Abstract
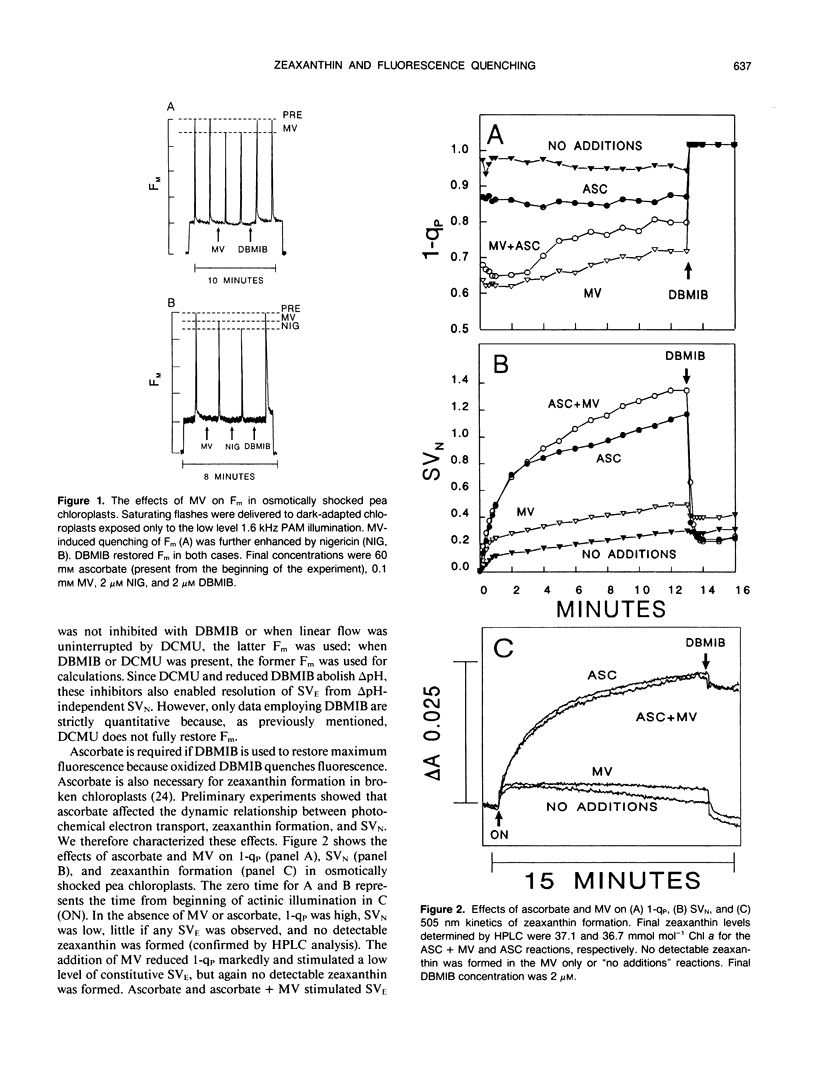
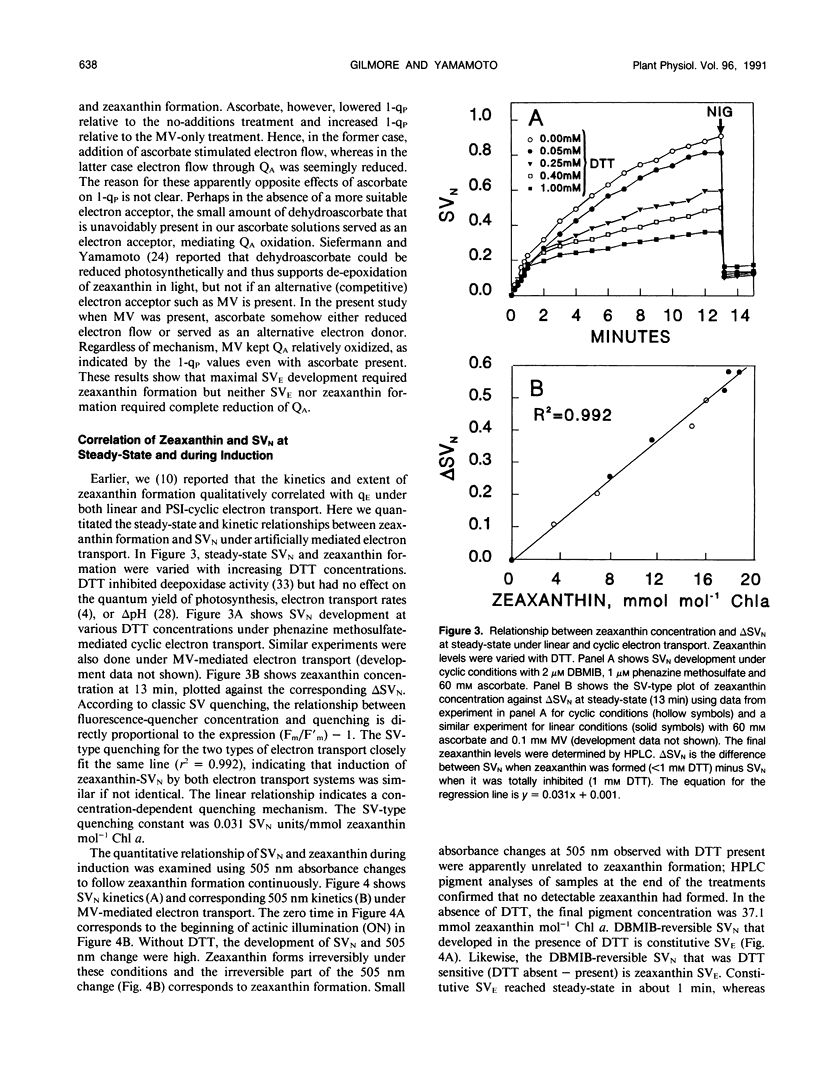
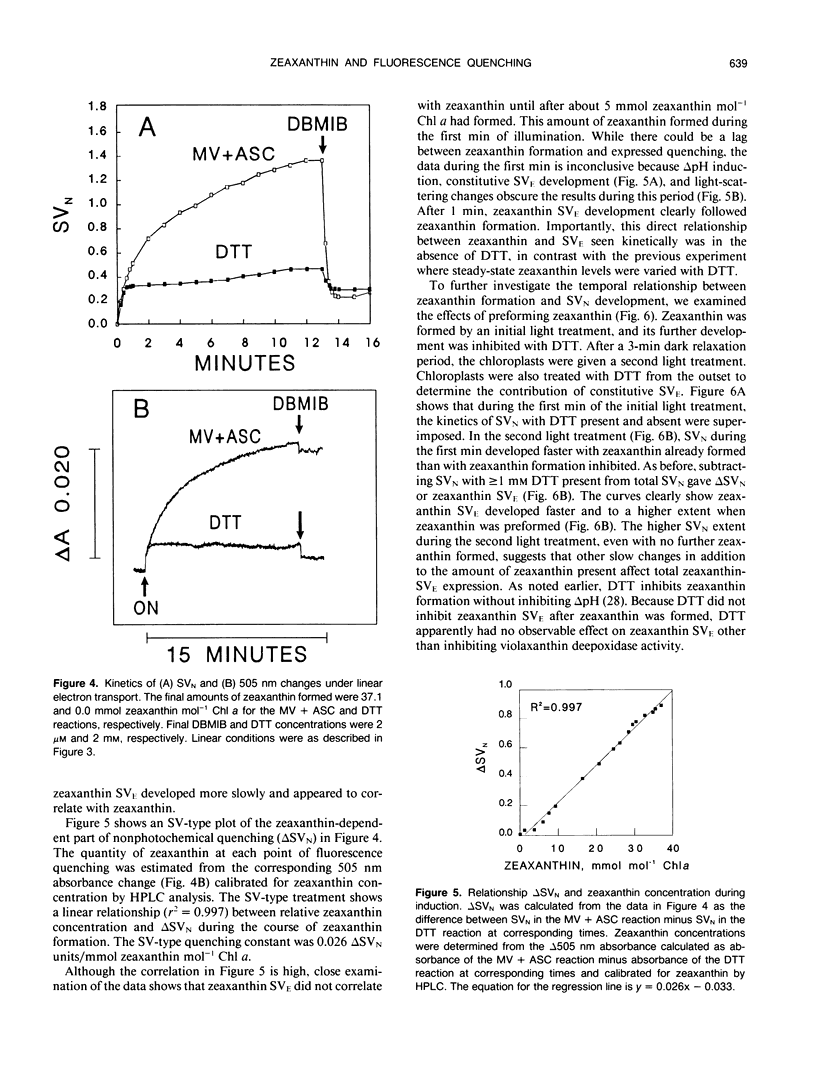
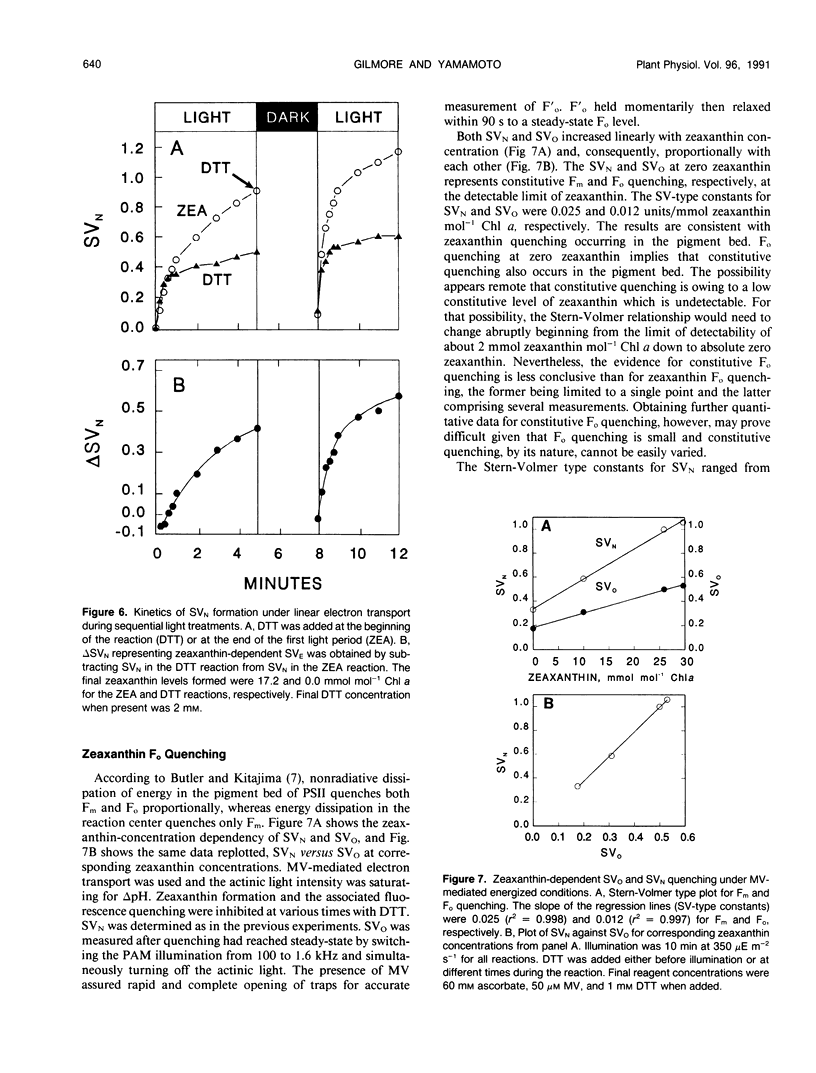
Artificially mediated linear (methylviologen) and cyclic (phenazine methosulfate) electron transport induced zeaxanthin-dependent and independent (constitutive) nonphotochemical quenching in osmotically shocked chloroplasts of pea (Pisum sativum L. cv Oregon). Nonphotochemical quenching was quantitated as Stern-Volmer quenching (SVN) calculated as (Fm/F′m)-1 where Fm is the fluorescence intensity with all PSII reaction centers closed in a nonenergized, dark-adapted state and F′m is the fluorescence intensity with all PSII reaction centers closed in an energized state. Reversal of quenching by nigericin and electron-transport inhibitors showed that both quenching types were energy-dependent SVN. Under light-induced saturating ΔpH, constitutive-SVN reached steady-state in about 1 minute whereas zeaxanthin-SVN continued to develop for several minutes in parallel with the slow kinetics of violaxanthin deepoxidation. SVN above the constitutive level and relative zeaxanthin concentration showed high linear correlations at steady-state and during induction. Furthermore, Fo quenching, also treated as Stern-Volmer quenching (SVO) and calculated as (Fo/F′o)-1, showed high correlation with zeaxanthin and consequently with SVN (Fo and F′o are fluorescence intensities with all PSII reaction centers in nonenergized and energized states, respectively). These results support the view that zeaxanthin increases SVN above the constitutive level in a concentration-dependent manner and that zeaxanthin-dependent SVN occurs in the pigment bed. Preforming zeaxanthin increased the rate and extent of SVN, indicating that slow events other than the amount of zeaxanthin also affect final zeaxanthin-SVN expression. The redox state of the primary electron acceptor of photosystem II did not appear to determine SVN. Antimycin, when added while chloroplasts were in a dark-adapted or nonenergized state, inhibited both zeaxanthin-SVN and constitutive-SVN induced by linear and cyclic electron transport. These similarities, including possible constitutive Fo quenching, suggest that zeaxanthin-dependent and constitutive SVN are mechanistically related.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams W. W., Demmig-Adams B., Winter K. Relative contributions of zeaxanthin-related and zeaxanthin-unrelated types of ;high-energy-state' quenching of chlorophyll fluorescence in spinach leaves exposed to various environmental conditions. Plant Physiol. 1990 Feb;92(2):302–309. doi: 10.1104/pp.92.2.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilger W., Björkman O., Thayer S. S. Light-induced spectral absorbance changes in relation to photosynthesis and the epoxidation state of xanthophyll cycle components in cotton leaves. Plant Physiol. 1989 Oct;91(2):542–551. doi: 10.1104/pp.91.2.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradbury M., Baker N. R. Analysis of the slow phases of the in vivo chlorophyll fluorescence induction curve. Changes in the redox state of photosystem II electron acceptors and fluorescence emission from photosystems I and II. Biochim Biophys Acta. 1981 May 13;635(3):542–551. doi: 10.1016/0005-2728(81)90113-4. [DOI] [PubMed] [Google Scholar]
- Butler W. L., Kitajima M. Fluorescence quenching in photosystem II of chloroplasts. Biochim Biophys Acta. 1975 Jan 31;376(1):116–125. doi: 10.1016/0005-2728(75)90210-8. [DOI] [PubMed] [Google Scholar]
- Demmig-Adams B., Adams W. W., Heber U., Neimanis S., Winter K., Krüger A., Czygan F. C., Bilger W., Björkman O. Inhibition of zeaxanthin formation and of rapid changes in radiationless energy dissipation by dithiothreitol in spinach leaves and chloroplasts. Plant Physiol. 1990 Feb;92(2):293–301. doi: 10.1104/pp.92.2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demmig B., Winter K., Krüger A., Czygan F. C. Zeaxanthin and the Heat Dissipation of Excess Light Energy in Nerium oleander Exposed to a Combination of High Light and Water Stress. Plant Physiol. 1988 May;87(1):17–24. doi: 10.1104/pp.87.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton P., Black M. T. Light-dependent quenching of chlorophyll fluorescence in pea chloroplasts induced by adenosine 5'-triphosphate. Biochim Biophys Acta. 1981 Mar 12;635(1):53–62. doi: 10.1016/0005-2728(81)90006-2. [DOI] [PubMed] [Google Scholar]
- Mills J., Barber J. Energy-dependent cation-induced control of chlorophyll a fluorescence in isolated intact chloroplasts. Arch Biochem Biophys. 1975 Sep;170(1):306–314. doi: 10.1016/0003-9861(75)90122-8. [DOI] [PubMed] [Google Scholar]
- Siefermann-Harms D. The accumulation of neutral red in illuminated thylakoids. Biochim Biophys Acta. 1978 Nov 9;504(2):265–277. doi: 10.1016/0005-2728(78)90175-5. [DOI] [PubMed] [Google Scholar]
- Siefermann D., Yamamoto H. Y. Light-induced de-epoxidation of violaxanthin in lettuce chloroplasts. IV. The effects of electron-transport conditions on violaxanthin availability. Biochim Biophys Acta. 1975 Apr 14;387(1):149–158. doi: 10.1016/0005-2728(75)90059-6. [DOI] [PubMed] [Google Scholar]
- Slater E. C. The mechanism of action of the respiratory inhibitor, antimycin. Biochim Biophys Acta. 1973 Dec 7;301(2):129–154. doi: 10.1016/0304-4173(73)90002-5. [DOI] [PubMed] [Google Scholar]
- Sokolove P. M., Marsho T. V. Ascorbate-independent carotenoid de-epoxidation in intact spinach chloroplasts. Biochim Biophys Acta. 1976 May 14;430(2):321–326. doi: 10.1016/0005-2728(76)90088-8. [DOI] [PubMed] [Google Scholar]
- Vernotte C., Etienne A. L., Briantais J. M. Quenching of the system II chlorophyll fluorescence by the plastoquinone pool. Biochim Biophys Acta. 1979 Mar 15;545(3):519–527. doi: 10.1016/0005-2728(79)90160-9. [DOI] [PubMed] [Google Scholar]
- YAMAMOTO H. Y., NAKAYAMA T. O., CHICHESTER C. O. Studies on the light and dark interconversions of leaf xanthophylls. Arch Biochem Biophys. 1962 Apr;97:168–173. doi: 10.1016/0003-9861(62)90060-7. [DOI] [PubMed] [Google Scholar]
- Yamamoto H. Y., Kamite L. The effects of dithiothreitol on violaxanthin de-epoxidation and absorbance changes in the 500-nm region. Biochim Biophys Acta. 1972 Jun 23;267(3):538–543. doi: 10.1016/0005-2728(72)90182-x. [DOI] [PubMed] [Google Scholar]


