 10.1104/pp.57.5.760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Caboche M., Muller J. F., Chanut F., Aranda G., Cirakoglu S. Comparison of the growth promoting activities and toxicities of various auxin analogs on cells derived from wild type and a nonrooting mutant of tobacco. Plant Physiol. 1987 Apr;83(4):795–800. doi: 10.1104/pp.83.4.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chappell J., Nable R. Induction of sesquiterpenoid biosynthesis in tobacco cell suspension cultures by fungal elicitor. Plant Physiol. 1987 Oct;85(2):469–473. doi: 10.1104/pp.85.2.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dangl J. L., Hauffe K. D., Lipphardt S., Hahlbrock K., Scheel D. Parsley protoplasts retain differential responsiveness to u.v. light and fungal elicitor. EMBO J. 1987 Sep;6(9):2551–2556. doi: 10.1002/j.1460-2075.1987.tb02543.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis K. R., Hahlbrock K. Induction of defense responses in cultured parsley cells by plant cell wall fragments. Plant Physiol. 1987 Aug;84(4):1286–1290. doi: 10.1104/pp.84.4.1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis K. R., Lyon G. D., Darvill A. G., Albersheim P. Host-Pathogen Interactions : XXV. Endopolygalacturonic Acid Lyase from Erwinia carotovora Elicits Phytoalexin Accumulation by Releasing Plant Cell Wall Fragments. Plant Physiol. 1984 Jan;74(1):52–60. doi: 10.1104/pp.74.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ecker J. R., Davis R. W. Plant defense genes are regulated by ethylene. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5202–5206. doi: 10.1073/pnas.84.15.5202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamborg O. L., Miller R. A., Ojima K. Nutrient requirements of suspension cultures of soybean root cells. Exp Cell Res. 1968 Apr;50(1):151–158. doi: 10.1016/0014-4827(68)90403-5. [DOI] [PubMed] [Google Scholar]
- Grosset J., Meyer Y., Chartier Y., Kauffmann S., Legrand M., Fritig B. Tobacco Mesophyll Protoplasts Synthesize 1,3-beta-Glucanase, Chitinases, and "Osmotins" during in Vitro Culture. Plant Physiol. 1990 Feb;92(2):520–527. doi: 10.1104/pp.92.2.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauffmann S., Legrand M., Geoffroy P., Fritig B. Biological function of ;pathogenesis-related' proteins: four PR proteins of tobacco have 1,3-beta-glucanase activity. EMBO J. 1987 Nov;6(11):3209–3212. doi: 10.1002/j.1460-2075.1987.tb02637.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keen N. T. Specific elicitors of plant phytoalexin production: detenninants of race specificity in pathogens? Science. 1975 Jan 10;187(4171):74–75. doi: 10.1126/science.187.4171.74. [DOI] [PubMed] [Google Scholar]
- Keen N. T., Yoshikawa M. beta-1,3-Endoglucanase from Soybean Releases Elicitor-Active Carbohydrates from Fungus Cell Walls. Plant Physiol. 1983 Mar;71(3):460–465. doi: 10.1104/pp.71.3.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Legrand M., Kauffmann S., Geoffroy P., Fritig B. Biological function of pathogenesis-related proteins: Four tobacco pathogenesis-related proteins are chitinases. Proc Natl Acad Sci U S A. 1987 Oct;84(19):6750–6754. doi: 10.1073/pnas.84.19.6750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer Y., Aspart L., Chartier Y. Auxin-induced regulation of protein synthesis in tobacco mesophyll protoplasts cultivated in vitro: I. Characteristics of auxin-sensitive proteins. Plant Physiol. 1984 Aug;75(4):1027–1033. doi: 10.1104/pp.75.4.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer Y., Chartier Y., Alibert G. Auxin reduces the synthesis of major vacuolar proteins in tobacco mesophyl protoplast. Plant Physiol. 1987 Mar;83(3):713–718. doi: 10.1104/pp.83.3.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohnen D., Shinshi H., Felix G., Meins F. Hormonal regulation of beta1,3-glucanase messenger RNA levels in cultured tobacco tissues. EMBO J. 1985 Jul;4(7):1631–1635. doi: 10.1002/j.1460-2075.1985.tb03830.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nothnagel E. A., McNeil M., Albersheim P., Dell A. Host-Pathogen Interactions : XXII. A Galacturonic Acid Oligosaccharide from Plant Cell Walls Elicits Phytoalexins. Plant Physiol. 1983 Apr;71(4):916–926. doi: 10.1104/pp.71.4.916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinette D., Matthysse A. G. Inhibition by Agrobacterium tumefaciens and Pseudomonas savastanoi of development of the hypersensitive response elicited by Pseudomonas syringae pv. phaseolicola. J Bacteriol. 1990 Oct;172(10):5742–5749. doi: 10.1128/jb.172.10.5742-5749.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinshi H., Mohnen D., Meins F. Regulation of a plant pathogenesis-related enzyme: Inhibition of chitinase and chitinase mRNA accumulation in cultured tobacco tissues by auxin and cytokinin. Proc Natl Acad Sci U S A. 1987 Jan;84(1):89–93. doi: 10.1073/pnas.84.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi Y., Yoshikawa M., Takeba G., Tanaka K., Shibata D., Horino O. Molecular Cloning and Ethylene Induction of mRNA Encoding a Phytoalexin Elicitor-Releasing Factor, beta-1,3-Endoglucanase, in Soybean. Plant Physiol. 1990 Jun;93(2):673–682. doi: 10.1104/pp.93.2.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward E. W., Cahill D. M., Bhattacharyya M. K. Abscisic Acid Suppression of Phenylalanine Ammonia-Lyase Activity and mRNA, and Resistance of Soybeans to Phytophthora megasperma f.sp. glycinea. Plant Physiol. 1989 Sep;91(1):23–27. doi: 10.1104/pp.91.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]