Abstract
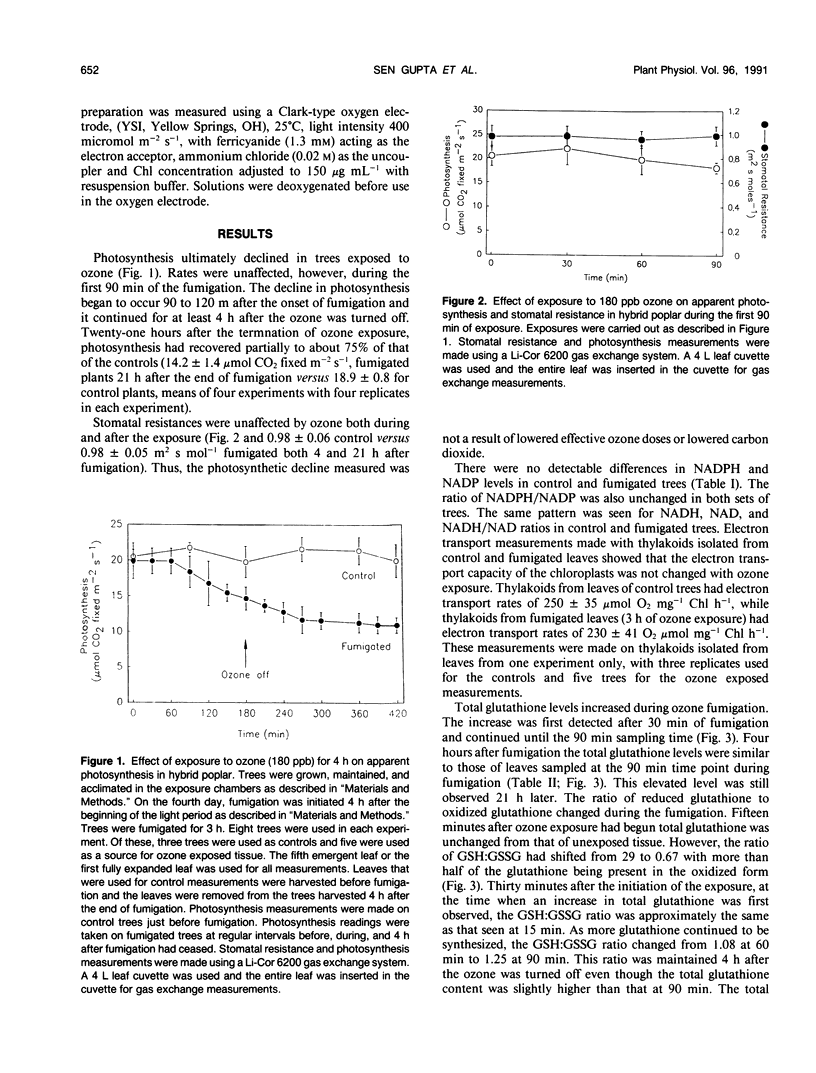
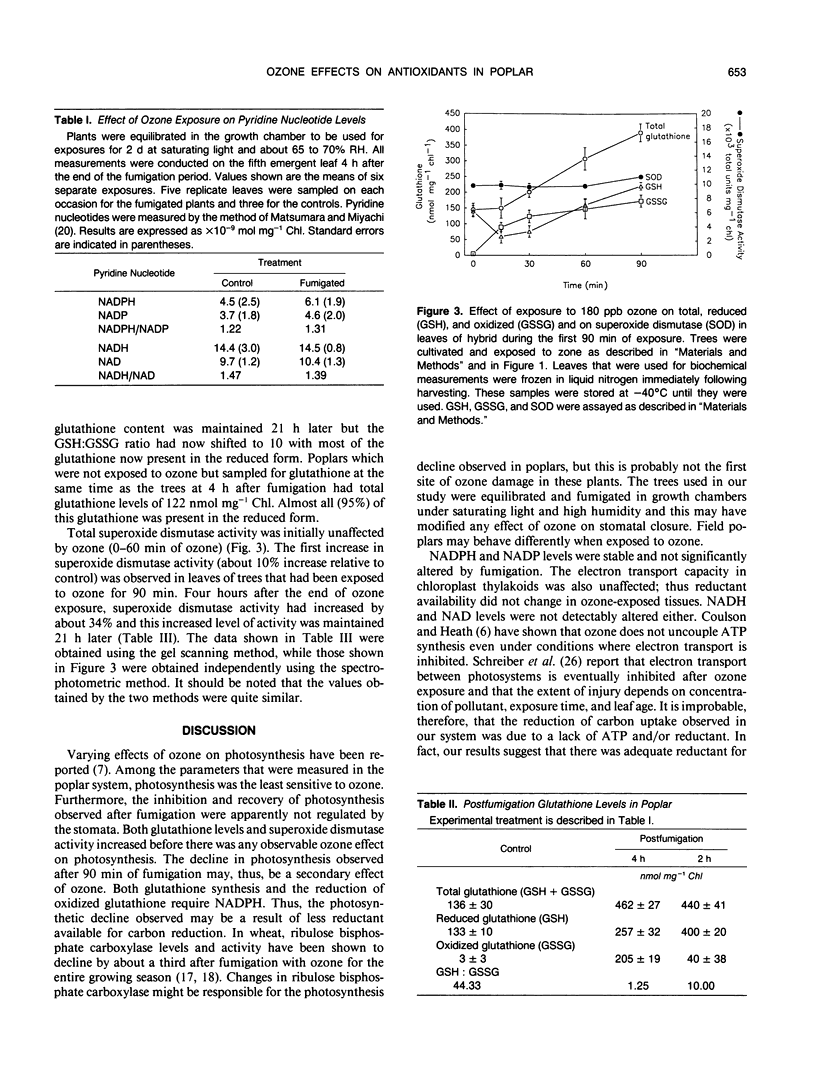
Atmospheric ozone causes formation of various highly reactive intermediates (e.g. peroxyl and superoxide radicals, H2O2, etc.) in plant tissues. A plant's productivity in environments with ozone may be related to its ability to scavenge the free radicals formed. The effects of ozone on photosynthesis and some free radical scavengers were measured in the fifth emergent leaf of poplars. Clonal poplars (Populus deltoides × Populus cv caudina) were fumigated with 180 parts per billion ozone for 3 hours. Photosynthesis was measured before, during, and after fumigation. During the first 90 minutes of ozone exposure, photosynthetic rates were unaffected but glutathione levels and superoxide dismutase activity increased. After 90 minutes of ozone exposure, photosynthetic rates began to decline while glutathione and superoxide dismutase continued to increase. Total glutathione (reduced plus oxidized) increased in fumigated leaves throughout the exposure period. The ratio of GSH/GSSG also decreased from 12.8 to 1.2 in ozone exposed trees. Superoxide dismutase levels increased twofold in fumigated plants. After 4 hours of ozone exposure, the photosynthetic rate was approximately half that of controls while glutathione levels and superoxide dismutase activity remained above that of the controls. The elevated antioxidant levels were maintained 21 hours after ozone exposure while photosynthetic rates recovered to about 75% of that of controls. Electron transport and NADPH levels remained unaffected by the treatment. Hence, elevated antioxidant metabolism may protect the photosynthetic apparatus during exposure to ozone.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchamp C. O., Fridovich I. Isozymes of superoxide dismutase from wheat germ. Biochim Biophys Acta. 1973 Jul 12;317(1):50–64. doi: 10.1016/0005-2795(73)90198-0. [DOI] [PubMed] [Google Scholar]
- Charles S. A., Halliwell B. Effect of hydrogen peroxide on spinach (Spinacia oleracea) chloroplast fructose bisphosphatase. Biochem J. 1980 Aug 1;189(2):373–376. doi: 10.1042/bj1890373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulson C., Heath R. L. Inhibition of the photosynthetic capacity of isolated chloroplasts by ozone. Plant Physiol. 1974 Jan;53(1):32–38. doi: 10.1104/pp.53.1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groden D., Beck E. H2O2 destruction by ascorbate-dependent systems from chloroplasts. Biochim Biophys Acta. 1979 Jun 5;546(3):426–435. doi: 10.1016/0005-2728(79)90078-1. [DOI] [PubMed] [Google Scholar]
- Jackson C., Dench J., Moore A. L., Halliwell B., Foyer C. H., Hall D. O. Subcellular localisation and identification of superoxide dismutase in the leaves of higher plants. Eur J Biochem. 1978 Nov 15;91(2):339–344. doi: 10.1111/j.1432-1033.1978.tb12685.x. [DOI] [PubMed] [Google Scholar]
- Kan B., London I. M., Levin D. H. Role of reversing factor in the inhibition of protein synthesis initiation by oxidized glutathione. J Biol Chem. 1988 Oct 25;263(30):15652–15656. [PubMed] [Google Scholar]
- Lee E. H., Bennett J. H. Superoxide Dismutase: A POSSIBLE PROTECTIVE ENZYME AGAINST OZONE INJURY IN SNAP BEANS (PHASEOLUS VULGARIS L.). Plant Physiol. 1982 Jun;69(6):1444–1449. doi: 10.1104/pp.69.6.1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehnherr B., Mächler F., Grandjean A., Fuhrer J. The Regulation of Photosynthesis in Leaves of Field-Grown Spring Wheat (Triticum aestivum L., cv Albis) at Different Levels of Ozone in Ambient Air. Plant Physiol. 1988 Dec;88(4):1115–1119. doi: 10.1104/pp.88.4.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewinsohn E., Gressel J. Benzyl viologen-mediated counteraction of diquat and paraquat phytotoxicities. Plant Physiol. 1984 Sep;76(1):125–130. doi: 10.1104/pp.76.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehlhorn H., Seufert G., Schmidt A., Kunert K. J. Effect of SO(2) and O(3) on Production of Antioxidants in Conifers. Plant Physiol. 1986 Sep;82(1):336–338. doi: 10.1104/pp.82.1.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pell E. J., Pearson N. S. Ozone-Induced Reduction in Quantity of Ribulose-1,5-bisphosphate Carboxylase in Alfalfa Foliage. Plant Physiol. 1983 Sep;73(1):185–187. doi: 10.1104/pp.73.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber U. Chlorophyll fluorescence assay for ozone injury in intact plants. Plant Physiol. 1978 Jan;61(1):80–84. doi: 10.1104/pp.61.1.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith I. K. Stimulation of glutathione synthesis in photorespiring plants by catalase inhibitors. Plant Physiol. 1985 Dec;79(4):1044–1047. doi: 10.1104/pp.79.4.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]


