Abstract
Antibody to pneumococcal surface protein A (PspA) has been shown to be protective for Streptococcus pneumoniae infections in mice. In an attempt to define a model for inducing protective antibody to PspA in the absence of adjuvant, we designed two genetic fusions, PspA–interleukin-2 [IL-2]) and PspA–granulocyte-macrophage colony-stimulating factor (GM-CSF). These constructs maintained high cytokine function in vitro, as tested by their activity on IL-2 or GM-CSF-dependent cell lines. While intranasal immunization with PspA induced no detectable anti-PspA response, both PspA–IL-2 and PspA–GM-CSF stimulated high immunoglobulin G1 (IgG1) antibody responses. Interestingly, only the PspA–IL-2, not the PspA–GM-CSF, construct stimulated IgG2a antibody responses, suggesting that this construct directed the response along a TH1-dependent pathway. Comparable enhancement of the anti-PspA response with similar isotype profiles was observed after subcutaneous immunization as well. The enhancement observed with PspA–IL-2 was dependent on IL-2 activity in that it was not seen in IL-2 receptor knockout mice, while PspA in alum induced high-titer antibody in these mice. The antibody was tested for its protective activity in a mouse lethality model using S. pneumoniae WU-R2. Passive transfer of 1:90 dilutions of sera from mice immunized with PspA–IL-2 and PspA–GM-CSF elicited protection of CBA/N mice against intravenous challenge with over 170 50% lethal doses of capsular type 3 strain WU2. Only 0.17 μg or less of IgG antibody to PspA was able to provide passive protection against otherwise fatal challenge with S. pneumoniae. The data demonstrate that designing protein-cytokine fusions may be a useful approach for mucosal immunization and can induce high-titer systemic protective antibody responses.
The use of cytokines as agents to enhance both humoral and cell-mediated responses has been proven to be effective in many different experimental systems. Whether these stimulatory molecules operate specifically at the level of the antigen-specific T or B cell and/or whether they act in a more nonspecific way by activating other cell types which may themselves enhance immune responses is unresolved. A number of investigators have demonstrated that the injection of cytokines when simply admixed with antigen results in enhanced immune responses (8, 19, 27, 30). In addition to their role as enhancers of immune responses, cytokines can also influence the isotype of antibody that is induced by influencing the balance of TH1- and TH2-mediated help (5, 20). One could thus specifically tailor the profile of the immune response that is stimulated depending on the cytokine that is used.
More recently, it has been shown that coinjection of a plasmid encoding granulocyte-macrophage colony-stimulating factor (GM-CSF), interleukin-2 (IL-2), or IL-12 can enhance humoral as well as cell-mediated responses to a simultaneously injected plasmid encoding a soluble protein (10, 31, 34). Other investigators have shown that for cytokines to be effective, they must be physically linked to the antigen. Thus, Tao and Levy (29) and Chen et al. (4) demonstrated that coinjection of IL-2 or GM-CSF with antigens had little, if any, enhancing effect on the immune response, and enhancement was observed only after direct linkage of the cytokine to the antigen. Another possible advantage of directly linking cytokine to protein is that it can extend the half-life of the cytokine in the circulation and thus prolong its stimulatory effect (11).
We wished to explore a role for protein-cytokine fusion in stimulating humoral antibody responses when administered mucosally as well as their role in inducing protective antibody responses for subsequent bacterial challenge. These two avenues have not yet been examined by others. To this end, we used pneumococcal surface protein (PspA) as a model antigen. This protein is a surface-exposed virulence factor of Streptococcus pneumoniae that can elicit protective antibody in mice (15, 17). PspA is approximately 590 amino acids in size (65 kDa). The N-terminal, approximately 290 amino acids are surface exposed and predict an α-helical coiled structure. The protection eliciting epitopes of PspA map to this α-helical domain. C terminal to the α-helical region is a proline-rich region and a choline-binding domain. The latter is responsible for the binding of PspA to the pneumococcal surface (16, 28, 31, 33). While native PspA is highly immunogenic in the absence of adjuvant (2), it is difficult to prepare in large quantities in purified form. The 245-amino-acid fragment of PspA induces protection but only when administered with an adjuvant such as complete Freund adjuvant or cholera toxin (CT) (33). When mice are immunized intranasally (i.n.) or orally with moderate doses of native PspA, little or no anti-PspA antibody response is stimulated unless it is coadministered with CT, or the B subunit of CT. When given with CT, the majority of total immunoglobulin (Ig) that is secreted is directed to the CT rather than to PspA. It would be useful to find ways to enhance anti-PspA responses without diverting a significant part of the antibody response to an antigen that is merely a bystander. We have found that in vitro, both IL-2 and GM-CSF can enhance Ig secretion by purified B cells over 100-fold (26). We therefore made constructs of IL-2 and GM-CSF with PspA and studied both the magnitude and isotype of antibody responses stimulated after i.n. and subcutaneous (s.c.) injection, as well as their protective efficacy after challenge with a lethal strain of S. pneumoniae. These studies indicate that protein-cytokine fusions can rapidly enhance antiprotein responses after both i.n. and s.c. injections and influence the isotype of antibody that is made and that this antibody is extremely protective. This is the first report of cytokine fusions being administered i.n. and inducing protective antibody responses.
MATERIALS AND METHODS
Mice.
Six- to eight-week-old DBA/2, C3H/HEJ, and C3H/HEN mice were housed in the pathogen-free facilities at the Uniformed Services University of the Health Sciences. CBA/CAHN-XID/J (CBA/N) mice were purchased from Jackson Laboratory, Bar Harbor, Maine.
Cell lines.
The IL-2-dependent CTEV cell line and the IL-3-dependent line 32DGR2 (Jacalyn H. Pierce, National Institutes of Health) were used to measure the cytokine activity of the recombinant proteins. 32DGR2 cells were converted to GM-CSF dependence by the gradual reduction of IL-3 in the media and the addition of GM-CSF (data not shown). Cells were cultured in RPMI 1640 medium (RPMI 1640, l-glutamine, 10% fetal calf serum, penicillin-streptomycin [100 U/ml], sodium pyruvate [1 mM], and 2-mercaptoethanol (50 μM]) with the addition of 20 U of recombinant human IL-2 or recombinant murine GM-CSF per ml to the respective cell lines. Cells were cultured at 37°C and 5% CO2.
Bacteria.
Escherichia coli DH10B (Gibco/BRL, Gaithersburg, Md.) was used for plasmid construction. Cells were cultured in Terrific Broth (12 g of Bacto Tryptone, 24 g of Bacto Yeast Extract, 4 ml of glycerol, and 100 ml of 0.17 M KH2PO4–0.7 M K2HPO4 per liter). S. pneumoniae virulent capsular type 3 strain WU2 was grown in Todd-Hewitt broth containing 5% yeast extract (Difco, Detroit, Mich.).
Yeast.
Saccharomyces cerevisiae BJ3505 provided with the Kodak YEpFLAG1 expression kit was used for the expression of recombinant proteins. Frozen stocks of transformed clones were prepared by growing the yeast in selective expansion medium (SEM; 8% dextrose, 0.67% yeast nitrogen base without amino acids, and 0.075% CSM-tryptophan, plus 1.5% agar for SEM plates) to stationary phase in an incubator shaker at 32°C and 175 rpm. The yeast was centrifuged at 5,000 × g, resuspended in 80% SEM plus 20% glycerol, aliquoted into 1-ml tubes, and frozen at −70°C.
Plasmid construction.
The YEpFLAG-1 expression vector (Kodak Scientific Imaging Systems, New Haven, Conn.), used for cloning and expression of the proteins, contains origin of replication in E. coli, gene for ampicillin resistance in E. coli, 2μm DNA for replication in S. cerevisiae, tryptophan marker for selection in yeast, regulated promoter, and α-factor sequence for leader peptide, providing secretion from yeast cells.
To construct the IL-2- and GM-CSF-containing plasmids, the following primer pairs with KpnI and SmaI restriction sites and specific for IL-2 and GM-CSF genes were used in the PCR: 5′ggggtacctttggataaaagagcacctacttcaagttct3′-5′gaccccgggaccaccaccagttagtgttgagatgat3′ and 5′ggggtacctttggataaaagagctccgacgcgtagcccg3′-5′gaccccgggaccaccacctttttggactggttttttgc3′, respectively. Plasmids carrying cDNA for human IL-2 (provided by Howard A. Young, Laboratory of Experimental Immunology, NCI-FCRDC) and murine GM-CSF (R&D Systems Europe Ltd.) were used as templates. PCR products were digested with restriction endonucleases KpnI and SmaI and ligated to the appropriate sites in YEpFLAG1. A few codons of the α-factor sequence (downstream of the KpnI site in YEpFLAG1) were eliminated by KpnI/SmaI cleavage of YEpFLAG1. They were restored by inclusion into 5′ primers. 3′ Primers contained three glycine codons for generation of a polyglycine linker between fused proteins. The resulting plasmids were named pYIL2 and pYGM, respectively.
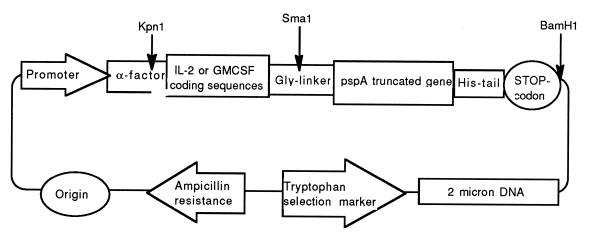
Genomic DNA from S. pneumoniae Rx1 was used as template in PCR with a primer pair containing SmaI and BamHI sites and specific to pspA gene. The 5′-primer contains three glycine codons, as part of the linker between fusion protein subunits (5′gtccccgggggtggtcccgtagccagtcagtctaaa3′). The 3′ primer contains translation stop codon downstream of six histidine codons (5′gacggatccttagtggtggtggtggtggtgtggagtttctggagctggagc3′). The six histidine codons generate a His tail at the C terminus of the protein molecule for purification purposes. The PCR product was digested with restriction endonucleases SmaI and BamHI and cloned between the appropriate sites of pYIL2 and pYGM. The new plasmids, called pIL2PA and pGMPA, contain an α-factor sequence followed by full coding regions for IL-2 and GM-CSF, respectively, glycine linker codons, truncated gene for PspA (coding for amino acid residues 4 through 299 of mature PspA), and six histidine codons at the end upstream of the translation stop codon (Fig. 1).
FIG. 1.
Structures of pIL2PA and pGMPA.
To construct a plasmid expressing only truncated PspA, the KpnI-SmaI fragment of pIL2PA was replaced by a synthetic linker with a KpnI cohesive end on the 5′ termini, a blunt SmaI end on the 3′ termini, and the codons for α-factor, removed by KpnI-SmaI cleavage of pIL2PA in between. This plasmid was named pPAF1.
Yeast electroporation.
A 100-ml culture of S. cerevisiae BJ3505 was grown in YPD medium (1% yeast extract, 2% peptone, 2% dextrose) until the optical density at 600 nm (OD600) was 1.3 to 1.5. The yeast was centrifuged at 2,000 rpm, the supernatant was removed, and the yeast was resuspended in 25 ml of sterile ice-cold 1 M sorbitol. After this wash step was performed three additional times, the yeast was centrifuged and as much sorbitol as possible was removed from the pellet. The yeast was vortexed, and 40 μl of the cells was transferred to an ice-cooled 0.2-cm electroporation cuvette. Then 2.5 μl of sheared salmon sperm DNA and 100 ng of plasmid DNA in a volume of 2.5 μl were mixed with the yeast, and the mixture was kept on ice for 30 min before electroporation. Cells were electroporated in a gene pulser (Bio-Rad) set at 1.5 kV, 200 Ω, and 25 μF. Immediately after electroporation, 0.4 ml of cold 1 M sorbitol was added to the cuvette. Transformed clones were selected by plating the cells onto SEM plates.
Protein production and purification.
Starter cultures of transformed yeast were prepared from frozen aliquots that were thawed and grown to saturation in SEM in an incubator shaker at 30°C and 175 rpm. Fifty milliliters of starter culture was added to 1 liter of YPHSF expression medium (1% dextrose, 3% glycerol, 1% yeast extract, 8% peptone, 20 mM CaCl2) and incubated for 72 h. The yeast was removed from the fusion protein containing medium by centrifugation at 6,000 rpm in an ultracentrifuge and filtration of the supernatant through a 0.45-μm-pore-size filter. The fusion protein supernatant was concentrated and dialyzed against 1× phosphate-buffered saline (PBS; pH 7.4) using a spiral membrane ultrafiltration cartridge (Amicon, Beverly, Mass.) with a molecular size cutoff of 30 kDa.
Recombinant proteins were purified with the QIAexpressionist system (Qiagen, Chatsworth, Calif.). This system uses Ni-nitrilotriacetic acid (NTA) agarose, which has a strong affinity for the six-His tail incorporated into the C-termini of the fusion proteins. Ni-agarose is added to the concentrated, dialyzed supernatant and mixed overnight at 4°C. The Ni-agarose is applied to a column and washed with 0.2 M sodium acetate (pH 6.5) until the OD280 is less than 0.01. Proteins were eluted with 0.2 M sodium acetate (pH 4.5) and collected as fractions. The OD280 of the fractions was measured, and samples with an OD280 of >0.05 were pooled and dialyzed against 1× PBS (pH 7.4). The purified proteins were then tested for cytokine activity and antibody reactivity.
SDS-PAGE and immunoblot analyses.
Proteins were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and stained with Coomassie blue or transferred to nitrocellulose for immunoblotting. Proteins were transferred to nitrocellulose by using a semidry transfer apparatus (Pharmacia). Membranes were probed with either mouse anti-PspA IgG1 monoclonal antibody (MAb) XIR278, anti-IL-2 IgG1 mouse MAb 5B1, or rat anti-GM-CSF MAb. Membranes were probed with rabbit anti-mouse IgG or rabbit anti-rat IgG followed by goat anti-rabbit alkaline phosphatase-labeled antibody. The immunoblots were developed with 5-bromo-4-chloro-3-indolylphosphate–nitroblue tetrazolium phosphatase substrate system (Kirkegaard & Perry, Gaithersburg, Md.).
Cytokine proliferation assays.
The biological activity of the fusion proteins was assayed by their ability to support the proliferation of the IL-2- and GM-CSF-dependent cell lines. In 96-well plates, 100 μl of cells at 5 × 104 cells/ml were added to 100 μl of protein-cytokine samples that were titered in 10-fold dilutions beginning at a dilution of 1:10. Cells were pulsed on day 2 with 1 μCi of [3H]thymidine in a volume of 10 μl of Hanks balanced saline solution plus HEPES and incubated for 6 h at 37°C. Cells were harvested with a cell harvester (LKB Wallac, Turku, Finland) onto glass fiber filters (LKB Wallac). Thymidine incorporation was measured with a liquid scintillation counter (1205 Betaplate; LKB Wallac). All samples were run in duplicate, and recombinant IL-2 (rIL-2) and rGM-CSF were used as controls.
Immunization.
Mice were immunized s.c. with various doses of protein-cytokine fusion prepared in PBS in a final volume of 0.2 ml/mouse or antigen in alhydrogel (Superfos Biosector, Vedbaek, Denmark). Mice were boosted 14 days after the first injection, and blood was collected from the tail vein on days 14 and 28 after boosting. Other groups of mice were immunized i.n. with various amounts of fusion protein in a total volume of 20 μl in PBS. Mice were anesthetized with dry ice, and DNA gel loading tips were used to apply antigen to each of the nares. Mice were boosted on days 7 and 14 after the initial immunization. Serum was collected from the mice 14, 21, and 28 days after the primary immunization.
ELISA.
Titers in mouse sera were determined for IgG1, IgG2a, and IgG3 anti-PspA and anti-IL-2 antibody by enzyme-linked immunosorbent assay (ELISA). Wells were coated with 10 μg of recombinant PspA per ml or with 1 μg of rIL-2 (National Cancer Institute) per ml. IgG1 mouse anti-human IL-2 MAb 5BI was used as the positive control for IL-2-coated plates. Rabbit anti-mouse IgG1 (Advanced ChemTech, Louisville, Ky.) or IgG2a or IgG3 (Serotec Ltd., Oxford, England) was used as the primary antibody, and alkaline phosphatase-conjugated goat anti-rabbit IgG (Calbiochem, La Jolla, Calif.) was used as the secondary antibody. The plates were developed with alkaline phosphatase substrate (p-nitrophenyl phosphate, disodium; Sigma) read at A405 with a Titertek Multiskan ELISA reader. Assays for IgG, IgA, and IgM antibody to PspA were done as described previously (25).
Mouse protection assay.
CBA/N mice were injected intraperitoneally with a 1:5, 1:10, 1:30, or 1:90 dilution of pooled immune serum in Ringer’s solution. Other mice received Ringer’s solution in place of diluted mouse serum. Pooled immune serum was obtained from mice immunized with the recombinant 245-amino-acid N-terminal fragment of PspA, PspA–IL-2, or PspA–GM-CSF. One hour after receiving serum from immunized mice, all mice were challenged intravenously with 1,700 CFU of strain WU2 in 0.2 ml. The lethal intravenous dose of WU2 in CBA/N mice is between 10 and 100 CFU. Deaths were monitored for a period of 21 days postchallenge. Strain WU2 was grown in 100 ml of THY medium until late log phase, adjusted to 3% glycerol, and frozen in 1-ml aliquots containing about 107 CFU/ml. CFU were predetermined by plating pneumococci from one of the thawed aliquots. For inoculation, a fresh aliquot was thawed and appropriately diluted (about 1,000-fold) for injection. The actual number of CFU injected was confirmed by plating.
RESULTS
Characterization of protein-cytokine fusions.
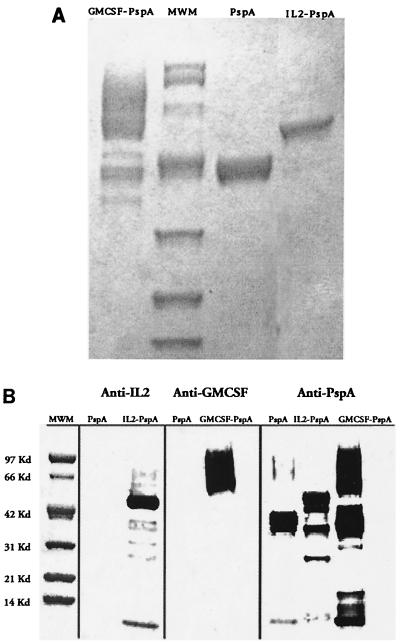
To test that the stability of PspA and that its antigenic integrity was not altered after fusion with cytokine, we analyzed the purified protein-cytokine by SDS-PAGE and Western blotting (immunoblotting) with anti-PspA and anti-IL-2 antibodies (Fig. 2). The protein-cytokine product showed a single major band at 47 kDa, and this pattern was not altered in a sample that had been stored for 4 months. On Western immunoblotting with anti-PspA, anti-IL-2 or anti-GM-CSF showed a major band at the predicted molecular weight region of the gel. This result demonstrates that the protein-cytokine was purified to relative homogeneity, was relatively stable, and interacted with anti-PspA to a degree comparable to the reactivity of unconjugated PspA.
FIG. 2.
Coomassie blue-stained SDS–4 to 20% polyacrylamide gel (A) and Western blot (B) of Ni-NTA-purified proteins. Filtered yeast supernatants containing His-tailed fusion proteins were concentrated, dialyzed, and purified by Ni-NTA affinity chromatography; 5-μl samples of purified proteins and a molecular mass marker (MWM) standard were size fractionated on an SDS–4 to 20% denaturing polyacrylamide gel. Membranes were probed with an MAb against either IL-2 or GM-CSF.
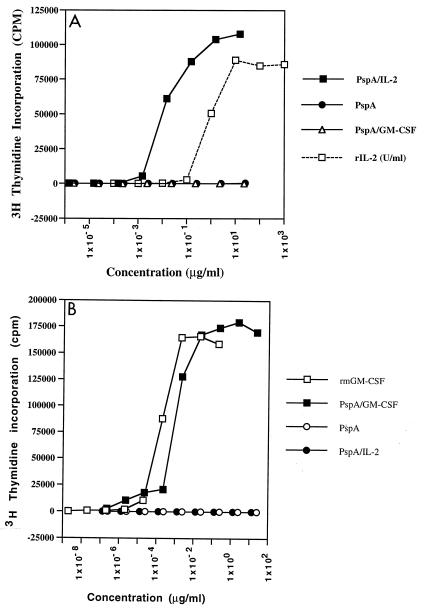
To test that the cytokine activity was intact, we cultured the IL-2-dependent cytotoxic T-lymphocyte cell line CTEV with the PspA–IL-2 and the GM-CSF-dependent cell line 32DGR2 with PspA–GM-CSF. The activity of the IL-2 and GM-CSF fusions was comparable to that of the unconjugated cytokine (Fig. 3). There was minimal variation in cytokine activity between the different batches of PspA-cytokine that were prepared. In general, 1 μg of protein was associated with 2,200 U of IL-2 activity or 1,300 U of GM-CSF activity.
FIG. 3.
Proliferation assay to measure cytokine activity of recombinant proteins. (A) 1:10 serial dilutions of Ni-NTA-purified proteins were added to CTEV IL-2-dependent cells at 5 × 104 cells/well with an rIL2 control at a starting concentration of 1,000 U/ml. (B) 32D6RZ GM-CSF-dependent cells at 5 × 104 cells/well were incubated for 48 h, then pulsed with 1 μCi of [3H]thymidine for 6 h, and harvested.
Immunogenicity of PspA cytokines after i.n. immunization.
Mice were immunized i.n. with 10 μg of PspA, PspA–IL-2, or PspA–GM-CSF, boosted on days 7 and 14, and bled 7 and 14 days after the last boost (Table 1). In five of five experiments, PspA failed to stimulate a detectable antibody response, while the protein-cytokine fusions always stimulated significant antibody titers. This response was dependent on IL-2 binding activity in that immunization of IL-2 receptor knockout mice did not stimulate significant antibody responses with PspA–IL-2 but did with PspA given in adjuvants (data not shown). This response was dose dependent, and i.n. administration of less than 10 μg did not stimulate a detectable response (Table 2). To demonstrate that this enhanced response did not reflect the activity of small amounts of immunoadjuvanting endotoxin that could potentially be present in our preparations, we examined the activity of PspA–IL-2 in lipopolysaccharide nonresponder C3H/HeJ mice (Table 3). Equivalent anti-PspA responses were observed in C3H/HeJ and control C3H/HeN mice, demonstrating that enhanced responses did not reflect lipopolysaccharide activity. Pathologic examination of the lungs 2 weeks after three i.n. inoculations showed no abnormal pathology (not shown). These results demonstrate that protein-cytokine fusions may provide a practical approach to inducing immunity via the i.n. route.
TABLE 1.
Anti-PspA responses in mice immunized i.n. with PspA–GM-CSF or PspA–IL-2a
| Immunogen | IgG1 anti-PspA titer on indicated day postboost
|
||
|---|---|---|---|
| 14 | 28 | 44 | |
| PspA | <20 | <20 | <20 |
| PspA–IL-2 | 24,249 | 33,248 | 26,191 |
| PspA–GM-CSF | 94,240 | 93,114 | ND |
Groups of five DBA/2 mice were immunized i.n. with PspA–IL-2 (10 μg), PspA–GM-CSF (10 μg), or PspA (10 μg) on days 0, 7, and 14. Mice were bled 14, 28, and 44 days later, and anti-PspA titers were determined by ELISA. Results are from one of three representative experiments. ND, not done.
TABLE 2.
Dose-response profile of PspA–GM-CSF injected i.n.a
| Immunogen | Amt (U) of cyto- kine injected | Amt (μg) of protein) | Mean IgG1 anti-PspA titer (SE) |
|---|---|---|---|
| PspA | 37 | 246 | |
| PspA–GM-CSF | 80,000 | 37 | 65,476 (1.40) |
| 40,000 | 18 | 29,060 (2.71) | |
| 20,000 | 9 | 12,823 (1.65) | |
| 10,000 | 4.5 | 143 (2.79) |
Groups of five DBA/2 mice were immunized i.n. with PspA (37 μg) or different doses of PspA–GM-CSF on days 0, 7, and 14. Mice were bled 14 days later, and anti-PspA titers were determined by ELISA.
TABLE 3.
Immunogenicity of PspA–IL-2 conjugates in C3H/HeJ micea
| Immunogen | IgG1 anti-PspA antibody titer
|
|
|---|---|---|
| C3H/HeJ | C3H/HeN | |
| PspA | 1,209 | 867 |
| PspA–IL-2 | 30,932 | 74,870 |
| PspA–GM-CSF | 103,790 | 34,226 |
Groups of five mice were immunized with 10 μg of PspA, PspA–IL-2, or PspA–GM-CSF and boosted 14 days later. Titers reflect averages of individual serum titrations obtained 14 days after the boost.
Isotype profile of antibody stimulated after i.n. immunization.
IL-2 has been shown to be instrumental in stimulating TH1 cells and consequently a gamma interferon-dominated response (5, 20). Gamma interferon is the critical cytokine in influencing the pattern from one that is predominantly IgG1 to one that is predominantly IgG2a (25). To test whether this predicted pattern is observed after injection of PspA–IL-2, we tested the isotype profile of anti-PspA after i.n. injection with the PspA-cytokine fusions. We compared these responses to that which is seen after PspA is given s.c. in alhydrogel (Table 4). Both GM-CSF and IL-2 constructs stimulated high levels of IgG1 anti-PspA antibody, but only the IL-2-containing construct stimulated IgG2a antibody. Both constructs stimulated lower but significant levels of IgG3 and nondetectable levels of IgA. To determine whether the isotype profile or magnitude of the response may have been influenced by the fraction of antigen that was introduced by aspiration into the pulmonary tree compared to that which remained in the local nasal lymphoid tissue after placement in the nares, we compared the responses of anesthetized and nonanesthetized mice. The expectation was that with anesthetization, a greater fraction of the antigen would reach the pulmonary tissues after deeper inspiration and less would be eliminated by swallowing. The responses of the two groups of mice were quantitatively and qualitatively comparable (Table 5).
TABLE 4.
Stimulation of different IgG isotypes after i.n. immunization with PspA–IL-2 or PspA–GM-CSFa
| Immunogen | Anti-PspA titer
|
|||
|---|---|---|---|---|
| IgG1 | IgG2a | IgG3 | IgA | |
| PspA | <20 | <20 | <20 | <200 |
| PspA–IL-2 | 42,256 | 8,419 | 2,237 | <200 |
| PspA–GM-CSF | 142,411 | 131 | 708 | <200 |
| PspA (alhydrogel) | 23,705 | 78 | 1,203 | <200 |
Groups of five DBA/2 mice were immunized i.n. with PspA–IL-2 (5 μg), PspA–GM-CSF (5 μg), or PspA (5 μg) on days 0, 7, and 14. Another group of mice were immunized s.c. with PspA (5 μg) in alhydrogel on day 0 and boosted with PspA (5 μg) on day 14. Mice were bled 14, 28, and 44 days later, and anti-PspA titers were determined by ELISA.
TABLE 5.
Comparison of i.n. immunization with PspA-cytokine fusions in anesthetized and nonanesthetized micea
| Immunogen | Mean IgG1 anti-PspA titer (SE)
|
|
|---|---|---|
| Anesthetizeda | Nonanesthetizedb | |
| PspA | <1,000 | <1,000 |
| PspA–IL-2 | 31,655 (1.44) | 16,656 (1.51) |
| PspA–GM-CSF | 22,730 (1.75) | 9,363 (1.49) |
Groups of five DBA/2 mice were immunized i.n. with PspA–IL-2 (5 μg), PspA–GM-CSF (5 μg), or PspA (5 μg) on days 0, 7, and 14. Mice were bled 14, 28, and 44 days later, and anti-PspA titers were determined by ELISA.
Mice were anesthetized with CO2.
Enhanced antibody responses after s.c. administration of protein-cytokine fusions.
We wished to test whether comparable enhancement and similar isotype profiles would be stimulated after s.c. injection. Mice were injected on days 0 and 14 and bled 14 days later. In contrast to i.n. immunizations, which failed to stimulate anti-PspA responses, s.c. immunization with PspA often stimulated antibody responses even in the absence of adjuvants or cytokines. This response was dramatically enhanced when IL-2 or GM-CSF was fused to the protein (Table 6). Enhanced antibody responses to s.c.-injected antigen were seen after two injections, while optimal responses induced by intranasal administration required three injections (data not shown). The profile of IgG isotypes was similar to that seen with the i.n. immunization. There was a predominance of IgG1, with the appearance of IgG2a after injection of PspA–IL-2 but not PspA–GM-CSF (Table 7).
TABLE 6.
Anti-PspA responses in mice stimulated by s.c. injection of different doses of PspA–IL-2 or PspA–GM-CSFa
| Immunogen | Dose (μg/mouse) | IgG1 anti-PspA antibody titer
|
|
|---|---|---|---|
| Day 14 | Postboost day 14 | ||
| PspA | 5 | <50 | <50 |
| 0.05 | <5 | <5 | |
| PspA–IL-2 prepn 1 | 5 | 559 | >40,000 |
| 0.5 | 188 | 11,639 | |
| 0.05 | 15 | 183 | |
| PspA–GM-CSF | 5 | 3,883 | 35,852 |
| 0.5 | <10 | 6,032 | |
| 0.05 | <10 | 137 | |
Groups of five DBA/2 mice were immunized with PspA–IL-2 or PspA–GM-CSF at 5, 0.5, or 0.05 μg/mouse. Mice were boosted on day 14 and bled 14 days later. Results represent arithmetic means of ELISA titers determined for individual sera. Results are from one of four representative experiments.
TABLE 7.
Stimulation of different IgG isotypes by PspA–IL-2 and PspA–GM-CSF after s.c. injectiona
| Immunogen | Anti-PspA ELISA titer
|
||
|---|---|---|---|
| IgG1 | IgG2a | IgG3 | |
| PspA | 1,407 | <50 | 62 |
| PspA–IL2 | 35,000 | 14,632 | 3,056 |
| PspA–GM-CSF | 17,387 | 617 | 894 |
Groups of five DBA/2 mice were immunized s.c. with PspA–IL-2 (5 μg), PspA–GM-CSF (10 μg), or PspA (10 μg) on days 0, 7, and 14. Mice were bled 14, 28, and 44 days later, and anti-PspA titers were determined by ELISA. Results are from one of three representative experiments.
Enhanced antipolysaccharide responses with PspA–IL-2–polysaccharide conjugates.
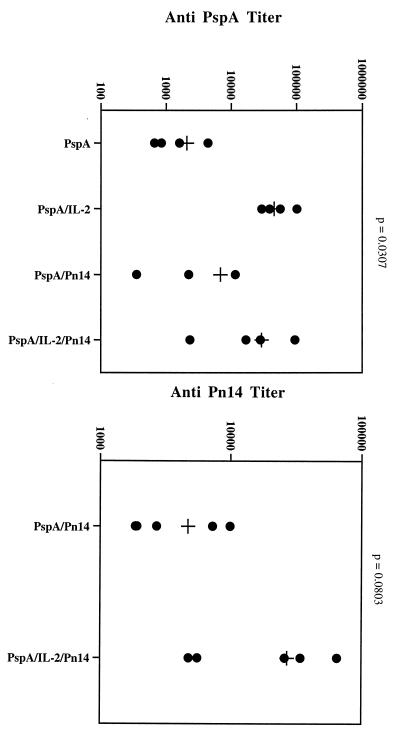
To test whether cytokine could also enhance antipolysaccharide responses, we conjugated PspA–IL-2 or PspA only to pneumococcal polysaccharide type 14 (Pn14). Mice were immunized with either PspA-Pn14 or PspA–IL-2–Pn14, boosted on day 14, and bled 14 days later (Fig. 4). As was observed in previous experiments, anti-PspA responses were enhanced in mice immunized with the protein-cytokine fusion. Likewise, anti-Pn14 antibody responses were higher in mice given PspA–IL-2–Pn14 than in those given PspA-Pn14 only (Fig. 4).
FIG. 4.
Effect of IL-2 on anti-Pn14 responses to PspA-Pn14. Groups of five DBA/2 mice were immunized s.c. with either PspA-Pn14 (5 μg) or PspA–IL-2–Pn14 (5 μg), boosted on day 14, and bled 14 days later.
Protective efficacy of antibody elicited by PspA–IL-2 and PspA–GM-CSF.
To evaluate the ability of antibody elicited by PspA–IL-2 and PspA–GM-CSF against pneumococcal infection, pooled sera from immunized DBA/2 mice were used to passively protect CBA/N mice from infection with encapsulated type 3 strain WU2. As in past studies, the recombinant α-helical fragment of PspA was not able to elicit protection when injected without adjuvant (reference 2 and Table 8). Sera from mice immunized with PspA–IL-2 or PspA–GM-CSF, however, were able to protect mice from fatal infection even when diluted 1:90 (Table 8). In the case of antibody elicited by PspA–GM-CSF, no more than 0.17 μg of antibody per mouse was required for protection. This was about roughly 1/1,800 of the amount of antibody in the blood volume of each mouse contributing to the serum pool.
TABLE 8.
Protective efficacy of sera from mice immunized with PspA-cytokine fusions after challenge with capsular type 3 strain WU2a
| Route of injection | Antigen | Serum dilutionb | Results of pneumococcal challenge
|
||
|---|---|---|---|---|---|
| No. of days alive | No. alive:no. dead | P value vs PspA onlyc | |||
| s.c. | PspA (amino acids 1–243) | 1:5 | 2, 2, 2, 2 | 0:4 | NA |
| 1:10 | 2, 2, 2 | 0:3 | |||
| 1:30 | 2, 2, 2, 2 | 0:4 | |||
| PspA–IL-2 | 1:5 | >21, >21, >21, >21 | 4:0 | 0.0007 | |
| 1:10 | 2, >21, >21 | 2:1 | 0.033 | ||
| 1:30 | >21, >21, >21, >21 | 4:0 | 0.0007 | ||
| 1:90 | >21, >21, >21, >21 | 4:0 | 0.0007 | ||
| i.n. | PspA (amino acids 1–243) | 1:5 | 2, 2, 2, 3 | 0:4 | NA |
| 1:10 | 2, 2, 2 | 0:3 | |||
| 1:30 | 2, 2, 2, 2 | 0:4 | |||
| PspA–GM-CSF | 1:5 | >21, >21, >21, >21 | 4:0 | 0.0007 | |
| 1:10 | >21, >21, >21 | 3:0 | 0.033 | ||
| 1:30 | 2, >21, >21, >21 | 3:1 | 0.088 | ||
| 1:90 | >21, >21, >21, >21 | 4:0 | 0.0007 | ||
| None (Ringer’s solution) | 2, 2, 2, 2, 3 | 0:4 | NS | ||
IgG antibody levels (micrograms/milliliter) to PspA in immune serum produced by immunization with PspA (s.c.), PspA-IL2 (s.c.), PspA (i.n.), and PspA-GMCSF (i.n.) were <0.3, 400, <0.3, and 156, respectively. Levels for IgA were <0.3, 8.5, <0.3, and 14.5; levels for IgM were <0.5, 2.7, <0.5, and 2.6.
Sera were diluted in Ringer’s solution.
Calculated versus pooled PspA only for the same injection route by Fisher’s exact test; NA, not applicable; NS, not significant, P ≥ 0.05.
DISCUSSION
Cytokines have been shown to be important regulators of antibody responses to both T-cell-dependent (TD) (19, 30) and T-cell-independent (TI) antigens (18). In the recent past, many groups have exploited this property and demonstrated that the humoral and cell-mediated responses of mice to both TD and TI antigens can be enhanced by the coadministration of cytokines. An early demonstration of this phenomenon was reported by Reed et al. (23), who showed that IL-1 enhanced the antibody response to coadministered bovine serum albumin. The adjuvanticity of IL-1 was not dependent on the presence of T cells and could also enhance responses to the TI antigen pneumococcal polysaccharide (1, 21). GM-CSF has also been shown to enhance humoral antibody responses to soluble and particulate antigens as well as to peptide antigens (9, 32).
More recently, a number of investigators have improved this system by directly linking the cytokine to the experimental antigen via genetic engineering (4, 12, 22, 29). This offers the advantage of specifically targeting the cytokine to the antigen-specific B cell as well as prolonging the in vivo half-life of the cytokine (11). Using this approach of fusing cytokine to protein, Chen et al. demonstrated that an antibody response to an Ig idiotype could be enhanced when GM-CSF or IL-2 was fused to the idiotype, and this provided a model vaccine system for B-cell lymphoma (4). Hinuma et al. created a genetic fusion of the herpes simplex virus glycoprotein D with IL-2 and found that it stimulated high humoral and cell-mediated responses (13). Furthermore, mice immunized with these constructs were protected against herpes simplex virus type 1 infection. Cytokine fusions can also be constructed by using only the biological active relevant portion of the cytokine molecule. Thus, Rao and Nayak chemically coupled the immunostimulatory nonapeptide sequence of IL-1β to amino acids 12 to 32 of hepatitis B surface antigen. This construct also stimulated enhanced antibody responses to the experimental antigen (22).
We have extended these studies in a number of ways to show that an E. coli-expressed protein, PspA (15), when genetically fused with GM-CSF or IL-2, can induce enhanced anti-PspA responses. This protein has been shown by McDaniel et al. to elicit protection, in a mouse model, to lethal challenge with various serotypes of S. pneumoniae (17). In these studies, protection against the truncated PspA could be elicited by immunization of mice with PspA in adjuvant but not in the absence of adjuvant (2, 3). In this study, we show that serum from PspA-cytokine-immunized mice, when passively transferred to recipient mice, was protective even at serum dilutions of 1:90, a serum dilution larger than has been used successfully with other PspA immunization procedures. This is the first demonstration that protective antibacterial antibody responses can be induced by this approach.
We show that when this protein-cytokine conjugate is covalently linked to pneumococcal polysaccharide, one can induce high-titer antibodies to both the protein and polysaccharide components. This result suggests that IL-2 can be targeted to the polysaccharide-specific B cell by using this approach and that this results in enhanced antibody responses as well. While the mechanism of action of the GM-CSF and IL-2 fusions is unresolved, the data suggest that they stimulate B cells via different pathways. Thus, the IL-2 fusion stimulated high IgG1 and IgG2a antibody titers, while the GM-CSF fusion stimulated predominantly IgG1 antibody, with extremely low levels of IgG2a. This result suggests that the GM-CSF fusion stimulated a predominant TH2-dominated response and the IL-2 fusion stimulated a response reflecting a TH1 component as well. This observation supports the idea that the cytokines were not merely functioning as carrier molecules for PspA independent of their cytokine activity. In other studies, we injected PspA into IL-2 receptor knockout mice and found that PspA in alhydrogel stimulated a significant anti-PspA response; PspA-IL2 stimulated no detectable response (not shown).
The data presented here are also the first to demonstrate that protein-cytokine fusions can stimulate enhanced systemic responses even when administered i.n. Under these conditions, the TH1-versus-TH2 profile of the response followed the same pattern as when the antigens were given s.c. While for i.n. immunizations 10 μg of antigen was placed on the nares, the fraction that was actually delivered to an immune response-relevant site is unknown. In an attempt to enhance intrapulmonary localization, we anesthetized mice, and we found no obvious difference in the antibody titers stimulated in anesthetized versus nonanesthetized mice. We were able to detect only extremely low levels of serum, or salivary IgA (not shown), suggesting either that this specific protein may be unable to stimulate IgA+ antigen-specific B cells or that the attached cytokines may not be the appropriate ones for stimulating a mucosal IgA response. We are attempting to determine whether the described IgA switch factor with transforming growth factor β might be a better fusion partner to induce this isotype.
Immunizations by the i.n. route have been demonstrated by many groups to provide an effective mode of antigen delivery. In all of the published reports, however, antigens had to be administered either daily or in an adjuvanted form to be effective (6, 7, 8, 14, 24). Liposomes, proteasomes, CT, and E. coli labile toxin have all been used to enhance the magnitude of the antibody responses when antigens are given i.n. The use of adjuvants has the disadvantage that after they induce unwanted polyclonal Ig secretion, undesired inflammatory responses are unstable. The use of cytokines as the mucosal adjuvant provides the benefit that it is a physiologic immune modulator that is being targeted to an antigen-specific cell. The use of protein-cytokine fusions provides a way of inducing rapid immunity while being able to modulate the isotype being produced without diverting the immune response to producing antibody to the administered adjuvant. Thus, for example, when CT is used as the adjuvant for i.n. immunization, more than 50% of the antibody that is made has specificity for CT. Exactly how this may affect subsequent immunizations with CT is not yet known.
Since cytokines are both immune system stimulatory and proinflammatory, we examined pathological specimens of pulmonary tissue from mice given three to four i.n. immunizations with protein-cytokine. No difference from control lung specimens was noted (not shown). Furthermore, since we were injecting the cytokines in an altered, potentially immunogenic presentation, we tested for the presence of anticytokine antibody. In an assay so sensitive that we could detect as little as 0.1 ng of anti-IL-2 antibody per ml, we detected no anti-IL-2 in mice given three to four injections of PspA–IL-2 even when administered in complete Freund adjuvant (not shown).
Taken together, these data suggest that protective immunity can be induced to bacterial challenge by proteins fused to GM-CSF as well as IL-2 and that protection can be achieved even after i.n. immunizations.
ACKNOWLEDGMENTS
The first two authors contributed equally to this work.
This work was supported by NIH grant 1RO1-A136588. We gratefully acknowledge the financial and scientific support of SmithKline Beecham, Rixensart, Belgium.
We are particularly grateful to Jean Paul Prieels, SmithKline Beecham, for continuing support and sage advice.
REFERENCES
- 1.Boraschi D, Nencioni L, Villa L, Censini S, Bossù P, Ghiara P, Presentini R, Perin F, Frasca D, Doria G, Forni G, Musso T, Giovarelli M, Ghezzi P, Bertini R, Besedovsky H O, Del Ray A, Sipe J D, Antoni G, Silvestri S, Tagliabue A. In vivo stimulation and restoration of the immune response by the noninflammatory fragment 163-171 of human interleukin 1β. J Exp Med. 1988;168:675–686. doi: 10.1084/jem.168.2.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Briles D E, King J D, Gray M A, McDaniel L S, Swiatlo E, Benton K A. PspA, a protection-eliciting pneumococcal protein: immunogenicity of isolated native PspA in mice. Vaccine. 1996;14:858–867. doi: 10.1016/0264-410x(96)82948-3. [DOI] [PubMed] [Google Scholar]
- 3.Briles D E, Tart R C, Wu H-Y, Ralph B A, Russell M W, McDaniel L S. Systemic and mucosal protective immunity to pneumococcal surface protein A. N Y Acad Sci. 1996;797:118–126. doi: 10.1111/j.1749-6632.1996.tb52954.x. [DOI] [PubMed] [Google Scholar]
- 4.Chen T T, Tao M-H, Levy R. Idiotype-cytokine fusion proteins as cancer vaccines. Relative efficacy of IL-2, IL-4, and granulocyte-macrophage colony-stimulating factor. J Immunol. 1994;153:4775–4787. [PubMed] [Google Scholar]
- 5.Constant S L, Bottomly K. Induction of Th1 and Th2 CD4+ T cell responses: the alternative approaches. Annu Rev Immunol. 1997;15:297–322. doi: 10.1146/annurev.immunol.15.1.297. [DOI] [PubMed] [Google Scholar]
- 6.Dale J B, Chiang E C. Intranasal immunization with recombinant group A streptococcal M protein fragment fused to the B subunit of Escherichia coli labile toxin protects mice against systemic challenge infections. J Infect Dis. 1995;171:1038–1041. doi: 10.1093/infdis/171.4.1038. [DOI] [PubMed] [Google Scholar]
- 7.Debard N, Buzoni-Gatel D, Bout D. Intranasal immunization with SAG1 protein of Toxoplasma gondii in association with cholera toxin dramatically reduces development of cerebral cysts after oral infection. Infect Immun. 1996;64:2158–2166. doi: 10.1128/iai.64.6.2158-2166.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Haan A, Tomee J F, Huchshorn J P, Wilschut J. Liposomes as an immunoadjuvant system for stimulation of mucosal and systemic antibody responses against inactivated measles virus administered intranasally to mice. Vaccine. 1995;13:1320–1324. doi: 10.1016/0264-410x(95)00037-2. [DOI] [PubMed] [Google Scholar]
- 9.Disis M L, Bernhard H, Shiota F M, Hand S L, Grolow J R, Huseby E S, Gillis S, Cheever M A. Granulocyte-macrophage colony-stimulating factor: an effective adjuvant for protein and peptide-based vaccines. Blood. 1996;88:202–210. [PubMed] [Google Scholar]
- 10.Geissler M, Gesien A, Tokushige K, Wands J R. Enhancement of cellular and humoral immune responses to hepatitis C virus core protein using DNA-based vaccines augmented with cytokine-expressing plasmids. J Immunol. 1997;158:1231–1237. [PubMed] [Google Scholar]
- 11.Harvill E T, Fleming J M, Morrison S L. In vivo properties of an IgG3-IL-2 fusion protein. A general strategy for immune potentiation. J Immunol. 1996;157:3165–3170. [PubMed] [Google Scholar]
- 12.Hazama M, Mayumi-Aono A, Asakawa N, Kuroda S, Hinuma S, Fujisawa Y. Adjuvant-independent enhanced immune responses to recombinant herpes simplex virus type 1 glycoprotein D by fusion with biologically active interleukin-2. Vaccine. 1993;11:629–637. doi: 10.1016/0264-410x(93)90308-k. [DOI] [PubMed] [Google Scholar]
- 13.Hinuma S, Hazama M, Mayumi A, Fujisawa Y. A novel strategy for converting recombinant viral protein into high immunogenic antigen. FEBS Lett. 1991;288:138–142. doi: 10.1016/0014-5793(91)81020-9. [DOI] [PubMed] [Google Scholar]
- 14.Lowell G H, Kaminski R W, Grate S, Hunt R E, Charney C, Zimmer S, Colleton C. Intranasal and intramuscular proteosome-staphylococcal enterotoxin B (SEB) toxoid vaccines: immunogenicity and efficacy against lethal SEB intoxication in mice. Infect Immun. 1996;64:1706–1713. doi: 10.1128/iai.64.5.1706-1713.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McDaniel L S, Scott G, Widenhofer K, Carroll J M, Briles D E. Analysis of a surface protein of Streptococcus pneumoniae recognized by protective monoclonal antibodies. Microb Pathog. 1986;1:519–531. doi: 10.1016/0882-4010(86)90038-0. [DOI] [PubMed] [Google Scholar]
- 16.McDaniel L S, Ralph B A, McDaniel D O, Briles D E. Localization of protection-eliciting epitopes on PspA of Streptococcus pneumoniae between amino acid residues 192 and 260. Microb Pathog. 1994;17:323–337. doi: 10.1006/mpat.1994.1078. [DOI] [PubMed] [Google Scholar]
- 17.McDaniel L S, Sheffield J S, Delucchi P, Briles D E. PspA, a surface protein of Streptococcus pneumoniae, is capable of eliciting protection against pneumococci of more than one capsular type. Infect Immun. 1991;59:222–228. doi: 10.1128/iai.59.1.222-228.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mond J J, Lees A, Snapper C M. T cell-independent antigens type 2. Annu Rev Immunol. 1995;13:655–692. doi: 10.1146/annurev.iy.13.040195.003255. [DOI] [PubMed] [Google Scholar]
- 19.Morrissey P J, Bressler L, Park L S, Alpert A, Gillis S. Granulocyte-macrophage colony-stimulating factor augments the primary antibody response by enhancing the function of antigen presenting cells. J Immunol. 1987;139:1113–1119. [PubMed] [Google Scholar]
- 20.Mosmann T R, Sad S. The expanding universe of T-cell subsets: Th1, Th2 and more. Immunol Today. 1996;17:138–146. doi: 10.1016/0167-5699(96)80606-2. [DOI] [PubMed] [Google Scholar]
- 21.Nencioni L, Villa L, Tagliabue A, Antoni G, Presentini R, Perin F, Silvestri S, Boraschi D. In vivo immunostimulating activity of the 163-171 peptide of human IL-1β. J Immunol. 1987;139:800–804. [PubMed] [Google Scholar]
- 22.Rao K V S, Nayak A R. Enhanced immunogenicity of a sequence derived from hepatitis B virus surface antigen in a composite peptide that includes the immunostimulatory region from human interleukin 1. Proc Natl Acad Sci USA. 1990;87:5519–5522. doi: 10.1073/pnas.87.14.5519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reed S G, Pihl D L, Conlon P J, Grabstein K H. IL-1 as adjuvant: role of T cells in the augmentation of specific antibody production by recombinant human IL-1α. J Immunol. 1989;142:3129. [PubMed] [Google Scholar]
- 24.Russell M W, Moldoveanu Z, White P L, Sibert G J, Mestecky J, Michalek S M. Salivary, nasal, genital, and systemic antibody responses in monkeys immunized intranasally with a bacterial protein antigen and the cholera toxin B subunit. Infect Immun. 1996;64:1272–1283. doi: 10.1128/iai.64.4.1272-1283.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Snapper C M, Paul W E. Interferon-γ and B cell stimulatory factor-1 reciprocally regulate Ig isotype production. Science. 1987;236:944–947. doi: 10.1126/science.3107127. [DOI] [PubMed] [Google Scholar]
- 26.Snapper C M, Moorman M A, Rosas F R, Kehry M R, Maliszewski C R, Mond J J. IL-3 and granulocyte-macrophage colony-stimulating factor strongly induce Ig secretion by sort-purified murine B cells activated through the membrane Ig, but not the CD40, signaling pathway. J Immunol. 1995;154:5842–5850. [PubMed] [Google Scholar]
- 27.Tagliabue A, Boraschi D. Cytokines as vaccine adjuvants: interleukin 1 and its synthetic peptide 163-171. Vaccine. 1993;11:594–595. doi: 10.1016/0264-410x(93)90240-x. [DOI] [PubMed] [Google Scholar]
- 28.Talkington D F, Voellinger D C, McDaniel L S, Briles D E. Analysis of pneumococcal PspA microheterogeneity in SDS polyacrylamide gels and the association of PspA with the cell membrane. Microb Pathog. 1992;13:343–355. doi: 10.1016/0882-4010(92)90078-3. [DOI] [PubMed] [Google Scholar]
- 29.Tao M-H, Levy R. Idiotype/granulocyte-macrophage colony-stimulating factor fusion protein as a vaccine for B-cell lymphoma. Nature. 1993;362:755–758. doi: 10.1038/362755a0. [DOI] [PubMed] [Google Scholar]
- 30.Weinberg A, Merigan T C. Recombinant interleukin 2 as an adjuvant for vaccine-induced protection: immunization of guinea pigs with herpes simplex virus subunit vaccines. J Immunol. 1988;140:294–299. [PubMed] [Google Scholar]
- 31.Xiang Z, Ertl H C J. Manipulation of the immune response to a plasmid-encoded viral antigen by coinoculation with plasmids expressing cytokines. Immunity. 1995;2:129–135. doi: 10.1016/s1074-7613(95)80001-8. [DOI] [PubMed] [Google Scholar]
- 32.Yother J, Briles D E. Structural properties and evolutionary relationships of PspA, a surface protein of Streptococcus pneumoniae, as revealed by sequence analysis. J Bacteriol. 1992;174:601–609. doi: 10.1128/jb.174.2.601-609.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yother J, Handsome G L, Briles D E. Truncated forms of PspA that are secreted from Streptococcus pneumoniae and their use in functional studies and cloning of the PspA gene. J Bacteriol. 1992;174:610–618. doi: 10.1128/jb.174.2.610-618.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yother J, White J M. Novel surface attachment mechanism for the Streptococcus pneumoniae protein PspA. J Bacteriol. 1994;176:2976–2985. doi: 10.1128/jb.176.10.2976-2985.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]