Abstract
Background: In the last years growing evidences on the role of radiomics and machine learning (ML) applied to different nuclear medicine imaging modalities for the assessment of thyroid diseases are starting to emerge. The aim of this systematic review was therefore to analyze the diagnostic performances of these technologies in this setting. Methods: A wide literature search of the PubMed/MEDLINE, Scopus and Web of Science databases was made in order to find relevant published articles about the role of radiomics or ML on nuclear medicine imaging for the evaluation of different thyroid diseases. Results: Seventeen studies were included in the systematic review. Radiomics and ML were applied for assessment of thyroid incidentalomas at 18 F-FDG PET, evaluation of cytologically indeterminate thyroid nodules, assessment of thyroid cancer and classification of thyroid diseases using nuclear medicine techniques. Conclusion: Despite some intrinsic limitations of radiomics and ML may have affect the results of this review, these technologies seem to have a promising role in the assessment of thyroid diseases. Validation of preliminary findings in multicentric studies is needed to translate radiomics and ML approaches in the clinical setting.
Keywords: Thyroid, Machine learning, Radiomics, Texture analysis, Positron emission tomography
Introduction
Thyroid diseases are a heterogeneous group of conditions which includes both benign and malignant diseases, with an increasing in the incidence of both of them in the recent years. Notably, differentiated thyroid cancer (DTC) is the most common endocrine malignant neoplasm worldwide, while Hashimoto’s thyroiditis is the most common autoimmune form of thyroid disease [1].
The diagnosis of thyroid pathologies is based primarily on the assessment of its function with laboratory blood test, in particular for thyroiditis or hyperthyroidism [2]. Moreover, the anatomical evaluation of the organ with the well-established ultrasonography (US) is mandatory to assess the presence of nodules, possible expression of thyroid cancer, or other pathological conditions and therefore this imaging modality has experienced an increasing relevance in the last decades [3].
In this scenario, the role of nuclear medicine for the diagnosis and treatment of thyroid conditions is central. Thyroid scintigraphy with 99mTc-pertechnetate enables the functional evaluation of the gland, allowing the differentiation between thyroiditis and hyperthyroidism, but also detecting the presence of ectopic thyroid tissue [4]. Moreover, 131I is mandatory for the management of DTC, since its pivotal role for the therapy but also the stage or the restage of the disease [5–9]. Beside single photon imaging, positron emission tomography (PET) has continuously spread its indication for the evaluation of DTC and associated conditions, and in the recent years many different tracers have been proposed for the assessment of such diseases [7–12].
Recently, an increase in the extraction of specific quantitative features from PET and scintigraphic images, called radiomics or texture analysis, is being experienced and researches in this field are focusing on its diagnostic and prognostic role in a wide range of pathological conditions, and the thyroid does not make any exception [13–14]. Similarly, machine learning (ML) is a hot topic of recent clinical research and focuses on the development of algorithms that can use different combinations of features in order to predict a specific target [15–16].
The aim of this systematic review is therefore to evaluate the role of radiomics and ML for the assessment of thyroid diseases.
Materials and methods
Search strategy
A wide literature search of the PubMed/MEDLINE, Scopus and Web of Science databases was made in order to find significant published articles concerning the role of radiomics and ML for the assessment of thyroid diseases. The algorithm used for the research was the following: (“thyroid”) AND (“radiomics” OR “texture” OR “textural” OR “machine learning”).
No beginning date limit was applied to the search, and it was updated until 01 February 2023. Only articles in the English language were considered and preclinical studies, conference proceedings, reviews or editorials were excluded. To expand our search, the references of the retrieved articles were also screened for additional papers.
Study selection
Two researchers (F.D. and R.G.) independently reviewed the titles and abstracts of the retrieved articles. The same two researchers then independently reviewed the full-text version of the remaining articles to determine their eligibility for the inclusion.
Quality assessment
The quality assessment of these studies, including the risk of bias and applicability concerns, was carried out using Quality Assessment of Diagnostic Accuracy Studies version 2 (QUADAS-2) evaluation [17].
Data extraction
For each included study, data concerning the basic study (author names, year of publication, country of origin, design of the study, radiotracer used and number of patients), the type of scan used and its setting were collected. The main findings of the articles included in this review are reported in the Results section.
Results
Literature search
A total of 1643 articles were extrapolated with the computer literature search and, by reviewing the titles and abstracts, 1627 of them were excluded because the reported data were not within the field of interest of this review. Sixteen articles were therefore selected and retrieved in full-text version [18–33] and one additional study was also found screening the references of these articles (Fig. 1) [34]; as a consequence, the total number of studies evaluated in the review was 17.
Fig. 1.
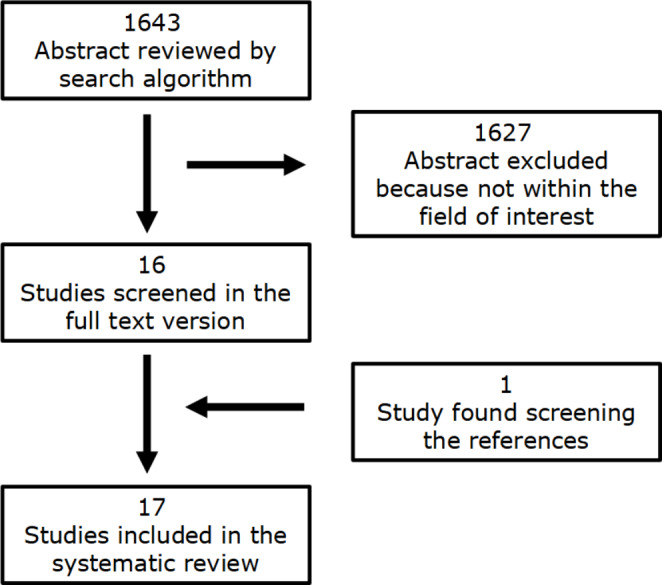
Flowchart of the research of eligible studies on the role of radiomics or ML for the assessment of pathological conditions of thyroid
In general, the quality assessment using QUADAS-2 evaluation underlined the presence of unclear risk of bias and applicability concerns in some of the studies for what concerns patients selection, index test, reference standard and flow and timing. Nevertheless, only a small amount of studies were characterized by the presence of high risks of bias or applicability (Fig. 2).
Fig. 2.
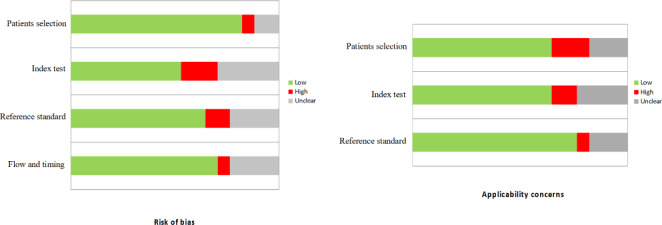
QUADAS-2 quality assessment for risk of bias and applicability concerns for the studies considered in the review
Among the total number of studies included in the systematic review, 14 were of retrospective nature [18–24, 26–28, 30, 32–34], whereas one had a prospective design [25] and in two cases it was not specified the nature of the study [29, 31]. Ten studies focused on PET imaging [18–26, 34], 8 with PET/computed tomography (PET/CT) hybrid tomographs [19–20, 22–26, 34] and 2 with both PET/CT and PET [18, 21]. Furthermore, 7 studies focused on single photon imaging [27–33] and in particular 3 were performed with single photon emission computed tomography (SPECT) [27, 29, 31] while 4 were performed with planar scintigraphic scans [28, 30, 32–33].
Speaking about radiopharmaceuticals, 9 of the studies that focused on PET imaging were performed with 18 F-fluorodeoxyglucose (18 F-FDG) [19–26, 34], while in 1 case both 68Ga-DOTATOC or 68Ga-DOTATATE were used [18]. Moreover, in the case of scintigraphic imaging, 131I was used in 2 studies [28–31] while in 4 other it was reported the use of 99mTc-pertechnetate [29–[30, 32]–33]; lastly, in 1 case the radiotracer was not specified [27].
The main characteristics of the studies and their results are briefly presented in Tables 1 and 2.
Table 1.
Characteristics of the studies considered for the review
| First author | Ref. N. | Year | Country | Study design | Radiopharmaceuticals | N. Pts. | Sex M:F |
|---|---|---|---|---|---|---|---|
| PET studies | |||||||
| Kim SJ | 34 | 2015 | Korea | Retrospective | 18 F-FDG | 200 | 43:157 |
| Lapa C | 18 | 2015 | Germany | Retrospective |
68Ga-DOTATOC 68Ga-DOTATATE |
12 | 9:3 |
| Sollini M | 19 | 2017 | Italy | Retrospective | 18 F-FDG | 55 | 20:35 |
| Nakajo M | 20 | 2018 | Japan, Germany | Retrospective | 18 F-FDG | 114 | 33:81 |
| Werner RA | 21 | 2018 | Germany, USA, Japan, Hungary | Retrospective | 18 F-FDG | 18 | 12:6 |
| Aksu A | 22 | 2020 | Turkey | Retrospective | 18 F-FDG | 60 | ns |
| Ceriani L | 23 | 2020 | Switzerland, Italy | Retrospective | 18 F-FDG | 104 | 37:67 |
| Giovanella L | 24 | 2021 | Switzerland, Italy | Retrospective | 18 F-FDG | 78 | 20:58 |
| De Koster EJ | 25 | 2022 | The Netherlands, Italy | Prospective | 18 F-FDG | 123 | 21:102 |
| Dondi F | 26 | 2022 | Italy | Retrospective | 18 F-FDG | 221 | 72:149 |
| Other studies | |||||||
| Ma L | 27 | 2019 | China | Retrospective | ns | 2888 | ns |
| Kavitha M | 28 | 2020 | Japan, South Korea | Retrospective | 131I | 230 | ns |
| Liu Y | 29 | 2020 | China | ns | 99mTc-pertechnetate | 136 | ns |
| Currie G | 30 | 2021 | Australia, USA, Pakistan | Retrospective | 99mTc-pertechnetate | 123 | 12:111 |
| Guo Y | 31 | 2021 | China | ns | 131I | 446 | 87:359 |
| Qiao T | 32 | 2021 | China | Retrospective | 99mTc-pertechnetate | 1430 | ns |
| Yang P | 33 | 2021 | China | Retrospective | 99mTc-pertechnetate | 3389 | 168:3221 |
N.: number; Pts: patients; Ref: reference; M: male; F: female; ns: not specified
Table 2.
Results and main findings of the studies considered for the review
| First author | Type of scan | Number of scanners | Setting | Patients characteristics | Performance validation methods | ML/statistical techniques | Features with best performances | Main findings |
|---|---|---|---|---|---|---|---|---|
| PET studies | ||||||||
| Kim SJ | PET/CT | 1 | Evaluation of citologically indeterminate thyroid nodules | 151 without malignancy and 49 with malignancy | Training set | Kruskall-Wallis | HF | Intratumoral heterogeneity could characterize thyroid nodules with inconclusive FNAB. |
| Lapa C | PET and PET/CT | 3 | Evaluation of iodine refractory DTC or MTC treated with PRRT | 4 MTC and 8 iodine refractory DTC | Training set | Survival models | Contrast, Entropy, Grey Level Non Uniformity, High Grey Level Zone Emphasis, Intensity Variation, Short Run Emphasis, Short Run High Level Grey Emphasis, Short Zone High Grey Level Emphasis, Short Zone Low Grey Level Emphasis | Tumor heterogeneity could be a predictor of response to PRRT. |
| Sollini M | PET/CT | 2 | Assessment of thyroid incidentalomas at PET/CT | 32 without malignancy and 18 with malignancy | Training set | Fisher test, ANOVA | Skewness, Kurtosis, CorrelationGLCM | Texture analysis seems to be able to stratify thyroid incidentalomas with respect to the risk of malignancy. |
| Nakajo M | PET/CT | 1 | Prediction of the risk of recurrence of DTC | 88 18 F-FDG avid tumors and 26 non-avid | Training set | Mann Whitney, Chi square | IV, SVZ, ZP | When MTV is higher than 10, the combined use of SUV-related, volumetric and texture parameters increases the identification of patients with high risk of recurrence. |
| Werner RA | PET and PET/CT | 3 | Prognostic evaluation in patients with MTC treated with TKI | 1 with hereditary MTC and 17 without | Training set | Survival models | Complexity, Contrast | Baseline complexity and TLG are independent prognosticators for OS. |
| Aksu A | PET/CT | 1 | Assessment of thyroid incidentalomas at PET/CT | 32 without malignancy and 28 with malignancy | Cross-fold validation | RF, KNN, naive bayes, DT and support vector machine | GLRLMRLNU |
Texture analysis may be more useful than SUVmax in predicting the malignancy of thyroid incidentalomas. |
| Ceriani L | PET/CT | 2 of the same model | Assessment of thyroid incidentalomas at PET/CT | 77 without malignancy and 30 with malignancy | Cross-fold validation | Logistic regression | Shape_Sphericity | The proposed multiparametric radiomics model showed good performance in stratifying the risk of malignancy of incidentalomas. |
| Giovanella L | PET/CT | 2 | Evaluation of citologically indeterminate thyroid nodules | 55 without malignancy and 23 with malignancy | Cross-fold validation | LASSO logistic regression | Shape_Sphericity, GLCM_Autocorrelation | The proposed multiparametric model increased the accuracy of risk stratification compared to Bethesda system and PET/CT alone. |
| De Koster EJ | PET/CT | 20 | Evaluation of citologically indeterminate thyroid nodules | 99 without malignancy and 24 with malignancy | Cross-fold validation | Elastic net regression | / |
Radiomic analysis did not contribute to the additional differentiation of thyroid nodules. |
| Dondi F | PET/CT | 2 | Assessment of thyroid incidentalomas at PET/CT | 150 without malignancy and 71 with malignancy | Cross-fold validation | Logistic regression | GLCM-related features | Some radiomics features were able to predict with certain good accuracy the final diagnosis of incidentalomas. A good overlap in the extraction of these features between two different scanner was reported. |
| Other studies | ||||||||
| Ma L | SPECT | 1 | Classification of thyroid diseases at scintigraphy | 780 with Grave’s disease, 438 with Hashimoto disease, 810 with subacute thyroidits and 860 normal | Independent internal testing set | 8 different ANNs | / | The proposed network is efficient for the diagnosis of thyroid diseases with SPECT images. |
| Kavitha M | Planar whole-body | 1 | Recognition of metastatic lymph nodes in DTC | 2481 remnant tissue regions and 500 metastatic lymph nodes regions | Cross-fold validation | ANN | / | The proposed model offers excellent diagnostic performances for the assessment of metastatic lymph nodes. |
| Liu Y | SPECT | 1 | Classification of thyroid diseases at scintigraphy | 65 with hyperthyroidism, 43 with hypothyroidism and 28 normal | Independent internal testing set | 4 different ANNs | / |
The four deep learning models are efficient for the classification of thyroid diseases with SPECT images. |
| Currie G | Planar | ns | Classification of thyroid diseases at scintigraphy | 9 with hypothyroidism, 22 with Grave’s disease, 9 with multinodular goitres, 2 with nodular thyroids, 3 with goitres, 11 with reduced or absent uptake, 7 with autonomous glands with contralateral suppression, 24 with cold nodules, 8 with hot nodules and 28 normal | Independent internal testing set | ML and deep learning ANNs | / | The proposed ML algorithm can improve accuracy as second readers system. DL algorithms can be developed to improve accuracy in the absence of biochemistry results. |
| Guo Y | SPECT | 1 | Classify and diagnose the residual thyroid tissue after thyroidectomy | 346 with residual tissue and 100 without residual tissue | Independent internal testing set | ANN | / | The proposed method has good performances for the assessment of residual thyroid tissue. |
| Qiao T | Planar | 1 | Classification of thyroid diseases at scintigraphy | 175 with no thyroid disease, 834 with Grave’s disease and 421 with subacute thyroiditis | Independent internal testing set | 3 different ANNs | / | Deep learning models perform well in the diagnosis of Grave’s disease and subacute thyroiditis. |
| Yang P | Planar | 2 of the same model | Classification of thyroid diseases at scintigraphy | 1420 diffusely increased uptake, 1177 diffusely decreased uptake, 135 focal increased uptake and 657 heterogeneous uptake | Independent internal and external testing set | 4 different ANNs | / | The proposed AI model has good accuracy in the classification of thyroid disease . |
N.: number; FNAB: fine needle aspiration biopsy; DTC: differentiated thyroid cancer; MTC: medullary thyroid cancer; PRRT: peptide receptor radionuclide therapy; MTV: metabolic tumor volume; OS: overall survival; SUV: standardized uptake value; AI: artificial intelligence; ML: machine learning; RF: random forest; KNN: k nearest neighbor; DT: decision tree; LASSO: least absolute shrinkage and selection operator; 18 F-FDG: 18 F-fluorodeoxyglucose; ANN: artificial neural network; HF: heterogeneity factor; GLCM: grey level co-occurrence matrix; IV: intensity variability; SVZ: site-zone variability; ZP: zone%; GLRLMRLNU: gray-level run-length matrix run length non-uniformity
PET/CT studies
As mentioned, several studies evaluated different thyroid conditions by applying radiomics and ML to positron imaging [18–26, 34], demonstrating the possible role of such techniques in these areas of research. A list and a legend of the radomics features with best performances are presented in Table 3.
Table 3.
Legend of the radomics features with best performances
| Feature’s full name | Feature’s abbreviation used in the original work | Feature’s meaning |
|---|---|---|
| Heterogeneity factor | HF | Variation in glucose metabolism over the entire volume consiedered |
| Contrast | / | Measures the difference of the grey value when going to the next voxel |
| Entropy | / | A measure for the grade of derangement |
| Grey Level Non Uniformity | / | Measures the similarity of grey level values |
| High Grey Level Zone Emphasis | / | Measures the distribution of high grey level values |
| Intensity Variation | / | Describes the variation of the intensity of different substructures (zones) |
| Short Run Emphasis | / | Describes the distribution of runs |
| Short Run High Level Grey Emphasis | / | Measures the joint distribution of small runs and low grey level values |
| Short Zone High Grey Level Emphasis | / | Measures the joint distribution of short zones and high grey level values |
| Short Zone Low Grey Level Emphasis | / | Measures the joint distribution of small zones and low grey level values |
| Skewness | / | Represents the symmetry of distribution of grey levels |
| Kurtosis | / | Is a measure of peakedness in the grey level distribution |
| Correlation grey level co-occurrence – related | GLCM – related, CorrelationGLCM, GLCM_Autocorrelation | The GLCM is a matrix that expresses how combinations of discretised grey levels of neighbouring pixels are distributed along one of the image directions |
| Intensity variability | IV | Measures the similarity in pixel intensities throughout the image |
| Size-zone variability | SVZ | Measures the similarity in zone sizes |
| Zone% | ZP | Measures the homogeneity |
| Complexity | / |
Refers to the visual information content of a texture; a texture is considered complex if the information content is high |
| Contrast | / | Measures the intensity difference between neighboring regions |
| Gray-level run-length matrix run length non-uniformity | GLRLMRLNU | Assesses the distribution of runs over the run lengths |
| Shape_Sphericity | Depicts the geometric properties of the lesion in terms of sphericity |
Assessment of thyroid incidentalomas
One of the most explored field of application of radiomics in thyroid PET/CT imaging is the assessment of 18 F-FDG avid thyroid incidentalomas (TI). In this setting, the first research on this topic was proposed by Sollini et al. [19], reporting that some specific radiomics feature (RF) related to “Compacity” were significantly different between TIR categories and that “Skewness” was different between benign and malignant nodules. Moreover 3 RF (“Skewness”, “Kurtosis”, “CorrelationGLCM”) were selected, in addition to standardized uptake value (SUV)-related and volumetric parameters, as potential predictors with high sensitivity. In the same setting Aksu et al. [22] revealed that 21 RF were significantly different between malignant and benign nodules and “GLRLMRLNU” was reported as the feature with the best discriminating power with high specificity, positive predictive value (PPV) and negative predictive value (NPV). Moreover, the authors proposed a random forest model including this feature and SUVmax with good performances in the classification of TI (area under the curve [AUC] 0.849). More recently, Ceriani et al. [23] performed a similar analysis, including however the evaluation of the influence of different scanner on the extraction of RF. In this setting, only 54/107 RF were statistically reproducible between the two PET/CT scanner included in the study and “Shape_Sphericity” was reported as an affordable classificator. Furthermore, a predictive model with total lesion glycolysis (TLG), SUVmax and “Shape_Sphericity” was built by the authors. Similarly, Dondi et al. [26] evaluated the influence of different scanner on the extraction of RF and their ability to predict the final diagnosis of TI. In this setting, they reported that 9/42 RF had apparent correlation with the scanner used for their extraction, with cross-correlation maps that were quite similar between the two scanners. After bivariate analysis performed for single scanners and considering both of them together, none of the RF obtained an optimal AUC above 0.8 and, in general, higher AUCs value were visible on a particular scanner. Generally speaking, GLCM-related features were the ones with best perfomances.
Evaluation of citologically indeterminate thyroid nodules
Fine-needle aspiration biopsy is an accurate and essential method for the assessment of thyroid nodules, however in about 30% of the cases its results remain inconclusive or indeterminate. In this setting, Kim et al. [34] were the first to propose a paper to evaluate the predictive role of distributive 18 F-FDG heterogeneity to characterize such indeterminate nodules. Even if not characterized by a proper texture analysis, this work revealed that this parameter could be an affordable predictor. More recently, Giovanella et al. [24] revealed that “Shape_Sphericity” and “GLCM_Autocorrelation” were non redundant predictors for malignancy and a combination of the two features had an AUC of 0.733. Moreover, the authors performed different analysis considering only patients with non-Hürthle cell lesions and all the cohort of the study. In the first group, the two aforementioned RF were independently associated with higher risk of malignancy, with an accuracy for the identification of thyroid cancer of 75%, and an effective predictive model with such parameters was built. When considering all the cohort, the accuracy of the RF was 72% and the association with malignancy and the good performances of the model were confirmed. Lastly, De Koster et al. [25] performed a similar study including both Hürtle and non-Hürtle cell nodules and revealed that radiomics did not contribute to the additional differentiation of such nodules, compared to SUV-related parameters.
Thyroid cancer
PET/CT is an imaging tool that can be used for the assessment of the biological behavior of thyroid cancer. Particularly, 18 F-FDG PET can properly restage aggressive forms of DTC and medullary thyroid cancer (MTC) [35–36]. In this scenario Lapa et al. [18] investigated the prognostic value of textural parameters for the assessment of iodine refractory DTC or MTC treated with peptide receptor radionuclide therapy (PRRT). The authors reported a significant correlation for several RF with progression free survival (PFS) and in particular “Grey level non uniformity” was reported as the feature with best performance (AUC 0.930) even if other RF had higher AUCs values. Regarding overall survival (OS), non-significant prognostic RF were reported. Interestingly, in a per-lesion based analysis, only the parameter “Entropy” was able to predict the progression of the lesions (AUC 0.730).
Nakajo et al. [20] evaluated the role of radiomics, together with classical SUV-related and volumetric parameters of primary DTC, in the prediction of the risk of recurrence after total thyroidectomy. They reported that patients with high risk of recurrence had higher “IV” and “SVZ” and lower “ZP” values compared to non-high risk subjects and moreover this observation was confirmed in the group of patients with higher metabolic tumor volume (MTV). Furthermore, the same parameters had high AUCs values in the prediction of patients with high risk of recurrence, findings confirmed also in the group of patients with high MTV. Lastly, the authors developed a scoring system for the discrimination between high and non-high risk with a high accuracy.
The pretherapeutic role of radiomics in MTC subjects treated with tyrosine kinase inhibitor was evaluated by Werner et al. [21], reporting that a high value of “Complexity” was associated with a reduced OS and that a high value of “Contrast” was correlated with lower PFS; these parameters were also confirmed as affordable prognosticators at multivariate analysis.
Scintigraphic studies
As previously underlined, ML was also applied to scintigraphic studies in order to assess thyroid pathologies [27–33].
Classification of thyroid pathologies
First Ma et al. [27] developed a deep convolutional neural network (DCNN) in order to perform thyroid diagnosis based on SPECT images. This method revealed high performances in the differential diagnosis of Grave’s disease, Hashimoto’s thyroiditis and subacute thyroiditis; better performances compared to other method were demonstrated, with higher precision and less classification errors. Similar studies were also proposed by Qiao et al. [32] and Liu et al. [29] by proposing different DCNN models, revealing high performances for all of them, with AUCs ranging from 0.850 to 0.996. In this setting, an interesting work by Currie et al. [30] revealed that ML artificial neural network (ANN) were able to improve the accuracy of the evaluation of thyroid scintigraphy as second readers systems when biochemistry results were available and moreover, deep learning (DL) algorithms were developed to improve the accuracy in the absence of biochemistry results.
A dual center study with similar purpose was performed by Yang et al. [33] revealing that a specific DCNN model had the best performances, also confirmed at the external validation. In this setting, the pattern of “heterogeneous uptake” was the most likely to be misclassified and at the external validation this insight was experienced for the “focal increased” uptake pattern.
Miscellaneous
An interesting study was performed by Kavitha et al. [28] that applied (DL) on post-ablation 131I whole body scans in order to assess the presence of metastatic lymph node of DTC. The proposed method revealed the best performances in comparison with the manual detection for both the evaluation of metastatic lymph nodes and the recognition of thyroid remnant tissue. Interestingly, the performances of this method were similar with or without the application of post-processing and had better performances for the recognition of metastatic lymph nodes than physicians at SPECT images.
Lastly, Guo et al. [31] evaluated the role of a DCNN model for the classification and diagnosis of residual thyroid tissue at SPECT images, reporting higher performances in comparison to other computer aided diagnosis models with statistically significant differences in particular for sensitivity and accuracy.
Discussion
Radiomics is defined as the application of different tools for the extraction of quantitative imaging features that reflect the heterogeneity in an image [14, 37], while ML is the scientific discipline that focuses on how computers learn from data and identify some features that are believed to be important for making a final diagnosis [37‒39]. Generally speaking, their role for the assessment of thyroid diseases has been proved in several studies [18–24, 26–34]. Starting with PET/CT imaging, one of the most explored field of application of these technologies was the assessment of TI. In this setting, the studies included in the review [19, 22–23, 26] revealed the selection of some RF as predictor of the final diagnosis of such TIs, with good performances. Moreover, different predictive models with different RF were built and in general high performances for such differential diagnosis were demonstrated. Some attempts to compare different scanner for the assessment of these features and its influence on the final diagnosis were also performed.
Cytologically indeterminate thyroid nodules were also evaluated with radiomics in some studies [24–25, 34], that revealed how some RF were good predictors for the characterization of these nodules even when performing different analysis for Hürthle or non-Hürthle cell lesions. Interestingly, a single study [25] revealed that radiomics did not have a significant role in this field.
In this setting, it is important to mention that in clinical practice there is the option to perform molecular tests, that are useful to define the nature of such nodules. Furthermore, these tests allow the modification of the therapeutic recommendation based on an individualized approach; it has been reported that they could have the ability to rule out the presence of malignancy with great specificity and PPV, avoiding therefore the need to perform unnecessary surgery [39–41].
The role of radiomics analysis on PET/CT was also explored in the field of thyroid carcinomas [18, 20–21] revealing that, in case of iodine refractory DTC or MTC under PRRT therapy, some RF were significantly correlated with the prognosis [18]. Furthermore, some RF were reported as predictive of high risk of recurrence in DTC after total thyroid [20] and some of them were significant prognosticators for OS and PFS in MTC [21].
The role of ML has been also evaluated in scintigraphic imaging [27–33]. In this setting, most of the studies focused on the automatic classification of thyroid disease at scintigraphy applying different DCNN models, reporting in general high performances even in the case of dual center analysis [27, 32–33]. Lastly, good performances of ML models were also obtained when assessing the presence of metastatic lymph nodes and the classification of residual thyroid tissue in DTC patients [28, 31].
Even if our data suggest a role for radiomics and ML in thyroid diseases, these new diagnostic approaches need to be inserted and compared with the current clinical practice. In the case of TI, the high amount of thyroid nodules, the first steps of their evaluation comprehend the measurement of thyroid-stimulating hormone (TSH) levels and US evaluation of the thyroid and the cervical lymph nodes. In this setting, it is known that US is the gold standard for the assessment of thyroid nodules and is therefore mandatory to evaluate the presence of malignant features that will enable the use of fine needle aspiration (FNA), with an accuracy near 95% in determine the presence of malignancy [42–43]. In particular, hypoechoic echogenicity, solid composition, irregular margins, microcalcifications, height greater than width, extrathyroidal extension, disrupted rim calcification, and cervical lymph nodes with suspicious features are elements suspicious for the presence of malignancy. It is worth to underline the fact that our investigation was focused on TI discovered at 18 F-FDG PET/CT and in this scenario it has been reported that tracer uptake within a US confirmed thyroid nodule conveys an increased risk of thyroid cancer [44]. In these cases, radiomics could help in the identification of nodules with higher risk.
As mentioned, in the case of indeterminate thyroid nodules (approximately 25% of thyroid FNA samples are classified as Bethesda category 3 or 4), molecular tests can be used to assess the possible presence of malignancy, even if their role in guiding therapeutic decision-making is currently lacking. Moreover, the use of such tests should not be intended to replace other sources of information or clinical judgment [44]. Therefore, even though a benign pattern on molecular testing significantly decreases the risk of malignancy, US surveillance is still required [43]. Again, radiomics and ML could help to underline some features that, together with other information, could be useful to better define the risk of such nodules.
Speaking about patients affected by DTC, total-body scintigraphy with 131I, neck US and serum thyrogloblulin measurement are the cornerstones for their clinical follow-up, with high diagnostic accuracy and sensitivity, even in the case of thyroid remnants assessment [44]. However, we should underline that some patients could be classified in the “indeterminate” response group during follow-up and are therefore at risk of relapse [45]. In this setting, radiomics could give some important information able to better classify these patients and setup a specific follow-up.
Lastly, in the case of hyperthyrodidism, a clinical assessment of the patients with subsequent US evaluation, serum assessment of specific antibodies and hormones and scintigraphic evaluation are able to reach a specific diagnosis with proved elevated diagnostic accuracy [2]. The possible role of ML and radiomics in this field seem therefore marginal and wider and stronger studies need to be performed in order to strengthen the value of such diagnostic modalities in daily clinical setting.
Even if, as mentioned, radiomics and ML seems to have a role for the evaluation of thyroid diseases, not all that glitters is gold and many different points and limitations of these technologies and this review need to be underlined. First of all, the problem of repeatability and reproducibility of RF extraction and their subsequent analysis is well known and many efforts in this direction need to be performed in order to clarify this issue. In this setting, it is known that for PET images different scanners used for the acquisition, partial volume effect, reconstruction protocol, tumor segmentation and uptake time are able to affect the textural features extraction and ML [13–14, 26, 37, 38, 46–49]. Another important point that needs to be addressed is the fact that most of the studies included in the review lacked the presence of external validation that is mandatory to strengthen the result obtained in a single center and therefore to establish the clinical significance of radiomics and ML [38, 46–48]. Moreover, it is important to underline that the studies included in the review were performed including different features type and different analysis for their selection, which is an important limit in order to compare their results [37, 49]. Lastly, one of the most important limitations of most of the study evaluated in the review, is the fact that they were performed with limited cohort, which is a big limitation for the evaluation of radiomics potential.
Conclusion
In conclusion, radiomics and ML seem to have a promising role in the assessment of thyroid diseases. However, many open issues are still present in these fields of research and therefore these results need to be confirmed and standardized in other multicentric settings.
Funding
Open access funding provided by Università degli Studi di Brescia within the CRUI-CARE Agreement.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Dias Lopes NM, Mendonça Lens HH, Armani A, Marinello PC, Cecchini AL. Thyroid cancer and thyroid autoimmune disease: A review of molecular aspects and clinical outcomes. Pathol Res Pract. 2020 Sep;216(9):153098. doi: 10.1016/j.prp.2020.153098. Epub 2020 Jul 4. PMID: 32825964. [DOI] [PubMed]
- 2.Kravets I, Hyperthyroidism. Diagnosis and Treatment. Am Fam Physician. 2016 Mar 1;93(5):363 – 70. PMID: 26926973. [PubMed]
- 3.Russell MD, Orloff LA. Ultrasonography of the thyroid, parathyroids, and beyond. HNO. 2022 May;70(5):333–44. 10.1007/s00106-022-01162-0. Epub 2022 Apr 1. PMID: 35364686; PMCID: PMC8974803. [DOI] [PMC free article] [PubMed]
- 4.Giovanella L, Avram AM, Iakovou I, Kwak J, Lawson SA, Lulaj E, Luster M, Piccardo A, Schmidt M, Tulchinsky M, Verburg FA, Wolin E. EANM practice guideline/SNMMI procedure standard for RAIU and thyroid scintigraphy. Eur J Nucl Med Mol Imaging 2019 Nov;46(12):2514–25. doi: 10.1007/s00259-019-04472-8. Epub 2019 Aug 7. PMID: 31392371. [DOI] [PubMed]
- 5.Cistaro A, Quartuccio N, Garganese MC, Villani MF, Altini C, Pizzoferro M, Piccardo A, Cabria M, Massollo M, Maghnie M, Campennì A, Siracusa M, Baldari S, Panareo S, Urso L, Bartolomei M, De Palma D, Grossi A, Mazzoletti A, Dondi F, Bertagna F, Giubbini R, Albano D. Prognostic factors in children and adolescents with differentiated thyroid carcinoma treated with total thyroidectomy and RAI: a real-life multicentric study. Eur J Nucl Med Mol Imaging. 2022 Mar;49(4):1374–85. 10.1007/s00259-021-05586-8. Epub 2021 Oct 19. PMID: 34664092; PMCID: PMC8921094. [DOI] [PMC free article] [PubMed]
- 6.Albano D, Dondi F, Zilioli V, Panarotto MB, Galani A, Cappelli C, Bertagna F, Giubbini R, Casella C. The role of Hashimoto thyroiditis in predicting radioiodine ablation efficacy and prognosis of low to intermediate risk differentiated thyroid cancer. Ann Nucl Med. 2021 Oct;35(10):1089–99. 10.1007/s12149-021-01644-1. Epub 2021 Jun 21. PMID: 34152569; PMCID: PMC8408084. [DOI] [PMC free article] [PubMed]
- 7.Albano D, Tulchinsky M, Dondi F, Mazzoletti A, Lombardi D, Bertagna F, Giubbini R. Thyroglobulin doubling time offers a better threshold than thyroglobulin level for selecting optimal candidates to undergo localizing [18F]FDG PET/CT in non-iodine avid differentiated thyroid carcinoma. Eur J Nucl Med Mol Imaging. 2021 Feb;48(2):461–8. Epub 2020 Aug 13. PMID: 32794104; PMCID: PMC7835169. [DOI] [PMC free article] [PubMed]
- 8.Albano D, Tulchinsky M, Dondi F, Mazzoletti A, Bertagna F, Giubbini R. The role of Tg kinetics in predicting 2-[18F]-FDG PET/CT results and overall survival in patients affected by differentiated thyroid carcinoma with detectable Tg and negative 131I-scan. Endocrine. 2021 Nov;74(2):332–339. doi: 10.1007/s12020-021-02755-5. Epub 2021 May 20. PMID: 34014437; PMCID: PMC8497300. [DOI] [PMC free article] [PubMed]
- 9.Albano D, Dondi F, Mazzoletti A, Bellini P, Rodella C, Bertagna F. Prognostic role of 2-[18F]FDG PET/CT metabolic volume parameters in patients affected by differentiated thyroid carcinoma with high Thyroglobulin Level, negative 131I WBS and positive 2-[18F]-FDG PET/CT. Diagnostics (Basel). 2021 Nov 25;11(12):2189. doi: 10.3390/diagnostics11122189. PMID: 34943426; PMCID: PMC8700137. [DOI] [PMC free article] [PubMed]
- 10.Rizzo A, Racca M, Dall’Armellina S, Delgado Bolton RC, Albano D, Dondi F, Bertagna F, Annunziata S, Treglia G. Potential Role of PSMA-Targeted PET in Thyroid Malignant Disease: A Systematic Review. Diagnostics (Basel). 2023 Feb 3;13(3):564. doi: 10.3390/diagnostics13030564. PMID: 36766670; PMCID: PMC9914698. [DOI] [PMC free article] [PubMed]
- 11.Dittmann M, Gonzalez Carvalho JM, Rahbar K, Schäfers M, Claesener M, Riemann B, Seifert R. Incremental diagnostic value of [18F]tetrafluoroborate PET-CT compared to [131I]iodine scintigraphy in recurrent differentiated thyroid cancer. Eur J Nucl Med Mol Imaging. 2020 Oct;47(11):2639–46. 10.1007/s00259-020-04727-9. Epub 2020 Apr 4. PMID: 32248325; PMCID: PMC7515952. [DOI] [PMC free article] [PubMed]
- 12.Fu H, Wu J, Huang J, Sun L, Wu H, Guo W, Qiu S, Chen H. 68Ga fibroblast activation protein inhibitor PET/CT in the detection of metastatic thyroid Cancer: comparison with 18F-FDG PET/CT. Radiology. 2022 Aug;304(2):397–405. doi: 10.1148/radiol.212430. Epub 2022 May 10. PMID: 35536131. [DOI] [PubMed]
- 13.Dondi F, Gatta R, Albano D, Bellini P, Camoni L, Treglia G, Bertagna F. Role of Radiomics features and machine learning for the histological classification of stage I and Stage II NSCLC at [18F]FDG PET/CT: a comparison between two PET/CT scanners. J Clin Med 2022 Dec 29;12(1):255. doi: 10.3390/jcm12010255. PMID: 36615053; PMCID: PMC9820870. [DOI] [PMC free article] [PubMed]
- 14.Albano D, Gatta R, Marini M, Rodella C, Camoni L, Dondi F, Giubbini R, Bertagna F. Role of 18F-FDG PET/CT Radiomics features in the Differential diagnosis of Solitary Pulmonary Nodules: diagnostic accuracy and comparison between two different PET/CT scanners. J Clin Med 2021 Oct 29;10(21):5064. doi: 10.3390/jcm10215064. PMID: 34768584; PMCID: PMC8584460. [DOI] [PMC free article] [PubMed]
- 15.Choi RY, Coyner AS, Kalpathy-Cramer J, Chiang MF, Campbell JP. Introduction to Machine Learning, Neural Networks, and Deep Learning. Transl Vis Sci Technol. 2020 Feb 27;9(2):14. doi: 10.1167/tvst.9.2.14. PMID: 32704420; PMCID: PMC7347027. [DOI] [PMC free article] [PubMed]
- 16.Deo RC. Machine Learning in Medicine. Circulation. 2015 Nov 17;132(20):1920-30. doi: 10.1161/CIRCULATIONAHA.115.001593. PMID: 26572668; PMCID: PMC5831252. [DOI] [PMC free article] [PubMed]
- 17.Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, Leeflang MM, Sterne JA, Bossuyt PM, QUADAS-2 Group. ;. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011 Oct 18;155(8):529 – 36. doi: 10.7326/0003-4819-155-8-201110180-00009. PMID: 22007046. [DOI] [PubMed]
- 18.Lapa C, Werner RA, Schmid JS, Papp L, Zsótér N, Biko J, Reiners C, Herrmann K, Buck AK, Bundschuh RA. Prognostic value of positron emission tomography-assessed tumor heterogeneity in patients with thyroid cancer undergoing treatment with radiopeptide therapy. Nucl Med Biol. 2015 Apr;42(4):349–54. Epub 2014 Dec 20. PMID: 25595135. [DOI] [PubMed]
- 19.Sollini M, Cozzi L, Pepe G, Antunovic L, Lania A, Di Tommaso L, Magnoni P, Erba PA, Kirienko M. [18F]FDG-PET/CT texture analysis in thyroid incidentalomas: preliminary results. Eur J Hybrid Imaging. 2017;1(1):3. doi: 10.1186/s41824-017-0009-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nakajo M, Jinguji M, Shinaji T, Tani A, Nakabeppu Y, Nakajo M, Nakajo A, Natsugoe S, Yoshiura T. 18F-FDG-PET/CT features of primary tumours for predicting the risk of recurrence in thyroid cancer after total thyroidectomy: potential usefulness of combination of the SUV-related, volumetric, and heterogeneous texture parameters. Br J Radiol. 2019 Feb;92(1094):20180620. 10.1259/bjr.20180620. Epub 2018 Nov 7. PMID: 30273012; PMCID: PMC6404810. [DOI] [PMC free article] [PubMed]
- 21.Werner RA, Bundschuh RA, Higuchi T, Javadi MS, Rowe SP, Zsótér N, Kroiss M, Fassnacht M, Buck AK, Kreissl MC, Lapa C. Volumetric and texture analysis of pretherapeutic 18F-FDG PET can predict overall survival in medullary thyroid cancer patients treated with Vandetanib. Endocrine. 2019 Feb;63(2):293–300. Epub 2018 Sep 11. PMID: 30206772; PMCID: PMC6394453. [DOI] [PMC free article] [PubMed]
- 22.Aksu A, Karahan Şen NP, Acar E, Çapa Kaya G. Evaluating focal 18F-FDG uptake in thyroid gland with Radiomics. Nucl Med Mol Imaging. 2020 Oct;54(5):241–8. 10.1007/s13139-020-00659-2. Epub 2020 Jul 28. PMID: 33088353; PMCID: PMC7560675. [DOI] [PMC free article] [PubMed]
- 23.Ceriani L, Milan L, Virili C, Cascione L, Paone G, Trimboli P, Giovanella L. Thyroid. 2021 Jan;31(1):88–95. Epub 2020 Jul 6. PMID: 32517585. Radiomics Analysis of [18F]-Fluorodeoxyglucose-Avid Thyroid Incidentalomas Improves Risk Stratification and Selection for Clinical Assessment. [DOI] [PubMed]
- 24.Giovanella L, Milan L, Piccardo A, Bottoni G, Cuzzocrea M, Paone G, Ceriani L. Radiomics analysis improves 18FDG PET/CT-based risk stratification of cytologically indeterminate thyroid nodules. Endocr 2022 Jan;75(1):202–10. doi: 10.1007/s12020-021-02856-1. Epub 2021 Sep 1. PMID: 34468949; PMCID: PMC8763930. [DOI] [PMC free article] [PubMed]
- 25.de Koster EJ, Noortman WA, Mostert JM, Booij J, Brouwer CB, de Keizer B, de Klerk JMH, Oyen WJG, van Velden FHP, de Geus-Oei LF, Vriens D. ; EfFECTS trial study group. Quantitative classification and radiomics of [18F]FDG-PET/CT in indeterminate thyroid nodules. Eur J Nucl Med Mol Imaging 2022 Jun;49(7):2174–88. doi: 10.1007/s00259-022-05712-0. Epub 2022 Feb 9. PMID: 35138444; PMCID: PMC9165273. [DOI] [PMC free article] [PubMed]
- 26.Dondi F, Pasinetti N, Gatta R, Albano D, Giubbini R, Bertagna F. Comparison between Two Different Scanners for the Evaluation of the Role of 18F-FDG PET/CT Semiquantitative Parameters and Radiomics Features in the Prediction of Final Diagnosis of Thyroid Incidentalomas. J Clin Med. 2022 Jan 26;11(3):615. doi: 10.3390/jcm11030615. PMID: 35160067; PMCID: PMC8836668. [DOI] [PMC free article] [PubMed]
- 27.Ma L, Ma C, Liu Y, Wang X. Thyroid diagnosis from SPECT images using convolutional neural network with optimization. Comput Intell Neurosci 2019 Jan 15;2019:6212759. doi: 10.1155/2019/6212759. PMID: 30766599; PMCID: PMC6350547. [DOI] [PMC free article] [PubMed]
- 28.Kavitha M, Lee CH, Shibudas K, Kurita T, Ahn BC. Deep learning enables automated localization of the metastatic lymph node for thyroid cancer on 131I post-ablation whole-body planar scans. Sci Rep. 2020 May 8;10(1):7738. doi: 10.1038/s41598-020-64455-w. Erratum in: Sci Rep. 2020 Jul 9;10(1):11579. PMID: 32385375; PMCID: PMC7211007. [DOI] [PMC free article] [PubMed]
- 29.Liu Y, Xu Y, Meng X, Wang X, Bai T. A Study on the Auxiliary Diagnosis of Thyroid Disease Images Based on Multiple Dimensional Deep Learning Algorithms. Curr Med Imaging. 2020;16(3):199–205. doi: 10.2174/1573405615666190115155223. PMID: 32133949. [DOI] [PubMed]
- 30.Currie GM, Iqbal BM. Re-modelling 99m-Technetium pertechnetate thyroid uptake; Statistical, Machine Learning and Deep Learning Approaches. J Nucl Med Technol 2021 Dec 7:jnmt.121.263081. doi: 10.2967/jnmt.121.263081. Epub ahead of print. PMID: 34876477. [DOI] [PubMed]
- 31.Guo Y, Xu J, Li X, Zheng L, Pan W, Qiu M, Mao S, Huang D, Yang X. Classification and diagnosis of residual thyroid tissue in SPECT images based on fine-tuning deep convolutional neural network. Front Oncol 2021 Oct 28;11:762643. doi: 10.3389/fonc.2021.762643. PMID: 34778083; PMCID: PMC8581297. [DOI] [PMC free article] [PubMed]
- 32.Qiao T, Liu S, Cui Z, Yu X, Cai H, Zhang H, Sun M, Lv Z, Li D. Deep learning for intelligent diagnosis in thyroid scintigraphy. J Int Med Res 2021 Jan;49(1):300060520982842. doi: 10.1177/0300060520982842. PMID: 33445994; PMCID: PMC7812409. [DOI] [PMC free article] [PubMed]
- 33.Yang Y, Zheng B, Li Y, Li Y, Ma X. Computer-aided diagnostic models to classify lymph node metastasis and lymphoma involvement in enlarged cervical lymph nodes using PET/CT. Med Phys. 2023 Jan;50(1):152–62. 10.1002/mp.15901. Epub 2022 Aug 17. PMID: 35925871. [DOI] [PubMed]
- 34.Kim SJ, Chang S. Predictive value of intratumoral heterogeneity of F-18 FDG uptake for characterization of thyroid nodules according to Bethesda categories of fine needle aspiration biopsy results. Endocrine. 2015 Dec;50(3):681–8. 10.1007/s12020-015-0620-z. Epub 2015 May 7. PMID: 25948075. [DOI] [PubMed]
- 35.Treglia G, Rufini V, Piccardo A, Imperiale A. Update on Management of Medullary Thyroid Carcinoma: Focus on Nuclear Medicine. Semin Nucl Med. 2023 Jan 24:S0001-2998(23)00003-X. doi: 10.1053/j.semnuclmed.2023.01.003. Epub ahead of print. PMID: 36702731. [DOI] [PubMed]
- 36.Piccardo A, Trimboli P, Foppiani L, Treglia G, Ferrarazzo G, Massollo M, Bottoni G, Giovanella L. PET/CT in thyroid nodule and differentiated thyroid cancer patients. The evidence-based state of the art. Rev Endocr Metab Disord. 2019 Mar;20(1):47–64. doi: 10.1007/s11154-019-09491-2. PMID: 30900067. [DOI] [PubMed]
- 37.Sollini M, Cozzi L, Chiti A, Kirienko M. Texture analysis and machine learning to characterize suspected thyroid nodules and differentiated thyroid cancer: where do we stand? Eur J Radiol. 2018 Feb;99:1–8. 10.1016/j.ejrad.2017.12.004. Epub 2017 Dec 7. PMID: 29362138. [DOI] [PubMed]
- 38.Deo RC. Machine Learning in Medicine. Circulation. 2015 Nov 17;132(20):1920-30. 10.1161/CIRCULATIONAHA.115.001593. PMID: 26572668; PMCID: PMC5831252. [DOI] [PMC free article] [PubMed]
- 39.Lee JW, Lee SM. Radiomics in Oncological PET/CT: clinical applications. Nucl Med Mol Imaging. 2018 Jun;52(3):170–89. 10.1007/s13139-017-0500-y. Epub 2017 Oct 20. PMID: 29942396; PMCID: PMC5995782. [DOI] [PMC free article] [PubMed]
- 40.Roth MY, Witt RL, Steward DL. Molecular testing for thyroid nodules: Review and current state. Cancer. 2018 Mar 1;124(5):888–898. doi: 10.1002/cncr.30708. Epub 2017 Dec 26. PMID: 29278433. [DOI] [PubMed]
- 41.Livhits MJ, Zhu CY, Kuo EJ, Nguyen DT, Kim J, Tseng CH, Leung AM, Rao J, Levin M, Douek ML, Beckett KR, Cheung DS, Gofnung YA, Smooke-Praw S, Yeh MW. Effectiveness of Molecular Testing Techniques for Diagnosis of Indeterminate Thyroid Nodules: A Randomized Clinical Trial. JAMA Oncol. 2021 Jan 1;7(1):70–77. doi: 10.1001/jamaoncol.2020.5935. PMID: 33300952; PMCID: PMC7729582. [DOI] [PMC free article] [PubMed]
- 42.Bose S, Sacks W, Walts AE. Update on Molecular Testing for Cytologically Indeterminate Thyroid Nodules. Adv Anat Pathol. 2019 Mar;26(2):114–123. doi: 10.1097/PAP.0000000000000211. PMID: 30664001. [DOI] [PubMed]
- 43.Burguera B, Gharib H. Thyroid incidentalomas. Prevalence, diagnosis, significance, and management. Endocrinol Metab Clin North Am. 2000 Mar;29(1):187–203. doi: 10.1016/s0889-8529(05)70123-7. PMID: 10732271. [DOI] [PubMed]
- 44.Kant R, Davis A, Verma V. Thyroid Nodules: Advances in Evaluation and Management. Am Fam Physician. 2020 Sep 1;102(5):298–304. PMID: 32866364. [PubMed]
- 45.Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, Pacini F, Randolph GW, Sawka AM, Schlumberger M, Schuff KG, Sherman SI, Sosa JA, Steward DL, Tuttle RM, Wartofsky L. 2015 american thyroid Association Management Guidelines for adult patients with thyroid nodules and differentiated thyroid Cancer: the american thyroid Association Guidelines Task Force on thyroid nodules and differentiated thyroid Cancer. Thyroid. 2016 Jan;26(1):1–133. 10.1089/thy.2015.0020. PMID: 26462967; PMCID: PMC4739132. [DOI] [PMC free article] [PubMed]
- 46.Albano D, Bellini P, Dondi F, Calabrò A, Casella C, Taboni S, Lombardi D, Treglia G, Bertagna F. Temporal Evolution and Prognostic Role of Indeterminate Response Sub-Groups in Patients with Differentiated Thyroid Cancer after Initial Therapy with Radioiodine. Cancers (Basel). 2023 Feb 16;15(4):1270. doi: 10.3390/cancers15041270. PMID: 36831612; PMCID: PMC9954717. [DOI] [PMC free article] [PubMed]
- 47.Gul M, Bonjoc KC, Gorlin D, Wong CW, Salem A, La V, Filippov A, Chaudhry A, Imam MH, Chaudhry AA. Diagnostic utility of Radiomics in thyroid and Head and Neck cancers. Front Oncol 2021 Jul 7;11:639326. doi: 10.3389/fonc.2021.639326. PMID: 34307123; PMCID: PMC8293690. [DOI] [PMC free article] [PubMed]
- 48.Cao Y, Zhong X, Diao W, Mu J, Cheng Y, Jia Z. Radiomics in Differentiated Thyroid Cancer and Nodules: Explorations, Application, and Limitations. Cancers (Basel). 2021 May 18;13(10):2436. doi: 10.3390/cancers13102436. PMID: 34069887; PMCID: PMC8157383. [DOI] [PMC free article] [PubMed]
- 49.Gherghe M, Lazar AM, Mutuleanu MD, Stanciu AE, Martin S. Radiomics Analysis of [18F]FDG PET/CT Thyroid Incidentalomas: How Can It Improve Patients’ Clinical Management? A Systematic Review from the Literature. Diagnostics (Basel). 2022 Feb 12;12(2):471. doi: 10.3390/diagnostics12020471. PMID: 35204561; PMCID: PMC8870948. [DOI] [PMC free article] [PubMed]
- 50.Morland D, Triumbari EKA, Boldrini L, Gatta R, Pizzuto D, Annunziata S. Radiomics in Oncological PET Imaging: A Systematic Review-Part 1, Supradiaphragmatic Cancers. Diagnostics (Basel). 2022 May 27;12(6):1329. doi: 10.3390/diagnostics12061329. PMID: 35741138; PMCID: PMC9221970. [DOI] [PMC free article] [PubMed]


