Abstract
Background
Obesity and underweight represent classical risk factors for outcome in patients treated for cardiovascular disease. This study describes the impact of different body mass index (BMI) categories on 1-year clinical outcome in patients with tricuspid regurgitation (TR) undergoing transcatheter-edge-to-edge repair (TEER).
Methods
We analyzed 211 consecutive patients (age 78.3 ± 7.2 years, 55.5% female, median EuroSCORE II 9.6 ± 6.7) with tricuspid regurgitation undergoing TEER from June 2015 until May 2021. Patients were prospectively enrolled in our single center registry and were retrospectively analyzed. Patients were stratified according to body mass index (BMI) into 4 groups: BMI < 20 kg/m2 (underweight), BMI 20.0 to < 25.0 kg/m2 (normal weight), BMI 25.0 to > 30.0 kg/m2 (overweight) and BMI ≥ 30 kg/m2 (obese).
Results
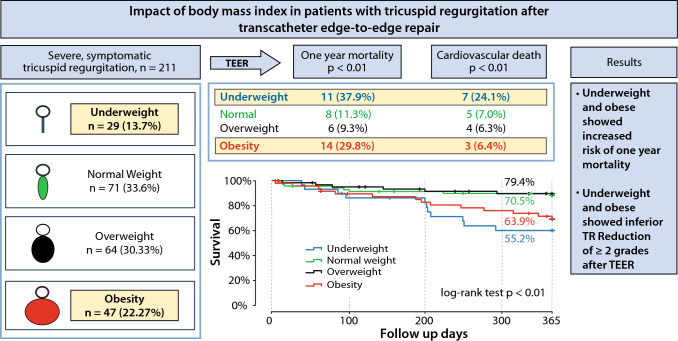
Kaplan–Meier survival curves demonstrated inferior survival for underweight and obese patients, but comparable outcomes for normal and overweight patients (global log rank test, p < 0.01). Cardiovascular death was significantly higher in underweight patients compared to the other groups (24.1% vs. 7.0% vs. 6.3% vs. 6.4%; p < 0.01). Over all, there were comparable rates of bleeding, stroke and myocardial infarction. Multivariable Cox regression analysis (adjusted for age, gender, coronary artery disease, chronic obstructive pulmonary disease, tricuspid annular plane systolic excursion, left-ventricular ejection fraction) confirmed underweight (HR 3.88; 95% CI 1.64–7.66; p < 0.01) and obesity (HR 3.24; 95% CI 1.37–9.16; p < 0.01) as independent risk factors for 1-year all-cause mortality.
Conclusions
Compared to normal weight and overweight patients, obesity and underweight patients undergoing TEER display significant higher 1-year all-cause mortality.
Graphical abstract
Supplementary Information
The online version contains supplementary material available at 10.1007/s00392-023-02312-2.
Keywords: Tricuspid valve disease, Tricuspid regurgitation, Transcatheter edge-to-edge repair, Body mass index
Introduction
Tricuspid regurgitation is a common finding in routine practice as population-based studies showed that the prevalence of tricuspid regurgitation of any grade amounts to > 80% of the population, particularly affecting people at older age and of female gender [1, 2]. Consequently, and with regard to the elderly (> 70 years), a significant TR (moderate) was present in 1.5% of male and 5.6% of female patients, respectively [2]. Thus, clinically relevant TR can be anticipated in approximately 3 Mill. individuals in Europe and 1.5 Mill. individuals in the USA [2–4]. Moreover, prevalence of 3 moderate TR in patients with chronic heart failure and reduced left ventricular ejection fraction is even higher with approximately 26% [2, 5, 6]. The importance of TR for prognosis has long been underrated and treatment has subsequently been neglected in accordance with the initial recommendations to handle TR with optimal heart failure therapy [4, 7, 8]. However, numerous studies recently underlined congruently the negative impact of significant TR on morbidity and mortality if left untreated [4, 6, 9–13].
Underweight and obesity are known risk factors for adverse outcome in patients with cardiovascular diseases [14, 15]. With regard to valvulopathies, recent studies showed an increased morbidity and mortality for the overweight and obese patient cohort after transcatheter aortic valve replacement (TAVR) as well as for underweight patients after transcatheter edge-to-edge repair (TEER) for mitral regurgitation, respectively [16, 17].
Concerning the prognostic impact of significant TR for the clinical course, identification and characterization of relevant risk constellations have more recently come to the fore—especially in the light of selecting the suitable treatment strategy for each patient. In terms of patient selection, established scores for left-sided valvulopathies and/or CABG procedures such as STS Score and the EUROScore II are not validated and imprecise concerning accurate risk stratification of tricuspid valve procedures although still recommended for perioperative risk assessment especially in the elderly and high-risk population [18]. Currently, the TRISCORE is most frequently used to assess procedural risk for tricuspid valve procedures as it includes and addresses parameters of right heart failure such as impaired liver function as a result of an increased central venous backlog, daily dosage of diuretics and right heart failure signs [19, 20]. However, parameters such as frailty which are associated with adverse outcome and BMI are not included into this score. While defining frailty still remains vague and without a predefined gold standard, BMI acquisition and interpretation is simple to attain and less faulty. But up to now, little is known regarding the impact of underweight and obesity on the postinterventional course after edge-to-edge repair for significant symptomatic TR.
Methods
Study population
This study was designed as a retrospective analysis of data from the Bonn registry, which is a prospective, consecutive collection of patient data from the Heart Center Bonn. We identified patients with symptomatic TR who underwent TEER interventions from June 2015 to May 2021. All included patients were considered as inoperable or at high-risk for surgery by the interdisciplinary heart team. After a standardized diagnostic workup, including transthoracic (TTE) echocardiography, left- and right-heart catheterization, the patient’s anatomical suitability for a TEER system was assessed using CT images and transesophageal echocardiography (TEE).
The indication for a tricuspid valve intervention was evaluated based on the current guidelines, and the decision to perform transcatheter tricuspid valve interventions as well as the device selection was made by the interdisciplinary heart team. The registry was approved by the local ethics committee. This study was conducted in accordance with the Declaration of Helsinki and its amendment, and all patients provided written informed consent.
Procedure
The TEER procedure was performed with either the MitraClip System (Abbott Structural Heart, Santa Clara, CA, USA), TriClip System (Abbott Structural Heart, Santa Clara, CA, USA) or PASCAL Implant System (Edwards Lifesciences, Irvine, CA, USA). The details of each device system and procedure have previously been well described [3, 21–24].
Echocardiographic assessments
Comprehensive TTE and TEE were performed at baseline, and at discharge and 1-year follow-up a comprehensive TTE was performed according to the current guidelines. The severity of TR was graded by the current recommendations using the five-scaled grading scheme as described in detail before [25]. All measurements were reviewed by two independent cardiologists dedicated to echocardiographic evaluation.
BMI—measurements and definitions
Underweight was defined as BMI < 20 kg/m2 based on the Academic Research Consortium and 2 [26] and 3 [27] definition of frailty. Four groups were defined, after calculating the preprocedural BMI for each patient: underweight (BMI < 20 kg/m2), normal weight (BMI 20 to < 25 kg/m2, reference group), overweight (BMI 25 to < 30 kg/m2) and obesity (BMI ≥ 30 kg/m2).
Endpoints
The primary endpoint of this study was all-cause mortality within 1 year after the procedure. Secondary outcomes were the reduction of TR, New York Heart Association class (NYHA), major adverse cardiovascular and cerebrovascular events (MACCE) (myocardial infarction and stroke) and changes in right ventricular function, measured by TAPSE and right ventricular fractional area change (RVFAC) at 1-year follow-up.
All patients were followed through interviews at scheduled hospital visits, telephone, or documentation from the referring general practitioners.
Statistics
Statistical analysis
Continuous variables are expressed as the mean ± standard deviation or the median (with a 25–75% interquartile range) depending on the distribution of the variables. Categorical variables are presented as numbers and percentages. Continuous variables were compared with a Student’s t test or Mann–Whitney U test, while the Chi-squared or Fisher exact tests were used for categorical variables. Cox proportional regression model was conducted to calculate hazard ratios (HR) and 95% confidence intervals (CI) for the primary endpoint for each variable. We entered variables with a p value < 0.05 upon univariate analysis into the multivariate model. Survival curves for 1 year after TEER procedure are depicted using Kaplan–Meier method. Two-tailed p values < 0.05 were considered statistically significant. All statistical analyses were performed using SSPS statistics version 27.0.
Results
Study population
All patients (n = 211) who underwent TEER were stratified according to their baseline BMI into four groups: underweight (n = 29), normal weight (n = 71), overweight (n = 64) and obesity (n = 47). Overall, the mean age was 78.3 ± 7.2, 55.5% of patients were female and the median EuroSCORE II was 9.6 ± 6.7% and the median TRI-SCORE was 5.2 ± 2.0. 182 (86.3%) of subjects were classified as NYHA functional class III and more. The prevalence of hyperlipidemia (p < 0.001) and diabetes (p = 0.01) was significantly higher in obese patients. All other comorbidities were without significant differences and in addition there were no statistically significant differences in echocardiographic and laboratory assessment between the four groups at baseline (Table 1).
Table 1.
Baseline patient characteristics
| All | Underweight | Normal weight | Overweight | Obesity | p value | |
|---|---|---|---|---|---|---|
| n = 211 | n = 29 | n = 71 | n = 64 | n = 47 | ||
| Age, years | 78.3 ± 7.2 | 78.3 ± 6.32 | 79.5 ± 7.5 | 78.1 ± 6.8 | 76.6 ± 7.4 | 0.18 |
| Female, n (%) | 117 (55.5) | 19 (65.6) | 39 (54.9) | 34 (53.1) | 25 (53.2) | 0.71 |
| BMI, kg/m2 | 26.1 ± 5.5 | 18.7 ± 1.0 | 22.9 ± 1.4 | 27.2 ± 1.3 | 34.1 ± 3.9 | < 0.001 |
| Risk stratification | ||||||
| TRI-SCORE | 5.2 ± 2.0 | 5.8 ± 1.8 | 5.0 ± 2.1 | 5.1 ± 2.0 | 5.5 ± 1.8 | 0.63 |
| EuroSCORE II, % | 9.6 ± 6.7 | 10.6 ± 7.8 | 10.4 ± 7.0 | 8.9 ± 6.5 | 8.9 ± 5.8 | 0.39 |
| Comorbidities | ||||||
| Hypertension, n (%) | 175 (82.9) | 23 (79.3) | 56 (78.9) | 53 (82.8) | 43 (91.4) | 0.28 |
| Hyperlipidemia, n (%) | 110 (52.1) | 17 (58.6) | 29 (40.8) | 28 (43.8) | 36 (76.6) | < 0.001 |
| Diabetes mellitus, n (%) | 51 (24.2) | 5 (17.2) | 10 (14.1) | 17 (26.6) | 19 (40.4) | 0.01 |
| Peripheral artery disease, n (%) | 81 (17.1) | 13 (44.8) | 27 (38.0) | 21 (32.8) | 2042.6) | 0.63 |
| CAD, n (%) | 117 (55.5) | 15 (51.7) | 39 (54.5) | 32 (50.0) | 31 (66.0) | 0.39 |
| Prior MI, n (%) | 57 (27.0) | 9 (31.0) | 19 (26.8) | 16 (25.0) | 13 (27.7) | 0.95 |
| COPD, n (%) | 39 (18.5) | 7 (24.1) | 8 (11.3) | 14 (21.9) | 10 (21.3) | 0.26 |
| NYHA ≥ III, n (%) | 182 (86.3) | 28 (15.4) | 58 (31.9) | 57 (31.3) | 39 (21.4) | 0.19 |
| Atrial fibrillation, n (%) | 195 (92.4) | 26 (89.7) | 64 (90.1) | 61 (95.3) | 44 (93.6) | 0.70 |
| CIED, n (%) | 71 (33.6) | 10 (34.5) | 19 (26.8) | 23 (35.9) | 19 (40.4) | 0.46 |
| Smoking, n (%) | 9 (4.3) | 2 (6.9) | 3 (4.2) | 3 (4.7) | 1 (2.1) | 0.74 |
| Smoking historya, n (%) | 47 (22.3) | 9 (31.0) | 15 (21.1) | 12 (18.8) | 11 (23.4) | 0.61 |
| Prior surgery/interventions | ||||||
| Prior cardiac surgery, n (%) | 128 (59.2) | 17 (58.6) | 45 (63.3) | 35 (54.7) | 28 (59.6) | 0.77 |
| Prior TAVI, n (%) | 13 (6.2) | 1 (3.4) | 9 (12.7) | 2 (3.1) | 1 (2.1) | 0.08 |
| Previous MV intervention, n (%) | 0.13 | |||||
| None | 148 (70.4) | 25 (86.2) | 48 (67.6) | 40 (62.5) | 35 (74.5) | |
| TEER | 61 (28.9) | 4 (13.8) | 23 (32.4) | 22 (34.4) | 12 (25.5) | |
| Replacement | 1 (0.5) | 0 | 1 (1.4) | 0 | 0 | |
| Other | 1 (0.5) | 1 (3.4) | 0 | 0 | 0 | |
| Heart failure medication, n (%) | ||||||
| Beta-blockers | 200 (94.8) | 26 (89.7) | 68 (95.8) | 61(95.3) | 45 (95.7) | 0.80 |
| ACEi/ARB | 209 (99.1) | 28 (96.6) | 71 (100.0) | 63 (98.4) | 47 (100.0) | 0.86 |
| Loop diuretic | 200 (94.8) | 25 (86.2) | 68 (95.8) | 61 (95.3) | 46 (97.8) | 0.15 |
| MRA | 191 (90.5) | 26 (89.7) | 65 (91.5) | 59 (92.2) | 41 (87.2) | |
| SGLT-2 inhibitor | 172 (81.5) | 23 (79.3) | 58 (81.7) | 51 (79.7) | 40 (85.1) | |
| Laboratory parameters | ||||||
| Creatinine, mg/dl | 1.5 ± 0.9 | 1.4 ± 0.6 | 1.4 ± 0.7 | 1.6 ± 0.9 | 1.8 ± 1.1 | 0.11 |
| eGFR, ml/min/m2 | 50.0 ± 23.9 | 55.4 ± 25.8 | 53.8 ± 25.7 | 48.8 ± 21.8 | 43.6 ± 21.7 | 0.10 |
| NT-proBNP, pg/ml | 4296 [3094, 5498] | 3526 [1568, 5484] | 5282 [2716, 7847] | 3082 [2232, 3931] | 4779 [1494, 8065] | 0. 48 |
| Bilirubin, mg/dl | 0.88 ± 0.6 | 1.0 ± 0.6 | 0.9 ± 0.6 | 0.8 ± 0.6 | 0.8 ± 0.6 | 0.39 |
| Echocardiographic findings | ||||||
| LVEF, % | 54.4 ± 10.3 | 53.2 ± 11.2 | 54.4 ± 11.2 | 53.8 ± 10.5 | 55.9 ± 7.8 | 0.66 |
| RVFAC, % | 43.2 ± 9.6 | 42.7 ± 11.9 | 43.1 ± 9.7 | 43.1 ± 9.3 | 43.1 ± 9.3 | 1.00 |
| TAPSE, mm | 17.9 ± 5.1 | 17.2 ± 5.9 | 18.9 ± 5.4 | 18.0 ± 4.3 | 16.8 ± 5.0 | 0.16 |
| TR severity, n (%) | 0.48 | |||||
| Severe | 96 (45.5) | 13 (13.7) | 36 (50.7) | 26 (40.6) | 21 (44.7) | |
| Massive | 69 (32.7) | 8 (40.8) | 24 (33.8) | 19 (29.7) | 18 (38.3) | |
| Torrential | 46 (21.8) | 8 (27.6) | 11 (15.5) | 19 (29.7) | 8 (17.0) | |
| SPAP, mmHg | 36.8 ± 14.2 | 34.3 ± 9.7 | 37.3 ± 15.6 | 35.4 ± 13.9 | 39.4 ± 14.7 | 0.51 |
Values are either n (%), mean ± SD, or median [interquartile range]
BMI body mass index, EuroSCORE European System for Cardiac Operative Risk Evaluation, CAD coronary artery disease, MI myocardial infarction, COPD chronic obstructive pulmonary disease, NYHA New York Heart Association, CIED cardiac implantable electronic device, TAVI transcatheter aortic valve implantation, MV mitral valve, TEER transcatheter edge-to-edge repair, ACEi/ARB angiotensin-converting enzyme inhibitor/angiotensin receptor blocker, MRA mineralcorticoid receptor antagonist, SGLT Sodium-glucose cotransporter 2, eGFR estimated glomerular filtration rate, NT-proBNP N-terminal pro-B-type natriuretic peptide, LVEF left-ventricular ejection fraction, RVFAC right ventricular fractional area change, TAPSE tricuspid annular plane systolic excursion, TR tricuspid regurgitation, SPAP systolic pulmonary artery pressure
aSmoking history: defined as a smoking cessation of ≥ 5 years
Periprocedural findings
Periprocedural findings are summarized in Table 2. Most cases of TEER were treated with the TriClip system (51.2%, Abbott Structural, Santa Clara, CA, USA), followed by the PASCAL Implant System (25.1%, Edwards Lifesciences, Irvine, CA, USA) and the MitraClip system (23.7%, Abbott Structural, Santa Clara, CA, USA). The mean number of implanted devices and implantation failure was comparable between the groups with 1.8 ± 0.8 devices per procedure (p = 0.06 and p = 0.07). Extensive bleeding events occurred in two patients (one patient with normal weight and one patient with overweight) without fatal outcome. These patients required blood transfusion. There was a tendency towards more failed intervention in the obese group, mainly driven through a lesser postprocedural reduction of TR grade (definition of failed procedure: reduction of TR grade < 2 grades or no device implantation)—without statistical significance (p = 0.06).
Table 2.
Outcome parameters
| All | Underweight | Normal weight | Overweight | Obesity | p value | |
|---|---|---|---|---|---|---|
| n = 211 | n = 29 | n = 71 | n = 64 | n = 47 | ||
| Procedural findings | ||||||
| Failed intervention, n (%) | 17 (8.1) | 2 (6.9) | 5 (7.0) | 2 (3.1) | 8 (17.0) | 0.06 |
| Implanted devices | 1.8 ± 0.8 | 1.7 ± 0.9 | 1.8 ± 0.8 | 2.0 ± 0.8 | 1.6 ± 0.9 | 0.07 |
| Procedure time, min | 50.6 ± 25.8 | 47.8 ± 24.3 | 47.1 ± 21.7 | 51.9 ± 25.4 | 56.3 ± 32.3 | 0.28 |
| Bleedings, n (%) | 0.12 | |||||
| Minor | 8 (3.8) | 2 (6.9) | 1 (1.4) | 4 (6.2) | 1 (2.1) | |
| Major | 8 (3.8) | 3 (10.3) | 2 (2.8) | 3 (4.7) | 0 | |
| Extensive | 2 (0.9) | 0 | 1 (1.4) | 1 (1.6) | 0 | |
| Myocardial infarction, n (%) | 0 | 0 | 0 | 0 | 0 | 1.0 |
| Stroke, n (%) | 0 | 0 | 0 | 0 | 0 | 1.0 |
| 1-Year mortality | ||||||
| All-cause mortality, n (%) | 39 (18.5) | 11 (37.9) | 8 (11.3) | 6 (9.3) | 14 (29.8) | < 0.001 |
| Cardiovascular death, n (%) | 19 (48.7) | 7 (24.1) | 5 (7.0) | 4 (6.3) | 3 (6.4) | < 0.01 |
| Non-cardiovascular death, n (%) | ||||||
| Unknown | 10 | 3 | 1 | 1 | 5 | |
| Sepsis/MODS | 6 | 2 | 4 | |||
| Gastrointestinal bleeding | 2 | 2 | ||||
| Pneumonia | 1 | 1 | ||||
| Malignancy | 1 | 1 |
| All | Underweight | Normal weight | Overweight | Obesity | p value | |
|---|---|---|---|---|---|---|
| n = 172 | n = 18 | n = 63 | n = 58 | n = 33 | ||
| Outcome at 1-year follow-up | ||||||
| NYHA class, n (%) | 0.86 | |||||
| I | 68 (68.4) | 8 (44.4) | 27 (44.3) | 19 (32.8) | 14 (29.8) | |
| II | 99 (57.6) | 10 (55.5) | 33 (52.4) | 37 (63.8) | 19 (40.4) | |
| III | 5 | 0 | 3 | 2 | 0 | |
| Echocardiographic findings | ||||||
| TR severity, n (%) | 0.67 | |||||
| Mild | 67 (40.0) | 5 (27.8) | 27 (42.9) | 22 (37.9) | 13 (39.4) | |
| Moderate | 84 (48.8) | 11 (61.1) | 27 (42.9) | 30 (51.7) | 16 (34.0) | |
| Severe | 19 (9.0) | 1 (5.6) | 8 (12.7) | 6 (10.3) | 4 (12.1) | |
| Massive | 1 (0.6) | 1 (5.6) | 0 | 0 | ||
| Torrential | 0 | 0 | 0 | 0 | 0 | |
| RVFAC, % | 47.6 ± 6.9 | 47.7 ± 7.2 | 46.9 ± 6.8 | 47.2 ± 6.9 | 49.6 ± 6.9 | 0.33 |
| TAPSE, mm | 18.2 ± 3.3 | 16.9 ± 3.2 | 18.6 ± 3.2 | 18.2 ± 3.2 | 17.9 ± 3.7 | 0.36 |
| SPAP, mmHg | 36.0 ± 15.6 | 28.9 ± 11.3 | 36.0 ± 15.6 | 35.1 ± 12.0 | 42.2 ± 22.9 | 0.29 |
Values are either n (%), mean ± SD, or median [interquartile range]
MODS multiple organ dysfunction syndrome, NYHA New York Heart Association, TR tricuspid regurgitation, SPAP systolic pulmonary artery pressure, RVFAC right ventricular fractional area change, TAPSE tricuspid annular plane systolic excursion
TR reduction
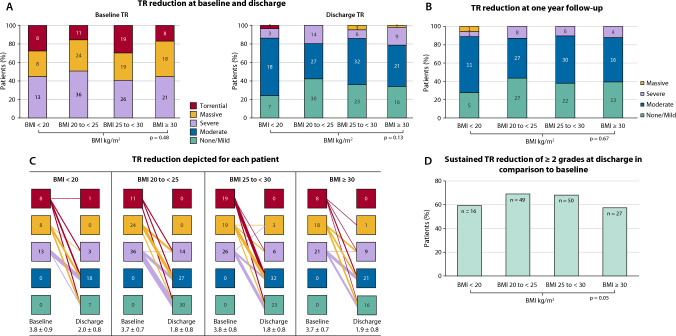
All patients had ≥ severe TR at baseline according to the five-scale grading scheme: 21.8% were graded torrential, 32.7% massive and 45.5% severe. There were no significant differences between the stratified BMI subgroups (p = 0.48). At discharge, TR severity was significantly reduced in all subgroups (Fig. 1a, b). In addition, there were no differences between the groups in TR severity at discharge and at 1 year-follow-up (p = 0.13 and p = 0.67, respectively) (Fig. 1c). 143 (67.8%) of subjects had a sustained TR reduction of ≥ 2 grades at discharge in comparison to baseline. Stratified according to the four BMI classes, underweight and obese patients showed significant lower rates of TR reduction ≥ 2 grades at discharge: 55.2% vs. 70.4% vs. 78.1%. vs. 57.4%; p = 0.05 (Fig. 1d).
Fig. 1.
Tricuspid regurgitation (TR) reduction after transcatheter edge-to-edge repair (TEER) stratified according to body mass index (BMI) classes at discharge a TR reduction at baseline and discharge, b TR reduction depicted for each patient, c TR reduction at one year follow-up, d sustained TR reduction of ≥ 2 grades at discharge in comparison to baseline
NYHA functional class
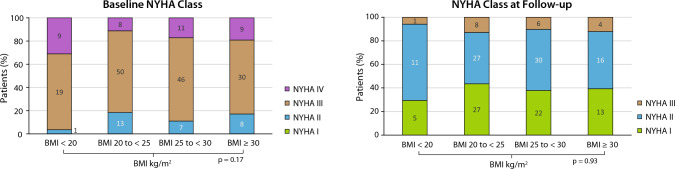
Stable and significant improvements in NYHA functional class were found for all patients without significant differences among the subgroups at baseline (p = 0.17). At 12 months, 167 of 172 patients (97.1%) were in NYHA functional classes I or II, similar according to BMI subgroups (p = 0.93) (Fig. 2).
Fig. 2.
NYHA classes at baseline and one year follow-up stratified according to body mass index (BMI)
Clinical outcomes
In the overall cohort, the primary endpoint (all-cause mortality at 1-year follow-up) was observed in 18.5% (39/211) of the patients. Kaplan–Meier curves showed that underweight and obese patients appeared to have a significantly higher all-cause mortality within 1 year after the procedure, compared to normal and overweight patients (p log rank < 0.001), 37.9% of the underweight group and 29.8% of the obese group died within 1 year (Fig. 3).
Fig. 3.
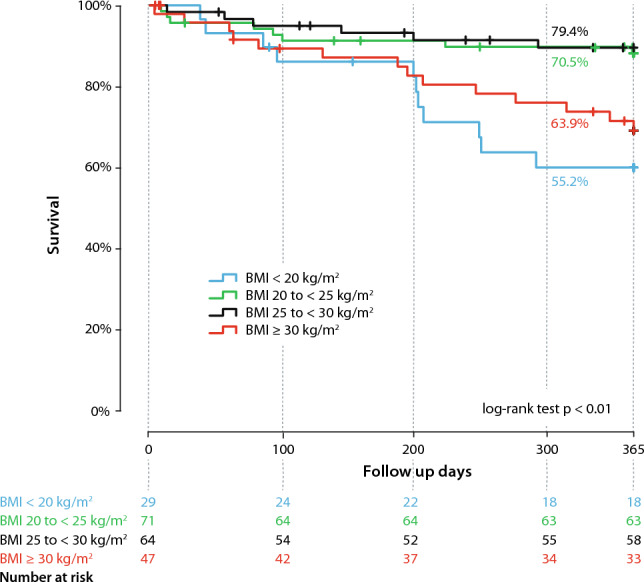
Kaplan–Meier curves for the endpoint of mortality in patients with tricuspid regurgitation (TR) after transcatheter edge-to-edge repair (TEER) stratified by body mass index (BMI)
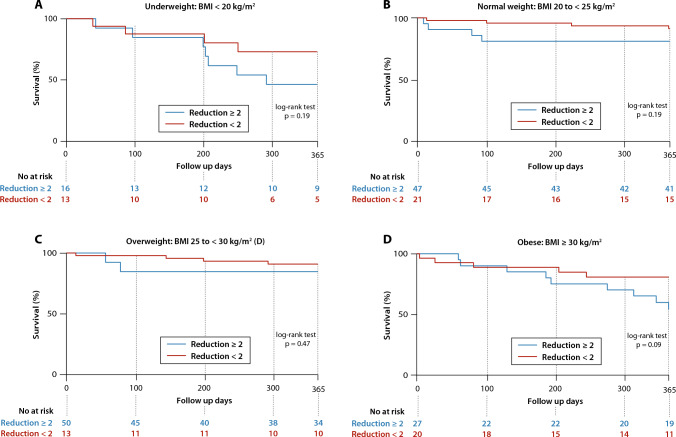
Patients with a TR reduction of ≤ 2 grades showed a tendency towards a higher 1-year mortality stratified by BMI groups (Fig. 4).
Fig. 4.
Kaplan–Meier curves for the endpoint of mortality in patients with tricuspid regurgitation (TR) after transcatheter edge-to-edge repair (TEER) stratified by body mass index (BMI) according to TR reduction of of ≥ 2 grades at discharge a underweight: BMI < 20 kg/m2, b normal weight: BMI 20 to < 25 kg/m2, c overweight: BMI 25 to < 30 kg/m2, d obese: BMI ≥ 30 kg/m2
In a multivariable cox proportional hazard model, underweight patients (3.88, 95% CI 1.64–7.66; p < 0.01) and obese patients (HR 3.24, 95% CI 1.37–9.16; p < 0.01) significantly revealed to be associated with an increased risk of reaching the primary endpoint. COPD (HR 2.52, 95% CI 1.14–5.56; p = 0.02) and a depressed left-ventricular ejection fraction (HR 3.28, 95% CI 1.21–8.86; p = 0.02) were also identified as predicting factors for reaching the primary endpoint in our cohort (Table 3).
Table 3.
Cox regression of prediction for 1-year mortality
| Univariable | Multivariable | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p value | HR | 95% CI | p value | |
| Demographic parameters | ||||||
| Age | 1.00 | 0.98–1.04 | 0.01 | 1.06 | 0.99–1.13 | 0.08 |
| Female sex | 0.72 | 0.38–1.34 | 0.31 | |||
| Coronary artery disease | 1.39 | 0.73–2.66 | 0.31 | |||
| COPD | 2.80 | 1.44–5.44 | 0.002 | 2.52 | 1.14–5.56 | 0.02 |
| CIED | 1.60 | 0.85–3.01 | 0.16 | |||
| Hyperlipidemia | 1.16 | 0.61–2.18 | 0.66 | |||
| Diabetes | 1.33 | 0.66–2.67 | 0.42 | |||
| Echocardiographic parameters | ||||||
| LVEF < 40% | 2.46 | 1.08–5.57 | 0.02 | 3.28 | 1.21–8.86 | 0.02 |
| TAPSE, over 15 mm | 1.47 | 0.25–0.91 | 0.02 | |||
| RVFAC_path | 1.72 | 0.85–3.49 | 0.14 | |||
| TR, torrential | 1.01 | 0.47–2.0 | 0.98 | |||
| BMI | ||||||
| BMI under 20 kg/m2 | 2.66 | 1.32–5.35 | 0.006 | 3.88 | 1.64–7.66 | < 0.01 |
| BMI over 30 kg/m2 | 1.98 | 1.03–3.81 | 0.01 | 3.24 | 1.37–9.16 | < 0.01 |
Significant parameters are depicted bold
COPD chronic obstructive pulmonary disease, LVEF left-ventricular ejection fraction, TAPSE tricuspid annular plane systolic excursion, RVFAC right ventricular fractional area change, TR tricuspid regurgitation, BMI body mass index
Discussion
Up to now, little is known regarding the influence of BMI on procedural outcome after TEER in patients with symptomatic TR. This study reports the implications of different BMI categories on the outcome of TEER procedures in patients with TR which to the best of our knowledge has not been published before. Our findings can be summarized as follows:
The prevalence of underweight in patients with symptomatic TR receiving TEER treatment was 13.7% (n = 29), additionally, prevalence of obese patients measured 22.3% (n = 47).
Except for a significant higher prevalence of hyperlipidemia and diabetes mellitus type 2 in the obese subcohort, baseline characteristics were comparable throughout the four BMI subcohorts.
For patients with symptomatic TR undergoing TEER, underweight (BMI < 20 kg/m2) and obesity (BMI > 30 kg/m2) were associated with significantly increased risk of 1-year all-cause mortality.
Regarding postprocedural complications, no differences in vascular and bleeding complications were documented.
Underweight and obese patients showed inferior rates of TR reduction of ≥ 2 grades after TEER.
BMI is a comparatively easy-to-asses parameter and routinely documented during hospital admission. Both underweight and overweight have previously been reported to be strong predictors for mortality in cardiovascular disease [14]. In our analysis, the absolute number of underweight patients was 29 (13.7%) and 47 (22.3%) of patients were obese. There were no significant differences regarding age, gender and surgical risk scores at baseline, however, concerning relevant co-morbidities, hyperlipidemia and diabetes mellitus were more frequent in obese patients compared the other subcohorts (Table 1). The prevalence of underweight and obesity and a potential influence on outcome have not been reported in previously published trials examining patients with TR undergoing TEER or other interventional reconstruction systems in the treatment of TR, for example in TRILUMINATE [3, 22], TRI-Repair [28], and studies focusing on TEER with PASCAL Implant System [29] or the MitraClip System [30] systems BMI values were not reported, respectively. Aurich et al. [31] reported BMI values in a small cohort of 16 patients with ≥ severe TR who underwent TEER with the PASCAL Ace Implant System: the mean BMI was 26 ± 3 kg/m2 in the complete cohort, there were no differences between the failed (n = 5) and successful (n = 11) intervention groups (BMI 24 ± 3 kg/m2 vs. BMI 26 ± 4 kg/m2; p = 0.24).
Obesity
Obesity has become an increasingly common chronic condition in the western civilization which is associated with significant morbidity and mortality [32–34]. It has also been characterized as a major and also modifiable risk factor for cardiovascular morbidity and mortality by the American Heart Association/American College of Cardiology and the Nutrition Council of the American Heart Association [35]. However, a considerable number of studies reported a beneficial effect of overweight and obesity on survival in patients with established heart disease in general (e.g. CAD, atrial fibrillation) and specifically in patients undergoing interventional procedures [36–39], such as transcatheter aortic valve replacement [40], TEER for symptomatic mitral regurgitation [17] and in patients with heart failure [41, 42]—in these studies, obesity was characterized inconsistently but mainly as a BMI ≥ 30 kg/m2. The phenomenon of a beneficial effect of overweight and obesity on survival was termed the obesity paradox, and its validity is still under discussion in the literature.
In our analysis, obesity—defined as a BMI ≥ 30 kg/m2—was associated with inferior survival (HR 3.24, 95% CI 1.37–9.16; p < 0.01) compared to normal weight and overweight patients. One reason in this regard might trace back to the fact that the underlying pathology mainly consists of a leading right heart disease and right heart failure whereas the studies showing a beneficial effect of obesity examined leading left heart diseases and valvulopathies, respectively. Thus, a different effect of overweight on outcome in patients with right heart failure and TR does not categorically contradict the proposed obesity paradox as it merely stresses the different disease entities of right and left sided valvulopathies and heart failure and their different clinical presentation and pathophysiology, respectively.
The investigators against the validity of the obesity paradox in the aforementioned cohorts argue that the obese population is younger, seeks medical care earlier, is treated medically more aggressively, and therefore, benefits more from medical and interventional treatment [36], thus obesity being merely a surrogate. Indeed, in our cohort obese patients were slightly but not statistically significant younger (mean age in: obesity 76.6 ± 7.4 years vs. overweight 78.1 ± 6.8 years vs. normal weight 79.5 ± 7.5 years vs. underweight age 78.3 ± 6.3 years; p = 0.18), but did not show a beneficial outcome. A possible explanation could be inferior rates of pronounced TR reduction (≥ 2 TR grades to baseline) of 42.6% in patients with the highest BMI. Additionally, rates of failed procedures (no device implantation or TR reduction ≤ 2 grades to baseline) appeared to be slightly higher in obese patients (17%, 8/47)—mainly driven by fewer cases with a pronounced TR reduction postprocedurally compared to baseline, but without statistical significance (p = 0.06).
A possible explanation might also be a potential difference in leaflet characteristics in obese patients compared to normal- and overweight patients making grasping more difficult, but by now this cannot easily be examined. Moreover, there were no significant differences regarding occurrence of SLDA (single leaflet device attachment) or number of implanted devices.
Additionally, we hypothesize that increasing BMI may have associated independent confounders not demonstrated in our study population for which it was potentially not powered enough and will be addressed in follow-up studies in further detail—such as leaflet characteristics, difficulty with catheter—or device guiding and navigation, challenging imaging, or different atrial and ventricular volumetries and TV annulus anatomies and dimensions.
Lastly, our analysis documented an increased 1-year all-cause mortality of underweight and obese patients receiving TEER for significant TR. Obese patients showed a comparable cardiovascular mortality after 1 year compared to the normal and overweight cohorts. As a main reason we postulate a potential underestimation of cardiovascular mortality in the obese cohort as in 5 deaths (10.6%) reasons for death remained unknown (in comparison to 4.7%, 10.3%, 1.4% and 1.5% for all, underweight, normal- and overweight patients).
High underweight mortality
Underweight has been previously documented as a risk factor for cardiovascular mortality [14]. However, there is no generally accepted definition of underweight—especially in the elderly, thus limiting comparability throughout the studies. Kalbacher et al. [17] reported underweight patients with mitral regurgitation undergoing TEER procedure with the MitraClip system to be exposed to increased short- and long-term mortality. In our cohort, underweight was associated with inferior 1-year survival (HR 3.88; 95% CI 1.64–7.66; p < 0.01) compared to normal weight and overweight patients. A significantly higher rate of cardiovascular death was documented in the underweight cohort compared to the other groups (24.1% vs. 7.0% vs. 6.3% vs. 6.4%; p < 0.01). Overall, only limited data exist about potential reasons as to why underweight is associated with increased mortality rates in cardiovascular patients. It cannot be excluded that underweight might act as a surrogate parameter for frailty as the most common definition used for frailty includes underweight and weight-loss, respectively—as well as serum-albumin levels, grip strengths, cognitive function and mobility (6 min-walking test) [26]. Moreover, underweight might effect a decreased capacity for reconvalescence and an overall reduced physiological reserve which might partly be an explanation for an increased short-term mortality. In addition, increased vulnerability to stressors has been discussed.
Limitations
Several limitations have to be taken into account. The modest sample size, the retrospective and single-center character reduce the generalizability and thus, this study should be considered rather hypothesis generating underlining the need for validation in a prospective and multi-center trial design.
Conclusions
In patients undergoing TEER for significant symptomatic TR, underweight (BMI < 20 kg/m2) and obesity (BMI > 30 kg/m2) were associated with significantly higher 1-year all-cause mortality compared to normal weight and overweight patients with no striking differences in baseline characteristics. There were no differences in bleeding and vascular complications. Changes in NYHA Functional Class and over-all TR reduction were comparable across all subgroups.
Moreover, after interventional treatment for significant TR, lower rates of pronounced TR reduction (≥ 2 TR grades reduction to baseline) were observed in underweight and obese patients compared to normal weight (BMI 20 to < 25 kg/m2) patients.
Considering the well‐documented association between obesity and cardiovascular morbidity and mortality [34, 43, 44] and the expanding nature of obesity as an endemic healthcare problem, it is reasonable to expect an increasing number of obese patients with severe and symptomatic TR being referred for TEER. Thorough preinterventional evaluation should be considered to identify those patients who would benefit most from interventional TR treatment. Identification and incorporation of potential risk factors such as obesity and underweight, frailty and malnutrition, respectively, should be included during patient evaluation and might contribute regarding optimal patient selection. However, there is a great need for detailed multi-center and prospective trials to validate the impact of potential risk factors.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
This research was not supported by grants from funding agencies in the public, commercial, or not-for-profit sectors.
Abbreviations
- LVEF
Left-ventricular ejection fraction
- LV
Left ventricle
- MR
Mitral regurgitation
- NYHA
New York Heart Association
- RVFAC
Right-ventricular fractional area change
- TAPSE
Tricuspid annular plane systolic excursion
- TEER
Transcatheter edge-to-edge repair
- TMVR
Transcatheter mitral valve replacement
- TEE
Transesophageal echocardiography
- TR
Tricuspid regurgitation
- TTE
Transthoracic echocardiography
- BMI
Body mass index
Funding
Open Access funding enabled and organized by Projekt DEAL. There was no funding through external sources.
Data availability
The dataset supporting the conclusions of this article are included within the article. The datasets during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Conflict of interest
Tetsu Tanaka has been financially supported in part by a Fellowship from the Japanese College of Cardiology and the Uehara Memorial Foundation. Atsushi Sugiura has received honoraria for lectures from Edwards Lifesciences. Georg Nickenig and Sebastian Zimmer have received research funding from the Deutsche Forschungsgemeinschaft, the German Federal Ministry of Education and Research, the EU, Abbott, Edwards Lifesciences, and Medtronic and have received honoraria for lectures or advisory boards from Abbott, Edwards Lifesciences, and Medtronic. Marcel Weber has received lecture or proctoring fees from Abbott and Edwards Lifesciences. The other authors have no conflicts of interest.
References
- 1.Prihadi EA. Tricuspid valve regurgitation: no longer the “forgotten valve”. E-J Cardiol Pract. 2018;16:30. [Google Scholar]
- 2.Singh JP, Evans JC, Levy D, et al. Prevalence and clinical determinants of mitral, tricuspid, and aortic regurgitation (the Framingham Heart Study) Am J Cardiol. 1999;83:897–902. doi: 10.1016/S0002-9149(98)01064-9. [DOI] [PubMed] [Google Scholar]
- 3.Nickenig G, Weber M, Lurz P, et al. Transcatheter edge-to-edge repair for reduction of tricuspid regurgitation: 6-month outcomes of the TRILUMINATE single-arm study. Lancet. 2019;394:2002–2011. doi: 10.1016/S0140-6736(19)32600-5. [DOI] [PubMed] [Google Scholar]
- 4.Hahn RT. Tricuspid regurgitation: finally unforgettable! Eur Heart J Cardiovasc Imaging. 2020;21(2):166–167. doi: 10.1093/ehjci/jez249. [DOI] [PubMed] [Google Scholar]
- 5.Mangieri A, Pagnesi M, Regazzoli D, et al. Future perspectives in percutaneous treatment of tricuspid regurgitation. Front Cardiovasc Med. 2020;7:581211. doi: 10.3389/fcvm.2020.581211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benfari G, Antoine C, Miller WL, et al. Excess mortality associated with functional tricuspid regurgitation complicating heart failure with reduced ejection fraction. Circulation. 2019;140:196–206. doi: 10.1161/CIRCULATIONAHA.118.038946. [DOI] [PubMed] [Google Scholar]
- 7.Braunwald NS, Ross J, Jr, Morrow AG. Conservative management of tricuspid regurgitation in patients undergoing mitral valve replacement. Circulation. 1967;35:I63–I69. doi: 10.1161/01.CIR.35.4S1.I-63. [DOI] [PubMed] [Google Scholar]
- 8.Essayagh B, Antoine C, Benfari G, et al. Functional tricuspid regurgitation of degenerative mitral valve disease: a crucial determinant of survival. Eur Heart J. 2020;41:1918–1929. doi: 10.1093/eurheartj/ehaa192. [DOI] [PubMed] [Google Scholar]
- 9.Prihadi EA, van der Bijl P, Gursoy E, et al. Development of significant tricuspid regurgitation over time and prognostic implications: new insights into natural history. Eur Heart J. 2018;39:3574–3581. doi: 10.1093/eurheartj/ehy352. [DOI] [PubMed] [Google Scholar]
- 10.Topilsky Y, Nkomo VT, Vatury O, et al. Clinical outcome of isolated tricuspid regurgitation. JACC Cardiovasc Imaging. 2014;7:1185–1194. doi: 10.1016/j.jcmg.2014.07.018. [DOI] [PubMed] [Google Scholar]
- 11.Chorin E, Rozenbaum Z, Topilsky Y, et al. Tricuspid regurgitation and long-term clinical outcomes. Eur Heart J Cardiovasc Imaging. 2020;21:157–165. doi: 10.1093/ehjci/jez216. [DOI] [PubMed] [Google Scholar]
- 12.Yzeiraj E, Bijuklic K, Tiburtius C, et al. Tricuspid regurgitation is a predictor of mortality after percutaneous mitral valve edge-to-edge repair. EuroIntervention. 2017;12:e1817–e1824. doi: 10.4244/EIJ-D-16-00909. [DOI] [PubMed] [Google Scholar]
- 13.Schueler R, Ozturk C, Sinning JM, et al. Impact of baseline tricuspid regurgitation on long-term clinical outcomes and survival after interventional edge-to-edge repair for mitral regurgitation. Clin Res Cardiol. 2017;106:350–358. doi: 10.1007/s00392-016-1062-1. [DOI] [PubMed] [Google Scholar]
- 14.Park D, Lee JH, Han S. Underweight: another risk factor for cardiovascular disease?: A cross-sectional 2013 Behavioral Risk Factor Surveillance System (BRFSS) study of 491,773 individuals in the USA. Medicine (Baltimore) 2017;96:e8769. doi: 10.1097/MD.0000000000008769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Demir A, Aydinli B, Guclu CY, et al. Obesity and postoperative early complications in open heart surgery. J Anesth. 2012;26:702–710. doi: 10.1007/s00540-012-1393-7. [DOI] [PubMed] [Google Scholar]
- 16.van der Boon RM, Chieffo A, Dumonteil N, et al. Effect of body mass index on short- and long-term outcomes after transcatheter aortic valve implantation. Am J Cardiol. 2013;111:231–236. doi: 10.1016/j.amjcard.2012.09.022. [DOI] [PubMed] [Google Scholar]
- 17.Kalbacher D, Tigges E, Boekstegers P, et al. Underweight is associated with inferior short and long-term outcomes after MitraClip implantation: results from the German TRAnscatheter mitral valve interventions (TRAMI) registry. Am Heart J. 2020;222:73–82. doi: 10.1016/j.ahj.2019.12.022. [DOI] [PubMed] [Google Scholar]
- 18.Vahanian A, Beyersdorf F, Praz F, et al. 2021 ESC/EACTS guidelines for the management of valvular heart disease. Eur J Cardiothorac Surg. 2021;60:727–800. doi: 10.1093/ejcts/ezab389. [DOI] [PubMed] [Google Scholar]
- 19.Dreyfus J, Audureau E, Bohbot Y, et al. TRI-SCORE: a new risk score for in-hospital mortality prediction after isolated tricuspid valve surgery. Eur Heart J. 2022;43:654–662. doi: 10.1093/eurheartj/ehab679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Omran H, Pfister R, Ehrenfels MA, et al. Prognostic performance of the surgical TRI-SCORE risk score in patients undergoing transcatheter tricuspid valve treatment. JACC Cardiovasc Interv. 2022;15:1996–1998. doi: 10.1016/j.jcin.2022.05.031. [DOI] [PubMed] [Google Scholar]
- 21.Kodali S, Hahn RT, Eleid MF, et al. Feasibility study of the transcatheter valve repair system for severe tricuspid regurgitation. J Am Coll Cardiol. 2021;77:345–356. doi: 10.1016/j.jacc.2020.11.047. [DOI] [PubMed] [Google Scholar]
- 22.Lurz P, Stephan von Bardeleben R, Weber M, et al. Transcatheter edge-to-edge repair for treatment of tricuspid regurgitation. J Am Coll Cardiol. 2021;77:229–239. doi: 10.1016/j.jacc.2020.11.038. [DOI] [PubMed] [Google Scholar]
- 23.Mehr M, Taramasso M, Besler C, et al. 1-Year outcomes after edge-to-edge valve repair for symptomatic tricuspid regurgitation: results from the TriValve registry. JACC Cardiovasc Interv. 2019;12:1451–1461. doi: 10.1016/j.jcin.2019.04.019. [DOI] [PubMed] [Google Scholar]
- 24.Nickenig G, Kowalski M, Hausleiter J, et al. Transcatheter treatment of severe tricuspid regurgitation with the edge-to-edge MitraClip technique. Circulation. 2017;135:1802–1814. doi: 10.1161/CIRCULATIONAHA.116.024848. [DOI] [PubMed] [Google Scholar]
- 25.Hahn RT, Zamorano JL. The need for a new tricuspid regurgitation grading scheme. Eur Heart J Cardiovasc Imaging. 2017;18:1342–1343. doi: 10.1093/ehjci/jex139. [DOI] [PubMed] [Google Scholar]
- 26.Kappetein AP, Head SJ, Genereux P, et al. Updated standardized endpoint definitions for transcatheter aortic valve implantation: the Valve Academic Research Consortium-2 consensus document. J Thorac Cardiovasc Surg. 2013;145:6–23. doi: 10.1016/j.jtcvs.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 27.Varc-3 Writing C. Genereux P, Piazza N, et al. Valve academic research consortium 3: updated endpoint definitions for aortic valve clinical research. J Am Coll Cardiol. 2021;77:2717–2746. doi: 10.1016/j.jacc.2021.02.038. [DOI] [PubMed] [Google Scholar]
- 28.Nickenig G, Weber M, Schuler R, et al. Tricuspid valve repair with the Cardioband system: two-year outcomes of the multicentre, prospective TRI-REPAIR study. EuroIntervention. 2021;16:e1264–e1271. doi: 10.4244/EIJ-D-20-01107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kitamura M, Fam NP, Braun D, et al. 12-Month outcomes of transcatheter tricuspid valve repair with the PASCAL system for severe tricuspid regurgitation. Catheter Cardiovasc Interv. 2021;97:1281–1289. doi: 10.1002/ccd.29583. [DOI] [PubMed] [Google Scholar]
- 30.Ruf TF, Hahn RT, Kreidel F, et al. Short-term clinical outcomes of transcatheter tricuspid valve repair with the third-generation MitraClip XTR system. JACC Cardiovasc Interv. 2021;14:1231–1240. doi: 10.1016/j.jcin.2021.03.033. [DOI] [PubMed] [Google Scholar]
- 31.Aurich M, Volz MJ, Mereles D, et al. Initial experience with the PASCAL ace implant system for treatment of severe tricuspid regurgitation. Circ Cardiovasc Interv. 2021;14:e010770. doi: 10.1161/CIRCINTERVENTIONS.121.010770. [DOI] [PubMed] [Google Scholar]
- 32.Adams KF, Schatzkin A, Harris TB, et al. Overweight, obesity, and mortality in a large prospective cohort of persons 50 to 71 years old. N Engl J Med. 2006;355:763–778. doi: 10.1056/NEJMoa055643. [DOI] [PubMed] [Google Scholar]
- 33.Batty GD, Shipley MJ, Jarrett RJ, Breeze E, Marmot MG, Davey SG. Obesity and overweight in relation to disease-specific mortality in men with and without existing coronary heart disease in London: the original Whitehall study. Heart. 2006;92:886–892. doi: 10.1136/hrt.2005.072637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Haslam DW, James WP. Obesity. Lancet. 2005;366:1197–1209. doi: 10.1016/S0140-6736(05)67483-1. [DOI] [PubMed] [Google Scholar]
- 35.Poirier P, Giles TD, Bray GA, et al. Obesity and cardiovascular disease: pathophysiology, evaluation, and effect of weight loss: an update of the 1997 American Heart Association Scientific Statement on Obesity and Heart Disease from the Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism. Circulation. 2006;113:898–918. doi: 10.1161/CIRCULATIONAHA.106.171016. [DOI] [PubMed] [Google Scholar]
- 36.Chrysant SG, Chrysant GS. New insights into the true nature of the obesity paradox and the lower cardiovascular risk. J Am Soc Hypertens. 2013;7:85–94. doi: 10.1016/j.jash.2012.11.008. [DOI] [PubMed] [Google Scholar]
- 37.Gurm HS, Whitlow PL, Kip KE, Investigators B The impact of body mass index on short- and long-term outcomes inpatients undergoing coronary revascularization. Insights from the bypass angioplasty revascularization investigation (BARI) J Am Coll Cardiol. 2002;39:834–840. doi: 10.1016/S0735-1097(02)01687-X. [DOI] [PubMed] [Google Scholar]
- 38.Mehta L, Devlin W, McCullough PA, et al. Impact of body mass index on outcomes after percutaneous coronary intervention in patients with acute myocardial infarction. Am J Cardiol. 2007;99:906–910. doi: 10.1016/j.amjcard.2006.11.038. [DOI] [PubMed] [Google Scholar]
- 39.Romero-Corral A, Montori VM, Somers VK, et al. Association of bodyweight with total mortality and with cardiovascular events in coronary artery disease: a systematic review of cohort studies. Lancet. 2006;368:666–678. doi: 10.1016/S0140-6736(06)69251-9. [DOI] [PubMed] [Google Scholar]
- 40.Konigstein M, Havakuk O, Arbel Y, et al. The obesity paradox in patients undergoing transcatheter aortic valve implantation. Clin Cardiol. 2015;38:76–81. doi: 10.1002/clc.22355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Horwich TB, Fonarow GC, Hamilton MA, MacLellan WR, Woo MA, Tillisch JH. The relationship between obesity and mortality in patients with heart failure. J Am Coll Cardiol. 2001;38:789–795. doi: 10.1016/S0735-1097(01)01448-6. [DOI] [PubMed] [Google Scholar]
- 42.Lavie CJ, Alpert MA, Arena R, Mehra MR, Milani RV, Ventura HO. Impact of obesity and the obesity paradox on prevalence and prognosis in heart failure. JACC Heart Fail. 2013;1:93–102. doi: 10.1016/j.jchf.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 43.Allison DB, Fontaine KR, Manson JE, Stevens J, VanItallie TB. Annual deaths attributable to obesity in the United States. JAMA. 1999;282:1530–1538. doi: 10.1001/jama.282.16.1530. [DOI] [PubMed] [Google Scholar]
- 44.Grundy SM, Pasternak R, Greenland P, Smith S, Jr, Fuster V. Assessment of cardiovascular risk by use of multiple-risk-factor assessment equations: a statement for healthcare professionals from the American Heart Association and the American College of Cardiology. Circulation. 1999;100:1481–1492. doi: 10.1161/01.CIR.100.13.1481. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The dataset supporting the conclusions of this article are included within the article. The datasets during and/or analyzed during the current study are available from the corresponding author on reasonable request.