Abstract
A successful anti-blood stage malaria vaccine trial based on a leading vaccine candidate, the major merozoite surface antigen-1 (MSP1), is reported here. The trial was based on Plasmodium cynomolgi, which is a primate malaria parasite which is highly analogous to the human parasite Plasmodium vivax, in its natural host, the toque monkey, Macaca sinica. Two recombinant baculovirus-expressed P. cynomolgi MSP1 proteins, which are analogous to the 42- and 19-kDa C-terminal fragments of P. falciparum MSP1, were tested by immunizing three groups of three animals each with either p42, p19, or both together. The vaccines were delivered subcutaneously in three doses at 4-week intervals with complete and incomplete Freund’s adjuvants. Very high antibody titers were obtained against both vaccinating antigens as measured by enzyme-linked immunosorbent assay (106 and above) and against whole parasites as measured by indirect immunofluorescence assay (>105), achieving, in most animals, about a 10-fold increase from the first to the last immunization. A blood stage challenge with P. cynomolgi parasites led, in three adjuvant-treated and three naive control animals, to blood infections which were patent for at least 44 days, reaching peak densities of 0.6 and 3.8%, respectively. In contrast, all except one of the nine animals in the three vaccinated groups were highly protected, showing either no parasitemia at all or transient parasitemias which were patent for only 1 or 2 days. When the three p19-vaccinated monkeys were rechallenged 6 months later, the protective efficacy was unchanged. The success of this trial, and striking analogies of this natural host-parasite system with human P. vivax malaria, suggests that it could serve as a surrogate system for the development of a human P. vivax malaria vaccine based on similar recombinant analogs of the P. vivax MSP1 antigen.
Plasmodium vivax, one of the two major human malaria parasites, accounts for a large proportion of the world’s clinical malaria infections due to its prevalence in much of Asia and South America; in Sri Lanka, P. vivax is responsible for 60 to 80% of the 300,000 to 400,000 clinical malaria infections which occur annually (1). Despite its wide reputation as the benign malaria parasite, it is not uncommon for it to lead to a severe and complicated pathology, including cerebral malaria and death (19, 25, 43). Thus, although it has been the subject of much less study than Plasmodium falciparum, due mainly to the lack of a continuous in vitro culture system, the threat to health from P. vivax in parts of the world where malaria is common is high. In addition, there is growing resistance of P. vivax parasite strains to chloroquine in many countries (12, 16, 17, 38). The situation therefore calls for extending to P. vivax the vaccine development efforts which are currently focused almost exclusively on P. falciparum malaria.
Our own efforts at vaccine development for P. vivax have focused on the major merozoite surface protein MSP1 (9, 29, 42), which is a leading candidate for an asexual blood stage vaccine against P. falciparum malaria (10). MSP1 is synthesized in hepatic and erythrocytic schizonts as a 200-kDa membrane-bound precursor (reviewed in references 6 and 21), which, around the time of merozoite release, is processed in P. falciparum in two steps leading first to 42-kDa and then to 19-kDa glycosylphosphatidylinositol-anchored C-terminal molecules. An immunization trial with the native MSP1 protein of P. falciparum conferred a highly protective immunity against homologous challenge in Aotus monkeys (39). Subsequently, protection was also elicited by Escherichia coli-expressed recombinant C-terminal MSP1 p19 analogs in rodent malaria models (7, 27). MSP1 p19 C-terminal recombinant antigens of P. falciparum produced in yeast (26) and p42 antigens produced in baculovirus (3) have also given protection in monkeys, indicating their potential as components of a subunit vaccine.
One of the greatest impediments to malaria vaccine development concerns the lack of in vitro correlates and suitable in vivo models of protective immunity against malaria in humans. Thus, the efficacy of candidate vaccines is assessed only at advanced stages of clinical testing, and the process from selection of the antigen through development of the product could take up to several years. This uncertainty of efficacy, combined with the high costs involved in product development and testing, has deterred potential investors from the field and slowed considerably the process of malaria vaccine development.
We describe here the use of a natural host-parasite system which appears to be highly analogous to human P. vivax malaria. The toque monkey, Macaca sinica, is one of the natural hosts of P. cynomolgi; it lives in regions of Sri Lanka which include the enzootic foci where the parasite abounds (11, 35). The analogy between the simian parasite P. cynomolgi and its human counterpart P. vivax is substantiated by biological (5, 8), genetic (13–15, 30, 44), and evolutionary (18) evidence (reviewed in references 28 and 31). Studies that we have carried out with this natural nonhuman primate system involving transmission-blocking immunity (34), relapses (unpublished data), and pathogenesis (33) have found direct application to human P. vivax malaria (31). P. cynomolgi and P. vivax MSP1 C-terminal recombinant proteins produced in the baculovirus expression system are highly homologous and appear to reproduce the physical and antigenic characteristics of the native proteins (22, 28). This report presents a highly successful vaccine trial with the toque monkey, showing excellent immunogenicity and protective efficacy of the P. cynomolgi MSP1 recombinant proteins.
MATERIALS AND METHODS
Animals.
Adult toque monkeys (M. sinica sinica) were captured from their natural habitats in the dry zone of Sri Lanka and were quarantined in the animal house for 1 month prior to experimental use. All animals were screened for the presence of a current malaria infection by microscopic examination of thick blood films and for previous exposure to malaria by detection of serum antimalarial antibodies by the indirect immunofluorescence assay (IFA). An animal was considered naive with respect to malaria when thick blood films failed to detect parasites for 7 consecutive days and anti-P. cynomolgi antibodies were undetectable. We have shown that such animals are free of subpatent malaria infections because they are incapable of transmitting malaria infections to other naive recipients by blood passage (20, 32).
Recombinant immunogens.
P. cynomolgi MSP1 recombinant proteins corresponding approximately to the P. falciparum MSP1 42- and 19-kDa C-terminal processing fragments (28, 29) were produced in the baculovirus expression system and purified by immunoaffinity chromatography with monoclonal antibodies as described previously (22).
Immunization of animals.
Fifteen malaria-naive animals comprising five groups of three animals each, matched by sex and weight (Table 1), were used for the trial. Animals in the first three groups (groups I, II, and III) were vaccinated with either p42, p19, or a combination of the two antigens, respectively, and those in group IV were vaccinated with normal saline; group V served as unvaccinated controls. Each inoculation consisted of 100 μg of the p42 antigen and/or 35 to 50 μg of the p19 antigen administered subcutaneously in three doses at 4-week intervals. Each dose was suspended in 0.5 ml of normal saline and emulsified in an equal volume of adjuvant consisting of Freund’s complete adjuvant (FCA) and Freund’s incomplete adjuvant (FIA) in proportions of 1:1 (first immunization) and 1:4 (second immunization). Only FIA was used for the third and last immunization. Immunized control animals in group IV were administered normal saline with the same adjuvant protocol. No clinically adverse reactions to vaccination were evident in any of the animals, even at the sites of subcutaneous vaccine injection. Serum samples were taken prior to the first immunization and 2 to 3 weeks after each immunization.
TABLE 1.
Relation between the course of infection in vaccinated toque monkeys and immune responses
| Group (vaccine) | Monkey
|
Prepatent period (days) | Peak parasitemia (%) | Duration of patency (days) | Reciprocal titera
|
||||
|---|---|---|---|---|---|---|---|---|---|
| No. | Wt (kg) | Sexb | IFA | ELISA
|
|||||
| 19 kDa | 42 kDa | ||||||||
| I (42 kDa + ADJ)c | T434 | 2.8 | M | 5 | 0.004 | 1 | >105 | 107 | 106 |
| T435 | 2.6 | F | 6 | 0.06 | 17 | >105 | 106 | 106 | |
| T428 | 1.95 | F | 0 | 0 | 0 | >105 | 106 | 106 | |
| II (19 kDa + ADJ) | T429 | 1.9 | M | 0 | 0 | 0 | >105 | 107 | 106 |
| T426 | 2.2 | M | 6 | 0.002 | 2 | >105 | 106 | 106 | |
| T427 | 2.04 | M | 8 | 0.002 | 1 | >105 | 107 | 106 | |
| III (19 kDa + 42 kDa + ADJ) | T430 | 1.5 | F | 6 | 0.02 | 2 | >105 | 106 | 106 |
| T431 | 1.9 | M | 13 | 0.002 | 1 | >105 | 107 | 107 | |
| T433 | 2.7 | M | 5 | 0.004 | 2 | >105 | 106 | 106 | |
| IV (normal saline + ADJ) | T425 | 3.5 | M | 5 | 0.1 | 54 | Negative | 102 | 102 |
| T436 | 2.2 | M | 7 | 0.4 | 39 | Negative | 102 | 102 | |
| T438 | 1.7 | F | 5 | 0.6 | 39 | Negative | 102 | 102 | |
| V (unvaccinated controls) | T437 | 2.4 | M | 5 | 0.4 | 39 | Negative | <102 | 102 |
| T440 | 2.0 | F | 5 | 1.5 | 39 | Negative | <102 | 102 | |
| T441 | 1.6 | M | 5 | 3.8 | 54 | Negative | <102 | 102 | |
Sera were obtained 3 weeks after the third immunization.
M, male; F, female.
ADJ, adjuvant.
Challenge infections and evaluation of protective efficacy.
Animals in all five groups were challenged 4 weeks after the third immunization with an inoculum of 2 × 105 asexual blood stage P. cynomolgi ceylonensis parasites obtained from a donor monkey. Parasites are maintained in the laboratory by blood and mosquito passage and cryopreservation (32, 34). Commencing from the fourth day after challenge, all 15 animals were screened for asexual blood stage parasites by microscopic examination of Giemsa-stained thick and thin smears prepared from an earprick. Blood film examinations were conducted daily for 6 weeks and weekly up to 8 weeks postchallenge.
On the 30th day of patency, blood samples from all animals were analyzed by PCR with standard methods and primers for small-subunit rRNA derived from P. vivax sequences (40). These primers amplify P. cynomolgi DNA with equal efficiency, as expected from the high degree of sequence homology between the two species (reviewed in reference 28). A second blood challenge with 106 parasites was given to animals in the p19-vaccinated (group II) and adjuvant-treated control (group IV) groups 6 months later.
ELISA.
Ninety-six-well microtiter plates (Nunc, Roskilde, Denmark) were coated with 0.1 μg of either p19 or p42 antigen per ml and incubated at 4°C overnight, and enzyme-linked immunosorbent assays (ELISAs) were carried out as previously described (37) with horseradish peroxidase-conjugated goat anti-human immunoglobulin (1/1,000 dilution) (DAKO Immunoglobulins, Copenhagen, Denmark). The optical density was read at 492 nm and the ELISA end point was taken as the highest serum dilution which gave an optical density greater than twice that of control sera.
IFA.
P. cynomolgi schizont-infected erythrocytes were purified from infected blood by a Percoll density gradient method as described previously for P. vivax (23). The infected-erythrocyte suspension was spotted onto 12-well antigen slides, air dried, and stored at −70°C. The IFA was performed as previously described (42) with fluorescein isothiocyanate-conjugated goat anti-human immunoglobulin G (Organon Teknika Corp., West Chester, Pa.).
SDS-polyacrylamide gel electrophoresis and Western blotting.
Purified P. cynomolgi schizonts were extracted in sodium dodecyl sulfate (SDS) sample buffer with reducing agents, the polypeptides were electrophoretically separated on SDS–10% polyacrylamide gels and transferred to nitrocellulose paper by electroelution, and the Western blot was processed as described previously (36).
Statistical analysis.
Log transformations of parasitemias were obtained, and comparison of means was done by analysis of variance.
RESULTS
Immunogenicity.
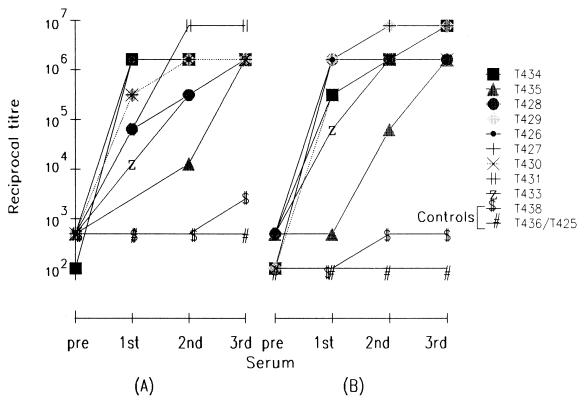
Both the recombinant p19 and p42 antigens, either alone or in combination, were highly immunogenic, since animals in all three MSP1-vaccinated groups developed very high antibody titers against the vaccinating antigens as detected by ELISA (106 and above) and against P. cynomolgi schizont-infected erythrocytes as measured by IFA (>105) (Table 1). Although antibody titers were already quite high after a single injection of the recombinant antigen, these titers increased in postimmunization sera in most animals by about 10-fold or more from the first to the last injections (Fig. 1). IFA titers of these sera also showed a similar increase through consecutive immunizations (data not shown).
FIG. 1.
ELISA end point titers of all pre- and post-immunization sera against p42 (A) and p19 (B) recombinant proteins.
Protection against parasite challenge.
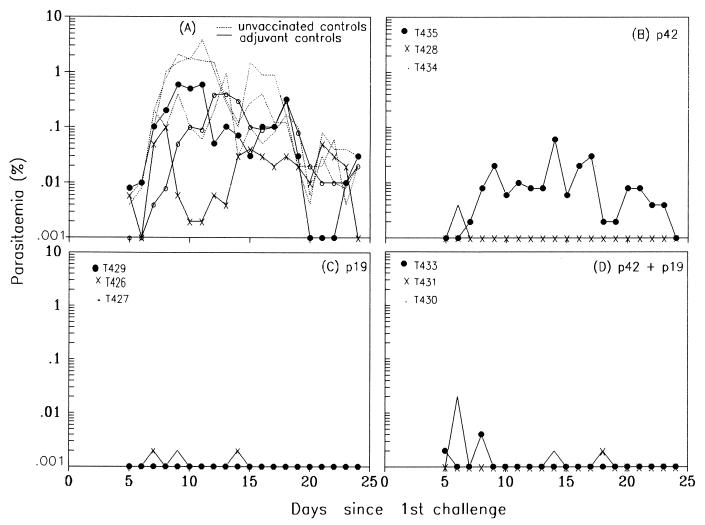
Following challenge, adjuvant-treated control animals of group IV developed P. cynomolgi parasitemias similar to those in the unvaccinated controls. All animals in both groups had patent infections for 39 to 54 days, although the parasite densities in the adjuvant-treated controls were significantly lower than those in the unvaccinated controls (P = 0.0006). These adjuvant-treated controls also experienced lower peak parasitemias (0.1 to 0.6%) than unvaccinated controls (0.4 to 3.8%) (Fig. 2A; Table 1), indicating some effect of adjuvant alone. In contrast, the three animals immunized with p19 (group II) developed either no parasitemia at all (T429) or a transient infection of 0.002% which was patent for either 1 (T427) or 2 (T426) days (Fig. 2C; Table 1). All but one of the six animals in the other two vaccinated groups were similarly protected, with peak parasitemias of 0.002 to 0.02% and 0- to 2-day patencies (Fig. 2B and D; Table 1). One animal in the p42-vaccinated group (group I, animal T435) developed a parasitemia that peaked at 0.06% and persisted for 17 days. The parasite densities from day 5 to 32 of challenge infections in each of the three vaccinated groups were significantly lower than those in both the adjuvant-treated control group and the unvaccinated control group (F = 23.7788; P < 0.0001).
FIG. 2.
Courses of blood parasitemia following challenge infection with P. cynomolgi in naive unvaccinated (group V) and adjuvant-treated control (group IV) animals (A) and in animals immunized with p42 (group I) (B), with p19 (group II) (C), and with both p42 and p19 (group III) (D).
PCR analysis was performed on blood samples from all animals 34 days after challenge in order to detect subpatent parasitemias. A positive PCR was obtained for all control animals (groups IV and V), while no PCR signal was observed in the vaccinated animals (groups I to III), indicating that any low-grade, microscopically subpatent blood infection was below the limits of detection by PCR (data not shown). All six control animals continued to have a patent parasitemia until day 44 after challenge; four of the six animals then had cleared their blood parasitemias, but the other two (one in each group) still had parasitemias of 0.004%. On the 68th day after challenge, all animals (vaccinated and control) were treated with a 3-day curative regime of chloroquine (25 mg per kg of body weight) administered intramuscularly to eliminate any remaining blood infection.
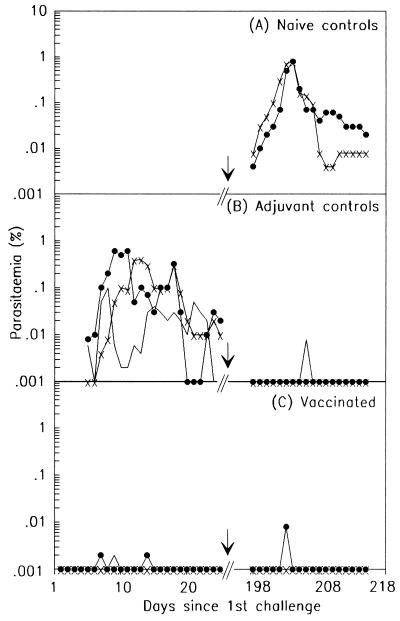
To examine the state of protective immunity 6 months after the immunization and the first challenge, a second blood challenge of 106 parasites but otherwise identical to the first was given to three groups, as follows: (i) the three animals vaccinated with p19 (group II), two of which had experienced a transient blood infection during the first challenge; (ii) the three animals in the adjuvant-treated control group (group IV) that were unprotected during the first challenge and therefore developed a complete blood infection; and (iii) a new control group of two naive unvaccinated animals. The two naive animals developed a normal blood infection with a peak 0.8% parasitemia which was still patent on day 20 after challenge, when daily screening was discontinued (Fig. 3A). In contrast, all animals in the other two groups were very well protected after the second challenge, with only one of the three animals in each group (T426 and T425) developing transient parasitemias of 0.008%, which were patent for only 1 day (Fig. 3B and C). These findings indicate that the animals vaccinated with p19 and only minimally stimulated by the first challenge infection, which resulted in either very low or no (T429) detectable infection, were able to resist a second challenge infection as well as the adjuvant-treated controls, which were protected by exposure to a much higher complete blood infection during the first challenge. Western blotting was performed with sera taken from both groups of animals, just before the second challenge, using schizont extracts of P. cynomolgi. While the sera of the adjuvant-treated control animals reacted with parasite antigens with a wide range of molecular weights, the sera of all three p19 vaccinees reacted strongly and only with the MSP1 processing products on the Western blot (data not shown). These p19 vaccinees also had very high titers of antibodies (106) against the recombinant p19 antigen when tested by ELISA. These findings indicate that the continued protected state in the p19 group must have been mediated by this sustained or boosted anti-MSP1 p19 immune response.
FIG. 3.
Courses of blood parasitemia following the first and second challenge infections with P. cynomolgi in naive control animals (A), in adjuvant-treated control animals (group IV) (B), and in p19-vaccinated animals (group II) (C).
DISCUSSION
Vaccination with the baculovirus-expressed p42 and p19 C-terminal P. cynomolgi MSP1 recombinant proteins resulted in a very high antibody response and very effective protection against a challenge infection in eight of nine animals, with an apparently sterile immunity induced in two. The low transient blood parasitemias of challenge infections seem to have been completely cleared, since parasite DNA could not be detected by PCR 34 days after challenge. The three animals in the p19-vaccinated group were also able to resist a second challenge 6 months later.
The high degree of protection elicited in this trial could be due to the nature of the recombinant proteins themselves, which were produced in a higher-order eukaryotic system and appear to reproduce the complex conformational structure of the native protein. We have, in fact, shown that the reactivity of human antisera from P. vivax-immune individuals is completely dependent on the integrity of reduction-sensitive conformational epitopes present in the p19 recombinant protein (22).
It is noteworthy that the smaller MSP1 p19 antigen containing only the two EGF domains conferred at least as good protection as the larger p42 antigen, suggesting that this part of the molecule is sufficient to generate protective immunity. This clearly indicates that the p19 antigen must have its own functional B- and T-cell epitopes that mediate a strong effective immune response without the addition of extraneous non-MSP1 peptides. We have previously shown that the baculovirus-expressed recombinant MSP1 p42 and p19 antigens have distinct physical properties and appear to display epitope subsets that are specific to each (22). In addition, the p42 protein appears to have a hypervariable region which may play a role in immune evasion, assuming that it is the focus of an effective antiparasite immune response (28). The presence of this region in a subunit vaccine might therefore be detrimental in a malaria-endemic environment where the risk of exposure to polymorphic variants in the hypervariable region may render vaccination with a single variant less effective.
The potent protective immunity observed in this trial might have been contingent upon the exceptionally high antibody titers that were elicited in the vaccinated animals by using Freund’s adjuvant. Indeed, the serum of the least protected vaccinee (T435) showed the slowest rise in ELISA titers against both the recombinant antigens, even though the titers were still quite high (106). In the primate P. falciparum trials involving recombinant MSP1 antigens, the protection observed did not appear to be dependent on antibody titers (3, 26). However, if this level of vaccine-induced protection does indeed require the presence of such high antibody titers, then the challenge ahead for preparation of an analogous human P. vivax vaccine will be to replace the potent Freund’s adjuvant. Indeed, parasitemias after challenge in the Freund’s adjuvant-treated controls were generally somewhat lower than those in the unvaccinated controls, suggesting that this strong adjuvant may contribute nonspecifically to the immunity generated in this model. Nevertheless, preliminary results do in fact suggest that significant protection can be obtained in this host-parasite system with the p19 antigen by using alum, which is the only adjuvant currently acceptable for human use, even though antibody titers were not as high as those observed with FCA and FIA (36a). The p19-vaccinated animals were still protected against a second challenge 6 months later, despite very low or undetectable parasitemias following the first challenge infection. Similar resistance to a second homologous challenge in the adjuvant-treated controls is attributable to immunity induced by much higher sustained parasitemias observed after the first challenge. Several observations indicate that protection against homologous Plasmodium strains can be induced by a single complete blood stage infection (see below). We have evidence to suggest that the sustained protection of the p19 vaccinees to a second challenge was due specifically to an anti-MSP1 p19 immune response which may have been either sustained following the first immunization itself or, more likely, boosted by a minimal exposure to parasites during the first challenge. If the latter is the case, it implies that vaccine-induced immunity could be boosted by natural infection, a feature which could be of considerable importance for the deployment of an MSP1 p19-based vaccine in malaria-endemic areas.
Protection as complete as that elicited in this trial has rarely, if ever, been achieved in primates with a recombinant subunit malaria vaccine against blood stage parasites. These results are now being confirmed in a trial with larger numbers of animals. Consistent success has, however, recently been achieved by immunization with baculovirus-expressed recombinant products of this antigen, both in P. vivax with MSP1 p42 and p19 in a nonsplenectomized squirrel monkey (Saimiri sciureus boliviensis) model (1a) and in P. falciparum with MSP1 p42 in Aotus monkeys (3), even though both used FCA and FIA as adjuvants. A recent successful clinical trial of a preerythrocytic stage recombinant malaria vaccine in conjunction with an oil-in-water adjuvant containing monophosphoryl lipid A and QS21 (41) has considerably heightened the prospects for replacing Freund’s adjuvant for human use.
The results of this trial have demonstrated that a high degree of protection against a malaria blood infection can be achieved in this natural primate host-parasite system by vaccinating with a recombinant subunit vaccine. Two points need to be considered in assessing the relevance and stringency of this model for vaccine evaluation. First, in the course of an uninterrupted challenge infection of P. cynomolgi in the toque monkey, parasitemias peak at less than 5% and last for at least 44 days, and probably more than 58 days, after which they are self-cured. These characteristics are similar to those of untreated P. vivax infections in human volunteers, which reached parasitemias of less than 10% and remained patent for 60 days or more prior to being self-cured (4).
Second, a complete P. cynomolgi blood infection in the adjuvant-treated control animals following the first challenge led to almost complete protection against a second challenge with the homologous strain 6 months later. Although this might suggest that protective immunity is too easily achieved in this system for it to be a valid immunization model for human malaria, two previous vaccine trials that were conducted with this system, using some of the same antigens under different conditions, failed to provide any protection, for a variety of possible reasons (36a). However, more important, it is likely that protection against a homologous strain of P. vivax in humans can also be induced by a single blood infection provided that the infection is allowed to run its natural course without drug treatment. Evidence for this comes from reports of early investigators who studied malaria infections which were therapeutically induced in neurosyphilitic patients (2, 24). Untreated P. vivax infection gave rise to complete clinical protection against a subsequent challenge with the identical strain but not with a different strain of the same species (2); since no parasitological observations were made in these studies, it is not possible to determine the extent to which antiparasite protection was achieved by these infections. Similar studies reported by Jeffery (24), in which all of the induced infections were drug cured, did not give rise to such a striking immunity, implying that the early termination of infection interferes with the development of immunity. The results of these early experiments also emphasize that the difficulty in acquiring natural protection against malaria must be largely attributable to polymorphism in target antigens or epitopes of protective immunity among natural parasite populations.
To test a malaria vaccine destined for use in humans, an analogous natural primate malaria may be superior to artificial experimental systems based on the human malaria parasites themselves in unnatural hosts. Nevertheless, the real relevance of the results reported here will be determined only by clinical trials with the P. vivax MSP1 recombinant analogs. In the meantime, however, this system will serve to address several important questions raised by this promising trial. These include, principally, the need for testing candidate vaccine antigens under statistically valid conditions and the search for effective adjuvants which are acceptable for human use.
ACKNOWLEDGMENTS
We acknowledge the expert services of Kamal Perera, Anura Jayasinghe, Kamal Gamage, and the support staff of the Animal House of the Faculty of Medicine for the care and maintenance of animals. We are very grateful to Peter David for sharing with us the early work and interest in the development of P. vivax vaccines and to Richard Carter for drawing our attention to some important aspects of immunity to human malaria. We are also grateful to Sunil Premawansa and Jayantha Wattewidanage for their help with the diagnostic aspects of this study, to G. M. G. Kapilananda for SDS-polyacrylamide gel electrophoresis and Western blot analysis, to Farida Nato (Hybridolab, Institut Pasteur, Paris) for providing the monoclonal antibodies used in antigen purification, and to Stephane Petres and Lucien Cabanie (Technologie Cellulaire, Institut Pasteur) for help with the baculovirus system. We are very grateful to Rajitha Wickremasinghe for helpful discussion and advice.
This work received financial support from the UNDP/World Bank/WHO Special Programme for Research and Training in Tropical Diseases. S.L. was funded by the Institut Pasteur and the Centre National Recherche Scientifique (CNRS) and by Direction de l’Application de la Recherche (DAR), Institute Pasteur.
REFERENCES
- 1.Anonymous. Annual administrative report of the anti-malaria campaign. Colombo Sri Lanka: Ministry of Health; 1992. [Google Scholar]
- 1a.Barnwell, J. W., et al. Unpublished data.
- 2.Boyd M F. Criteria of immunity and susceptibility in naturally acquired and induced vivax malaria infections. Am J Trop Med Hyg. 1942;22:217–227. [Google Scholar]
- 3.Chang S P, Case S E, Gosnell W L, Hashimoto A, Kramer K J, Tam L Q, Hashiro C Q, Nikaido C M, Gibson H L, Lee-Ng C T, Barr P J, Yokota B T, Hui G S N. A recombinant baculovirus 42-kilodalton C-terminal fragment of Plasmodium falciparum merozoite surface protein 1 protects Aotus monkeys against malaria. Infect Immun. 1996;64:253–261. doi: 10.1128/iai.64.1.253-261.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coatney G R, Collins W E, Warren M, Contacos P G. The primate malarias. Washington, D.C: U.S. Government Printing Office; 1971. [Google Scholar]
- 5.Collins W E, Aikawa M. Plasmodia of nonhuman primates. In: Kreier J P, editor. Parasitic protozoa. Vol. 5. San Diego, Calif: Academic Press; 1993. pp. 105–133. [Google Scholar]
- 6.Cooper J A. Merozoite surface antigen-1 of Plasmodium. Parasitol Today. 1993;9:50–54. doi: 10.1016/0169-4758(93)90031-a. [DOI] [PubMed] [Google Scholar]
- 7.Daly T M, Long C A. A recombinant 15-kilodalton carboxyl-terminal fragment of Plasmodium yoelii 17XL merozoite surface protein 1 induces a protective immune response in mice. Infect Immun. 1993;61:2462–2467. doi: 10.1128/iai.61.6.2462-2467.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.David P H, Barnwell J W, Mendis K N. Vivax malaria—strategies for vaccine development based on the hepatic, asexual erythrocytic, and sexual stages. In: Woodrow G C, Levine M M, editors. New generation vaccines. New York, N.Y: Marcel Dekker; 1990. pp. 531–543. [Google Scholar]
- 9.Del Portillo H A, Longacre S, Khouri E, David P H. Primary structure of the merozoite surface antigen 1 of Plasmodium vivax reveals sequences conserved between different Plasmodium species. Proc Natl Acad Sci USA. 1991;88:4030–4034. doi: 10.1073/pnas.88.9.4030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Diggs C L, Ballou W R, Miller L H. The major merozoite surface protein as a malaria vaccine target. Parasitol Today. 1993;9:300–302. doi: 10.1016/0169-4758(93)90130-8. [DOI] [PubMed] [Google Scholar]
- 11.Dissanaike A S, Nelson P, Garnham P C C. Two new malaria parasites, Plasmodium cynomolgi ceylonensis subsp. nov., and Plasmodium fragile sp. nov., from monkeys in Ceylon. Ceylon Med J Sci. 1965;14:1–9. [Google Scholar]
- 12.Dua V K, Kar P K, Sharma V P. Chloroquine resistant Plasmodium vivax malaria in India. Trop Med Int Health. 1996;1:816–819. doi: 10.1111/j.1365-3156.1996.tb00116.x. [DOI] [PubMed] [Google Scholar]
- 13.Escalante A A, Ayala F J. Phylogeny of the malarial genus Plasmodium, derived from rRNA gene sequences. Proc Natl Acad Sci USA. 1994;91:11373–11377. doi: 10.1073/pnas.91.24.11373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fang X, Kaslow D C, Adams J H, Miller L H. Cloning of the Plasmodium vivax Duffy receptor. Mol Biochem Parasitol. 1991;44:125–132. doi: 10.1016/0166-6851(91)90228-x. [DOI] [PubMed] [Google Scholar]
- 15.Galinski M R, Arnot D E, Cochrane A H, Barnwell J W, Nussenzweig R S, Enea V. The circumsporozoite gene of the Plasmodium cynomolgi complex. Cell. 1987;48:311–319. doi: 10.1016/0092-8674(87)90434-x. [DOI] [PubMed] [Google Scholar]
- 16.Garavelli P L, Corti E. Chloroquine resistance in Plasmodium vivax: the first case in Brazil. Trans R Soc Trop Med Hyg. 1992;86:128. doi: 10.1016/0035-9203(92)90535-k. [DOI] [PubMed] [Google Scholar]
- 17.Garg M, Gopinathan N, Bodhe P, Kshirsagar N. Vivax malaria resistant to chloroquine: case report from Bombay. Trans R Soc Trop Med Hyg. 1995;89:656–657. doi: 10.1016/0035-9203(95)90432-8. [DOI] [PubMed] [Google Scholar]
- 18.Garnham P C C. Malaria parasites and other hemosporidia. Oxford, United Kingdom: Blackwell; 1966. [Google Scholar]
- 19.Hamilton D K, Pikacha D. Ruptured spleen in a malarious area. Aust N Zealand Surg. 1982;52:310–313. doi: 10.1111/j.1445-2197.1982.tb05407.x. [DOI] [PubMed] [Google Scholar]
- 20.Handunnetti S M. An exploration into immunity in a natural malaria infection: Plasmodium fragile in the toque monkey Macaca sinica. Ph.D. thesis. Colombo, Sri Lanka: University of Colombo; 1986. [Google Scholar]
- 21.Holder A A. The precursor to major merozoite surface antigens: structure and role in immunity. Prog Allergy. 1988;41:72–97. [PubMed] [Google Scholar]
- 22.Holm I, Nato F, Mendis K N, Longacre S. Characterization of C-terminal merozoite surface protein 1 baculovirus recombinant proteins from Plasmodium vivax and Plasmodium cynomolgi as recognised by the natural anti-parasite immune response. Mol Biochem Parasitol. 1997;89:313–319. doi: 10.1016/s0166-6851(97)00128-x. [DOI] [PubMed] [Google Scholar]
- 23.Ihalamulla R L, Mendis K N. Plasmodium vivax: isolation of mature asexual stages and gametocytes from infected human blood by colloidal silica (Percoll) gradient centrifugation. Trans R Soc Trop Med Hyg. 1987;81:25–28. doi: 10.1016/0035-9203(87)90271-9. [DOI] [PubMed] [Google Scholar]
- 24.Jeffery G M. Epidemiological significance of repeated infections with homologous and heterologous strains and species of Plasmodium. Bulletin W H O. 1966;35:873–882. [PMC free article] [PubMed] [Google Scholar]
- 25.Jiang J B, Sy Y, Chen J M. A new strain of Plasmodium vivax endemic to China. Acta Sci Nat Univ Sunyatseni. 1965;1:131–132. [Google Scholar]
- 26.Kumar S, Yadava A, Keister D B, Tian J H, Ohl M, Perdue-Greenfield K A, Miller L H, Kaslow D C. Immunogenicity and in vivo efficacy of recombinant Plasmodium falciparum merozoite surface protein-1 in Aotus monkeys. Mol Med. 1995;1:325–332. [PMC free article] [PubMed] [Google Scholar]
- 27.Ling I T, Ogun S A, Holder A A. Immunization against malaria with a recombinant protein. Parasite Immunol. 1994;16:63–67. doi: 10.1111/j.1365-3024.1994.tb00324.x. [DOI] [PubMed] [Google Scholar]
- 28.Longacre S. The Plasmodium cynomolgi merozoite surface protein 1 C-terminal sequence and its homologies with other Plasmodium species. Mol Biochem Parasitol. 1995;74:105–111. doi: 10.1016/0166-6851(95)02477-8. [DOI] [PubMed] [Google Scholar]
- 29.Longacre S, Mendis K N, David P H. Plasmodium vivax merozoite surface protein 1 C-terminal recombinant proteins in baculovirus. Mol Biochem Parasitol. 1994;64:191–205. doi: 10.1016/0166-6851(94)00002-6. [DOI] [PubMed] [Google Scholar]
- 30.McCutchan T F, Dame J B, Miller L H, Barnwell J W. Evolutionary relatedness of Plasmodium species as determined by the structure of DNA. Science. 1984;225:808–811. doi: 10.1126/science.6382604. [DOI] [PubMed] [Google Scholar]
- 31.Mendis K N. Simian malaria models for research on human disease. Ceylon Med J Sci. 1995;38(2):31–35. [Google Scholar]
- 32.Naotunne T D S. A study of factors modulating infectivity of sexual stage malaria parasites: Plasmodium cynomolgi ceylonensis in Macaca sinica sinica. Ph.D. thesis. Colombo, Sri Lanka: University of Colombo; 1990. [Google Scholar]
- 33.Naotunne T D S, Karunaweera N D, Del Giudice G, Kularatne B D M U, Grau G E, Carter R, Mendis K N. Cytokines kill malaria parasites during infection crisis: extracellular complementary factors are essential. J Exp Med. 1991;173:523–529. doi: 10.1084/jem.173.3.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Naotunne T D S, Rathnayake K D L, Jayasinghe A, Carter R, Mendis K N. Plasmodium cynomolgi: serum-mediated blocking and enhancement of infectivity to mosquitoes during infections in the natural host, Macaca sinica. Exp Parasitol. 1990;71:305–313. doi: 10.1016/0014-4894(90)90035-b. [DOI] [PubMed] [Google Scholar]
- 35.Nelson P, Jayasuriya J M R, Bandarawatta B V P C. The establishment of Anopheles elegans as the natural vector of simian malaria in Ceylon. Ceylon Med J Sci. 1971;20:46–51. [Google Scholar]
- 36.Peiris J S M, Premawansa S, Ranawaka M B R, Udagama P V, Munesinghe Y D, Nanayakkara M V, Gamage C P, Carter R, David P H, Mendis K N. Monoclonal and polyclonal antibodies both block and enhance transmission of human Plasmodium vivax malaria. Am J Trop Med Hyg. 1988;39:26–32. doi: 10.4269/ajtmh.1988.39.26. [DOI] [PubMed] [Google Scholar]
- 36a.Perera, K. L. R. L., et al. Unpublished data.
- 37.Riley E M, Allen S J, Wheeler J G, Blackman M J, Bennett S, Takacs B, Schoenfeld H J, Holder A A, Greenwood B M. Naturally acquired cellular and humoral immune responses to the major merozoite surface antigen of Plasmodium falciparum are associated with reduced malaria morbidity. Parasite Immunol. 1992;14:321–337. doi: 10.1111/j.1365-3024.1992.tb00471.x. [DOI] [PubMed] [Google Scholar]
- 38.Schuurkamp G J, Spicer P E, Kereu R K, Bulungol P K, Rieckmann K H. Chloroquine-resistant Plasmodium vivax in Papua New Guinea. Trans R Soc Trop Med Hyg. 1992;86:121–122. doi: 10.1016/0035-9203(92)90531-g. [DOI] [PubMed] [Google Scholar]
- 39.Siddiqui W A, Tam L Q, Kramer K J, Hui G S, Case S E, Yamaga K M, Chang S P, Chan E B. Merozoite surface coat precursor protein completely protects Aotus monkeys against Plasmodium falciparum malaria. Proc Natl Acad Sci USA. 1987;84:3014–3018. doi: 10.1073/pnas.84.9.3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Snounou G, Viriyakosol S, Zhu X P, Jarra W, Pinheiro L, Rosario V E D, Thaithong S, Brown K N. High sensitivity of detection of human malaria parasites by the use of nested polymerase chain reaction. Mol Biochem Parasitol. 1993;61:315–320. doi: 10.1016/0166-6851(93)90077-b. [DOI] [PubMed] [Google Scholar]
- 41.Stoute J A, Slaoui M, Heppner G, Momin P, Kester K E, Desmons P, Wellde B T, Garcon N, Krzych U, Marchand M, Ballou W R, Cohen J W. A preliminary evaluation of a recombinant circumsporozoite protein vaccine against Plasmodium falciparum malaria. N Engl J Med. 1997;336:86–91. doi: 10.1056/NEJM199701093360202. [DOI] [PubMed] [Google Scholar]
- 42.Udagama P V, David P H, Peiris J S M, Ariyaratne Y G, Perera K L R L, Mendis K N. Demonstration of antigenic polymorphism in Plasmodium vivax malaria with a panel of 30 monoclonal antibodies. Infect Immun. 1987;55:2604–2611. doi: 10.1128/iai.55.11.2604-2611.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Valecha N, Bagga A, Chandra J, Sharma D. Cerebral symptoms with P. vivax malaria. Indian Pediatr. 1992;29:1176–1178. [PubMed] [Google Scholar]
- 44.Waters A P, Higgins D G, McCutchan T F. Evolutionary relatedness of some primate models of Plasmodium. Mol Biol Evol. 1993;10:914–923. doi: 10.1093/oxfordjournals.molbev.a040038. [DOI] [PubMed] [Google Scholar]