Abstract
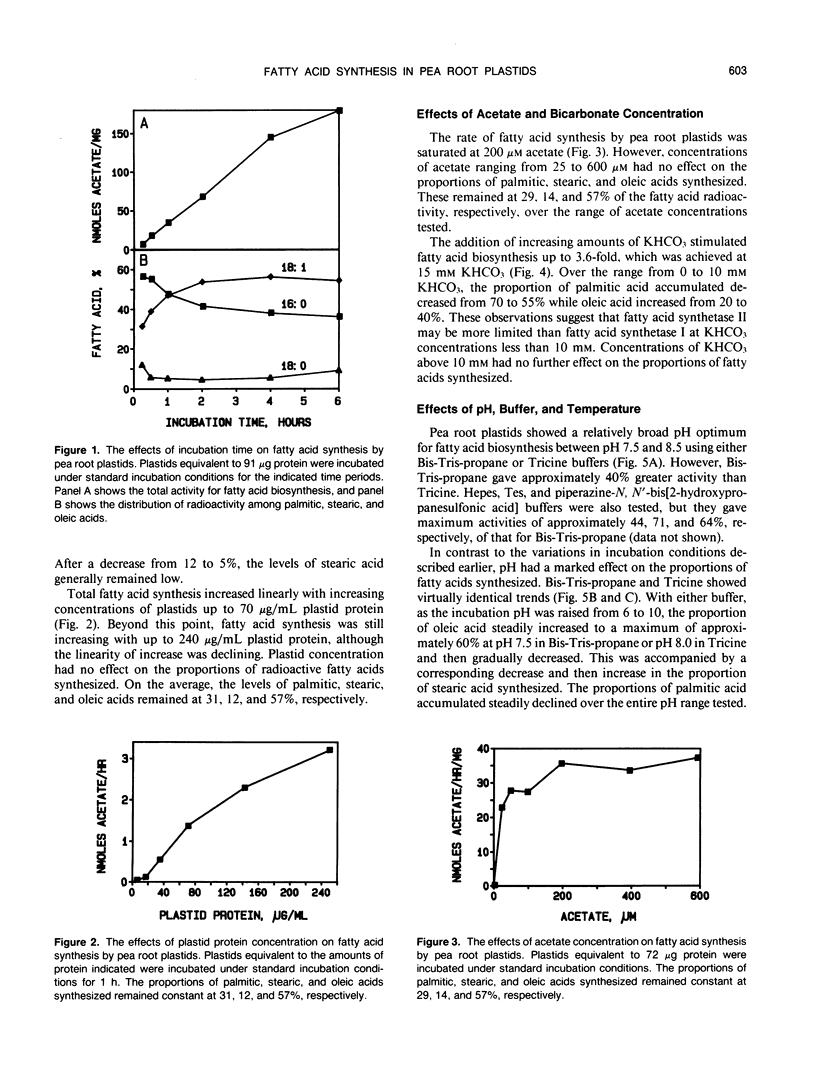
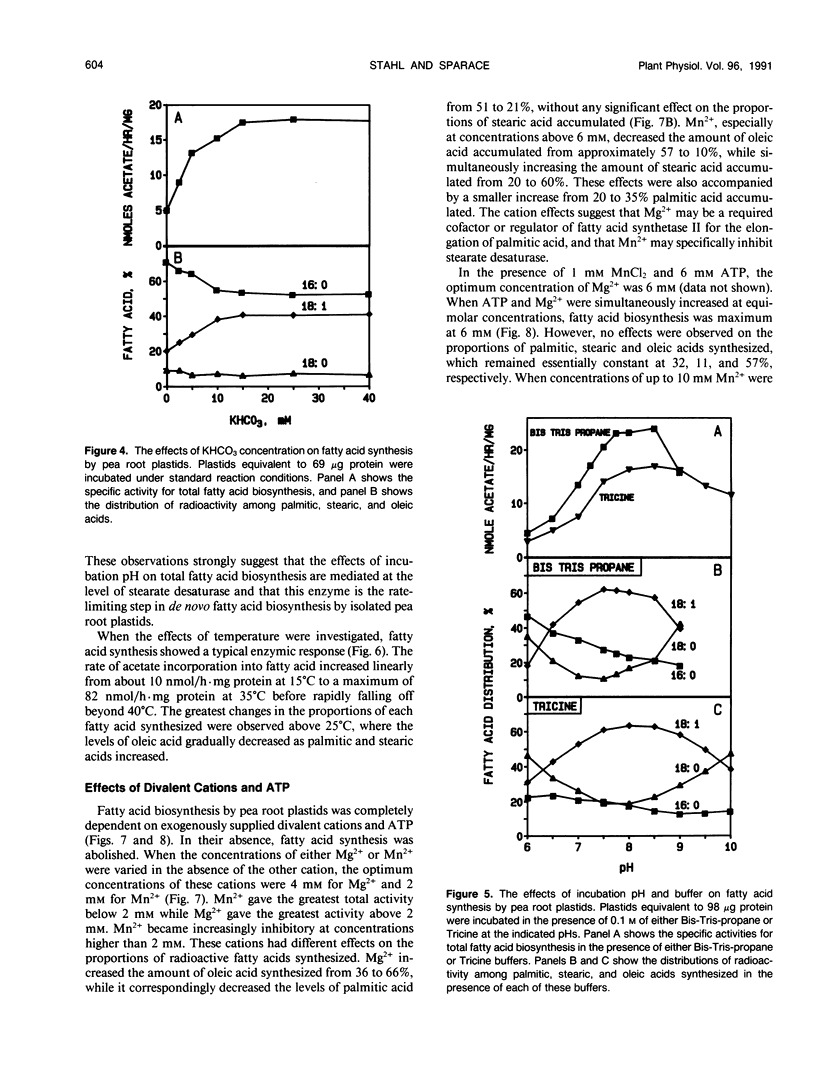
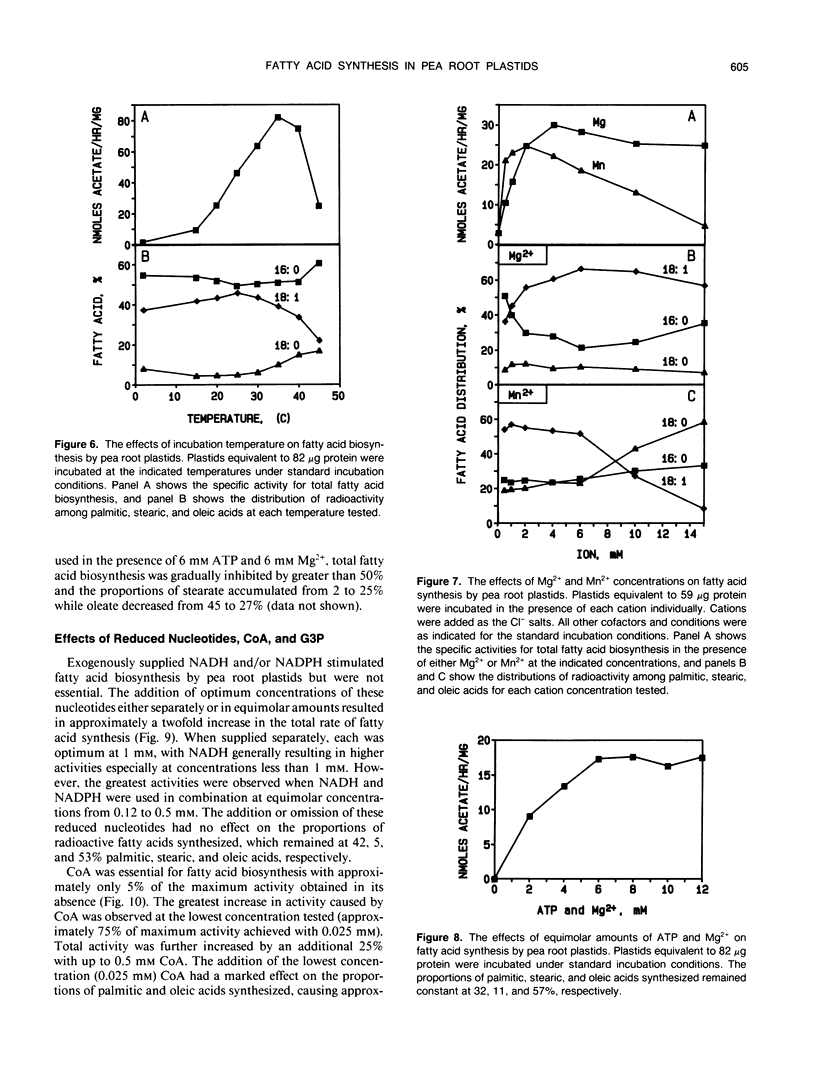
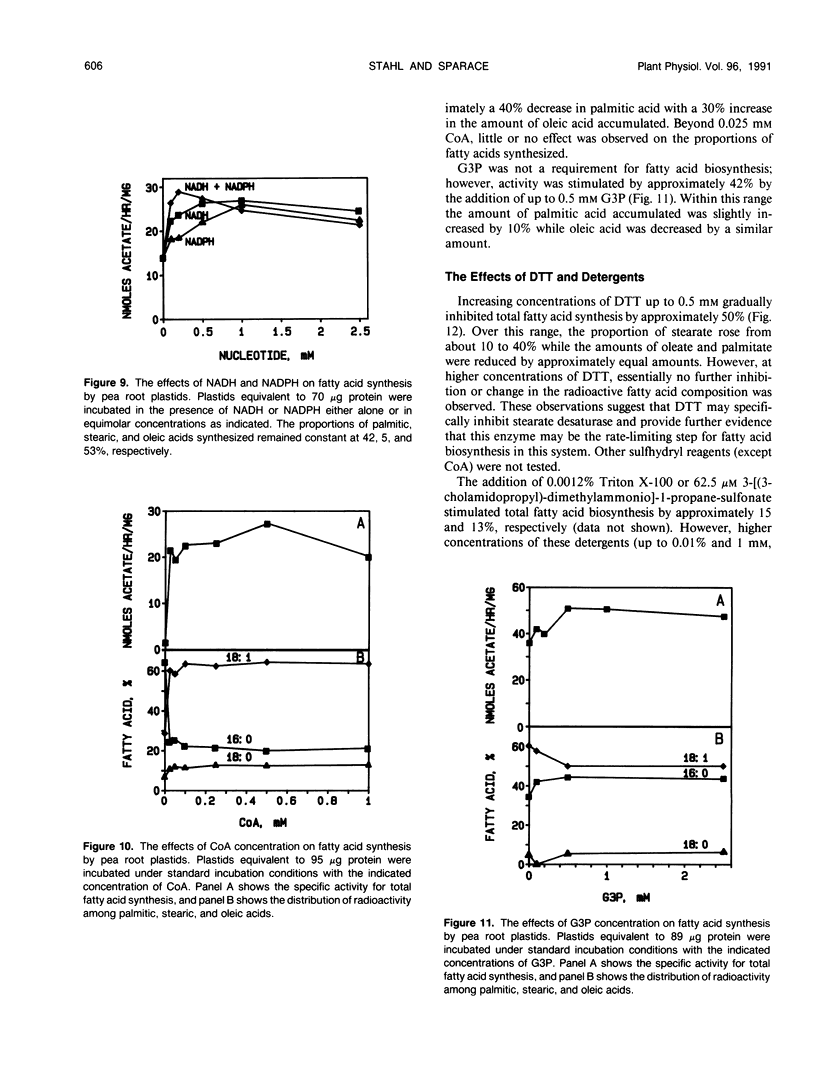
Fatty acid biosynthesis from Na[1-14C]acetate was characterized in plastids isolated from primary roots of 7-day-old germinating pea (Pisum sativum L.) seeds. Fatty acid synthesis was maximum at 82 nanomoles per hour per milligram protein in the presence of 200 micromolar acetate, 0.5 millimolar each of NADH, NADPH, and coenzyme A, 6 millimolar each of ATP and MgCl2, 1 millimolar each of MnCl2 and glycerol-3-phosphate, 15 millimolar KHCO3, 0.31 molar sucrose, and 0.1 molar Bis-Tris-propane, pH 8.0, incubated at 35°C. At the standard incubation temperature of 25°C, fatty acid synthesis was essentially linear for up to 6 hours with 80 to 120 micrograms per milliliter plastid protein. ATP and coenzyme A were absolute requirements, whereas divalent cations, potassium bicarbonate, and reduced nucleotides all variously improved activity two- to 10-fold. Mg2+ and NADH were the preferred cation and nucleotide, respectively. Glycerol-3-phosphate had little effect, whereas dithiothreitol and detergents generally inhibited the incorporation of [14C]acetate into fatty acids. On the average, the principal radioactive products of fatty acid biosynthesis were approximately 39% palmitic, 9% stearic, and 52% oleic acid. The proportions of these fatty acids synthesized depended on the experimental conditions.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Journet E. P., Douce R. Enzymic capacities of purified cauliflower bud plastids for lipid synthesis and carbohydrate metabolism. Plant Physiol. 1985 Oct;79(2):458–467. doi: 10.1104/pp.79.2.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MUDD J. B., McMANUS T. T. Metabolism of acetate by cellfree preparations from spinach leaves. J Biol Chem. 1962 Jul;237:2057–2063. [PubMed] [Google Scholar]
- Nothelfer H. G., Barckhaus R. H., Spener F. Localisation and characterization of the fatty acid synthesizing system in cells of Glycine max (soubean) suspension cultures. Biochim Biophys Acta. 1977 Dec 21;489(3):370–380. doi: 10.1016/0005-2760(77)90157-6. [DOI] [PubMed] [Google Scholar]
- Roughan P. G., Holland R., Slack C. R. Acetate is the preferred substrate for long-chain fatty acid synthesis in isolated spinach chloroplasts. Biochem J. 1979 Dec 15;184(3):565–569. doi: 10.1042/bj1840565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roughan P. G., Holland R., Slack C. R. On the control of long-chain-fatty acid synthesis in isolated intact spinach (Spinacia oleracea) chloroplasts. Biochem J. 1979 Nov 15;184(2):193–202. doi: 10.1042/bj1840193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roughan P. G., Slack C. R., Holland R. High rates of [1-14C]acetate incorporation into the lipid of isolated spinach chloroplasts. Biochem J. 1976 Sep 15;158(3):593–601. doi: 10.1042/bj1580593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simcox P. D., Reid E. E., Canvin D. T., Dennis D. T. Enzymes of the Glycolytic and Pentose Phosphate Pathways in Proplastids from the Developing Endosperm of Ricinus communis L. Plant Physiol. 1977 Jun;59(6):1128–1132. doi: 10.1104/pp.59.6.1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparace S. A., Menassa R., Kleppinger-Sparace K. F. A preliminary analysis of Fatty Acid synthesis in pea roots. Plant Physiol. 1988 May;87(1):134–137. doi: 10.1104/pp.87.1.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vick B., Beevers H. Fatty Acid synthesis in endosperm of young castor bean seedlings. Plant Physiol. 1978 Aug;62(2):173–178. doi: 10.1104/pp.62.2.173. [DOI] [PMC free article] [PubMed] [Google Scholar]


