Abstract
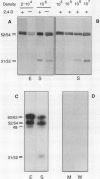
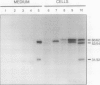
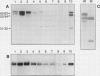
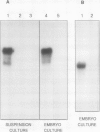
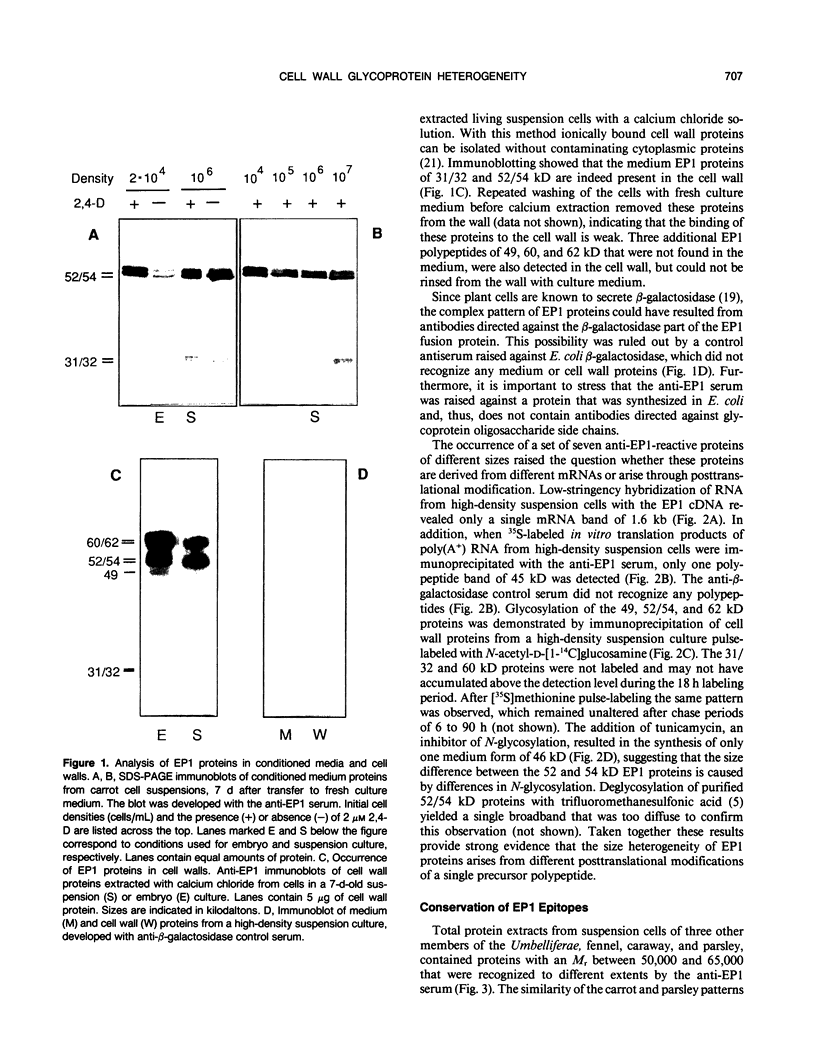
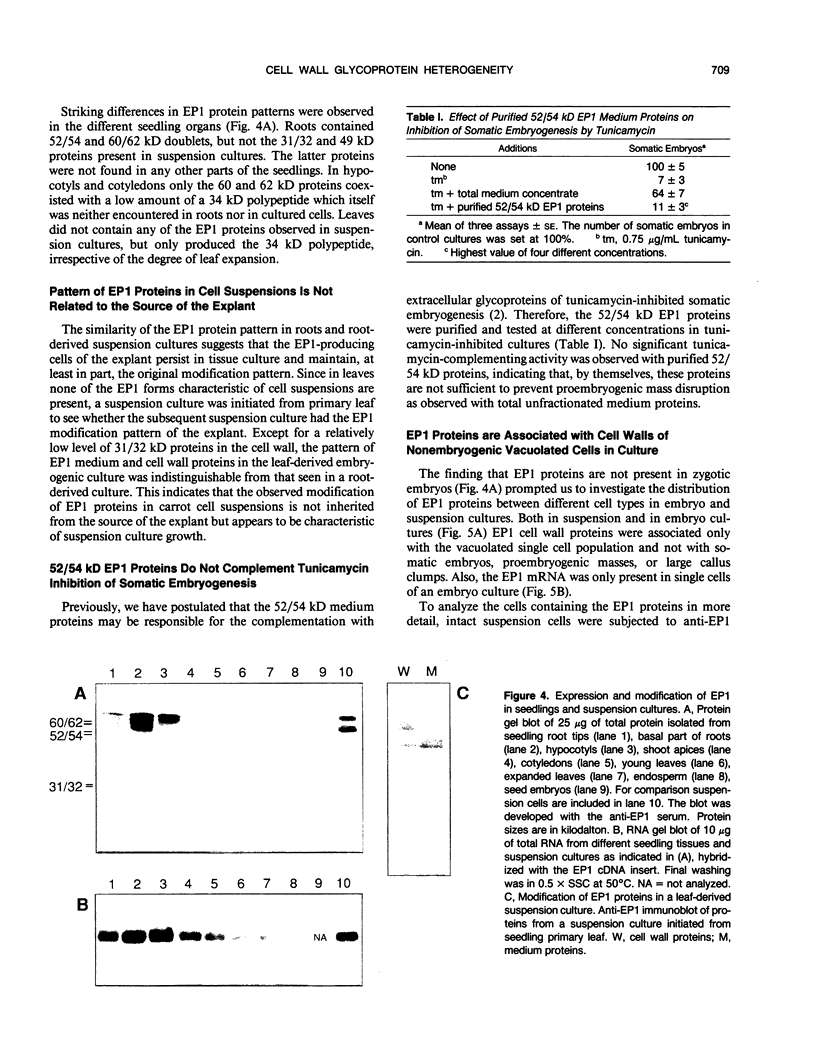
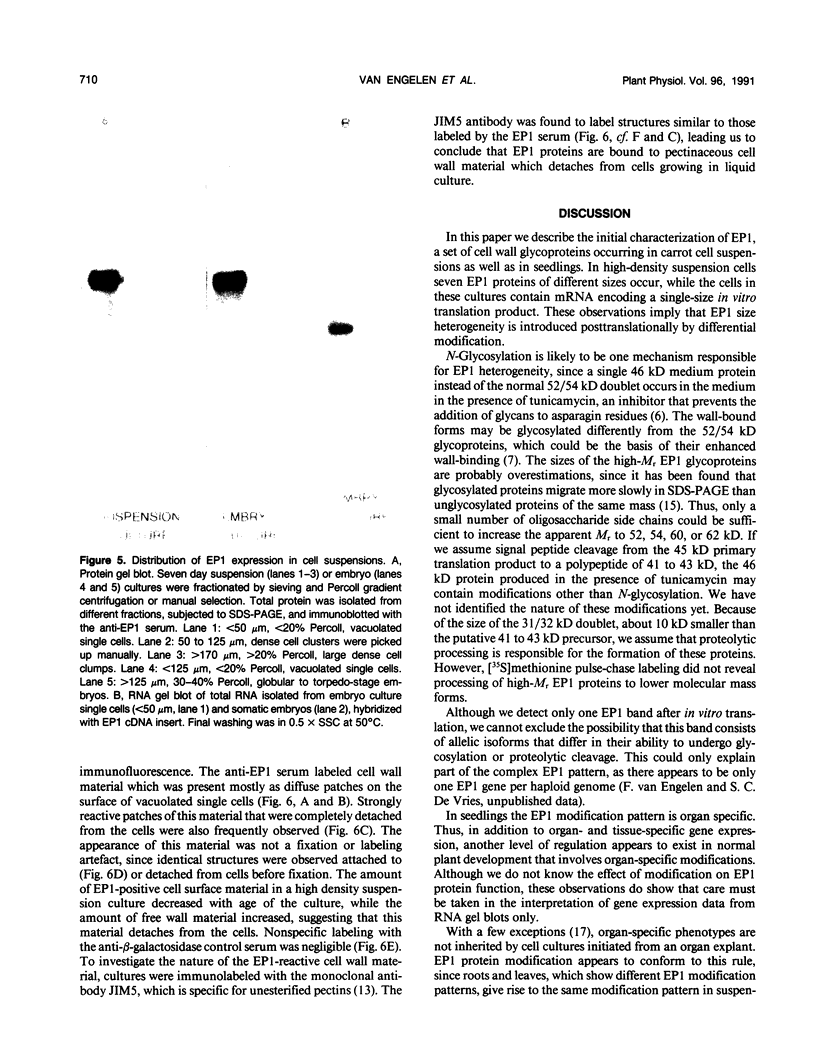
EP1, an extracellular protein from carrot (Daucus carota) cell suspensions, has been partially characterized by means of an antiserum and a cDNA clone. In both embryo and suspension cultures different molecular mass EP1 proteins were detected, some of which (31, 32, 52, and 54 kilodaltons) were bound to the cell wall and released into the medium, whereas others (49, 60, and 62 kilodaltons) were more firmly bound to the cell wall and could be extracted with a salt solution. Immunoprecipitation of in vitro translation products revealed a single primary translation product of 45 kilodaltons, suggesting that EP1 heterogeneity is due to differential posttranslational modification. In seedlings organ-specific modification of EP1 proteins was observed, a phenomenon which did not persist in suspension cultures initiated from different seedling organs. In culture EP1 proteins were only found to be associated with vacuolated, nonembryogenic cells, and on these cells they were localized in loosely attached, pectin-containing cell wall material. Purified 52/54 kilodaltons EP1 proteins did not alleviate the inhibitory effect of the glycosylation inhibitor tunicamycin on somatic embryogenesis.
Full text
PDF


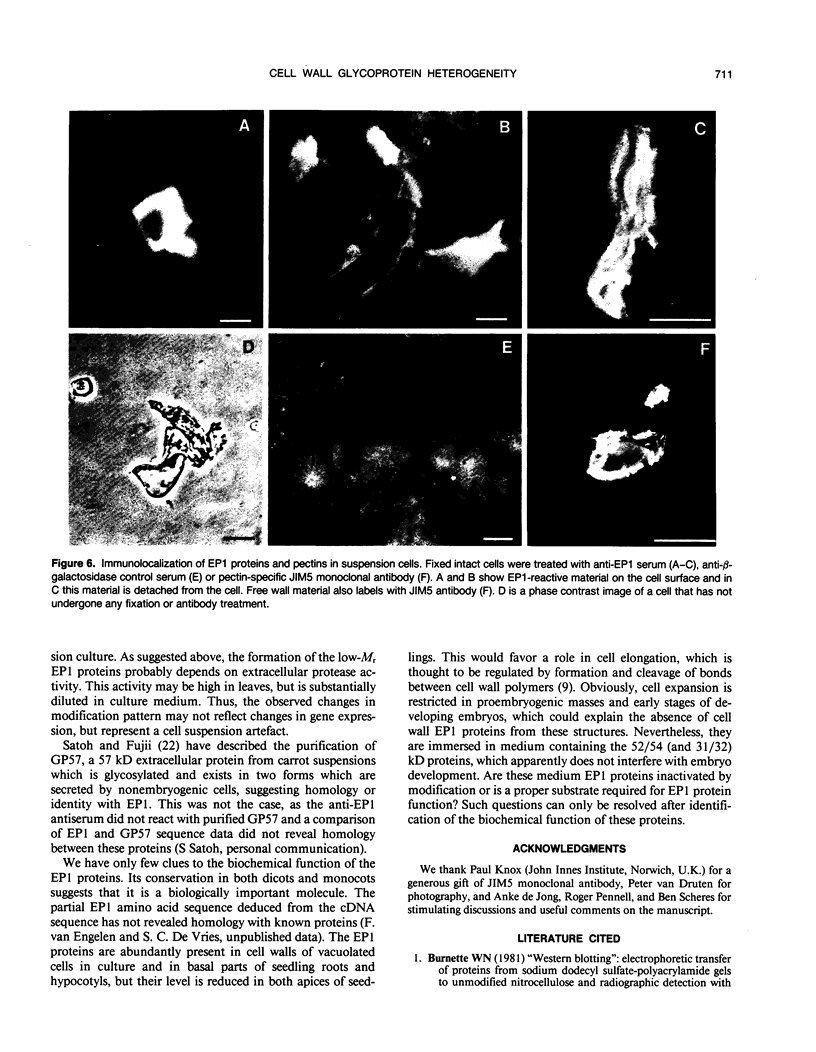

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Edge A. S., Faltynek C. R., Hof L., Reichert L. E., Jr, Weber P. Deglycosylation of glycoproteins by trifluoromethanesulfonic acid. Anal Biochem. 1981 Nov 15;118(1):131–137. doi: 10.1016/0003-2697(81)90168-8. [DOI] [PubMed] [Google Scholar]
- Elbein A. D. Inhibitors of the biosynthesis and processing of N-linked oligosaccharide chains. Annu Rev Biochem. 1987;56:497–534. doi: 10.1146/annurev.bi.56.070187.002433. [DOI] [PubMed] [Google Scholar]
- Franssen H., Goldbach R., Broekhuijsen M., Moerman M., van Kammen A. Expression of Middle-Component RNA of Cowpea Mosaic Virus: In Vitro Generation of a Precursor to Both Capsid Proteins by a Bottom-Component RNA-Encoded Protease from Infected Cells. J Virol. 1982 Jan;41(1):8–17. doi: 10.1128/jvi.41.1.8-17.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamborg O. L., Miller R. A., Ojima K. Nutrient requirements of suspension cultures of soybean root cells. Exp Cell Res. 1968 Apr;50(1):151–158. doi: 10.1016/0014-4827(68)90403-5. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leach B. S., Collawn J. F., Jr, Fish W. W. Behavior of glycopolypeptides with empirical molecular weight estimation methods. 1. In sodium dodecyl sulfate. Biochemistry. 1980 Dec 9;19(25):5734–5741. doi: 10.1021/bi00566a011. [DOI] [PubMed] [Google Scholar]
- O'neill R. A., Scott T. K. Rapid Effects of IAA on Cell Surface Proteins from Intact Carrot Suspension Culture Cells. Plant Physiol. 1987 Jun;84(2):443–446. doi: 10.1104/pp.84.2.443. [DOI] [PMC free article] [PubMed] [Google Scholar]