Summary
Background
Chronic cough is a common respiratory symptom with an impact on daily activities and quality of life. Global prevalence data are scarce and derive mainly from European and Asian countries and studies with outcomes other than chronic cough. In this study, we aimed to estimate the prevalence of chronic cough across a large number of study sites as well as to identify its main risk factors using a standardised protocol and definition.
Methods
We analysed cross-sectional data from 33,983 adults (≥40 years), recruited between Jan 2, 2003 and Dec 26, 2016, in 41 sites (34 countries) from the Burden of Obstructive Lung Disease (BOLD) study. We estimated the prevalence of chronic cough for each site accounting for sampling design. To identify risk factors, we conducted multivariable logistic regression analysis within each site and then pooled estimates using random-effects meta-analysis. We also calculated the population attributable risk (PAR) associated with each of the identifed risk factors.
Findings
The prevalence of chronic cough varied from 3% in India (rural Pune) to 24% in the United States of America (Lexington,KY). Chronic cough was more common among females, both current and passive smokers, those working in a dusty job, those with a history of tuberculosis, those who were obese, those with a low level of education and those with hypertension or airflow limitation. The most influential risk factors were current smoking and working in a dusty job.
Interpretation
Our findings suggested that the prevalence of chronic cough varies widely across sites in different world regions. Cigarette smoking and exposure to dust in the workplace are its major risk factors.
Funding
Wellcome Trust.
Keywords: Chronic cough, Epidemiology, Global health, Excess risk
Research in context.
Evidence before this study
We searched PubMed, from database inception to 30 July 2023, using terms such as “chronic cough,” “epidemiology,” “prevalence,” “risk factor,” “adult,” and “general population”. Prevalence estimates for chronic cough ranged from 2% to 18%. Smoking, asthma, obesity, chronic obstructive pulmonary disease (COPD), upper airway cough syndrome (UACS), gastro-oesophageal reflux disease (GORD), and low education level are some of the factors that have most commonly been associated with chronic cough. A systematic assessment of chronic cough and its risk factors across several world regions is lacking.
Added value of this study
This study provides population-based estimates of prevalence of chronic cough for 41 sites across several world regions using the same protocol and definition. It also provides a list of risk factors for chronic cough and the proportion of prevalence explained by each risk factor in each study site. Current smoking and working in a dusty job are the most influential risk factors for chronic cough worldwide. Besides a better understanding of the geographic variation in chronic cough prevalence and of the characteristics of people with chronic cough, a common finding across study sites and regions was that chronic cough may persist for several years.
Implications of all the available evidence
The available evidence should be used to better support public health strategies targeting chronic cough and attenuate its burden on global health.
Introduction
Chronic cough (CC) is one of the most common reasons why people seek medical attention.1 Regardless of the underlying cause, CC has a significant impact on daily activities2 and is linked to poorer health status in general populations.3,4 It has been associated with psychosocial conditions, urinary incontinence, and depression,5,6 as well as higher healthcare use and cost.7 Yet, little is known about its true prevalence in various regions of the world.
A systematic review published in 2015 reported a wide range of prevalence estimates for CC across world regions, varying from 2.3% in Africa to 18.1% in Oceania.8 However, less than a third of the studies were conducted in Africa and Asia, studies were not primarily designed to assess CC, and the definition was not the same across the studies, which poses challenges in comparing data across different studies. Even in more recent studies and within the same country the definition of CC varies.4,9
Several factors have been associated with CC, but with some exceptions the list of its established risk factors is limited. Smoking, chronic obstructive pulmonary disease (COPD), asthma, upper airway cough syndrome (UACS), and gastro-oesophageal reflux disease (GORD) are suggested as the most common causes of CC.10, 11, 12, 13 Recent reviews emphasise the importance of conducting extensive epidemiological studies that can identify the prevalence and relevant risk factors in general populations,11 and address the need to utilise a standardised definition of CC in representative populations from different countries.14 In this context, our study is well-suited and effectively fulfills this crucial need. Using a standardised protocol, we aimed to provide estimates of prevalence of CC for several sites across the world. It was also our aim to identify the most important risk factors for CC.
Methods
Study design
A detailed description of the BOLD cohort has been published elsewhere.15 In brief, non-institutionalised adults (≥40 years old) were identified and recruited from the general population in 41 sites with more than 150,000 inhabitants. In each site, the aim was to recruit a minimum of 600 participants, with equal number of males and females. Sampling strategies varied across sites, with some using cluster sampling and others using either simple random sampling or stratified random sampling. For each site and participant, weights were derived to account for sampling design and to preserve representativeness of prevalence estimates.
The 41 study sites were located across 34 countries and several world regions: 11 in Europe (Tirana in Albania, Salzburg in Austria, London in England, Tartu in Estonia, Hannover in Germany, Reykjavik in Iceland, Maastricht in the Netherlands, Bergen in Norway, Krakow in Poland, Lisbon in Portugal, and Uppsala in Sweden); 14 in Asia (Guangzhou in China, Kashmir, Mumbai, Mysore and Pune in India, Chui and Naryn in Kyrgyztan, Penang in Malaysia, Karachi in Pakistan, Manila and Nampicuan-Talugtug in the Philippines, Riyadh in Saudi Arabia, Sri Lanka, and Adana in Turkey); 11 in Africa (Annaba in Algeria, Seme-Kpodji in Benin, Limbe in Cameroon, Blantyre and Chikwawa in Malawi, Fes in Marocco, Ife in Nigeria, Uitsig-Ravensmead in South Africa, Gezeira and Khartoum in Sudan, and Sousse in Tunisia); 2 in North America (Vancouver in Canada, and Lexington, KY, in the United States of America); 2 in the Caribbean (Jamaica, and Trinidad and Tobago); and 1 in Australia (Sydney). Fourteen sites were in high-income countries, while 27 were in low- or middle-income countries.
Information on respiratory symptoms, health status, and exposure to potential risk factors was collected by trained fieldworkers, who administered standardised questionnaires translated into the local language. While prevalence data on CC is presented for 33,983 individuals who completed the core questionnaire and had data on CC, risk factor analyses are based on data from 28,639 participants who additionally provided acceptable quality post-bronchodilator spirometry. Recruitment occurred between Jan 2, 2003 and Dec 12, 2016.
Ethics
All sites received approval from their local ethics committee, and participants provided informed consent. The study was conducted as per good clinical practice (GCP) as well as local ethics regulations.
Definition of chronic cough
CC was defined as a cough, without having a cold, on most days for at least three months each year. It was assessed using two questions: “Do you usually cough when you don't have a cold?” and “Do you cough on most days for as much as three months each year?”, and participants who affirmatively responsed to both were classified as resporting CC.
Potential risk factors
Based on prior knowledge, we considered several potential risk factors for CC. These included age (in years), sex (males, females), and smoking status.11 The main question for smoking was “Have you ever smoked cigarettes? (‘Yes‘ means more than 20 packs of cigarettes in a lifetime or more than 1 cigarette each day for a year)”. Participants who responded with ‘No’ were classified as never smokers. In case of a ‘Yes’ response, a subsequent question asked at what age the participant had stopped smoking, if applicable. Participants who provided a numerical response were classified as former smokers, while those who did not provide an answer were categorised as current smokers. We also considered passive smoking (‘yes’ to the question whether anyone (other than the participant) had smoked a cigarette, pipe, or cigar in the participant's home during the past 2 weeks), body mass index (BMI; underweight: <18.5 kg m−2, normal weight: 18.5–24.9 kg m−2, overweight: 25.0–29.9 kg m−2, obese: ≥30.0 kg m−2), years worked in a dusty job (‘yes’ to “have you ever worked for a year or more in a dusty job?” and answer to “for how many years have you worked in a dusty job?”), education (based on ‘‘How many years of schooling have you completed?”), history of tuberculosis (‘yes’ to “has a doctor or health care provider ever told you that you had tuberculosis?”), and hypertension (‘yes’ to “has a doctor or health care provider ever told you that you had hypertension?”). We did not include the use of solid fuels as a factor in our analyses due to previous findings of the BOLD study, which showed no association with CC.16 Chronic airflow obstruction (CAO) was assessed using spirometry (EasyOneTM, ndd Medizintechnik AG, Zurich, Switzerland) and defined as post-bronchodilator forced expiratory volume in 1 s (FEV1) to forced vital capacity (FVC) less than the lower limit of normal (LLN) for age and sex, based on equations of the National Health and Nutrition Examination Survey (NHANES III).17
Statistical analysis
We used multivariable logistic regression to identify factors associated with CC. Our regression model included all potential risk factors listed above (i.e., age, sex, smoking status, passive smoking, BMI, years worked in a dusty job, education, history of tuberculosis, hypertension, and chronic airflow obstruction). We estimated the adjusted odds ratio for each factor within each site, and then pooled site-specific estimates using random effects meta-analysis.18 We used the I2 statistic to summarise heterogeneity across sites. Results were considered significant if the p-value was <0.05. Prevalence estimates and regression analysis were corrected for sampling weights. For each of the identified risk factors, we estimated the population attributable risk (PAR), i.e., the excess prevalence of CC that can be attributed to the risk factor.19 Analyses were conducted using Stata v.16 (Stata Corp., College Station, TX, USA), and a user-written program to call OpenBUGS into Stata.20
Role of the funding source
The funders of the study did not contribute to the study design, data collection, data analysis or writing of the manuscript. All authors had full access to the data and accept responsibility for the decision to submit for publication.
Results
Population characteristics
The mean age of participants was 55 years, with slightly more females (53.3%) than males, and the mean BMI was 26.5 kg m−2. About two thirds were never smokers, 18.9% were current smokers, and passive smoking was reported by 19.2%. About one third of the participants worked in a dusty job. The mean duration of working in a dusty job was 5.6 years. The mean duration of schooling was 9 years. History of tuberculosis was reported by 2.3% and hypertension by 25.3% (Table 1). For more details on the distribution of these characteristics please see Supplementary Table S1.
Table 1.
Characteristics and comparison of the BOLD participants with and without chronic cough.
| With chronic cough (n = 3444) | Without chronic cough (n = 30,539) | Total (n = 33,983) | |
|---|---|---|---|
| Age (years), mean (SD) | 57 (13) | 56 (11) | 55 (11) |
| Sex, n (%) | |||
| Females | 1873 (54.4%) | 16,255 (53.2%) | 18,128 (53.3%) |
| Males | 1571 (45.6%) | 14,284 (46.8%) | 15,855 (46.7%) |
| BMI (kg.m−2), mean (SD) | 27.2 (6.2) | 26.42 (5.6) | 26.5 (5.7) |
| Smoking status, n (%) | |||
| Current smokers | 1068 (31.0%) | 5353 (17.5%) | 6421 (18.9%) |
| Ex-smokers | 705 (20.5%) | 5772 (18.9%) | 6477 (19.1%) |
| Never smokers | 1671 (48.5%) | 19,414 (66.6%) | 21,085 (62.0%) |
| Passive smoking, n (%) | 885 (25.7%) | 5595 (18.3%) | 6480 (19.2%) |
| Ever worked in a dusty job, n (%) | 1497 (43.5%) | 9660 (31.6%) | 11,157 (32.8%) |
| Duration of work in a dusty job (years), mean (SD) | 8 (13) | 5 (11) | 6 (11) |
| Schooling (years), mean (SD) | 8 (5) | 9 (5) | 9 (5) |
| History of tuberculosis, n (%) | 153 (4.4%) | 624 (2.0%) | 777 (2.3%) |
| Hypertension, n (%) | 1178 (34.2%) | 7429 (24.3%) | 8607 (25.3%) |
| FEV1/FVC (%), mean (SD) | 73.7 (12.0) | 78.1 (8.2) | 77.7 (8.7) |
BMI: Body mass index. FEV1/FVC: Forced expiratory volume in 1 s to the forced vital capacity ratio. SD: Standard deviation.
Participants with CC were older, were more likely current smokers and had more frequent exposure to passive smoking and a dusty job. They also showed a higher BMI, a lower FEV1/FVC ratio, a lower education level (less schooling years) and a higher proportion of self-reported hypertension and tuberculosis history (Table 1).
Prevalence of chronic cough
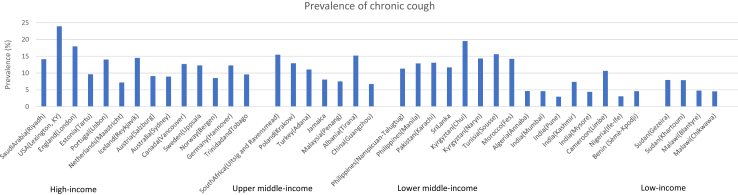
The pooled prevalence of CC in adults ≥ 40 years living in the study sites was 11.8%. However, variation across study sites was huge, ranging from 3% in India (rural Pune) to 24% in the United States of America (Lexington, KY). The lowest prevalence estimates were found in low- and middle-income countries (Fig. 1). The regions with the highest prevalence were North America (18.8%, 95% CI 16.5%–21%), Central Asia (18.4%, 95% CI 14.4%–22.5%), and Southern Africa (15.4%, 95% CI 12.6%–18.3%), whereas regions with the lowest prevalence were West Africa (3.6%, 95% CI 2.8%–4.3%), East Asia (6.7%, 95% CI 4.7%–8.7%), and East Africa (7.9%, 95% CI 6.5%–9.2%) (Table 2).
Fig. 1.
Prevalence of chronic cough across 41 sites of the BOLD study, by gross national income.
Table 2.
Prevalence and characteristics of study participants with chronic cough across world regions, sorted by highest overall prevalence.
| North America | Central Asia | Southern Africa | Central & Eastern Europe | North Africa & Middle East | Western Europe | Central Africa | South East Asia | Australia | South Asia | Caribbean | East Africa | East Asia | West Africa | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chronic cough prevalence (%) | 18.8 (16.5, 21) | 18.4 (14.4, 22.5) | 15.4 (12.6, 18.3) | 15.1 (11.1, 19) | 14.2 (11.5, 16.9) | 12.6 (11.1, 14) | 10.7 (7.3, 14) | 9.9 (7.5, 12.3) | 8.9 (6.6, 11.2) | 8.9 (7, 10.9) | 8.6 (7.3, 9.8) | 7.9 (6.5, 9.2) | 6.7 (4.7, 8.7) | 3.6 (2.8, 4.3) |
| Participants with chronic cough (n) | 247 | 314 | 135 | 275 | 488 | 696 | 45 | 393 | 52 | 304 | 194 | 176 | 40 | 85 |
| Cough duration (%) | ||||||||||||||
| <2 years | 20.1 (14.8, 25.4) | 20.8 (10.3, 31.3) | 37.8 (28.8, 46.8) | 19.1 (8.2, 29.9) | 27.3 (15.4, 39.1) | 21.2 (15.5, 26.8) | 60.7 (43.7, 77.8) | 54.8 (47.4, 62.3) | 17.5 (6.6, 28.5) | 28.5 (20.5, 36.6) | 46 (36.9, 55.1) | 20.1 (12.9, 27.4) | 22.5 (8.8, 36.2) | 46.9 (34.8, 58.9) |
| 2–5 years | 30.6 (24, 37.2) | 31.8 (23.8, 39.7) | 25.3 (17.1, 33.5) | 50 (37.3, 62.8) | 27.2 (20.1, 34.3) | 19.2 (12.2, 26.3) | 11.3 (1.6, 21) | 24.7 (16.9, 32.4) | 27.7 (15.1, 40.3) | 31.8 (19.9, 43.6) | 20.4 (11.2, 29.6) | 33 (21.4, 44.5) | 29.6 (15, 44.2) | 15.5 (8.3, 22.6) |
| >5 years | 49.3 (42.4, 56.2) | 47.4 (42.4, 52.5) | 36.9 (27.9, 46) | 30.9 (21.8, 39.9) | 45.5 (30.1, 60.9) | 59.6 (52.1, 67.1) | 28 (11.9, 44) | 20.5 (14.5, 26.5) | 54.8 (40.6, 69) | 39.7 (29.9, 49.5) | 33.6 (25.2, 42) | 46.9 (37.3, 56.5) | 48 (31.9, 64) | 37.7 (25.8, 49.6) |
| Female (%) | 52.3 (49.5, 55.2) | 54.3 (48.1, 60.6) | 56.1 (53.1, 59) | 50.9 (48.2, 53.7) | 50.6 (45.7, 55.5) | 54.2 (48.2, 60.3) | 40.6 (35, 46.2) | 51.6 (48.7, 54.5) | 52.9 (52.4, 53.5) | 42.7 (40.8, 44.6) | 51 (48.7, 53.3) | 47 (42.2, 51.7) | 49.4 (49.4, 49.4) | 49.3 (46.4, 52.1) |
| Age group (%) | ||||||||||||||
| 40–49 years | 34.2 (31.5, 37) | 39.6 (36.2, 43) | 43.7 (39.7, 47.7) | 35.6 (32.7, 38.6) | 44.4 (41, 47.8) | 28.4 (24.3, 32.4) | 50.9 (45.2, 56.6) | 42.8 (39.3, 46.3) | 31.8 (27.9, 35.7) | 44.9 (41.8, 48.1) | 36.3 (34.3, 38.4) | 44.5 (41.6, 47.3) | 44.9 (40.9, 48.8) | 47.1 (44, 50.2) |
| 50–59 years | 28.3 (25.9, 30.6) | 32.6 (29.1, 36.2) | 29.5 (26, 33) | 28.6 (24.8, 32.4) | 26.9 (24.9, 29) | 27.1 (25.4, 28.9) | 27.8 (22.8, 32.7) | 29.4 (26.5, 32.3) | 25.7 (22.2, 29.2) | 29.3 (26.5, 32) | 29.1 (26.9, 31.3) | 26.3 (24.1, 28.5) | 25.1 (21.6, 28.6) | 27 (24.7, 29.3) |
| 60–69 years | 17.8 (15.8, 19.8) | 14.1 (10.1, 18) | 17.8 (14.9, 20.7) | 19.2 (16.5, 21.8) | 16 (13.1, 18.9) | 23.2 (20.9, 25.4) | 17.7 (13.6, 21.7) | 17.3 (15.6, 19) | 18 (15.1, 20.9) | 16.5 (14.4, 18.6) | 18, 4 (16.7, 20.1) | 15, 9 (14.1, 17.7) | 19 (15.9, 22.2) | 15 (13.4, 16.7) |
| 70+ years | 19.7 (17.1, 22.3) | 13.7 (8.3, 19) | 9 (6.8, 11.1) | 16.6 (14.7, 18.4) | 12.6 (11, 14.3) | 21.3 (17.9, 24.8) | 3.7 (1.8, 5.5) | 10.5 (8, 12.9) | 24.6 (20.9, 28.2) | 9.3 (6.5, 12) | 16.2 (14.7, 17.6) | 13.3 (11, 15.7) | 11 (8.5, 13.5) | 10.9 (9, 12.8) |
| Education (%) | ||||||||||||||
| None/primary school | 2.9 (1.9, 3.9) | 6 (2.9, 9) | 44.7 (40.7, 48.6) | 19.9 (15.3, 24.5) | 69 (64.7, 73.3) | 23.2 (20.5, 25.8) | 57.1 (51.5, 62.8) | 25.1 (22.2, 27.9) | 3.6 (2, 5.1) | 42.9 (39.3, 46.6) | 30.7 (27.6, 33.8) | 60.7 (54.6, 66.8) | 28.9 (25.3, 32.5) | 53.5 (50, 57) |
| High school | 37.8 (35, 40.5) | 50 (44.4, 55.6) | 48.1 (44.3, 52) | 45.1 (38.1, 52.2) | 23.1 (19.1, 27.1) | 47.2 (45.1, 49.3) | 32 (26.7, 37.4) | 62 (59.5, 64.5) | 35.1 (31.2, 39) | 36.7 (33.6, 39.7) | 54.2 (51.3, 57.2) | 28.5 (24.1, 32.8) | 59.3 (55.4, 63.2) | 31.1 (28.2, 34) |
| College/University | 59.4 (56.6, 62.1) | 44 (38.9, 49.2) | 7.2 (5.2, 9.2) | 35 (30.5, 39.4) | 7.9 (3.7, 12) | 29.6 (26.4, 32.8) | 10.8 (7.4, 14.3) | 12.9 (11.1, 14.7) | 61.4 (57.4, 65.4) | 20.4 (17.6, 23.2) | 15.1 (12.8, 17.4) | 10.9 (8.2, 13.6) | 11.8 (9.2, 14.4) | 15.5 (12.6, 18.3) |
| Smoking status (%) | ||||||||||||||
| Never smokers | 39.2 (36.4, 42) | 63 (56.6, 69.4) | 30.5 (27, 33.9) | 63 (58.2, 67.7) | 71.7 (67.4, 76) | 48.6 (45.9, 51.3) | 78.6 (73.9, 83.3) | 62 (58.4, 65.5) | 47.4 (43.3, 51.4) | 81.2 (79, 83.4) | 63.4 (60.4, 66.4) | 76.6 (73.1, 80) | 56.6 (53.9, 59.3) | 89.6 (87.6, 91.5) |
| Current smokers | 21.7 (19.3, 24.1) | 26.7 (20.4, 33) | 47.8 (44.1, 51.6) | 22.3 (18.8, 25.8) | 10.7 (7.7, 13.7) | 20.4 (17.5, 23.2) | 6.8 (4, 9.6) | 26.5 (23.5, 29.5) | 14.9 (12, 17.9) | 11.8 (9.8, 13.7) | 18.1 (15.6, 20.7) | 8.8 (7.1, 10.5) | 29.7 (26.6, 32.8) | 3.4 (2.1, 4.7) |
| Ex-smokers | 39.1 (36.3, 41.9) | 10.3 (8.8, 11.8) | 21.7 (18.7, 24.7) | 14.8 (9.8, 19.7) | 17.6 (15.1, 20) | 31 (27.8, 34.3) | 14.6 (10.5, 18.7) | 11.5 (9.9, 13.1) | 37.7 (33.8, 41.6) | 7.1 (5.7, 8.4) | 18.4 (16.5, 20.3) | 14.6 (11.7, 17.5) | 13.7 (11.1, 16.3) | 7 (5.6, 8.5) |
| Passive smoking (%) | 18.7 (16.5, 21) | 7.2 (4.1, 10.4) | 50 (45.7, 54.2) | 36.7 (32.7, 40.7) | 10.8 (8.9, 12.7) | 18.8 (14.8, 22.8) | 3.6 (1.3, 6) | 35.4 (32.8, 38) | 11 (8.5, 13.6) | 7.1 (5.5, 8.6) | 17.6 (15.6, 19.7) | 9.1 (6.2, 12) | 23 (19.7, 26.4) | 1.2 (0.6, 1.7) |
| Body mass index (%) | ||||||||||||||
| Underweight (<18.5 kg/m2) | 0.3 (0, 0.6) | 1.2 (0.6, 1.8) | 7.1 (5.2, 9) | 0.1 (0, 0.3) | 1.8 (0.9, 2.6) | 0.3 (0.1, 0.5) | 1.1 (−0.1, 2.3) | 4.7 (3.2, 6.2) | 0.3 (−0.1, 0.8) | 6.7 (5.2, 8.2) | 3.5 (2.6, 4.5) | 4.3 (2.9, 5.8) | 4.7 (3, 6.4) | 4 (2.9, 5.1) |
| Normal (18.5–24.9 kg/m2) | 29.1 (26.7, 31.5) | 35.5 (32.9, 38.1) | 33.2 (29.7, 36.6) | 28.6 (25.9, 31.3) | 29.8 (23.8, 35.8) | 30.9 (28.5, 33.2) | 43.2 (37.6, 48.8) | 45.7 (43, 48.4) | 30.6 (26.7, 34.6) | 50.6 (47.3, 54) | 35.6 (33, 38.2) | 40.8 (37.8, 43.9) | 69 (65.3, 72.7) | 49.7 (46.7, 52.7) |
| Overweight (25–29.9 kg/m2) | 35.6 (32.8, 38.4) | 32.9 (28.6, 37.2) | 27 (23.8, 30.2) | 40.4 (38.2, 42.7) | 36.6 (33.3, 40) | 44.2 (42.3, 46.1) | 32.1 (26.8, 37.4) | 35.5 (32.8, 38.2) | 38.8 (34.7, 42.9) | 28.9 (26, 31.8) | 31.1 (29, 33.3) | 31.8 (29, 34.7) | 22.9 (19.5, 26.3) | 28 (25.5, 30.4) |
| Obese (≥30 kg/m2) | 35 (32.3, 37.7) | 30.4 (25, 35.9) | 32.8 (29.5, 36.1) | 30.8 (28.2, 33.4) | 31.8 (26.6, 36.9) | 24.6 (22.1, 27) | 23.6 (18.6, 28.6) | 14.1 (11.7, 16.5) | 30.2 (26.4, 34.1) | 13.8 (11.8, 15.8) | 29.7 (27.5, 31.9) | 23 (20.8, 25.2) | 3.4 (2, 4.9) | 18.3 (16.3, 20.4) |
| Ever worked in a dusty job (%) | 42.2 (39.4, 45) | 27.8 (18.7, 36.9) | 49 (45.5, 52.6) | 62.5 (55.8, 69.2) | 40.5 (37.1, 43.8) | 39.6 (34, 45.3) | 61.2 (55.6, 66.8) | 42 (38.8, 45.3) | 31.9 (28.2, 35.6) | 19.9 (17.1, 22.6) | 50.5 (48.1, 52.9) | 28 (25.4, 30.7) | 36.2 (32.3, 40.1) | 38.2 (35.5, 40.8) |
| Chronic airflow obstruction (%) | 14 (11.9, 16) | 11.5 (9, 13.9) | 19.6 (16.6, 22.6) | 8.8 (6.1, 11.6) | 8.2 (6.5, 9.8) | 10 (8.3, 11.7) | 4.3 (1.7, 6.9) | 6.2 (4.5, 7.9) | 11.1 (8.4, 13.8) | 8.4 (6.4, 10.3) | 7.7 (5.8, 9.5) | 8.5 (6.5, 10.5) | 7.9 (5.4, 10.3) | 7.1 (5.5, 8.8) |
| History of tuberculosis (%) | 2.4 (1.6, 3.3) | 1 (0.1, 1.9) | 15.2 (12.6, 17.8) | 0.8 (0.2, 1.4) | 1.4 (0.2, 2.5) | 3.7 (2.8, 4.6) | 0.9 (−0.2, 2.1) | 4.6 (2.9, 6.3) | 0.6 (0, 1.2) | 0.7 (0.3, 1.1) | 0.3 (0, 0.7) | 0.6 (0.2, 1) | 3.2 (1.8, 4.6) | 0.5 (0.1, 0.8) |
| Hypertension (%) | 36.4 (33.7, 39.2) | 27.9 (23.8, 32) | 37.4 (34, 40.9) | 24 (22.2, 25.8) | 29.1 (25, 33.3) | 32.8 (30.7, 35) | 9.8 (6, 13.5) | 24.5 (21.7, 27.3) | 32.7 (28.8, 36.5) | 20.6 (18.3, 22.9) | 31.7 (29.6, 33.8) | 17 (13.8, 20.2) | 16.7 (13.8, 19.7) | 10.7 (8.2, 13.3) |
Estimates were obtained through meta-analysis. 95% confidence intervals are given in brackets.
Sites included in each world region: North America–Canada (Vancouver), USA (Lexington, KY); Central Asia–Kyrgyztan (Chui), Kyrgyztan (Naryn); Southern Africa–South Africa (Uitsig and Ravensmead); Central and Eastern Europe–Albania (Tirana), Estonia (Tartu), Poland (Krakow); North Africa & Middle East–Algeria (Annaba), Morocco (Fes), Saudi Arabia(Riyadh), Tunisia (Sousse), Turkey (Adana); Western Europe–Austria (Salzburg), England (London), Germany (Hannover), Iceland (Reykjavik), Netherlands (Maastricht), Norway (Bergen), Portugal (Lisbon), Sweden (Uppsala); Central Africa–Cameroon (Limbe); South East Asia–Malaysia (Penang), Philippines (Manila), Philippines (Nampicuan-Talugtug), Sri Lanka; Australia–Australia (Sydney); South Asia–India (Mumbai), India (Mysore), India (Pune), India (Kashmir), Pakistan (Karachi); Caribbean–Jamaica, Trinidad and Tobago; Africa East–China (Guangzhou); East Asia–Malawi (Blantyre), Malawi (Chikwawa), Sudan (Gezeira), Sudan (Khartoum); West Africa–Benin (Sèmè-Kpodji), Nigeria (Ife-Ife).
From all the participants with CC, slightly more than a quarter (27.4%) reported having CC for less than two years, 29% for two to five years, and 43.6% for over five years. The region with the highest proportion of participants experiencing cough for over five years, in relation to all participants reporting CC, was Western Europe (59.6%, 95% CI 52.1%–67.1%). Amongst study sites, Bergen in Norway had the highest proportion (70.7%).
The region with the highest proportion of females experiencing CC was Southern Africa (56.1%, 95% CI 53.1%–59%), whereas the lowest proportion was found in Central Africa (40.6%, 9%%CI 35%–46.2%).
Regions with a higher proportion of younger participants with CC were primarily located in Africa and Asia, where most participants with CC were under 49 years old. The highest proportion of participants with CC aged 70 years or over were found in regions with mainly high-income study sites, i.e., Australia (24.6%), Western Europe (21.3%, 95% CI 17.9%–24.8%) and North America (19.7%, 95% CI 17.1%–22.3%).
The proportion of participants with lower level of education (none or primary school) amongst those with CC was highest in North Africa/Middle East (69%, 95% CI 64.7%–73.3%), East Africa (60.7%, 95% CI 54.6%–66.8%), and Central Africa (57.1%, 95% CI 51.5%–62.8%). The proportion of participants with a college or university degree amongst those with CC was highest in Australia (61.4%), North America (59.4%, 95% CI 56.6%–62.1%), and Central Asia (44%, 95% CI 38.9%–49.2%).
The proportion of current smokers amongst participants with CC were highest in Southern Africa (47.8%, 95% CI 44.1%–51.6%), East Asia (29.7%, 95% CI 26.6%–32.8%), and Central Asia (26.7%, 95% CI 20.4%–33%). Passive smoking amongst participants with CC was highest in Southern Africa (50%, 95% CI 45.7%–54.2%).
The proportion of obesity amongst participants with CC was highest in North America (35%, 95% CI 32.3%–37.7%), Southern Africa (32.8%, 95% CI 29.5%–36.1%) and North Africa/Middle East (31.8%, 95% CI 26.6%–36.9%).
Central and Eastern Europe had the highest proportion of dusty job exposure (62.5%, 95% CI 55.8%–69.2%) amongst participants with CC.
Regarding self-reported doctor-diagnoses amongst participants with CC, the prevalence of hypertension was highest in Southern Africa (37.4%, 95% CI 34%–40.9%), North America (36.4%, 95% CI 33.7%–39.2%), and Western Europe (32.8%, 95% CI 30.7%–35%), whereas a history of tuberculosis was most prevalent in Southern Africa (15.2%, 95% CI 12.6%–17.8%) followed by South East Asia (4.6%, 95% CI 2.9%–6.3%) and Western Europe (3.7%, 95% CI 2.8%–4.6%).
Factors associated with chronic cough
CC was associated with being a female, current smoking, passive smoking, working in a dusty job, obesity, lower education, tuberculosis as well as hypertension and airflow limitation (Table 3).
Table 3.
Adjusted estimates for the association of chronic cough with several risk factors.
| OR (95% CI) | p value | I2 (χ2 heterogeneity p value) | |
|---|---|---|---|
| Age/per 10 years | 0.99 (0.92–1.08) | 0.89 | 58.7 (<0.001) |
| Female sex | 1.50 (1.29–1.75) | <0.001 | 42 (<0.001) |
| Current smoker | 2.07 (1.73–2.48) | <0.001 | 44.2 (<0.001) |
| Ex-smoker | 1.15 (0.95–1.39) | 0.16 | 41.7 (<0.001) |
| Passive smoking | 1.29 (1.13–1.47) | <0.001 | 20.1 (0.15) |
| Working in a dusty job | 1.64 (1.47–1.84) | <0.001 | 23.3 (0.1) |
| Underweight | 1.02 (0.80–1.30) | 0.88 | 0 (0.73) |
| Overweight | 1.06 (0.95–1.18) | 0.28 | 0 (0.84) |
| Obesity | 1.20 (1.07–1.35) | <0.001 | 34.1 (0.02) |
| Schooling (per year) | 0.97 (0.96–0.99) | 0.01 | 50.4 (<0.001) |
| History of tuberculosis | 1.77 (1.30–2.40) | <0.001 | 42.6 (0.01) |
| Hypertension | 1.25 (1.14–1.38) | <0.001 | 58.9 (<0.001) |
| Post-BD FEV1/FVC | 0.96 (0.95–0.96) | <0.001 | 58.7 (<0.001) |
Underweight: <18.5 kg m−2, overweight: 25.0–29.9 kg m−2, obese: ≥30.0 kg m−2. Post-BD FEV1/FVC: Post bronchodilator Forced expiratory volume in 1 s as a ratio of the forced vital capacity.
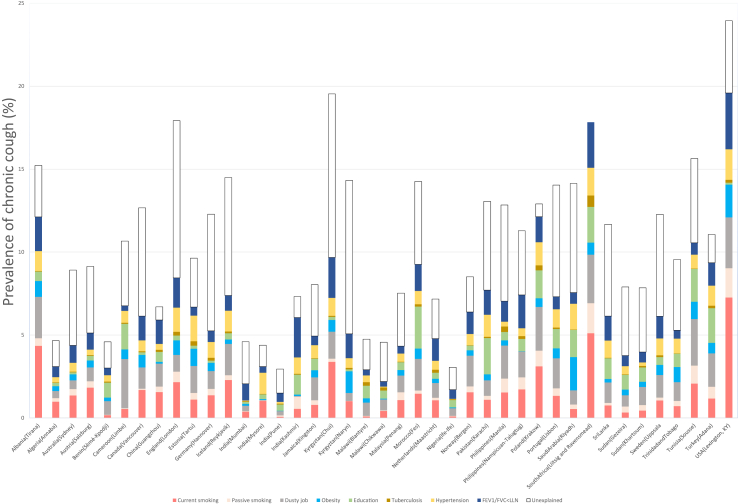
Site specific PAR estimates for each risk factor associated with CC are shown in Fig. 2. Globally, current smoking was the most important risk factor (PAR 1.47%) followed by working in a dusty job (PAR 1.32%) (Table 4). Current smoking was the most important factor in South Africa (Uitsig and Ravensmead), where 33.1% of the CC prevalence can be explained by current smoking (current smoking PAR 5.1%; CC prevalence 15.4%). Working in a dusty job was most influent in Cameron (Limbe) with 27.9% (PAR 2.97%; prevalence 10.7%), followed by China (Guangzhou) and Norway (Bergen) with 21.7% of the prevalence of CC explained by this factor (PAR 1.85%; prevalence 8.51%). Kyrgyzstan (Naryn) had the highest proportion of unexplained prevalence of CC, where almost two-thirds (65.6%) of reported prevalence could not be attributed to a specific risk factor. The next highest proportions of unexplained prevalence were reported in Germany (Hannover) at 57.2% and India (Mumbai) at 55.1%.
Fig. 2.
Population Attributable Risk for chronic cough (i.e., prevalence of chronic cough attributable to different risk factors) by BOLD study site. Each risk factor is represented by a different colour: red, current smoking; pink, passive smoking; grey, dusty job; light blue, obesity; green, education; brown, tuberculosis; yellow, hypertension; dark blue, FEV1/FVC < LLN; and white, unexplained.
Table 4.
Risk factors for chronic cough ranked by population attributable risk, expressed as percent of total population aged ≥ 40 years, with 95% credible intervals.
| PAR | Lcrl | Ucrl | |
|---|---|---|---|
| Current smoking | 1.47 | 0.07 | 7.26 |
| Dusty job | 1.32 | 0.01 | 1.81 |
| CAO | 1.15 | 0.04 | 3.08 |
| Hypertension | 0.8 | 0.12 | 2.52 |
| Education | 0.78 | 0.02 | 2.01 |
| Obesity | 0.53 | 0.02 | 0.69 |
| Passive smoking | 0.4 | 0.02 | 1.85 |
| Tuberculosis | 0.11 | −0.3 | 3.39 |
PAR: population attributable risk, Lcrl: Lower credible interval, Ucrl: Upper credible interval, CAO: chronic airflow obstruction.
Discussion
Using real-world data, we report a pooled prevalence of 11.8% for CC, with significant variation across world regions and study sites. CC was associated with being a female as well as current smoking, passive smoking, working in a dusty job, a history of tuberculosis, obesity, lower education level, hypertension and lower FEV1/FVC. The most influential of these factors were current smoking and working in a dusty job.
Reported prevalence in general populations can be up to 18%2,10,12,13,21, 22, 23, 24 and is lower in Asia, with representative data reported from China and Korea.3,4,9,13 Of note, potential causes for variation in prevalence estimates are due to the use of different study designs, sample populations, definitions and ethnicities.8 In our study, using one definition for all study sites, the pooled prevalence of CC was 11.8%, with wide variation ranging from 3% in India (Pune) to 24% in the United States of America (Lexington, KY). Overall, we found that low- and middle-income sites had lower prevalence of CC, and that site specific findings about CC can not easily be extrapolated to country or region level. However, one common feature among individuals with CC is the experience of coughing for several years which has been reported previously in numerous studies.2,21,25, 26, 27, 28, 29 Our study underlines this burden by showing that more than 70% of all participants have experienced CC for more than two years, with almost half (44.7%) of them having experienced CC for more than 5 years.
Most data about risk factors in general populations derive from Europe or Asia.11,14 In Europe, the Rotterdam study has identified current smoking, GORD, asthma, and chronic obstructive pulmonary disease (COPD) as independent risk factors for CC in the general population.10 In another population study from Copenhagen, the analysis of risk factors was stratified by smoking status and showed that female sex, asthma, and GORD were associated with CC in never smokers, abdominal obesity, low income, and asthma were associated in ex-smokers, and airflow limitation in current smokers.12 In Asia, current smoking, older age, and UACS were identified as risk factors by a recent cross-sectional general population study of the Korean National Health and Nutrition Examination Survey.3,4 Although several conditions are often suggested in the clinical literature as potential risk factors, some of them may only have limited evidence in general populations.11 We report a strong association of current smoking with CC, and identified it as the most influential risk factor for CC worldwide. Current smoking has been frequently linked to CC4,10,13,23, 24, 25 but to our knowledge, population attributable risk analysis in CC has been only applied in one study, which also found that smoking is the most important risk factor.12 Our study has also identified working in a dusty job as another important risk factor contributing to CC worldwide. Data about dust as a risk factor of CC are scarce. In a Danish study, exposure to dust was ranked as only the seventh most influential factor.12 Other associations had been described earlier in Poland30 and in Singapore.31 Another study from Norway reported that men with high exposure to dust had a higher incidence of CC.32 Our findings emphasise the importance of recognizing distinct risk factors in different regions to effectively address the needs of each population and further develop specific prevention and management strategies.
Our data indicate a slightly higher female prevalence in CC compared to the non-CC group (54.4% vs 53.2%), but we were unable to confirm a significant difference. Regarding regional patterns, we found higher proportions of CC among males in almost all Asian regions, as well as in some African regions. Several European population based studies conducted in the UK,33 Germany,23 Austria,25 and Denmark12 reported more women but did not state a significant sex- or gender-specific difference. The Rotterdam study reported a female predominance for the age under 70 years but no significant sex-specific differences in the total study population.10 One of the assumed explanations for the female predominance is a heightened cough reflex.34, 35, 36 In contrast to this, a systematic review reported higher male prevalence in CC.37 More recent studies from general populations in China4 and Korea3,13 as well as from Canada24 reported more CC in males.
While current smoking was the most important risk factor, we were unable to detect an association of former smoking with CC, which is consistent with findings from the Rotterdam study and results from a meta-analysis.10,11 However, studies conducted in Germany and Austria found that former smoking was a significant risk factor for CC.23,25 For smoking exposure in terms of passive smoking, we found a positive association with CC as previously described in other studies.38,39
Obesity is a significant risk factor in our study and literature suggests a positive association between CC and obesity.10,22,40,41 It has been reported that people with obesity may be at higher risk of developing CC as compared to people without obesity.10,24 From the Copenhagen General Population Study, an up to threefold higher risk in people with obesity has been reported.42 A partial explanation for this increased risk was attributed to GORD, which mediated up to one quarter of CC cases in obesity. This finding provides a potential explanation for the relationship between obesity and CC, where an increased BMI is linked to an elevated risk of GORD.43 Therefore, practical guidelines for managing CC due to GORD recommend dietary modifications to promote weight loss in people with obesity.44 However, it is important to note that a direct relationship between GORD and CC could not be analysed in this study.
In our study, age is not a significant global risk factor for CC which contrasts with literature stating that CC is age-related and typically found in middle-aged to elderly people.3,4,6,10,24,45 It is important to note, however, that our study considers data from regions beyond Europe and Asia, providing a more comprehensive view of the global landscape of CC.
Our study reveals a link between CC and level of education. In contrast to a German study that did not find any significant difference in education levels,23 a Norwegian community cohort study reported that people with a lower educational level had a higher risk of developing CC.46 Similar findings outside Europe were observed in China4 and Nigeria.47 Additionally, the findings of a recent systematic review are in line with our results, indicating that individuals with lower levels of education are at a higher risk of developing CC.11 In this study, a history of tuberculosis was identified as a significant risk factor. Tuberculosis is not generally considered one of the most common causes of CC, even in high prevalence countries.48 A Korean study found that history of tuberculosis was more prevalent among people with CC.13 However, contrary to our findings, tuberculosis was not identified as a significant risk factor in the Korean general population.3
We observed a significantly higher prevalence of hypertension among individuals with CC. Additionally, we found that the presence of hypertension was associated with CC. Studies from Asian populations, particularly from China and Korea, reported significantly higher proportions of hypertension amongst people with CC.4,13 Similar results were also reported in Austria, where the association between CC and hypertension was significant as well.25 Within the context of practical guidelines,49,50 various cardiac diseases including those involving pulmonary congestion, are identified as potential causes of CC. In addition to chronic left heart failure and the use of cardiac drugs (like ACE inhibitors, beta-blockers, and Amiodarone), arrhythmias have also been reported in contributing to CC.51, 52, 53 Given that our study did not assess or investigate these specific conditions, it is challenging to draw conclusions regarding the role of hypertension alone in CC so that further cardiovascular phenotyping is required.
Our study has several strengths. First, it is a large comprehensive study covering several sites across several world regions, making it representative of populations. Given that our study included only adults over the age of 40, it should be noted that the findings cannot be extrapolated across all age groups in the population. Data collection was conducted using a standardised protocol and one definition of CC for all study sites. Data collection was undertaken by trained interviewers in local language. The study also has limitations. The main one is its cross-sectional nature, which prevents us from inferring causal relationships between risk factors and CC. We acknowledge that the definition we used differs from the definition stated in the most recent guidelines, which specify a cough lasting for a minimum of 8 weeks. We adopted the 3-month cut-off as this duration has been used in the majority of epidemiological studies.8,37 The prevalence of CC may be affected by recall bias as it was self-reported. Since the presence of cough is a subjective state that was not quantitatively measured in this study but evaluated through questionnaires, the differences in prevalence may also indicate variations in the perception and interpretation of cough across different cultures and regions. Our study did not include information about antihypertensive drugs to evaluate the proportion of ACE-inhibitors as potential triggers of iatrogenic CC. Additionally, we were unable to provide proportions of reflux cough as we did not collect information on GORD which could lead to potential overestimation of the unexplained CC prevalence in PAR analysis.
In summary, CC is common in many parts of the world. However, its prevalence varies considerably across regions, with low- and middle-income countries showing the lowest estimates of CC prevalence. Besides current smoking, exposure to dust in the workplace was identified as an important risk factor for CC. Our study emphasises the need for a better understanding of CC and its risk factors to provide tailored relief strategies for this troublesome condition.
Contributors
HA, EFMW, and AFSA conceived the study. AFSA and JP accessed and verified the data. JP performed data analysis. Under the supervision of EFMW and AFSA, HA prepared the initial draft. All authors provided critical revision of the manuscript, as well as read and approved the final manuscript. All authors had full access to the data and accept responsibility for the decision to submit for publication.
Data sharing statement
De-identified participant data and questionnaires may be shared, after publication, on a collaborative basis upon reasonable request made to Dr Amaral (a.amaral@imperial.ac.uk). Requesting researchers will be required to submit an analysis plan.
Declaration of interests
Fatima Rodrigues declares grants and personal fees from A. Menarini, Boehringer Ingelheim, Teva Pharma, Novartis, GlaxoSmithKline, AstraZeneca, VitalAire and Nippon Gases outside the submitted work. Wan C. Tan received grants from the Canadian Institute of Heath Research (CIHR/Rx&D Collaborative Research Program Operating Grants- 93,326) with industry partners Astra Zeneca Canada Ltd., Boehringer-Ingelheim Canada Ltd, GlaxoSmithKline Canada Ltd, Merck, Novartis Pharma Canada Inc., Nycomed Canada Inc., Pfizer Canada Ltd. for conducting the longitudinal population-based Canadian Cohort of Obstructive Lung Disease (CanCOLD) study on COPD. David Mannino is a consultant to GSK, AstraZeneca, Regeneron, Genentech, COPD Foundation, and expert witness on behalf of people suing Tobacco Industry (Schlesinger Law Firm). Sonia Buist is Chair of the Data Safety & Monitoring Board for the RELIANCE Clinical Trial. Frits Franssen declares personal fees from AstraZeneca, Chiesi, GlaxoSmithKline, MSD, Pieris, and Verona Pharma. Robab Breyer-Kohansal declares consulting fees from AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Menarini, Novartis Pharma, and Sanofi, and participation on advisory boards for AstraZeneca, Menarini, and Sanofi. Thorarinn Gislason received a grant from the Icelandic Research Fund. Kevin Mortimer declares participation on advisory boards for AstraZeneca and GlaxoSmithKline. Sylvia Hartl declares grants from GSK, Chiesi Farma, Menarini Pharma, and AstraZeneca, and participation on advisory boards for Menarini Pharma and GSK. AFSA declares a grant from the COLT Foundation (CF/01/21).
Acknowledgements
We thank all participants and field workers for their time and effort dedicated to this study. Supported by Wellcome Trust grant 085790/Z/08/Z for the BOLD study. Additional local support for BOLD clinical sites was provided by: Boehringer Ingelheim China (GuangZhou, China); Turkish Thoracic Society, BoehringerIngelheim, and Pfizer (Adana, Turkey); Altana, AstraZeneca, Boehringer-Ingelheim, GlaxoSmithKline, Merck Sharpe & Dohme, Novartis, Salzburger Gebietskrankenkasse and Salzburg Local Government (Salzburg, Austria); Research for International Tobacco Control, the International Development Research Centre, the South African Medical Research Council, the South African Thoracic Society GlaxoSmithKline Pulmonary Research Fellowship, and the University of Cape Town Lung Institute (Cape Town, South Africa); and Landspıtali-University Hospital-Scientific Fund, GlaxoSmithKline Iceland, and AstraZeneca Iceland (Reykjavik, Iceland); GlaxoSmithKline Pharmaceuticals, Polpharma, Ivax Pharma Poland, AstraZeneca Pharma Poland, ZF Altana Pharma, Pliva Krakow, Adamed, Novartis Poland, Linde Gaz Polska, Lek Polska, Tarchominskie Zakłady Farmaceutyczne Polfa, Starostwo Proszowice, Skanska, Zasada, Agencja Mienia Wojskowego w Krakowie, Telekomunikacja Polska, Biernacki, Biogran, Amplus Bucki, Skrzydlewski, Sotwin, and Agroplon (Cracow, Poland); BoehringerIngelheim, and Pfizer Germany (Hannover, Germany); the Norwegian Ministry of Health’s Foundation for Clinical Research, and Haukeland University Hospital’s Medical Research Foundation for Thoracic Medicine (Bergen, Norway); AstraZeneca, Boehringer-Ingelheim, Pfizer, and GlaxoSmithKline (Vancouver, Canada); Marty Driesler Cancer Project (Lexington, Kentucky); Altana, Boehringer Ingelheim (Phil), GlaxoSmithKline, Pfizer, Philippine College of Chest Physicians, Philippine College of Physicians, and Underwriters Laboratories (Phil) (Manila, Philippines); Air Liquide Healthcare P/L, AstraZeneca P/L, Boehringer Ingelheim P/L, GlaxoSmithKline Australia P/L, Pfizer Australia P/L (Sydney, Australia), Department of Health Policy Research Programme, Clement Clarke International (London, UK); Boehringer Ingelheim and Pfizer (Lisbon, Portugal), Swedish Heart-Lung Foundation, The Swedish Association against Heart and Lung Diseases, GlaxoSmithKline (Uppsala, Sweden), Seed Money Grant (PF20/0512), Aga Khan University, and Chiesi Pakistan (Pvt.) Limited (Karachi, Pakistan).
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.eclinm.2024.102423.
Contributor Information
Hazim Abozid, Email: hazim@live.at.
BOLD Collaborative Research Group:
Hasan Hafizi, Anila Aliko, Donika Bardhi, Holta Tafa, Natasha Thanasi, Arian Mezini, Alma Teferici, Dafina Todri, Jolanda Nikolla, Rezarta Kazasi, Hamid Hacene Cherkaski, Amira Bengrait, Tabarek Haddad, Ibtissem Zgaoula, Maamar Ghit, Abdelhamid Roubhia, Soumaya Boudra, Feryal Atoui, Randa Yakoubi, Rachid Benali, Abdelghani Bencheikh, Nadia Ait-Khaled, Christine Jenkins, Guy Marks, Tessa Bird, Paola Espinel, Kate Hardaker, Brett Toelle, Michael Studnicka, Torkil Dawes, Bernd Lamprecht, Lea Schirhofer, Akramul Islam, Syed Masud Ahmed, Shayla Islam, Qazi Shafayetul Islam, Mesbah-Ul-Haque, Tridib Roy Chowdhury, Sukantha Kumar Chatterjee, Dulal Mia, Shyamal Chandra Das, Mizanur Rahman, Nazrul Islam, Shahaz Uddin, Nurul Islam, Luiza Khatun, Monira Parvin, Abdul Awal Khan, Maidul Islam, Herve Lawin, Arsene Kpangon, Karl Kpossou, Gildas Agodokpessi, Paul Ayelo, Benjamin Fayomi, Bertrand Mbatchou, Atongno Humphrey Ashu, Wan C. Tan, Wen Wang, NanShan Zhong, Shengming Liu, Jiachun Lu, Pixin Ran, Dali Wang, Jin-ping Zheng, Yumin Zhou, Rain Jogi, Hendrik Laja, Katrin Ulst, Vappu Zobel, Toomas-Julius Lill, Ayola Akim Adegnika, Tobias Welte, Isabelle Bodemann, Henning Geldmacher, Alexandra SchwedaLinow, Thorarinn Gislason, Bryndis Benedikdtsdottir, Kristin Jorundsdottir, Lovisa Gudmundsdottir, Sigrun Gudmundsdottir, Gunnar Gudmundsson, Mahesh Rao, Parvaiz A. Koul, Sajjad Malik, Nissar A. Hakim, Umar Hafiz Khan, Rohini Chowgule, Vasant Shetye, Jonelle Raphael, Rosel Almeda, Mahesh Tawde, Rafiq Tadvi, Sunil Katkar, Milind Kadam, Rupesh Dhanawade, Umesh Ghurup, Sanjay Juvekar, Siddhi Hirve, Somnath Sambhudas, Bharat Chaidhary, Meera Tambe, Savita Pingale, Arati Umap, Archana Umap, Nitin Shelar, Sampada Devchakke, Sharda Chaudhary, Suvarna Bondre, Savita Walke, Ashleshsa Gawhane, Anil Sapkal, Rupali Argade, Vijay Gaikwad, Sundeep Salvi, Bill Brashier, Jyoti Londhe, Sapna Madas, Althea Aquart-Stewart, Akosua Francia Aikman, Talant M. Sooronbaev, Bermet M. Estebesova, Meerim Akmatalieva, Saadat Usenbaeva, Jypara Kydyrova, Eliza Bostonova, Ulan Sheraliev, Nuridin Marajapov, Nurgul Toktogulova, Berik Emilov, Toktogul Azilova, Gulnara Beishekeeva, Nasyikat Dononbaeva, Aijamal Tabyshova, Kevin Mortimer, Wezzie Nyapigoti, Ernest Mwangoka, Mayamiko Kambwili, Martha Chipeta, Gloria Banda, Suzgo Mkandawire, Justice Banda, Li-Cher Loh, Abdul Rashid, Siti Sholehah, Mohamed C. Benjelloun, Chakib Nejjari, Mohamed Elbiaze, Karima El Rhazi, E.F.M. Wouters, G.J. Wesseling, Daniel Obaseki, Gregory Erhabor, Olayemi Awopeju, Olufemi Adewole, Amund Gulsvik, Tina Endresen, Lene Svendsen, Asaad A. Nafees, Muhammad Irfan, Zafar Fatmi, Aysha Zahidie, Natasha Shaukat, Meesha Iqbal, Luisito F. Idolor, Teresita S. de Guia, Norberto A. Francisco, Camilo C. Roa, Fernando G. Ayuyao, Cecil Z. Tady, Daniel T. Tan, Sylvia Banal-Yang, Vincent M. Balanag, Jr., Maria Teresita N. Reyes, Renato B. Dantes, Renato B. Dantes, Lourdes Amarillo, Lakan U. Berratio, Lenora C. Fernandez, Norberto A. Francisco, Gerard S. Garcia, Teresita S. de Guia, Luisito F. Idolor, Sullian S. Naval, Thessa Reyes, Camilo C. Roa, Jr., Flordeliza Sanchez, Leander P. Simpao, Ewa Nizankowska-Mogilnicka, Jakub Frey, Rafal Harat, Filip Mejza, Pawel Nastalek, Andrzej Pajak, Wojciech Skucha, Andrzej Szczeklik, Magda Twardowska, Cristina Barbara, Fatima Rodrigues, Herminia Dias, Joao Cardoso, João Almeida, Maria Joao Matos, Paula Simão, Moutinho Santos, Reis Ferreira, M. Al Ghobain, H. Alorainy, E. El-Hamad, M. Al Hajjaj, A. Hashi, R. Dela, R. Fanuncio, E. Doloriel, I. Marciano, L. Safia, Eric Bateman, Anamika Jithoo, Desiree Adams, Edward Barnes, Jasper Freeman, Anton Hayes, Sipho Hlengwa, Christine Johannisen, Mariana Koopman, Innocentia Louw, Ina Ludick, Alta Olckers, Johanna Ryck, Janita Storbeck, Kirthi Gunasekera, Rajitha Wickremasinghe, Asma Elsony, Hana A. Elsadig, Nada Bakery Osman, Bandar Salah Noory, Monjda Awad Mohamed, Hasab Alrasoul Akasha Ahmed Osman, Namarig Moham ed Elhassan, Abdel Mu’is El Zain, Marwa Mohamed Mohamaden, Suhaiba Khalifa, Mahmoud Elhadi, Mohand Hassan, Dalia Abdelmonam, Christer Janson, Inga Sif Olafsdottir, Katarina Nisser, Ulrike SpetzNystrom, Gunilla Hagg, GunMarie Lund, Terence Seemungal, Fallon Lutchmansingh, Liane Conyette, Imed Harrabi, Myriam Denguezli, Zouhair Tabka, Hager Daldoul, Zaki Boukheroufa, Firas Chouikha, Wahbi Belhaj Khalifa, Ali Kocabas, Attila Hancioglu, Ismail Hanta, Sedat Kuleci, Ahmet Sinan Turkyilmaz, Sema Umut, Turgay Unalan, Peter G.J. Burney, Anamika Jithoo, Louisa Gnatiuc, Hadia Azar, Jaymini Patel, Caron Amor, James Potts, Michael Tumilty, Fiona McLean, Risha Dudhaiya, A. Sonia Buist, Mary Ann McBurnie, William M. Vollmer, Suzanne Gillespie, Sean Sullivan, Todd A. Lee, Kevin B. Weiss, Robert L. Jensen, Robert Crapo, Paul Enright, David M. Mannino, John Cain, Rebecca Copeland, Dana Hazen, and Jennifer Methvin
Appendix ASupplementary data
References
- 1.Magni C., Chellini E., Zanasi A. Cough variant asthma and atopic cough. Multidiscip Respir Med. 2010;5:99–103. doi: 10.1186/2049-6958-5-2-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chamberlain S.A.F., Garrod R., Douiri A., et al. The impact of chronic cough: a cross-sectional European survey. Lung. 2015;193:401–408. doi: 10.1007/s00408-015-9701-2. [DOI] [PubMed] [Google Scholar]
- 3.Kang M.-G., Song W.-J., Kim H.-J., et al. Point prevalence and epidemiological characteristics of chronic cough in the general adult population: the Korean National Health and Nutrition Examination Survey 2010-2012. Medicine (Baltimore) 2017;96 doi: 10.1097/MD.0000000000006486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang K., Gu X., Yang T., et al. Prevalence and burden of chronic cough in China: a national cross-sectional study. ERJ Open Res. 2022;8 doi: 10.1183/23120541.00075-2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arinze J.T., Hofman A., de Roos E.W., et al. The interrelationship of chronic cough and depression: a prospective population-based study. ERJ Open Res. 2022;8:69–2022. doi: 10.1183/23120541.00069-2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morice A.H., Millqvist E., Bieksiene K., et al. ERS guidelines on the diagnosis and treatment of chronic cough in adults and children. Eur Respir J. 2020;55 doi: 10.1183/13993003.01136-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cho P.S.P., Shearer J., Simpson A., Campbell S., Pennington M., Birring S.S. Healthcare utilization and costs in chronic cough. Curr Med Res Opin. 2022;38:1251–1257. doi: 10.1080/03007995.2022.2065142. [DOI] [PubMed] [Google Scholar]
- 8.Song W.-J., Chang Y.-S., Faruqi S., et al. The global epidemiology of chronic cough in adults: a systematic review and meta-analysis. Eur Respir J. 2015;45:1479–1481. doi: 10.1183/09031936.00218714. [DOI] [PubMed] [Google Scholar]
- 9.Liang H., Ye W., Wang Z., et al. Prevalence of chronic cough in China: a systematic review and meta-analysis. BMC Pulm Med. 2022;22:62. doi: 10.1186/s12890-022-01847-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arinze J.T., de Roos E.W., Karimi L., Verhamme K.M.C., Stricker B.H., Brusselle G.G. Prevalence and incidence of, and risk factors for chronic cough in the adult population: the Rotterdam Study. ERJ Open Res. 2020;6 doi: 10.1183/23120541.00300-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang J., Perret J.L., Chang A.B., et al. Risk factors for chronic cough in adults: a systematic review and meta-analysis. Respirology. 2022;27:36–47. doi: 10.1111/resp.14169. [DOI] [PubMed] [Google Scholar]
- 12.Çolak Y., Nordestgaard B.G., Laursen L.C., Afzal S., Lange P., Dahl M. Risk factors for chronic cough among 14,669 individuals from the general population. Chest. 2017;152:563–573. doi: 10.1016/j.chest.2017.05.038. [DOI] [PubMed] [Google Scholar]
- 13.Koo H.-K., Jeong I., Lee S.W., et al. Prevalence of chronic cough and possible causes in the general population based on the Korean National Health and Nutrition Examination Survey. Medicine (Baltimore) 2016;95:e4595. doi: 10.1097/MD.0000000000004595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang X., Chung K.F., Huang K. Worldwide prevalence, risk factors and burden of chronic cough in the general population: a narrative review. J Thorac Dis. 2023;15:2300–2313. doi: 10.21037/jtd-22-1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Amaral A.F.S., Potts J., Knox-Brown B., et al. Cohort profile: burden of obstructive Lung disease (BOLD) study. Int J Epidemiol. 2023;52:e364–e373. doi: 10.1093/ije/dyad146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Amaral A.F.S., Patel J., Kato B.S., et al. Airflow obstruction and use of solid fuels for cooking or heating: BOLD results. Am J Respir Crit Care Med. 2018;197:595–610. doi: 10.1164/rccm.201701-0205OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hankinson J.L., Odencrantz J.R., Fedan K.B. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999;159:179–187. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- 18.Higgins J.P.T., Thompson S.G., Deeks J.J., Altman D.G. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burney P., Patel J., Minelli C., et al. Prevalence and population attributable risk for chronic airflow obstruction in a large multinational study. Am J Respir Crit Care Med. 2020;4(11):1353–1365. doi: 10.1164/rccm.202005-1990OC. published online Nov 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Palmer T., Thompson J., Moreno S. Stata Users Group, United Kingdom Stata Users’ Group Meetings 2007. vol. 6. 2007. Performing bayesian analysis in Stata using WinBUGS. [Google Scholar]
- 21.Lätti A.M., Pekkanen J., Koskela H.O. Defining the risk factors for acute, subacute and chronic cough: a cross-sectional study in a Finnish adult employee population. BMJ Open. 2018;8 doi: 10.1136/bmjopen-2018-022950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ford A.C., Forman D., Moayyedi P., Morice A.H. Cough in the community: a cross sectional survey and the relationship to gastrointestinal symptoms. Thorax. 2006;61:975–979. doi: 10.1136/thx.2006.060087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Virchow J.C., Li V.W., Fonseca E., et al. Chronic cough in Germany: results from a general-population survey. ERJ Open Res. 2021;8 doi: 10.1183/23120541.00420-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Satia I., Mayhew A.J., Sohel N., et al. Prevalence, incidence and characteristics of chronic cough among adults from the Canadian Longitudinal Study on Aging. ERJ Open Res. 2021;7 doi: 10.1183/23120541.00160-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abozid H., Baxter C.A., Hartl S., et al. Distribution of chronic cough phenotypes in the general population: a cross-sectional analysis of the LEAD cohort in Austria. Respir Med. 2022;192 doi: 10.1016/j.rmed.2021.106726. [DOI] [PubMed] [Google Scholar]
- 26.Everett C.F., Kastelik J.A., Thompson R.H., Morice A.H. Chronic persistent cough in the community: a questionnaire survey. Cough. 2007;3:5. doi: 10.1186/1745-9974-3-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yousaf N., Montinero W., Birring S.S., Pavord I.D. The long term outcome of patients with unexplained chronic cough. Respir Med. 2013;107:408–412. doi: 10.1016/j.rmed.2012.11.018. [DOI] [PubMed] [Google Scholar]
- 28.Kastelik J.A., Aziz I., Ojoo J.C., Thompson R.H., Redington A.E., Morice A.H. Investigation and management of chronic cough using a probability-based algorithm. Eur Respir J. 2005;25:235–243. doi: 10.1183/09031936.05.00140803. [DOI] [PubMed] [Google Scholar]
- 29.Meltzer E.O., Zeiger R.S., Dicpinigaitis P., et al. Prevalence and burden of chronic cough in the United States. J Allergy Clin Immunol Pract. 2021;9:4037–4044.e2. doi: 10.1016/j.jaip.2021.07.022. [DOI] [PubMed] [Google Scholar]
- 30.Krzyzanowski M., Jedrychowski W. Occupational exposure and incidence of chronic respiratory symptoms among residents of Cracow followed for 13 years. Int Arch Occup Environ Health. 1990;62:311–317. doi: 10.1007/BF00640839. [DOI] [PubMed] [Google Scholar]
- 31.LeVan T.D., Koh W.-P., Lee H.-P., Koh D., Yu M.C., London S.J. Vapor, dust, and smoke exposure in relation to adult-onset asthma and chronic respiratory symptoms: the Singapore Chinese Health Study. Am J Epidemiol. 2006;163:1118–1128. doi: 10.1093/aje/kwj144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Skorge T.D., Eagan T.M., Eide G.E., Gulsvik A., Bakke P.S. Occupational exposure and incidence of respiratory disorders in a general population. Scand J Work Environ Health. 2009;35:454–461. doi: 10.5271/sjweh.1352. [DOI] [PubMed] [Google Scholar]
- 33.Kaushik N., Smith J., Linehan M., Frank T. 6.1 epidemiology. European Respiratory Society; 2015. Prevalence of chronic cough in a population based survey; p. PA1139. [Google Scholar]
- 34.Kastelik J.A., Thompson R.H., Aziz I., Ojoo J.C., Redington A.E., Morice A.H. Sex-related differences in cough reflex sensitivity in patients with chronic cough. Am J Respir Crit Care Med. 2002;166:961–964. doi: 10.1164/rccm.2109061. [DOI] [PubMed] [Google Scholar]
- 35.Fujimura M., Kasahara K., Kamio Y., Naruse M., Hashimoto T., Matsuda T. Female gender as a determinant of cough threshold to inhaled capsaicin. Eur Respir J. 1996;9:1624–1626. doi: 10.1183/09031936.96.09081624. [DOI] [PubMed] [Google Scholar]
- 36.Kavalcikova-Bogdanova N., Buday T., Plevkova J., Song W.J. Chronic cough as a female gender issue. Adv Exp Med Biol. 2016;905:69–78. doi: 10.1007/5584_2015_182. [DOI] [PubMed] [Google Scholar]
- 37.Song W.J., Chang Y.S., Faruqi S., et al. Defining chronic cough: a systematic review of the epidemiological literature. Allergy Asthma Immunol Res. 2016;8:146–155. doi: 10.4168/aair.2016.8.2.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jordan R.E., Cheng K.K., Miller M.R., Adab P. Passive smoking and chronic obstructive pulmonary disease: cross-sectional analysis of data from the Health Survey for England. BMJ Open. 2011;1 doi: 10.1136/bmjopen-2011-000153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Robbins A.S., Abbey D.E., Lebowitz M.D. Passive smoking and chronic respiratory disease symptoms in non-smoking adults. Int J Epidemiol. 1993;22:809–817. doi: 10.1093/ije/22.5.809. [DOI] [PubMed] [Google Scholar]
- 40.Zeiger R.S., Schatz M., Butler R.K., Weaver J.P., Bali V., Chen W. Burden of specialist-diagnosed chronic cough in adults. J Allergy Clin Immunol Pract. 2020;8:1645–1657.e7. doi: 10.1016/j.jaip.2020.01.054. [DOI] [PubMed] [Google Scholar]
- 41.Guilleminault L. Chronic cough and obesity. Pulm Pharmacol Ther. 2019;55:84–88. doi: 10.1016/j.pupt.2019.01.009. [DOI] [PubMed] [Google Scholar]
- 42.Landt E.M., Çolak Y., Nordestgaard B.G., Lange P., Dahl M. Risk and impact of chronic cough in obese individuals from the general population. Thorax. 2021;77(3):223–230. doi: 10.1136/thoraxjnl-2020-216351. [DOI] [PubMed] [Google Scholar]
- 43.Irwin R.S., French C.L., Curley F.J., Zawacki J.K., Bennett F.M. Chronic cough due to gastroesophageal reflux. Clinical, diagnostic, and pathogenetic aspects. Chest. 1993;104:1511–1517. doi: 10.1378/chest.104.5.1511. [DOI] [PubMed] [Google Scholar]
- 44.Kahrilas P.J., Altman K.W., Chang A.B., et al. Chronic cough due to gastroesophageal reflux in adults: CHEST guideline and expert panel report. Chest. 2016;150:1341–1360. doi: 10.1016/j.chest.2016.08.1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Song W.-J., Morice A.H., Kim M.-H., et al. Cough in the elderly population: relationships with multiple comorbidity. PLoS One. 2013;8 doi: 10.1371/journal.pone.0078081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Eagan T.M.L., Gulsvik A., Eide G.E., Bakke P.S. The effect of educational level on the incidence of asthma and respiratory symptoms. Respir Med. 2004;98:730–736. doi: 10.1016/j.rmed.2004.02.008. [DOI] [PubMed] [Google Scholar]
- 47.Desalu O.O., Salami A.K., Fawibe A.E. Prevalence of cough among adults in an urban community in Nigeria. West Afr J Med. 2011;30:337–341. [PubMed] [Google Scholar]
- 48.Rosen M.J. Chronic cough due to tuberculosis and other infections. Chest. 2006;129:197S–201S. doi: 10.1378/chest.129.1_suppl.197S. [DOI] [PubMed] [Google Scholar]
- 49.Kardos P., Berck H., Fuchs K.-H., et al. Guidelines of the German respiratory society for diagnosis and treatment of adults suffering from acute or chronic cough. Pneumologie. 2010;64:701–711. doi: 10.1055/s-0030-1255526. [DOI] [PubMed] [Google Scholar]
- 50.Lai K., Shen H., Zhou X., et al. Clinical practice guidelines for diagnosis and management of cough-Chinese thoracic society (CTS) asthma consortium. J Thorac Dis. 2018;10:6314–6351. doi: 10.21037/jtd.2018.09.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Niimi A., Kihara Y., Sumita Y., Okano Y., Tambara K., Fujita M. Cough reflex by ventricular premature contractions. Int Heart J. 2005;46:923–926. doi: 10.1536/ihj.46.923. [DOI] [PubMed] [Google Scholar]
- 52.Stec S., Dabrowska M., Zaborska B., et al. Premature ventricular complex-induced chronic cough and cough syncope. Eur Respir J. 2007;30:391–394. doi: 10.1183/09031936.00062606. [DOI] [PubMed] [Google Scholar]
- 53.Stec S.M., Grabczak E.M., Bielicki P., et al. Diagnosis and management of premature ventricular complexes-associated chronic cough. Chest. 2009;135:1535–1541. doi: 10.1378/chest.08-1814. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.