Abstract
In the present investigation, a soil isolate Pseudomonas aeruginosa CSPS4 was used for retrieving the l-asparaginase encoding gene (Asn_PA) of size 1089 bp. The gene was successfully cloned into the pET28a (+) vector and expressed into E. coli BL21(DE3) for characterization of the protein. The recombinant rAsn_PA enzyme was purified by affinity chromatography using Ni-NTA2+ resins. Molecular weight analysis using SDS-PAGE unveiled rAsn_PA as a monomeric protein of molecular weight ~ 35 kDa. On characterization, the recombinant rAsn_PA showed optimum pH and temperature of 6.0 and 60 °C, respectively, along with significant stability at 50–70 °C, along with 50% residual activity at 80 °C after 3 h of incubation. Similarly, the rAsn_PA exhibited asparaginase activity over a broad pH range between 4 and 8. The enzyme was not significantly inhibited in the presence of detergents. The rAsn_PA was grouped into the asparaginase-glutaminase family II due to the glutaminase activity. The purified rAsn_PA showed antitumor activity by exhibiting a cytotoxic effect on three different cell lines, where IC50 of purified rAsn_PA was 2.3 IU, 3.7 IU, and 20.5 IU for HL-60, MOLM-13, and K-562 cell lines, respectively. Thus, recombinant rAsn_PA of P. aeruginosa CSPS4 may also be explored as an antitumor agent after reducing or minimizing the glutaminase activity. Thermo-acidophilic properties of rAsn_PA make it a novel enzyme that needs to be further investigated.
Keywords: L-Asparaginase, Heterologous cloning, Thermophilic enzymes, Anti-proliferative activity
Introduction
L-Asparaginases (EC.3.5.1.1) grabbed significant attention due to their applicability as a drug in treating cancer (Shrivastava et al. 2016; Qeshmi et al. 2018; Darvishi et al. 2022). The increasing demand for l-asparaginases in therapeutics and starch-based industries covers 40% of the global market of industrial enzymes (Qeshmi et al. 2022). The l-asparaginases are widely distributed among all three domains of life; however, microorganisms-derived l-asparaginases offer a rapid and cost-effective alternative to fulfill the industrial demand (Alrumman et al. 2019; Muneer et al. 2020). L-Asparaginases find two major biotechnological applications in industries. The antileukemic activity of l-asparaginases is one of the most promising applications for treating acute lymphoblastic leukemia (ALL) (Mahajan et al. 2014; Cachumba et al. 2016; Shrivastava et al. 2016). Acute lymphoblastic leukemia is a hematologic malignancy that is chiefly diagnosed among children between the age group of 2–10 years (Gaynon et al. 2012; Darvishi et al. 2022). Currently, Erwinia and Escherichia coli are the two major bacterial-derived commercial asparaginases such as Spectrila, Erwinase, and Oncaspar are available in the market for treating ALL type tumor. However, these enzymes come along with glutaminase activity (El-Bessoumy et al. 2004; El-Sharkawy et al. 2016; Gao et al. 2022). Due to the structural similarity between asparagine and glutamine, most of the bacterial asparaginases also exhibit affinity towards glutamine (Nagarethinam et al. 2012; El-Naggar et al. 2016; Goswami et al. 2015). Glutaminase imposes side effects by enhancing hypersensitivity and immunogenic responses (Duval et al. 2002; Mahajan et al. 2014). Starch-based food industries find the second major application of asparaginase, where this enzyme degrades the acrylamide present in the fried food products. Acrylamide is a product of the Maillard reaction when starch sugar reacts with asparagine at high temperatures during deep frying (Stadler et al. 2002; Kukurova et al. 2009; Jia et al. 2021). A non-permissible amount of acrylamide has been reported from various starch-based fried food products (Vinci et al. 2012). Thus, a significant amount of carcinogenic acrylamide is introduced into our body unintentionally through its regular consumption. Therefore, starch-based food industries demand asparaginases that exhibit stability at higher temperatures. In recent years, Pseudomonas, a member of phylum Proteobacteria has also been explored for harnessing asparaginase in search of desired characteristics. However, limited information is available on Pseudomonas-derived asparaginases. Therefore, in the present investigation, an environmental isolate of Pseudomonas aeruginosa CSPS4 has been explored for harnessing the l-asparaginase encoding gene (Asn_PA) and its heterologous expression for its biophysical and biochemical characterization. Besides, an attempt was also made to check the antitumor activity of the rAsn_PA enzyme.
Materials and methods
Bacterial strains, plasmids, and reagents
A soil isolate P. aeruginosa CSPS4 (accession no. CP113974.1) was used for amplification of l-asparaginase gene. Expression vector pET28a (+) (Novagen, Madison, WI) vector was used to construct a recombinant vector (pETAsn_PA) for expressing the asparaginase encoding gene (Asn_PA). E. coli DH5α and E. coli BL21(DE3) were used as hosts for propagating the recombinant plasmids. The plasmid preparation was done using a plasmid isolation kit (Thermo-Fisher Scientific). The other chemical reagents such as Taq DNA polymerase, PCR buffers, dNTPs, and T4 DNA ligase were used from New England Biolabs under standard conditions as per the instructions.
Genomic DNA isolation and retrieval of l-asparaginase encoding gene
5.0 ml of overnight-grown cells of P. aeruginosa CSPS4 was processed for genomic DNA isolation. Briefly, the bacterial pellet from the broth was obtained by high-speed centrifugation (10,000 rpm) followed by incubating the cells by adding 1 ml extraction buffer [(N-,N-,N-,N-cetyltrimethylammonium bromide (CTAB) 1% (w/v), polyvinylpolypyrrolidone (PVPP) 2% (w/v), 1.5 M NaCl, 100 mM EDTA, 0.1 M TE buffer (pH 8.0), 0.1 M sodium phosphate buffer (pH 8.0), and 100 μL RNaseA)], and 5 μL of proteinase-K (10 mg/ml) at 37 °C for 1 h. Thereafter, the cells were further lysed by adding 100 µL of 10% (w/v) SDS solution and incubated at 60 °C for 1 h. The cell lysate was treated with an equal volume of phenol: chloroform (24:24) and the aqueous phase was collected after high-speed centrifugation. The DNA was precipitated by adding 0.7 V isopropanol to the aqueous phase followed by incubation at room temperature for 1 h. The DNA pellet was obtained after high-speed centrifugation and washed with 70% (v/v) ethanol. The dried DNA pellet was dissolved in 50 μL sterile MilliQ water. Two sets of primers were designed in this study for amplifying asparaginase encoding genes (PA_AS-1F-5ʹATGCTCCCAGTCAAGAACC3ʹ; PA_AS-1R: 5ʹTCAGTCGGCGTTCTCGCCGC 3ʹ and PA_GASF: 5ʹATGAAGCCATTGCTCCACGC3ʹ, PA_GASR: 5ʹ TCAGTATTCCCAGAAGATCC3ʹ) to retrieve the l-asparaginase encoding gene in a thermocycler (Bio-Rad, USA). The conditions of the PCR reaction were as follows: initial denaturation of 95 °C followed by 29 cycles of denaturation (95 °C for 50 s), annealing (52–62 °C gradients for 60 s), and extension (72 °C for 1 min). A final extension of 10 min at 72 °C was performed. The amplicon was confirmed using 1.2% (w/v) gel electrophoresis and processed for sequence analysis.
Bioinformatics analysis
The sequence was analyzed using the BLASTn tool of NCBI (https://www.ncbi.nlm.nih.gov/). The gene was translated into protein using the ExPASy translation tool (https://web.expasy.org/translate/). The phylogenetic analysis was carried out using MEGA 11.0 software (https://www.megasoftware.net/).
Construction and expression of the recombinant vector pETAsn_PA
The recombinant vector pETAsn_PA was constructed into pET28a(+) vector. Restriction sites of EcoRI and XhoI were incorporated in the forward and reverse primers after checking the zero cutter sites in the asparaginase gene using the NEB cutter (https://nc3.neb.com/NEBcutter/). The amplified full-length gene of asparaginase and pET28a(+) vector were digested with EcoRI and XhoI. Digested genes and vectors were purified using the gel extraction method. Appropriate concentrations of digested gene and plasmid were mixed and ligated using T4 DNA ligase under a low temperature (16 °C) ligation chamber overnight. The recombinant vectors were transformed into competent E. coli DH5α cells using the heat shock method at 42 °C for 45 s in a water bath. Randomly, a few clones were picked from LB-kan agar plates and confirmed by colony PCR followed by a double digestion method. Three positive clones for having pETAsn_PA vectors were picked for plasmids isolation and confirmed for the presence of the insert. On confirmation of the gene sequence, ~ 5 to 10 ng of recombinant vector was transformed into E. coli BL21(DE3) cells for expressing the gene. The expression of the gene was induced by 1 mM IPTG to the E. coli BL21(DE3) cells (A600 of 0.4–0.5). The recombinant pETAsn_PA was allowed to express in induced cells by further cultivating the cells for another 4–5 h at 37 °C at 150 rpm.
Purification of rAsn_PA protein
The recombinant protein was purified using affinity chromatography with Ni2+-NTA resins (Anne Spriestersbach et al. 2015). For this, 5 h post-induced cells were harvested by high-speed centrifugation (10,000 rpm) followed by cell lysis. The cells were suspended in lysis buffer (10 mM MgCl2, 25 mM phosphate buffer (pH 6.5), 100 mM NaCl, and 10 mM imidazole) and sonicated in 30 s on/off conditions for 10 min in an ice bucket. The lysate was collected after centrifugation and loaded on pre-calibrated Ni2+-NTA resins using 0.1 M phosphate buffer (6.5). The rAsn_PA enzyme was allowed to bind with calibrated Ni2+-NTA resins for 1 h under low-speed shaking conditions on a rocker. Thereafter, the resins were washed with 5 V wash buffer [0.1 M phosphate buffer (pH 6.5), 100 mM NaCl, 20 mM imidazole) multiple times to remove the unbound and non-specific his-tagged proteins. The purified recombinant protein was collected using 1 ml elution buffer [0.1 M TE buffer (pH 6.5)] of varying concentrations of imidazole (100–400 mM). The protein profile was visualized on 12% (w/v) SDS-PAGE to check the purity and molecular weight of the rAsn_PA enzyme.
L-Asparaginase assay
Asparaginase activity of the rAsn_PA was assayed by measuring ammonia using Nessler’s reagent (Shifrin et al. 1974). Appropriately diluted rAsn_PA protein was mixed with an equal volume of 1% (w/v) asparagine substrate (dissolved in 0.1 M Tris–HCl; pH 6.0) and incubated for 15 min for substrate hydrolysis. One international unit (IU) of l-asparaginase was interpreted as the amount of rAsn_PA required for liberating 1 nmol of ammonia at pH 6.0 and temperature 60 °C. Besides, the glutaminase activity of the rAsn_PA enzymes was also performed to determine the substrate specificity under a similar condition by replacing the substrate with 1% (w/v) glutamine as substrate in place of asparagine. Besides, the substrate specificity of rAsn_PA enzyme was also assessed in the presence of casein and keratin. Enzymatic assays were performed in triplicates to calculate the standard deviation.
Biochemical and biophysical characterization of rAsn_PA
The optimum pH of rAsn_PA was determined by assaying the asparaginase under a broad range of pH buffers [0.1 M; citrate buffer (pH 3.0–6.0), Tris–HCl buffer (7.0–8.0), and glycine–NaOH buffer (9.0–12.0)]. Similarly, a broad range of temperatures (40–70 °C) was used to determine the optimum temperature of rAsn_PA. The pH stability and thermal stability were determined by preincubating the enzyme at various temperatures and pH, respectively. Residual l-asparaginase activity was measured by performing an asparaginase assay of the samples collected at varying time intervals. The biochemical characterization of the rAsn_PA enzyme was carried out by adding various modulators (Tween-20, Tween-80, SDS, and EDTA) into the enzyme followed by measuring l-asparaginase activity. Enzymatic assays were performed in triplicates to calculate the standard deviation to statistically validate the data.
Nucleotide sequence accession numbers
The nucleotide sequence of the l-asparaginase gene was submitted to the GenBank database under accession no. OR509736.
Applications of rAsn_PA
Antileukemic activity (MTT) assay
The rAsn_PA was assessed for antiproliferative activity on three different tumor cell lines (HL-60, MOLM-13, and K562) using MTT assay (Mahajan et al. 2014). The assay was performed in 96-well microtiter plates where cell density was maintained at 1 × 106 per well. Varying concentrations of rAsn_PA (0.75 µg/ml, 3 µg/ml, and 12 µg/ml) were added into the respective wells and incubated at 37 °C in a CO2 incubator for 24 h. The plates were centrifuged at 5000 rpm for 5 min at 4 °C to sediment the cells. 50 µL of MTT solution (5 mg/ml in phosphate buffer of pH 6.5) was added into each well and incubated for 4 h at 37 °C. Thereafter, 150 µL of DMSO (prepared in a 1:1 ratio with ethanol) was added into each well and wrapped on the plate for 15 min under shaking conditions for 20 min to dissolve purple-colored formazan precipitate. The antiproliferative activity of rAsn_PA was calculated by measuring the absorbance at 595 nm. The 0.1 M phosphate buffer saline was used in control in place of rAsn_PA in respective wells.
Hemolysis activity of rAsn_PA
Varying concentrations of rAsn_PA (0.75 µg/ml, 1.5 µg/ml, 3 µg/ml, 6 µg/ml, and 12 µg/ml) were added in 2% (v/v) heparinized blood cell suspension in phosphate buffer (collected from the rotatory blood bank). Normal saline (NS) and ddH2O were added in 2% blood cell suspension as negative control and positive control. The reaction setup was kept for 6 h at 37 °C. The absorbance of the supernatant was determined at 545 nm using a UV–Vis spectrophotometer (Yuan et al. 2012). The percentage of hemolysis was calculated using the following formula (Kumar et al. 2020). Hemolysis exceeding 5% indicates the unsuitability of the protein/drug for administration:
where ODTest is the absorbance of test sample; ODnc is the absorbance of negative control; ODpc is the absorbance of positive control.
Results
Genomic DNA isolation and retrieval of l-asparaginase encoding gene
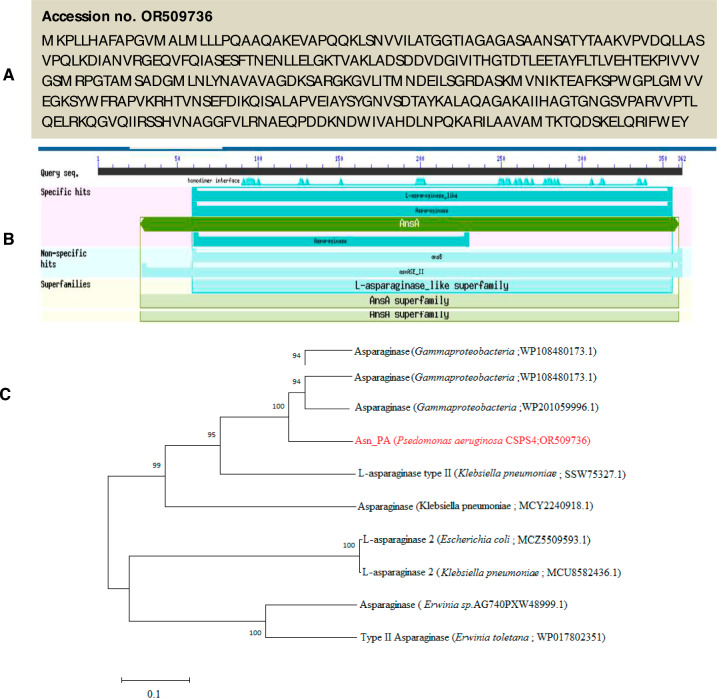
The genomic DNA from P. aeruginosa was successfully used for retrieving the l-asparaginase gene (Asn_PA) (Fig. 1A). On sequencing, a 1089 bp long full-length gene was achieved that was translated to 362 amino acids with a predictive molecular weight of ~ 40 kDa. The BLASTp analysis showed 100% sequence identity with an uncharacterized l-asparaginase (accession no. WP_003104093.1) of Pseudomonas followed by ~ 99% identity with several other l-asparaginases of Pseudomonas spp. (Fig. 1B). The phylogenetic tree showed that the Asn_PA is distantly related to highly common and commercial asparaginases of Erwinia chrysanthemum and Escherichia coli (Fig. 1C).
Fig. 1.
Translated amino acid sequence of Asn_PA gene (accession no. OR509736) (A). The BLASTp analysis of Asn_PA protein sequence showed homology with l-asparaginase like superfamily (B). The phylogenetic tree showed a close relatedness of rAsn_PA protein with asparaginases of different bacterial sources (C)
Construction and expression of the recombinant vector pETAsn_PA
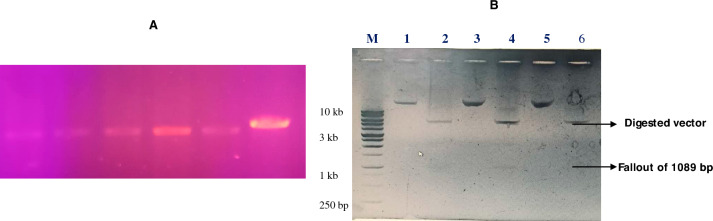
The desired insert of the l-asparaginase gene in the recombinant vector was confirmed by colony PCR as well as the double digestion method from randomly picked clones (Fig. 2A and B). The pETAsn_PA was successfully expressed in E. coli BL21 (DE3) cells. The recombinant l-asparaginase from the vector was expressed by 1 mM IPTG induction at 37 °C, where the major fraction (~ 90%) of the recombinant protein was obtained in the intracellular fraction.
Fig. 2.
The colony PCR showed the successful amplification of Asn_PA gene from the respective clones (A). Confirmation of the clones was checked by plasmids of randomly picking clones using respective restriction enzymes used in digestion. M Marker, Lane 1, 3, and 5 undigested clones; Lane 2, 4, and 6 showed fallout of desired length (1089 bp) (B)
Purification of rAsn_PA
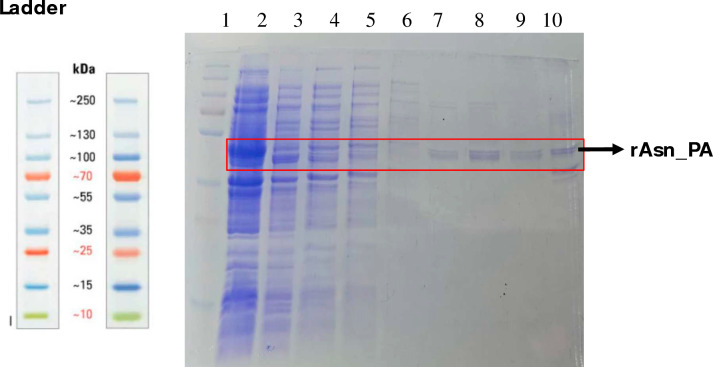
The purified rAsn_PA was eluted from Ni2+-NTA resin at 100–400 mM imidazole. The purified rAsn_PA was eluted at 300 mM imidazole as a single band of ~ 40 kDa on 12% SDS-PAGE, which was in agreement with the predicted molecular weight of the protein (Fig. 3A).
Fig. 3.
A SDS-PAGE [12% (w/v)] showed the protein profiling of recombinant rAsn_PA protein. L1 Protein ladder, L2 Induced protein 1 mM IPTG, L3 Uninduced cells, L4, 5, and 6 wash, L7–10 Eluted purified rASN_PA at 100 mM, 200 mM, 300 mM and 500 mM imidazole, respectively. L denotes lane here
Biochemical and biophysical characterization of rAsn_PA
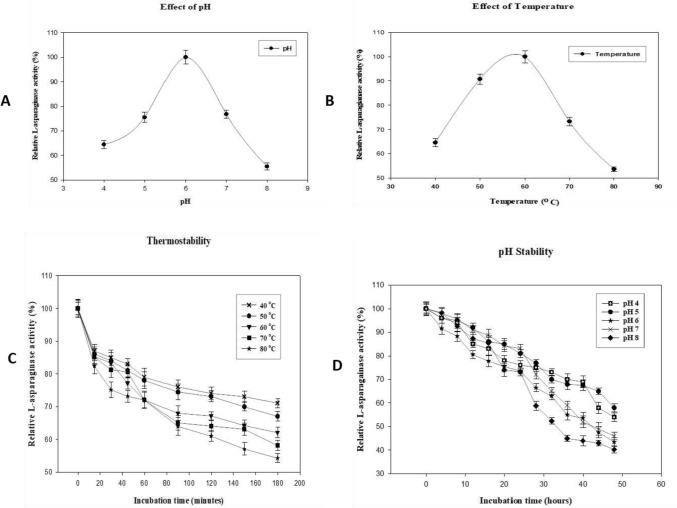
On characterization, purified rAsn_PA showed an optimum pH and temperature of 6.0 at 60 °C (Fig. 4A and B pH and temp opt.). Besides, the enzyme was stable for 3 h at 50 °C, 60 °C, and 70 °C (Fig. 4C). Whereas, at 80 °C, the purified rAsn_PA retained 50% activity after 3 h of incubation. The rAsn_PA showed enzyme activity at a broad pH of 4 to 8. The enzymes retained more than 50% activity even after 40 h at lower pH of 4 and 5 (Fig. 4D). A significant loss was observed in l-asparaginase activity from neutral to alkaline pH. The enzyme was able to retain more than 80% activity in the presence of detergents and slight stimulation was observed in the presence of 10% SDS. EDTA did not show significant inhibition on purified rAsn_PA activity (Table 1).
Fig. 4.
The purified rAsn_PA were characterized to determine the optimum pH (A), optimum temperature (B). The stability of the recombinant rAsn_PA enzymes was observed under varying pH (C), and temperatures (D)
Table 1.
Effect of modulators on rAsn_PA enzyme
| S. no | Modulators | Concentration | Residual enzyme activity (%) |
|---|---|---|---|
| 1 | Control | – | 100 |
| 2 | Tween-20 | 1% | 93.536 ± 1.08 |
| 3 | Tween-80 | 1% | 92.037 ± 2.05 |
| 4 | CHAPS | 1% | 100.310 ± 1.07 |
| 5 | SDS | 5% | 98.017 ± 2.10 |
| 6 | SDS | 10% | 102.016 ± 3.05 |
| 7 | EDTA | 1 mM | 94.449 ± 2.03 |
Substrate specificity of rAsn_PA
The purified rAsn_PA showed significant glutaminase activity of ~ 80% while assaying the enzyme at pH 6.0 and temperature of 60 °C in the presence of l-glutamine. However, other protein-based products such as casein and keratin did not show hydrolysis in the presence of l-asparaginase (Table 2).
Table 2.
Substrate specificity of rAsn_PA enzyme
| S. no | Substrates (1%) | Residual enzyme activity (%) |
|---|---|---|
| 1 | L-Asparagine | 100 ± 1.04 |
| 2 | L-Glutamine | 79.25 ± 2.01 |
| 3 | Casein | 0 |
| 4 | Keratin | 0 |
Antileukemic activity (MTT) assay
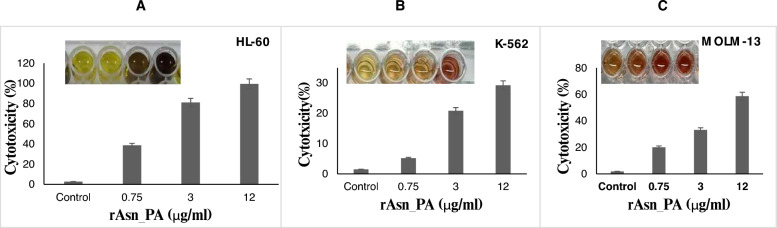
Cytotoxicity activity of rAsn_PA was observed on all three human leukemic cell lines (HL-60, MOLM-13, and K-562). Purified rAsn_PA (12 µg/ml) showed maximum cytotoxic activity of 97% against HL-60 cell lines, whereas MOLM-13 and K-562 cell lines were reduced by 56% and 26%, respectively, by rAsn_pA (Fig. 5A and B). The IC50 of purified l-asparaginase was 2.3 IU, 3.7 IU and 20.5 IU for HL-60, MOLM-13, and K-562, respectively.
Fig. 5.
The MTT assay showed antileukemic activity of the rAsn_PA enzyme on HL-60 (A), K-562 (B), and MOLM-13(C) cell lines. The inset picture depicts MTT assay on different cell lines by ELISA methods
Hemolysis test
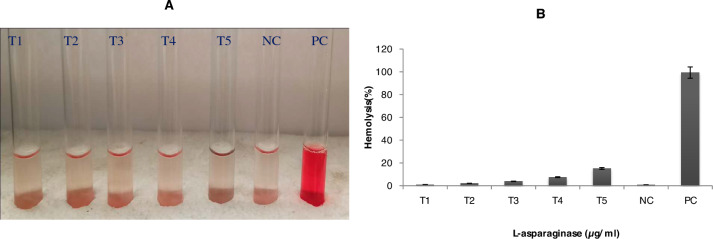
The purified l-asparaginase showed dose-dependent lysis activity on healthy blood cells. 3 µg/ml of rAsn_PA showed acceptable hemolysis of merely 3.86% for human administration (Fig. 6A and B). More than 5% hemolysis was observed at higher concentrations (6 µg/ml and 12 µg/ml) of l-asparaginase on blood cells.
Fig. 6.
In vitro hemolysis test for determining the toxicity of rAsn_PA protein was performed. T1 to T5 depict varying concentrations (0.75, 1.5, 3, 6, and 12) µg/ml rAsn_PA protein, respectively. NC Negative control (cells incubated in PBS), Lane PC indicates a positive control (cells in ddH2O) (A). The graph showing the % hemolysis in the presence of varying concentrations of rAsn_PA (B)
Discussion
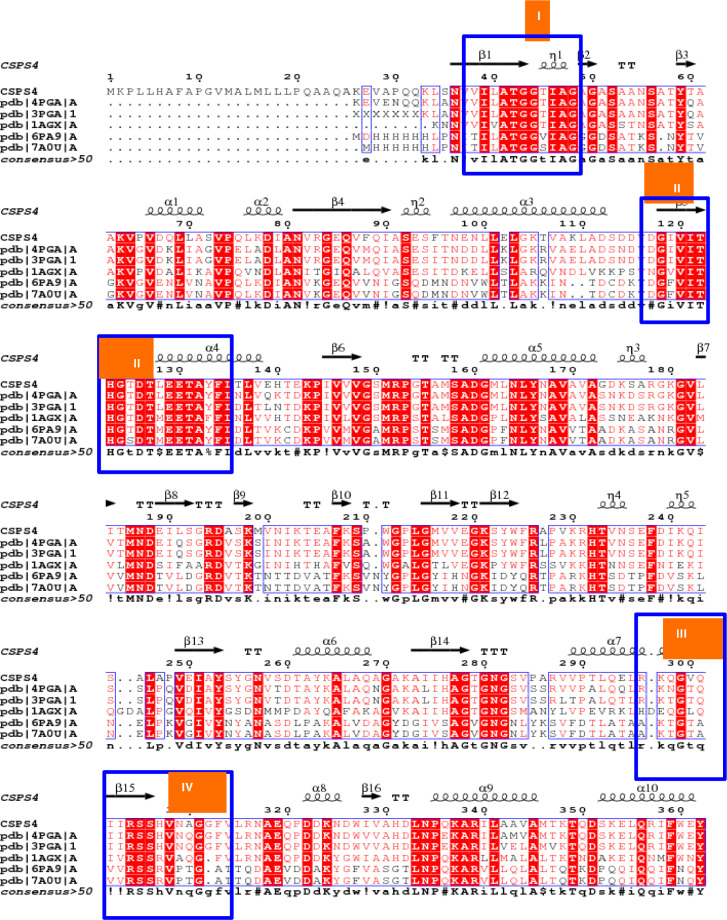
In the last few years, Pseudomonas-derived asparaginases have also been explored from various environmental sources (Badoei-Dalfard 2015; Fatima et al. 2019; Gao et al. 2022; Kumar et al. 2022a) to address existing issues with available l-asparaginases. Handful asparaginases have been characterized to date from various Pseudomonas spp. (Kamble et al. 2012; Audipudi et al. 2013; Shakambari et al. 2017; Vachhani et al. 2018; Fatima et al. 2019). The Asn_PA of this investigation showed 100% homology with asparaginase of P. aeruginosa POA1 (CP129519.1) during the BLASTp analysis. However, to the best of our knowledge, asparaginase of P. aeruginosa POA1 is not characterized to date. Besides, other asparaginases derived from different P. aeruginosa also showed a significant difference with the asparaginase of this investigation. Therefore, the recombinant Asn_PA was further explored for its extensive characterization. The ESPript 3.0 analysis identified highly conserved regions of asparaginase enzymes along with catalytically important residues (Fig. 7). In this investigation, we attempted to clone and express l-asparaginase encoding gene from a soil isolate P. aeruginosa CSPS4 in E. coli BL21(DE3) for characterizing its asparaginase. The gene size of Asn_PA was 1089 bp which was very close to the L-asnII (1 kb) and HR03Asnase (981 bp; partial) genes of Pseudomonas sp. PCH44 (Kumar et al. 2022a) and P. aeruginosa strain HR03 (Qeshmi et al. 2022), respectively. The pETAsn_PA was successfully expressed in E. coli BL21(DE3) and showed a molecular weight of ~ 40 kDa. It corroborates the previous findings on asparaginases by exhibiting similar molecular weight from Pseudomonas spp. (El-Sharkawy et al. 2016; Sindhu and Manonmani 2018; Saeed et al. 2018; Kumar et al. 2022a, 2022b). Rati et al. (2019) reported the size of 40 kDa recombinant asparaginase from P. fluorescens on SDS-PAGE; however, on native PAGE, it was identified as a homo-tetramer of 160 kDa. Husain et al. (2016) reported a homo-hexamer of asparaginase of ~ 205 kDa which appeared as a 37 kDa monomer on SDS-PAGE. It indicates that Pseudomonas-type asparaginases exist in multimeric forms and are highly diverse. On characterization, the rAsn_PA showed an optimum pH and temperature of 6.0 and 60 °C, respectively, which classify it as a thermo-acidophilic enzyme. Similar characteristics of optimum pH (5.0) and temperature (50 °C) were observed from the asparaginase of the P. aeruginosa strain (Badoei-Dalfard 2015). A thermo-acidophilic (Topt: 60 °C and pHopt: 5.6) asparaginase has also been reported from the fungus Aspergillus tubingensis SY1 (Yahya et al. 2016). Thus, only few thermo-acidophilic l-asparaginases exist from Pseudomonas spp. which makes Asn_PA a novel enzyme of Pseudomonas origin. Though, several thermophilic asparaginases have been reported from various Pseudomonas spp.; however, most of them exhibit their optimum pH either at neutral (Kamble et al. 2012; Fatima et al. 2019; Amany et al. 2021) or alkaline range (El-Bessoumy et al. 2004; Kumar et al. 2022a; Kumar et al. 2022a; Qeshmi et al. 2022). El-Sharkawy et al. (2016) also observed a thermophilic l-asparaginase (Topt. 45 °C) from P. aeruginosa strain EGYII DSM 101801; however, its optimum pH was 8.5. Similarly, a recombinant l-asparaginase from Pseudomonas sp. PCH44 also showed an optimum temperature of 45 °C under an alkaline pH of 8.5 (Kumar et al. 2022a). The rAsn_PA also exhibited stability for longer hours under acidic conditions at high temperatures which augment its suitability for industrial applications. Kumar et al. (2022a, b) also observed a fair thermostability in l-asparaginase of Pseudomonas sp. PCH44, where the enzyme was able to retain 76.53% residual activity at 45 °C after a longer incubation of 120 min. The asparaginase of P. aeruginosa HR03 also showed stability over a broad range (40–90 °C) of temperature along with 52% residual activity under a highly acidic pH of 4.0 (Qeshmi et al. 2022). Like wild-type/native asparaginase, recombinant rAsn_PA also showed similar characteristics while assessing the effect of modulators (detergents and metal inhibitors). The sustainable activity of rAsn_PA in the presence of EDTA and EGTA conforms that the enzyme does not belong to the family of metalloenzymes. However, Kumar et al. (2022a, b) observed a significant loss of 36% in asparaginase activity of PS44 ASNase II of Pseudomonas spp. PCH44 in the presence of EDTA (Kumar et al. 2022a). Thus, Pseudomonas spp. exhibit high diversity in their asparaginases. On substrate affinity, the rAsn_PA was categorized as asparaginase–glutaminase type due to significant activity on glutamine like several asparaginases from Pseudomonas spp. (Davidson et al. 1976; Ashok et al. 2019) as well as other bacterial sources (Mahajan et al. 2012; Prakash et al. 2020).
Fig. 7.
The ESPRIPT analysis of rAsn_PA protein showed high homology with other l-asparaginase proteins, where four highly conserved signature motifs were observed
The rAsn_PA was successfully employed for its application as an antileukemic activity like the asparaginases of other bacterial sources (Mahajan et al. 2014; El-Naggar et al. 2016; Darvishi et al. 2022). Here, 12 µg/ml enzyme from the stock of purified rAsn_PA (1.0 IU/ml) showed 97% cytotoxic activity against HL-60 cell lines. However, at this concentration, non-permissible blood cells were also depleted. Therefore, 3 µg/ml of asparaginase was found optimum to use for treating cancer cells, where 79% cytotoxic activity on HL-60 cell lines was achieved with an allowed limit (< 5%) of hemolysis of RBCs. Other cancerous cell lines (MOLM-13 and K-562) also showed a significant reduction in the presence of rAsn_PA which needs to be further optimized. The asparaginase of Pseudomonas sp. PCH199 showed a cytotoxic effect on K-562 blood cancer cell lines with an IC50 value of 0.309 U/ml (Darnal et al. 2023). Saeed et al. (2021) also observed the cytotoxic effect of recombinant asparaginase on various cell lines (THP-1, MDA-MB-231, A549, Caco2, and HCT-116). Here, IC50 of purified rAsn_PA was 2.3 IU, 3.7 IU, and 20.5 IU for HL-60, MOLM-13, and K-562, respectively. A similar range of IC50 values has been reported from recombinant l-ASNase of P. aeruginosa on MDA-MB-231 and THP-1 cell lies (Saeed et al. 2021) Pg-ASNase II of another Pseudomonas sp. PCH199 also showed IC50 of 0.169 U/ml towards MCF-7 cell lines (Kumar et al. 2022a). Thus, the recombinant rAsn_PA finds potential in treating tumor cells.
Conclusion
The Asn_PA gene of 1089 bp of P. aeruginosa CSPS4 was successfully cloned, expressed, and purified. The enzymes showed a complete domain of asparaginase during in-silico analysis and were categorized into the asparaginase–glutaminase family due to significant glutaminase activity. The phylogenetic analysis of rAsn_PA showed maximum similarity (100%) with several asparaginases of Pseudomonas spp. and was distantly related to the available commercial asparaginases of E. coli and Erwinia. On characterization, purified rAsn_PA was identified as thermo-acidophilic asparaginase type having optimum l-asparaginase activity at 60 °C under a slightly acidic pH of 6.0. Besides, the enzyme was stable for a longer duration at high temperatures and acidic pH. These properties make this enzyme novel and suitable for industrial applications. Besides, the rAsn_PA showed strong cytotoxic activity on various cancer cell lines along with non-significant hemolysis and suggests its applicability for treating cancer.
Acknowledgements
VK and DV are grateful to Dr. Shilpa Sharma, NSUT, New Delhi for accessing her laboratory for the cloning experiments. AS and CPC are acknowledged for providing facilities to perform cell line experiments at SGPGI, Lucknow. Besides, DV and VK are thankful to Babasaheb Bhimrao Ambedkar University, Lucknow for providing infrastructure and other useful resources.
Author contributions
DV conceived the idea and coordinated the complete study. VK performed all the experiments. RK and SS assisted in gene cloning. AS and CPC helped in cell line experiments. VK and DV wrote the manuscript. All the authors read, edited, and approved the final version of the manuscript.
Funding
There was no financial support received from any funding agency to carry out the work presented here.
Data availability
The full-length gene of l-asparaginase reported here can be retrieved using accession no. OR509736 at NCBI.
Declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Research involving human participants and/or animals
Not applicable.
Informed consent
Not applicable.
References
- Alrumman SA, Mostafa YS, Al-Izran KA, Alfaifi MY, Taha TH, Elbehairi SE. Production and anticancer activity of an l-asparaginase from Bacillus licheniformis isolated from the red sea, Saudi Arabia. Sci Rep. 2019;6:3756. doi: 10.1038/s41598-019-40512-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amany B, El-Aziz A, Hassanein WA, Mattar ZA, El-Didamony RA. Production of chemotherapeutic agent l-asparaginase from gamma-irradiated Pseudomonas aeruginosa WCHPA075019. Jordan J Biol Sci. 2021;14:403–412. doi: 10.54319/jjbs/140304. [DOI] [Google Scholar]
- Ashok A, Doriya K, Rao JV, Qureshi A, Tiwari AK, Kumar DS. Microbes producing l-asparaginase free of glutaminase and urease isolated from extreme locations of Antarctic soil and Moss. Sci Rep. 2019;9:1423. doi: 10.1038/s41598-018-38094-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Audipudi AV, Pallavi R, Supriya GNR. Characterization of l-asparaginase producing bacteria from mangrove soil. Chem Tech. 2013;1:109–112. [Google Scholar]
- Badoei-Dalfard A. Purification and characterization of l-asparaginase from Pseudomonas aeruginosa strain SN004: production optimization by statistical methods. Biocatal Agri Biotechnol. 2015;4:388–397. doi: 10.1016/j.bcab.2015.06.007. [DOI] [Google Scholar]
- Cachumba JJ, Antunes FA, Peres GF, Brumano LP, Santos JC, Da Silva SS. Current applications and different approaches for microbial l-asparaginase production. Braz J Microbiol. 2016;47:77–85. doi: 10.1016/j.bjm.2016.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darnal S, Patial V, Kumar V, Kumar S, Kumar V, Padwad YS, Singh D. Biochemical characterization of extremozyme l-asparaginase from Pseudomonas sp. PCH199 for therapeutics. AMB Express. 2023;13:22. doi: 10.1186/s13568-023-01521-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darvishi F, Jahanafrooz Z, Mokhtarzadeh A. Microbial l-asparaginase as a promising enzyme for treatment of various cancers. Appl Microbiol Biotechnol. 2022;106:5335–5347. doi: 10.1007/s00253-022-12086-8. [DOI] [PubMed] [Google Scholar]
- Davidson L, Brear DR, Wingard P, Hawkins J, Kitto GB. Purification and properties of an l-glutaminase-l-asparaginase from Pseudomonas acidovorans. J Bact. 1976;129:1379–1386. doi: 10.1128/jb.129.3.1379-1386.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duval M, Suciu S, Ferster A, Rialland X, Nelken B, Lutz P, Philippe N. Comparison of Escherichia coli–asparaginase with Erwinia-asparaginase in the treatment of childhood lymphoid malignancies: results of a randomized European organization for research and treatment of cancer: children's leukemia group phase 3 trial. Blood J Am Soc Hematol. 2002;99:2734–2739. doi: 10.1182/blood.v99.8.2734. [DOI] [PubMed] [Google Scholar]
- El-Bessoumy AA, Sarhan M, Mansour J. Production, isolation, and purification of l-asparaginase from Pseudomonas aeruginosa 50071 using solid-state fermentation. BMB Rep. 2004;37:387–393. doi: 10.5483/BMBRep.2004.37.4.387. [DOI] [PubMed] [Google Scholar]
- El- Naggar NE, El-Ewasy SM, El-Shweihy NM. Microbial l-asparaginase as a potential therapeutic agent for the treatment of acute lymphoblastic leukemia: the pros and cons. Int J Pharmacol. 2014;10:182–199. doi: 10.3923/ijp.2014.182.199. [DOI] [Google Scholar]
- El-Naggar NEA, Deraz SF, Soliman HM, El-Deeb NM, El-Ewasy SM. Purification, characterization, cytotoxicity, and anticancer activities of l–asparaginase, anti-colon cancer protein, from the newly isolated alkaliphilic Streptomyces fradiae NEAE-82. Sci Rep. 2016;6:32926. doi: 10.1038/srep32926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Sharkawy AS, Farag AM, Embaby AM, Saeed H, El-Shenawy M. Cloning, expression, and characterization of aeruginosa EGYII l-asparaginase from Pseudomonas aeruginosa strain EGYII DSM 101801 in E. coli BL21 (DE3) pLysS. J Mol Cat B: Enzym. 2016;132:16–23. doi: 10.1016/j.molcatb.2016.06.011. [DOI] [Google Scholar]
- Fatima N, Khan MM, Khan IA. L-asparaginase produced from soil isolates of Pseudomonas aeruginosa shows potent anti-cancer activity on HeLa cells. Saudi J Biol Sci. 2019;26:1146–1153. doi: 10.1016/j.sjbs.2019.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao C, Ma X, Zhang Z, Lu Q, Ashby CR, Jr, Wei L, Chen ZS. Asparaginase Erwinia chrysanthemi for acute lymphoblastic leukemia and lymphoblastic lymphoma. Drugs Today (barc) 2022;58:261–271. doi: 10.1358/dot.2022.58.6.3413459. [DOI] [PubMed] [Google Scholar]
- Goswami R, Hegde K, Veerank VD. Production and characterization of novel glutaminase free recombinant l-asparaginase II of Erwinia carotovora subsp. atroseptica SCRI 1043 in E. coli BL21(DE3) Br Microbiol Res J. 2015;6:95–112. doi: 10.9734/BMRJ/2015/13867. [DOI] [Google Scholar]
- Husain I, Sharma A, Kumar S, Malik F. Purification and characterization of glutaminase free asparaginase from Pseudomonas otitidis: induce apoptosis in human leukemia MOLT-4 cells. Biochimie. 2016;121:38–51. doi: 10.1016/j.biochi.2015.11.012. [DOI] [PubMed] [Google Scholar]
- Jia R, Wan X, Geng X, Xue D, Xie Z, Chen C. Microbial l-asparaginase for application in acrylamide mitigation from food: current research status and future perspectives. Microorganisms. 2021;9:1659. doi: 10.3390/microorganisms9081659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamble KD, Bidwe PR, Muley VY, Kamble LH, Bhadange DG, Musaddiq M. Characterization of l-asparaginase producing bacteria from water, farm, and saline soil. Biosci Disc. 2012;3:116–119. [Google Scholar]
- Kukurova K, Morales FJ, Bednáriková A, Ciesarová Z. Effect of l-asparaginase on acrylamide mitigation in a fried dough pastry model. Mol Nut Food Res. 2009;53:1532–1539. doi: 10.1002/mnfr.200800600. [DOI] [PubMed] [Google Scholar]
- Kumar SS, Haridas M, Abdulhameed S. A novel fibrinolytic enzyme from marine Pseudomonas aeruginosa KU1 and its rapid in vivo thrombolysis with little haemolysis. Int J Biol Macromol. 2020;162:470–479. doi: 10.1016/j.ijbiomac.2020.06.178. [DOI] [PubMed] [Google Scholar]
- Kumar S, Darnal S, Patial V, Kumar V, Kumar S, Singh D. Molecular cloning, characterization, and in-silico analysis of l-asparaginase from Himalayan Pseudomonas sp PCH44. 3 Biotech. 2022;12:162. doi: 10.1007/s13205-022-03224-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Darnal S, Patial V, Kumar V, Singh D. Molecular characterization of a stable and robust l-asparaginase from Pseudomonas sp. PCH199: evaluation of cytotoxicity and acrylamide mitigation potential. Fermentation. 2022;8:568. doi: 10.3390/fermentation8100568. [DOI] [Google Scholar]
- Mahajan RV, Saran S, Kameswaran K, Kumar V, Saxena RK. Efficient production of l-asparaginase from Bacillus licheniformis with low-glutaminase activity: optimization, scale up and acrylamide degradation studies. Biores Technol. 2012;125:11–16. doi: 10.1016/j.biortech.2012.08.086. [DOI] [PubMed] [Google Scholar]
- Mahajan RV, Kumar V, Rajendran V, Saran S, Ghosh PC, Saxena RK. Purification and characterization of a novel and robust l-asparaginase having low-glutaminase activity from Bacillus licheniformis: in vitro evaluation of anti-cancerous properties. PLoS ONE. 2014;6:99037. doi: 10.1371/journal.pone.0099037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muneer F, Siddique MH, Azeem F, Rasul I, Muzammil S, Zubair M, Afzal M, Nadeem H. Microbial l-asparaginase: purification, characterization, and applications. Arch Microbiol. 2020;202:967–981. doi: 10.1007/s00203-020-01814-1. [DOI] [PubMed] [Google Scholar]
- Nagarethinam S, Naik AN, Udupa N, Rao VJ, Vanathi MB. Microbial l-asparaginase and its future prospects. Asian J Med Res. 2012;1:159–160. [Google Scholar]
- Prakash O, Nimonkar Y, Desai D. A recent overview of microbes and microbiome preservation. Indian J Microb. 2020;60:297–309. doi: 10.1007/s12088-020-00880-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qeshmi IF, Homaei A, Fernandes P, Javadpour S. Marine microbial l- asparaginase: biochemistry, molecular approaches, and applications in tumor therapy and in food industry. Microbiol Res. 2018;208:99–112. doi: 10.1016/j.micres.2018.01.011. [DOI] [PubMed] [Google Scholar]
- Qeshmi FI, Homaei A, Khajeh K, Kamrani E, Fernandes P. Production of a novel marine Pseudomonas aeruginosa recombinant l-asparaginase: insight on the structure and biochemical characterization. Mar Biotechnol. 2022;24:599–613. doi: 10.1007/s10126-022-10129-9. [DOI] [PubMed] [Google Scholar]
- Saeed H, Soudan H, El Sharkawy A, Farag A, Embaby A, Ataya F. Expression and functional characterization of Pseudomonas aeruginosa recombinant l-asparaginase. Protein J. 2018;37:461–471. doi: 10.1007/s10930-018-9789-3. [DOI] [PubMed] [Google Scholar]
- Saeed H, Hemida A, Abdel-Fattah M, Eldoksh A, Shalaby M, Nematalla H, Elkewedi M. Pseudomonas aeruginosa recombinant L-asparaginase: large scale production, purification, and cytotoxicity on THP-1, MDA-MB-231, A549, Caco2 and HCT-116 cell lines. Protein Expr Purif. 2021;181:105820. doi: 10.1016/j.pep.2021.105820. [DOI] [PubMed] [Google Scholar]
- Shakambari G, Ashok Kumar B, Varalakshmi P. L-asparaginase – a promising biocatalyst for industrial and clinical applications. Biocat Agri Biotechnol. 2017;17:213–224. doi: 10.1016/j.bcab.2018.11.018. [DOI] [Google Scholar]
- Shifrin S, Parrott CL, Luborsky SW. Substrate binding and inter subunit interactions in l-asparaginase. J Biol Chem. 1974;249:1335–1340. doi: 10.1016/S0021-9258(19)42886-X. [DOI] [PubMed] [Google Scholar]
- Shrivastava A, Khan AA, Khurshid M, Kalam MA, Jain SK, Singhal PK. Recent developments in L-asparaginase discovery and its potential as anticancer agent. Crit Rev Oncol Hematol. 2016;100:1–10. doi: 10.1016/j.critrevonc.2015.01.002. [DOI] [PubMed] [Google Scholar]
- Sindhu R, Manonmani HK. Expression and characterization of recombinant l-asparaginase from Pseudomonas fluorescens. Protein Expr Purif. 2018;143:83–91. doi: 10.1016/j.pep.2017.09.009. [DOI] [PubMed] [Google Scholar]
- Stadler RH, Blank I, Varga N, Robert F, Hau J, Guy PA, Robert MC, Riediker S. Acrylamide from Maillard reaction products. Nature. 2002;3:449–450. doi: 10.1038/419449a. [DOI] [PubMed] [Google Scholar]
- Vachhani P, Al Yacoub R, Miller A, Zhang F, Cronin TL, Ontiveros EP, Wang ES. Intensive chemotherapy vs. hypomethylating agents in older adults with newly diagnosed high-risk acute myeloid leukemia: a single center experience. Leuk Res. 2018;75:29–35. doi: 10.1016/j.leukres.2018.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinci RM, Mestdagh F, Meulenaer BD. Acrylamide formation in fried potato products present and future, a critical review on mitigation strategies. Food Chem. 2012;133:1138–1154. doi: 10.1016/j.foodchem.2011.08.001. [DOI] [Google Scholar]
- Yahya S, Jahangir S, Shaukat SS, Sohail M, Khan SA. Production optimization by using Plackett–Burman design and partial characterization of amylase from Aspergillus tubingensis SY 1. Pak J Bot. 2016;48:2557–2561. [Google Scholar]
- Yuan J, Yang J, Zhuang Z, Yang Y, Lin L, Wang S. Thrombolytic effects of Douchi fibrinolytic enzyme from Bacillus subtilis LD-8547 in vitro and in vivo. BMC Biotechnol. 2012;12:1–9. doi: 10.1186/1472-6750-12-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The full-length gene of l-asparaginase reported here can be retrieved using accession no. OR509736 at NCBI.