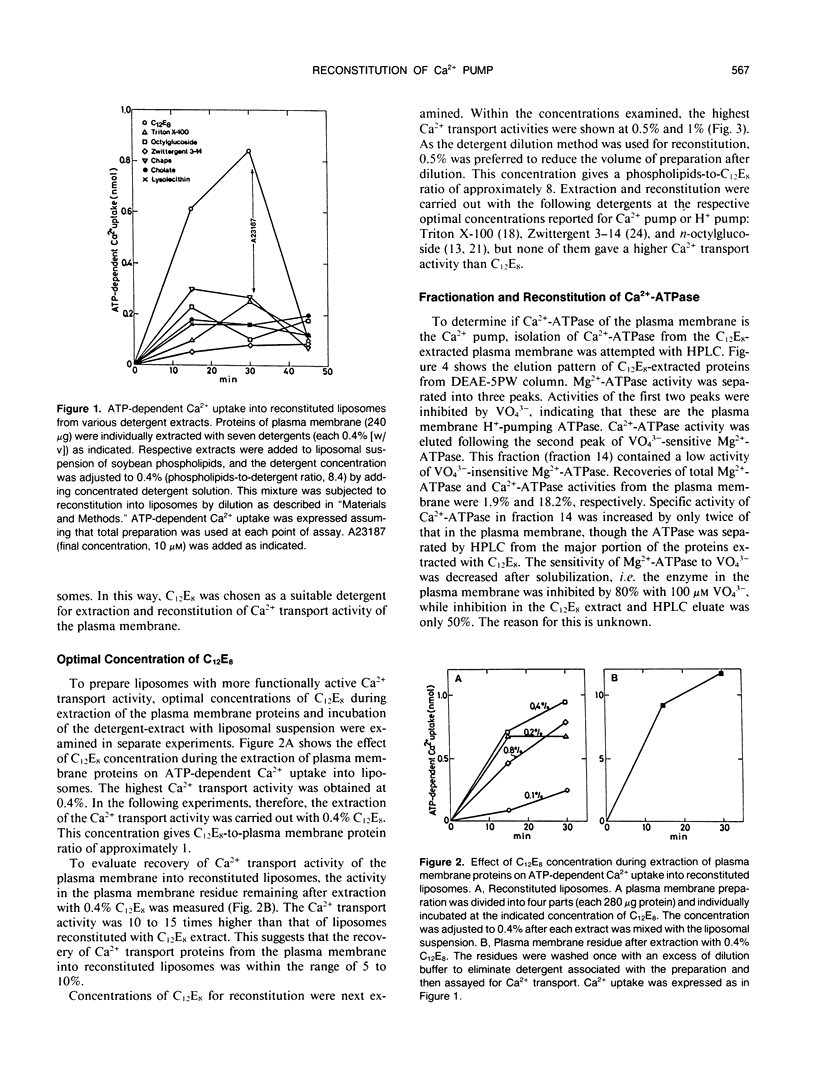
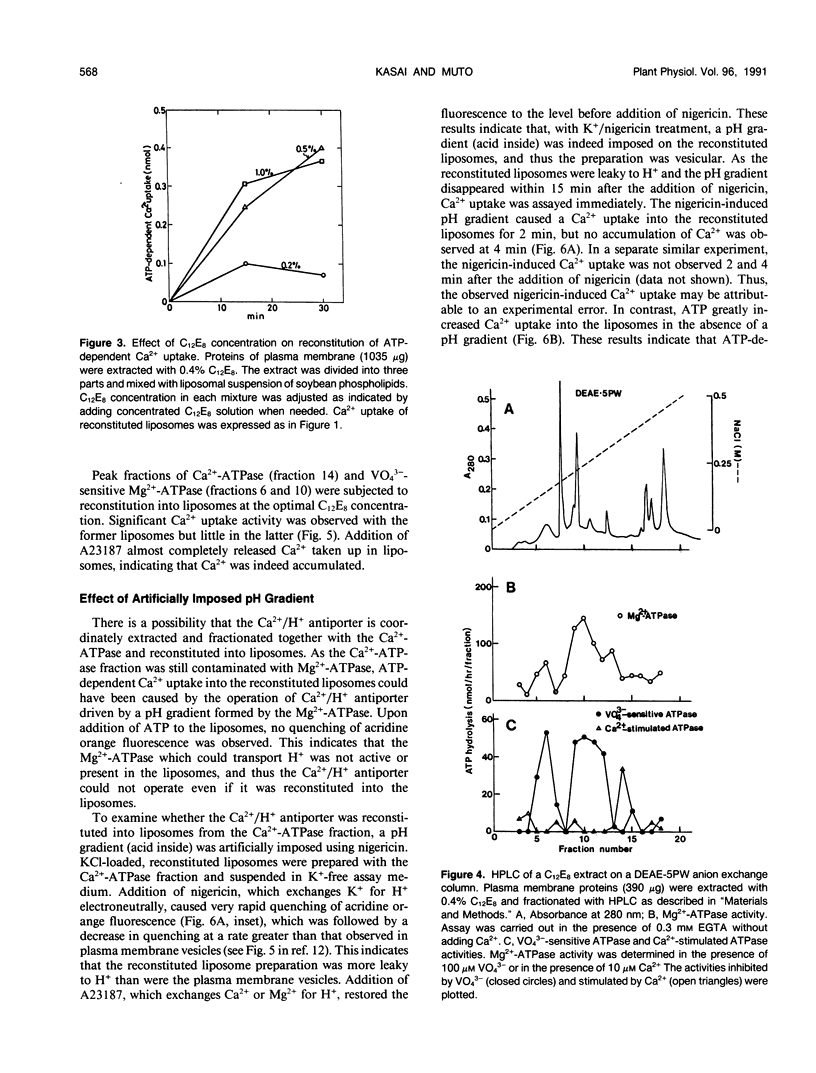
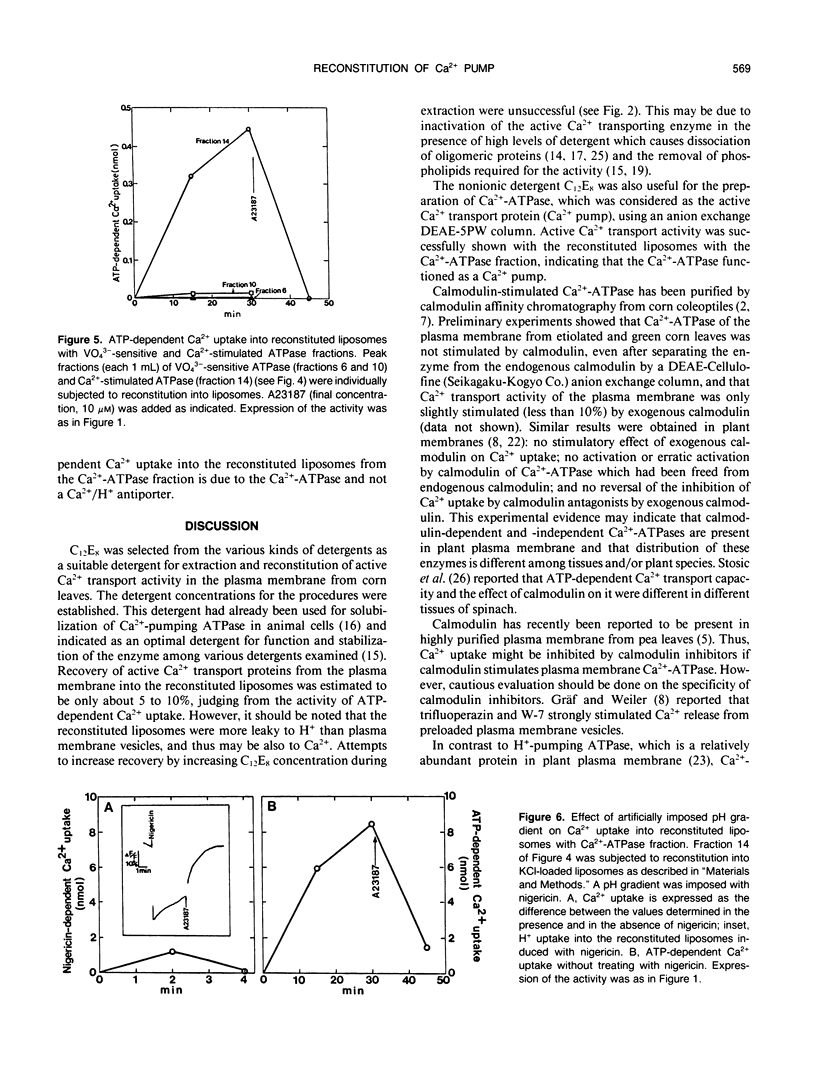
Abstract
The Ca2+ transport system of corn (Zea mays) leaf plasma membrane is composed of Ca2+ pump and Ca2+/H+ antiporter driven by H+ gradient imposed by a H+ pump (M Kasai, S Muto [1990] J Membr Biol 114: 133-142). It is necessary for characterization of these Ca2+ transporters to establish the procedure for their solubilization, isolation, and reconstitution into liposomes. We attempted to solubilize and reconstitute the Ca2+ pump in the present study. A nonionic detergent octaethyleneglycol monododecyl ether (C12E8) was the most effective detergent for a series of extraction and functional reconstitution of the Ca2+ pump among seven detergents examined. This was judged from activities of ATP-dependent 45Ca2+ uptake into liposomes reconstituted with the respective detergent-extract of the plasma membrane by the detergent dilution method. C12E8-extract of the plasma membrane was subjected to high performance liquid chromatography using a DEAE anion exchange column. Ca2+-ATPase was separated from VO43−-sensitive Mg2+-ATPase. These ATPases were separately reconstituted into liposomes, and their ATP-dependent Ca2+ uptake was measured. The liposomes reconstituted with the Ca2+-ATPase, but not with the VO43−-sensitive Mg2+-ATPase, showed ATP-dependent Ca2+ uptake. Nigericin-induced pH gradient (acid inside) caused only a little Ca2+ uptake into liposomes reconstituted with the Ca2+-ATPase, suggesting that the Ca2+/H+ antiporter was not present in the preparation. These results indicate that the Ca2+-ATPase actually functions as Ca2+ pump in the corn leaf plasma membrane.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Buckhout T. J. Characterization of Ca Transport in Purified Endoplasmic Reticulum Membrane Vesicles from Lepidium sativum L. Roots. Plant Physiol. 1984 Dec;76(4):962–967. doi: 10.1104/pp.76.4.962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carafoli E., Zurini M. The Ca2+-pumping ATPase of plasma membranes. Purification, reconstitution and properties. Biochim Biophys Acta. 1982 Dec 31;683(3-4):279–301. doi: 10.1016/0304-4173(82)90004-0. [DOI] [PubMed] [Google Scholar]
- Collinge M., Trewavas A. J. The location of calmodulin in the pea plasma membrane. J Biol Chem. 1989 May 25;264(15):8865–8872. [PubMed] [Google Scholar]
- Iggo R. D., Lane D. P. Nuclear protein p68 is an RNA-dependent ATPase. EMBO J. 1989 Jun;8(6):1827–1831. doi: 10.1002/j.1460-2075.1989.tb03577.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan R. S., Pedersen P. L. Determination of microgram quantities of protein in the presence of milligram levels of lipid with amido black 10B. Anal Biochem. 1985 Oct;150(1):97–104. doi: 10.1016/0003-2697(85)90445-2. [DOI] [PubMed] [Google Scholar]
- Kasai M., Muto S. Ca2+ pump and Ca2+/H+ antiporter in plasma membrane vesicles isolated by aqueous two-phase partitioning from corn leaves. J Membr Biol. 1990 Mar;114(2):133–142. doi: 10.1007/BF01869094. [DOI] [PubMed] [Google Scholar]
- Keresztes T., Jona I., Pikula S., Vegh M., Mullner N., Papp S., Martonosi A. Effect of calcium on the interactions between Ca2+-ATPase molecules in sarcoplasmic reticulum. Biochim Biophys Acta. 1989 Sep 18;984(3):326–338. doi: 10.1016/0005-2736(89)90300-3. [DOI] [PubMed] [Google Scholar]
- Lund S., Orlowski S., de Foresta B., Champeil P., le Maire M., Møller J. V. Detergent structure and associated lipid as determinants in the stabilization of solubilized Ca2+-ATPase from sarcoplasmic reticulum. J Biol Chem. 1989 Mar 25;264(9):4907–4915. [PubMed] [Google Scholar]
- Moore R. B., Manery J. F., Still J., Mankad V. N. The inhibitory effects of polyoxyethylene detergents on human erythrocyte acetylcholinesterase and Ca2+ + Mg2+ ATPase. Biochem Cell Biol. 1989 Feb-Mar;67(2-3):137–146. doi: 10.1139/o89-021. [DOI] [PubMed] [Google Scholar]
- Møller J. V., Lind K. E., Andersen J. P. Enzyme kinetics and substrate stabilization of detergent-solubilized and membraneous (Ca2+ + Mg2+)-activated ATPase from sarcoplasmic reticulum. Effect of protein-protein interactions. J Biol Chem. 1980 Mar 10;255(5):1912–1920. [PubMed] [Google Scholar]
- Niggli V., Adunyah E. S., Penniston J. T., Carafoli E. Purified (Ca2+-Mg2+)-ATPase of the erythrocyte membrane. Reconstitution and effect of calmodulin and phospholipids. J Biol Chem. 1981 Jan 10;256(1):395–401. [PubMed] [Google Scholar]
- Niggli V., Carafoli E. Interaction of the purified Ca2+, Mg2+-ATPase from human erythrocytes with phospholipids and calmodulin. Acta Biol Med Ger. 1981;40(4-5):437–442. [PubMed] [Google Scholar]
- Poovaiah B. W., Reddy A. S. Calcium messenger system in plants. CRC Crit Rev Plant Sci. 1987;6(1):47–103. doi: 10.1080/07352688709382247. [DOI] [PubMed] [Google Scholar]
- Racker E., Violand B., O'Neal S., Alfonzo M., Telford J. Reconstitution, a way of biochemical research; some new approaches to membrane-bound enzymes. Arch Biochem Biophys. 1979 Dec;198(2):470–477. doi: 10.1016/0003-9861(79)90521-6. [DOI] [PubMed] [Google Scholar]
- Rasi-Caldogno F., Pugliarello M. C., Olivari C., De Michelis M. I. Identification and Characterization of the Ca-ATPase which Drives Active Transport of Ca at the Plasma Membrane of Radish Seedlings. Plant Physiol. 1989 Aug;90(4):1429–1434. doi: 10.1104/pp.90.4.1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano R. Purification of the proton pumping ATPase from plant plasma membranes. Biochem Biophys Res Commun. 1984 Jun 15;121(2):735–740. doi: 10.1016/0006-291x(84)90243-2. [DOI] [PubMed] [Google Scholar]
- Soumarmon A., Robert J. C., Lewin M. J. Depolymerization of solubilized gastric (H+ + K+)-ATPase by n-octylglucoside or cholate. Biochim Biophys Acta. 1986 Aug 7;860(1):109–117. doi: 10.1016/0005-2736(86)90504-3. [DOI] [PubMed] [Google Scholar]
- Stosic V., Penel C., Marme D., Greppin H. Distribution of calmodulin-stimulated ca transport into membrane vesicles from green spinach leaves. Plant Physiol. 1983 Aug;72(4):1136–1138. doi: 10.1104/pp.72.4.1136. [DOI] [PMC free article] [PubMed] [Google Scholar]


