Abstract
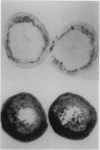
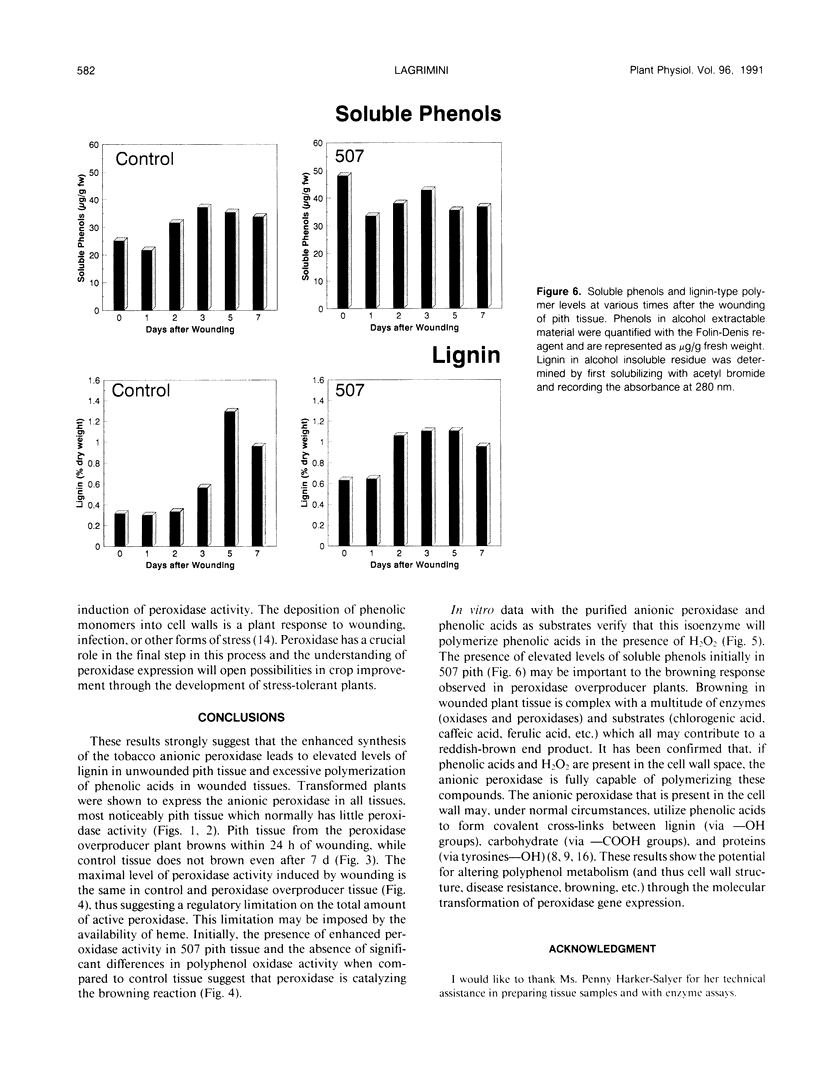
Tobacco (Nicotiana tabacum) plants transformed with a chimeric tobacco anionic peroxidase gene have previously been shown to synthesize high levels of peroxidase in all tissues throughout the plant. One of several distinguishable phenotypes of transformed plants is the rapid browning of pith tissue upon wounding. Pith tissue from plants expressing high levels of peroxidase browned within 24 hours of wounding, while tissue from control plants did not brown as late as 7 days after wounding. A correlation between peroxidase activity and wound-induced browning was observed, whereas no relationship between polyphenol oxidase activity and browning was found. The purified tobacco anionic peroxidase was subjected to kinetic analysis with substrates which resemble the precursors of lignin or polyphenolic acid. The purified enzyme was found to readily polymerize phenolic acids in the presence of H2O2 via a modified ping-pong mechanism. The percentage of lignin and lignin-related polymers in cell walls was nearly twofold greater in pith tissue isolated from peroxidase-overproducer plants compared to control plants. Lignin deposition in wounded pith tissue from control plants closely followed the induction of peroxidase activity. However, wound-induced lignification occurred 24 to 48 hours sooner in plants overexpressing the anionic peroxidase. This suggests that the availability of peroxidase rather than substrate may delay polyphenol deposition in wounded tissue.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Börner H., Grisebach H. Enzyme induction in soybean infected by Phytophthora megasperma f.sp. glycinea. Arch Biochem Biophys. 1982 Aug;217(1):65–71. doi: 10.1016/0003-9861(82)90479-9. [DOI] [PubMed] [Google Scholar]
- Cassab G. I., Varner J. E. Immunocytolocalization of extensin in developing soybean seed coats by immunogold-silver staining and by tissue printing on nitrocellulose paper. J Cell Biol. 1987 Dec;105(6 Pt 1):2581–2588. doi: 10.1083/jcb.105.6.2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espelie K. E., Franceschi V. R., Kolattukudy P. E. Immunocytochemical localization and time course of appearance of an anionic peroxidase associated with suberization in wound-healing potato tuber tissue. Plant Physiol. 1986 Jun;81(2):487–492. doi: 10.1104/pp.81.2.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everdeen D. S., Kiefer S., Willard J. J., Muldoon E. P., Dey P. M., Li X. B., Lamport D. T. Enzymic cross-linkage of monomeric extensin precursors in vitro. Plant Physiol. 1988 Jul;87(3):616–621. doi: 10.1104/pp.87.3.616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk T. K., Connors W. J., Bleam R. D., Hackett W. F., Zeikus J. G. Preparation and microbial decomposition of synthetic [14C]ligins. Proc Natl Acad Sci U S A. 1975 Jul;72(7):2515–2519. doi: 10.1073/pnas.72.7.2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagrimini L. M., Bradford S., Rothstein S. Peroxidase-Induced Wilting in Transgenic Tobacco Plants. Plant Cell. 1990 Jan;2(1):7–18. doi: 10.1105/tpc.2.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagrimini L. M., Burkhart W., Moyer M., Rothstein S. Molecular cloning of complementary DNA encoding the lignin-forming peroxidase from tobacco: Molecular analysis and tissue-specific expression. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7542–7546. doi: 10.1073/pnas.84.21.7542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagrimini L. M., Rothstein S. Tissue specificity of tobacco peroxidase isozymes and their induction by wounding and tobacco mosaic virus infection. Plant Physiol. 1987 Jun;84(2):438–442. doi: 10.1104/pp.84.2.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loschke D. C., Hadwiger L. A. Effects of Light and of Fusarium solani on Synthesis and Activity of Phenylalanine Ammonia-Lyase in Peas. Plant Physiol. 1981 Sep;68(3):680–685. doi: 10.1104/pp.68.3.680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothstein S. J., Lahners K. N., Lotstein R. J., Carozzi N. B., Jayne S. M., Rice D. A. Promoter cassettes, antibiotic-resistance genes, and vectors for plant transformation. Gene. 1987;53(2-3):153–161. doi: 10.1016/0378-1119(87)90003-5. [DOI] [PubMed] [Google Scholar]