Abstract
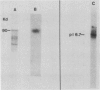
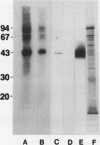
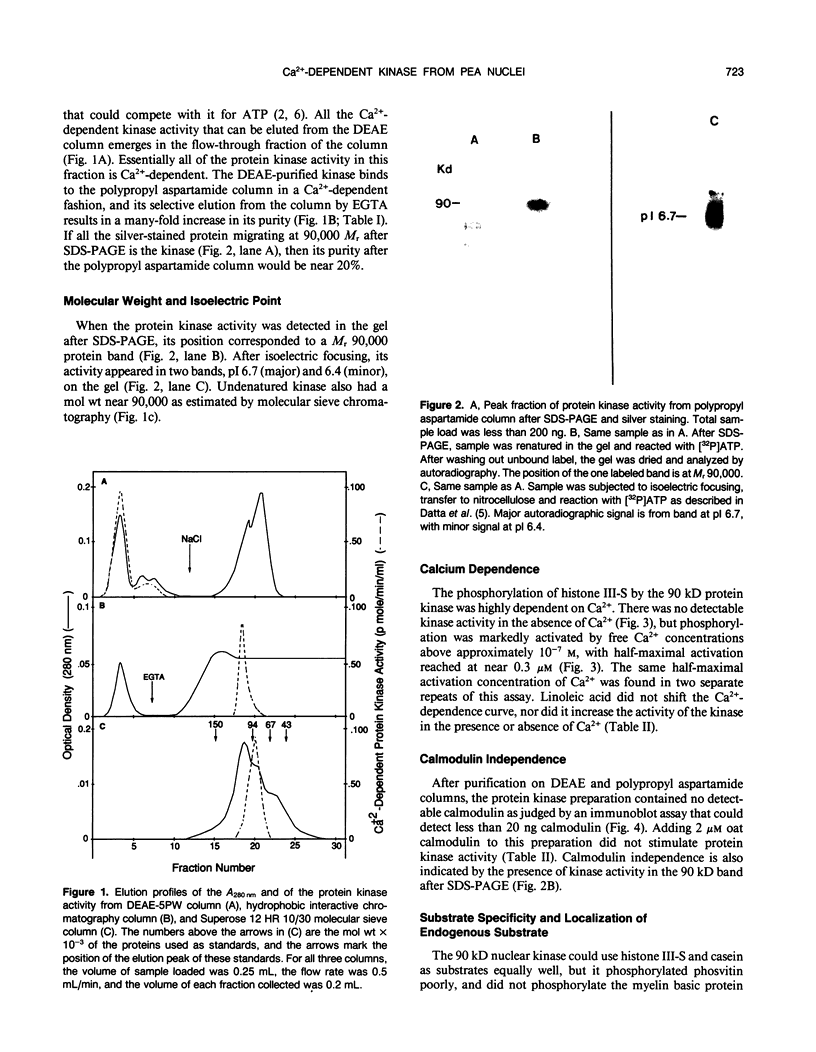
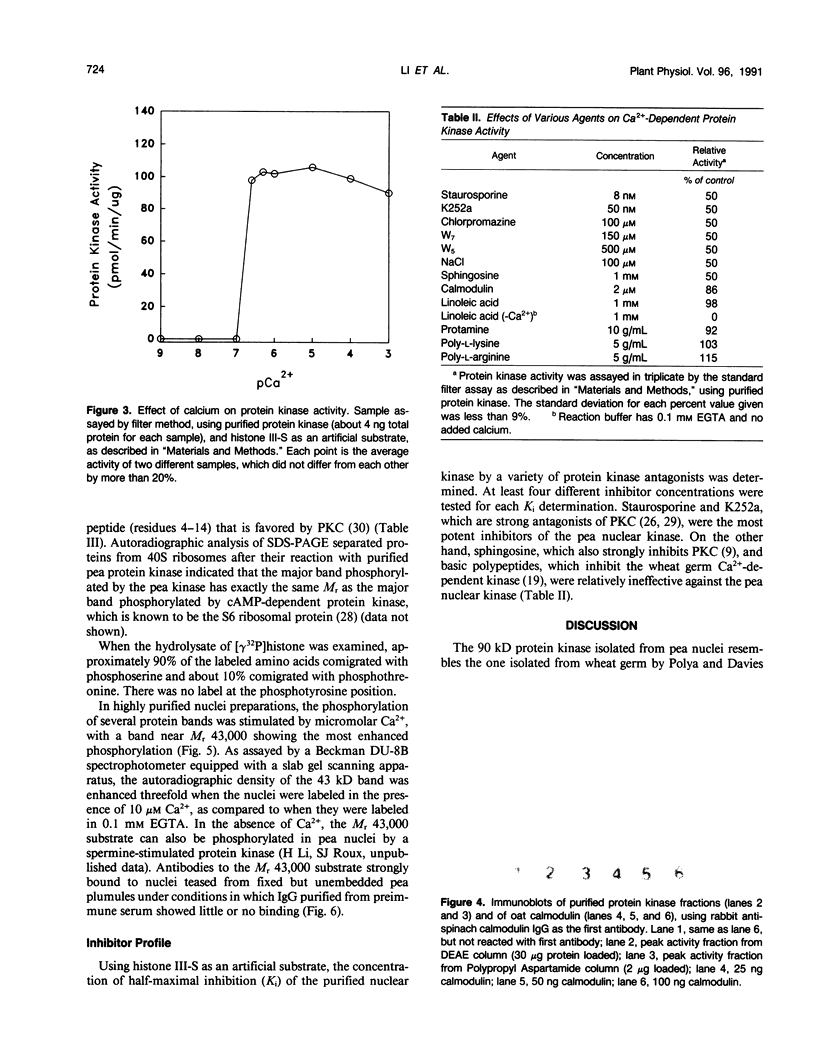
Almost all the Ca2+-dependent protein kinase activity in nuclei purified from etiolated pea (Pisum sativum, L.) plumules is present in a single enzyme that can be extracted from chromatin by 0.3 molar NaCl. This protein kinase can be further purified 80,000-fold by salt fractionation and high performance liquid chromatography, after which it has a high specific activity of about 100 picomoles per minute per microgram in the presence of Ca2+ and reaches half-maximal activation at about 3 ×10−7 molar free Ca2+, without calmodulin. It is a monomer with a molecular weight near 90,000. It can efficiently use histone III-S, ribosomal S6 protein, and casein as artificial substrates, but it phosphorylates phosvitin only weakly. Its Ca2+-dependent kinase activity is half-maximally inhibited by 0.1 millimolar chlorpromazine, by 35 nanomolar K-252a and by 7 nanomolar staurosporine. It is insensitive to sphingosine, an inhibitor of protein kinase C, and to basic polypeptides that block other Ca2+-dependent protein kinases. It is not stimulated by exogenous phospholipids or fatty acids. In intact isolated pea nuclei it preferentially phosphorylates several chromatin-associated proteins, with the most phosphorylated protein band being near the same molecular weight (43,000) as a nuclear protein substrate whose phosphorylation has been reported to be stimulated by phytochrome in a calcium-dependent fashion.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Biro R. L., Daye S., Serlin B. S., Terry M. E., Datta N., Sopory S. K., Roux S. J. Characterization of oat calmodulin and radioimmunoassay of its subcellular distribution. Plant Physiol. 1984 Jun;75(2):382–386. doi: 10.1104/pp.75.2.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y. R., Datta N., Roux S. J. Purification and partial characterization of a calmodulin-stimulated nucleoside triphosphatase from pea nuclei. J Biol Chem. 1987 Aug 5;262(22):10689–10694. [PubMed] [Google Scholar]
- Datta N., Cashmore A. R. Binding of a pea nuclear protein to promoters of certain photoregulated genes is modulated by phosphorylation. Plant Cell. 1989 Nov;1(11):1069–1077. doi: 10.1105/tpc.1.11.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta N., Chen Y. R., Roux S. J. Phytochrome and calcium stimulation of protein phosphorylation in isolated pea nuclei. Biochem Biophys Res Commun. 1985 May 16;128(3):1403–1408. doi: 10.1016/0006-291x(85)91096-4. [DOI] [PubMed] [Google Scholar]
- Datta N., Schell M. B., Roux S. J. Spermine stimulation of a nuclear NII kinase from pea plumules and its role in the phosphorylation of a nuclear polypeptide. Plant Physiol. 1987;84:1397–1401. doi: 10.1104/pp.84.4.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dauwalder M., Roux S. J., Hardison L. Distribution of calmodulin in pea seedlings: immunocytochemical localization in plumules and root apices. Planta. 1986;168:461–470. [PubMed] [Google Scholar]
- Guo Y-L, Roux S. J. Partial purification and characterization of a Ca(2+)-dependent protein kinase from the green alga, Dunaliella salina. Plant Physiol. 1990;94:143–150. doi: 10.1104/pp.94.1.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannun Y. A., Loomis C. R., Merrill A. H., Jr, Bell R. M. Sphingosine inhibition of protein kinase C activity and of phorbol dibutyrate binding in vitro and in human platelets. J Biol Chem. 1986 Sep 25;261(27):12604–12609. [PubMed] [Google Scholar]
- Harmon A. C., Putnam-Evans C., Cormier M. J. A calcium-dependent but calmodulin-independent protein kinase from soybean. Plant Physiol. 1987 Apr;83(4):830–837. doi: 10.1104/pp.83.4.830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lam E., Benedyk M., Chua N. H. Characterization of phytochrome-regulated gene expression in a photoautotrophic cell suspension: possible role for calmodulin. Mol Cell Biol. 1989 Nov;9(11):4819–4823. doi: 10.1128/mcb.9.11.4819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin L. N. Separation of guinea pig IgG subclasses by affinity chromatography on protein A-sepharose. J Immunol Methods. 1982 Jul 30;52(2):205–212. doi: 10.1016/0022-1759(82)90046-1. [DOI] [PubMed] [Google Scholar]
- Oakley B. R., Kirsch D. R., Morris N. R. A simplified ultrasensitive silver stain for detecting proteins in polyacrylamide gels. Anal Biochem. 1980 Jul 1;105(2):361–363. doi: 10.1016/0003-2697(80)90470-4. [DOI] [PubMed] [Google Scholar]
- Polya G. M., Davies J. R., Micucci V. Properties of a calmodulin-activated Ca2+-dependent protein kinase from wheat germ. Biochim Biophys Acta. 1983 Nov 22;761(1):1–12. doi: 10.1016/0304-4165(83)90355-0. [DOI] [PubMed] [Google Scholar]
- Polya G. M., Nott R., Klucis E., Minichiello J., Chandra S. Inhibition of plant calcium-dependent protein kinases by basic polypeptides. Biochim Biophys Acta. 1990 Feb 9;1037(2):259–262. doi: 10.1016/0167-4838(90)90177-h. [DOI] [PubMed] [Google Scholar]
- Putnam-Evans C. L., Harmon A. C., Cormier M. J. Purification and characterization of a novel calcium-dependent protein kinase from soybean. Biochemistry. 1990 Mar 13;29(10):2488–2495. doi: 10.1021/bi00462a008. [DOI] [PubMed] [Google Scholar]
- Roskoski R., Jr Assays of protein kinase. Methods Enzymol. 1983;99:3–6. doi: 10.1016/0076-6879(83)99034-1. [DOI] [PubMed] [Google Scholar]
- Silberman L. G., Datta N., Hoops P., Roux S. J. Characterization of monoclonal antibodies to oat phytochrome by competitive radioimmunoassays and comparative immunoblots of phytochrome peptides. Arch Biochem Biophys. 1985 Jan;236(1):150–158. doi: 10.1016/0003-9861(85)90614-9. [DOI] [PubMed] [Google Scholar]
- Suzuki H., Verma D. P. Nodule-specific kinases phosphorylating nuclear factors in isolated nuclei. Plant Cell. 1989 Mar;1(3):373–379. doi: 10.1105/tpc.1.3.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamaoki T., Nomoto H., Takahashi I., Kato Y., Morimoto M., Tomita F. Staurosporine, a potent inhibitor of phospholipid/Ca++dependent protein kinase. Biochem Biophys Res Commun. 1986 Mar 13;135(2):397–402. doi: 10.1016/0006-291x(86)90008-2. [DOI] [PubMed] [Google Scholar]
- Wettenhall R. E., Morgan F. J. Phosphorylation of hepatic ribosomal protein S6 on 80 and 40 S ribosomes. Primary structure of S6 in the region of the major phosphorylation sites for cAMP-dependent protein kinases. J Biol Chem. 1984 Feb 25;259(4):2084–2091. [PubMed] [Google Scholar]
- Yamada K., Iwahashi K., Kase H. K252a, a new inhibitor of protein kinase C, concomitantly inhibits 40K protein phosphorylation and serotonin secretion in a phorbol ester-stimulated platelets. Biochem Biophys Res Commun. 1987 Apr 14;144(1):35–40. doi: 10.1016/s0006-291x(87)80471-0. [DOI] [PubMed] [Google Scholar]
- Yasuda I., Kishimoto A., Tanaka S., Tominaga M., Sakurai A., Nishizuka Y. A synthetic peptide substrate for selective assay of protein kinase C. Biochem Biophys Res Commun. 1990 Feb 14;166(3):1220–1227. doi: 10.1016/0006-291x(90)90996-z. [DOI] [PubMed] [Google Scholar]