Abstract
Vaccination of mice with plasmid DNA carrying the gene for the major secreted mycobacterial antigen 85A (Ag85A) from Mycobacterium tuberculosis is a powerful technique for generating robust specific Th1 helper T-cell responses, CD8+-mediated cytotoxicity, and protection against M. tuberculosis challenge (K. Huygen et al., Nat. Med. 2:893–898, 1996). We have now analyzed in more detail the antigen-specific immune CD4+- and CD8+-T-cell responses induced in BALB/c mice vaccinated with Ag85A DNA and have compared these responses to those generated by intravenous infection with M. tuberculosis. T-cell-epitope mapping, as measured by interleukin-2 and gamma interferon secretion from splenic T cells restimulated in vitro with synthetic 20-mer peptides spanning the complete mature sequence of Ag85A, demonstrated that DNA vaccination stimulated a stronger and broader T-cell response than did M. tuberculosis infection. Moreover, elevated cytotoxic T lymphocyte (CTL) activity against Ag85A-transfected and peptide-pulsed P815 target cells could be generated exclusively by vaccination with plasmid DNA, not following M. tuberculosis infection. By using DNA vaccination, three Ag85A CTL epitopes with predicted major histocompatibility complex class I binding motifs were defined. One of them was previously reported as a dominant, promiscuously recognized T-cell epitope in healthy humans with primary infections. These data strengthen the potential of DNA vaccination with respect to inducing antituberculous immunity in humans.
Tuberculosis remains a major health problem affecting millions of people worldwide (2). Combination chemotherapy is very effective in curing this disease, but unfortunately, the treatment is long and expensive and requires stringent compliancy to avoid the development of multidrug-resistant forms. The only tuberculosis vaccine currently available is an attenuated strain of Mycobacterium bovis, termed bacillus Calmette-Guérin (BCG). BCG continues to be widely administered to children in developing countries, yet its efficacy remains controversial, particularly against the pulmonary form of the disease in adults (3). Clearly, the development of a more effective vaccine could be an effective solution to the global threat of tuberculosis.
Administration of plasmid DNA expression vectors has been shown to result in protein expression in vivo, the generation of humoral and cell-mediated immune responses, and protection in animal models of infectious diseases including influenza, human immunodeficiency virus infection, bovine herpes, rabies, malaria, leishmaniasis, herpes simplex, and cottontail papilloma (10, 32). Therefore, DNA vaccination seems to be a broadly applicable technique for generating protective immune responses against infectious pathogens without the need for live organisms, replicating vectors, or adjuvants.
Recently, we and others have shown that vaccination with plasmid DNA containing Mycobacterium tuberculosis genes encoding hsp65 (31), the 38-kDa PstS-1 homolog (35), and the fibronectin-binding antigen 85 (Ag85) complex (18) is an effective means for inducing protective immunity in animal models. The three components of the Ag85 complex, a 30-32-kDa family of proteins (Ag85A, Ag85B, and Ag85C), constitute a major portion of the secreted proteins in M. tuberculosis and BCG culture filtrate (33). The bacteriostatic drug isoniazid enhances the expression of this Ag85 complex in M. tuberculosis culture filtrate (15) and has been described as possessing enzymatic trehalose mycolyltransferase activity (1a). The Ag85 complex induces strong T-cell proliferation, gamma interferon (IFN-γ) production, and cytotoxic T lymphocyte (CTL) activity in most healthy individuals infected with M. tuberculosis or Mycobacterium leprae and in BCG-vaccinated mice and humans (16, 22, 25, 28, 30), making it a promising candidate as a protective antigen. Genetic vaccination with plasmid DNA encoding the Ag85A component of the Ag85 complex (Ag85A DNA) was found to generate robust specific Th1-type helper T-cell responses and CD8-mediated cytotoxic T-cell responses and induced protection in a mouse model against aerosol or intravenous M. tuberculosis challenge (reference 18 and unpublished data). Vaccination with plasmid DNA encoding the Ag85B component but not the Ag85C component was also found effective for generating strong Th1- and CD8+-mediated immune responses (23).
Here, we have analyzed in detail the immunogenicity of a DNA vaccine carrying the gene encoding Ag85A in comparison with the immunogenicity of intravenous M. tuberculosis infection. We show that in BALB/c mice, Ag85A DNA vaccination is more effective than live infection at inducing cellular immune responses, including Th1-type helper T-cell responses and CTL activity, against Ag85. Hence, DNA vaccination holds promise as a method of inducing immunity in humans.
MATERIALS AND METHODS
Plasmid construction.
Plasmid DNA encoding Ag85A was prepared as described previously. Briefly, the Ag85A gene of M. tuberculosis was amplified without the mycobacterial signal sequence from plasmid p85A.tub (4) by PCR with the BglII site containing primers. Amplified DNA was digested with BglII, isolated on a 1% agarose gel, and extracted on Prep a Gene (Bio-Rad). Fragments were ligated to the BglII-digested and dephosphorylated V1J-ns (24) or V1J-ns-tPA (29) vector, transformed into competent Escherichia coli DH5 (BRL) cells and plated on LB agar medium containing kanamycin (50 μg/ml). Recombinant plasmid DNA was amplified in E. coli DH5 and purified on two cesium chloride-ethidium bromide gradients, followed by extractions with 1-butanol and phenol-chloroform and ethanol precipitation. Plasmid DNA was adjusted to a final concentration of 1 mg/ml in saline and stored at −20°C. In these plasmids, the Ag85A gene is expressed under control of the promoter and intron A of the first immediate-early antigen IE1 from cytomegalovirus and followed by a polyadenylation site of the bovine growth hormone.
Mice.
BALB/c mice were purchased from Bantin and Kingman or bred in the Animal Facilities of the Pasteur Institute of Brussels, Belgium. Only female mice, 6 to 8 weeks old at the start of vaccination, were used.
DNA vaccination.
Mice were anesthesized by intraperitoneal injection of ketamine and xylazine (100 and 10 mg/kg, respectively) and injected intramuscularly three times (at 3-week intervals) in both quadriceps with Ag85A or control DNA (empty vector) in saline, by using a 0.3-ml insulin syringe (Becton Dickinson, Mountain View, Calif.). The mice received 100 μg of DNA at each injection and were analyzed 3 weeks after the last DNA vaccination.
M. tuberculosis and M. bovis BCG infection.
Mice were infected intravenously in the lateral tail vein with 104 CFU of M. tuberculosis H37Rv or 106 CFU of M. bovis BCG (strain GL2, derived from 1173P2 Paris) grown as a surface pellicle on synthetic Sauton medium for 14 days and stored as a concentrated stock solution at −20°C in glycerol. The mice were sacrificed after 4 or 12 weeks.
Antigens.
Ag85A was purified from M. bovis BCG culture filtrate (CF) as described previously by sequential chromatography on phenyl-Sepharose and DEAE-Sephacel ion exchange columns and molecular sieving on Sephadex G75 (8). Purified protein derivative (PPD), BCG CF, BCG cytoplasmic extract, and whole BCG bacilli were prepared as described before (16).
Peptide synthesis.
Peptides were synthesized on Tenta-Gel S-RAM and purified by high-pressure liquid chromatography as described before (17). Twenty-eight peptides spanning the complete mature Ag85A sequence from M. tuberculosis (295 amino acids [aa]) were synthesized as 20-mer peptides overlapping by 10 aa. Thirty-one peptides spanning the complete mature Ag85B sequence from M. tuberculosis (285 aa) were synthesized as 18-mer residues overlapping by 9 aa.
Cytokine production.
DNA-vaccinated or M. tuberculosis-infected mice were sacrificed, and their spleens were removed aseptically. The spleens from three mice were pooled in each group. Spleen cells were adjusted to a concentration of 4 × 106 cells/ml and grown in round-bottom microwell plates (Nunc) in RPMI 1640 (Gibco-BRL) supplemented with glutamine, HEPES, 50 μM 2-mercaptoethanol, antibiotics, and 10% heat-inactivated fetal calf serum (FCS; Gibco-BRL). A volume of 180 μl of cell suspension was added to a 20-μl volume of PPD, CF, BCG extract, whole BCG bacilli, purified Ag85A, or synthetic peptide. The cells were incubated at 37°C in a humidified CO2 incubator, and supernatants were harvested after 24 (interleukin-2 [IL-2]) and 72 (IFN-γ) h. Supernatants from three separate wells were pooled and stored frozen at −20°C until assay. Each experiment was performed at least twice.
Depletion of CD4+ or CD8+ T cells.
Mice were sacrificed 3 weeks after the third DNA injection, and their spleens were removed aseptically. The spleens from three mice were pooled in each group. Cells were isolated by use of a loosely fitting Dounce homogenizer, washed, and incubated with RL172 (anti-CD4) or 83.12.5 (anti-CD8) monoclonal antibody culture supernatant for 45 min at 37°C (5 × 106 cells/ml). Spleen cells were then pelleted and resuspended in low-toxicity rabbit serum (Cedarlane, Hornby, Ontario, Canada) as a complement source for 45 min at 37°C. Viable lymphocytes were purified on Lympholyte-M (Cedarlane). Finally, the cells were washed twice before their use in the cytokine induction experiment described above. The purity of these cell preparations was more than 95% on flow cytometric analysis in FACScalibur (Becton Dickinson).
IL-2 assay.
IL-2 activity was measured by a bioassay. Briefly, a volume of 100 μl of 24-h culture supernatant was added to 100 μl of CTLL-2 cells (105/ml) and incubated for 48 h (16). [3H]thymidine (Amersham; 8.3 Ci/ml) was added (0.4 μCi/well) during the last 6 h of culture. Cells were harvested on a Titertek cell harvester, and the radioactivity recovered on the fiber mats was counted in a Betaplate scintillation counter. Each sample was tested in duplicate. IL-2 levels are expressed as mean counts per minute. The standard deviation was below 10%. An internal laboratory control preparation of mouse IL-2 with a previously determined titer, based on NIBSC international standard 86/504, was run simultaneously in each assay. In this assay, 50,000 cpm corresponds to 3.12 IU/ml or about 600 pg/ml and the detection limit is around 10 pg/ml.
IFN-γ assay.
IFN-γ activity was quantified in duplicate for 72-h culture supernatants by using a mouse IFN-γ enzyme-linked immunosorbent assay (ELISA) (Intertest-γ; Genzyme catalog no. 80-3842-03). Titers are expressed as mean picograms per milliliter; the standard deviation was below 10%. The detection limit in this assay is 10 pg/ml. For comparison with previous results from an IFN bioassay (17), 1 log2 unit corresponds to 220 pg/ml.
In vitro stimulation of CTLs.
Spleen cells (5 × 106/well) from DNA-vaccinated or M. tuberculosis-infected mice (pool of three mice) were cocultured in 24-well plates with mitomycin-treated or gamma-irradiated (2,000 rads) P815-Ag85A (P815 cells transfected with the Ag85A cDNA [18]) stimulators (5 × 105/well) in RPMI 1640 containing 10% FCS, 2-mercaptoethanol, l-glutamine, and antibiotics. Cultures were maintained at 37°C in 5% CO2 for 6 days.
Cytolytic assay.
Lymphocytes harvested from the stimulated cultures were tested for cytotoxicity in a 4-h 51Cr release assay in round-bottom microwell plates with 104 51Cr-labeled P815 or P815-Ag85A-transfected target cells. Effector cells were added to target cells at various effector-cell-to-target-cell (E/T) ratios in RPMI 1640 supplemented with 10% FCS in a total volume of 0.2 ml. For CTL epitope mapping, peptides were added together with effector and target cells to a final concentration of 5 μg/ml. Spontaneous- and total-release samples were prepared by adding the targets to wells containing only medium or medium plus 2 M H2SO4. After 4 h, the plates were centrifuged, and 150 μl of supernatant was collected and counted in a gamma counter (LKB). Percent specific 51Cr release was calculated as 100 × [(release by CTLs − spontaneous release)/(total release − spontaneous release)]. Spontaneous release was generally 10 to 15% of the total release. Experiments were performed twice.
RESULTS
Ag85-specific IL-2 and IFN-γ production in BALB/c mice vaccinated with DNA encoding Ag85A or infected with M. tuberculosis H37Rv.
As shown in Table 1, significant IL-2 and IFN-γ production in response to purified native Ag85A protein could be measured in spleen cell culture supernatants from BALB/c mice vaccinated with Ag85A DNA, when tested 3 weeks after the third DNA injection. Mice vaccinated with control DNA produced only minimal amounts of these two cytokines. Significant cytokine levels were also detected in spleen cell cultures from Ag85A DNA-vaccinated mice stimulated with BCG culture filtrate (from which native Ag85A is purified), but not with PPD, cytoplasmic BCG extract, or whole BCG bacilli. In contrast, spleen cells from BALB/c mice infected with live M. tuberculosis secreted IL-2 and IFN-γ in response to all these inducers. Preliminary analysis at 2, 4, 6, 8, and 12 weeks after infection showed that peak values of IFN-γ in response to all inducers were observed early, i.e., 2 to 4 weeks after infection, and gradually declined over the next 2 months (data not shown). IL-2 levels in response to Ag85A were also maximal 2 to 4 weeks after infection, whereas IL-2 response to the other inducers increased up to 3 months after infection (data not shown). Therefore, peak levels of cytokine secretion in infected mice, i.e., at 4 and 12 weeks after infection, were compared with peak responses of DNA-vaccinated mice, i.e., at 3 weeks after the third DNA injection. IL-2 and IFN-γ levels in response to Ag85A were two and four times higher, respectively, in DNA-vaccinated than in M. tuberculosis-infected mice (Table 1).
TABLE 1.
Th1 cytokine production in BALB/c mice vaccinated with plasmid DNA encoding Ag85A or infected with M. tuberculosis H37Rv
| Spleen cell culturea | IL-2 production (mean cpm) in miceb
|
IFN-γ production (pg/ml) in micec
|
||||||
|---|---|---|---|---|---|---|---|---|
| Vaccinated with:
|
Infected with M. tuberculosis
|
Vaccinated with:
|
Infected with M. tuberculosis
|
|||||
| Control DNA | Ag85A DNA | Day 28 | Day 84 | Control DNA | Ag85A DNA | Day 28 | Day 84 | |
| Control | 419 | 482 | 799 | 172 | <10 | 198 | 161 | 20 |
| Ag85A | 220 | 12,990 | 7,425 | 8,739 | <10 | 1,628 | 419 | 113 |
| PPD | 124 | 3,335 | 7,875 | 81,690 | <10 | 342 | 2,089 | 1,280 |
| CF | 218 | 12,650 | 10,116 | 40,001 | <10 | 884 | 1,121 | 452 |
| Extract | 178 | 1,300 | 2,741 | 30,039 | <10 | 304 | 943 | 452 |
| BCG | 320 | 385 | 774 | 4,205 | <10 | 242 | 930 | 113 |
Cytokine production was determined in spleen cell cultures from BALB/c mice vaccinated with control DNA encoding Ag85A or infected with M. tuberculosis and either unstimulated (control) or stimulated in vitro with purified native Ag85A (5 μg/ml), PPD (25 μg/ml), BCG CF (25 μg/ml), BCG cytoplasmic extract (25 μg/ml), or whole BCG bacilli (25 μg/ml).
IL-2 production was assayed in 24-h culture supernatants and determined by a bioassay.
IFN-γ production was assayed in 72-h culture supernatants from pooled contents of three wells and determined by an ELISA.
IL-2 secretion in response to synthetic overlapping peptides from Ag85A in BALB/c mice vaccinated with plasmid DNA encoding Ag85A or infected with M. tuberculosis.
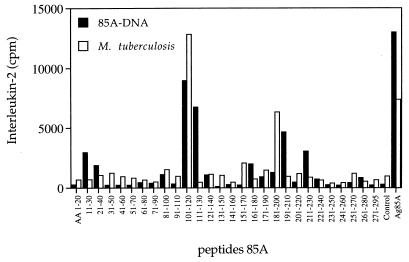
As shown in Fig. 1, strong IL-2 production could be measured in spleen cell culture supernatant from Ag85A DNA-vaccinated mice upon 24 h of in vitro stimulation with synthetic 20-mer peptides spanning the complete Ag85A protein. The strongest IL-2 production was observed in response to peptide 11 (p11; aa 101 to 120), the T-cell epitope that was also the most reactive in M. tuberculosis-infected mice and that we previously identified as the dominant Th1 epitope in BALB/c and DBA/2 mice vaccinated with live M. bovis BCG (17). Ag85A DNA-vaccinated mice, but not M. tuberculosis-infected or BCG-vaccinated mice, also consistently reacted to the overlapping p12 (aa 111 to 130). Lower-level IL-2 production was found following DNA vaccination in response to other peptides previously defined by BCG vaccination, i.e., p7 (aa 61 to 80), p9 (aa 81 to 100), and p17 (aa 161 to 180). Interestingly, though, DNA vaccination also generated a substantial IL-2 response against peptides which were not recognized following BCG vaccination or M. tuberculosis infection, i.e., p2 and p3 (aa 11 to 40) and two regions in the carboxy-terminal part of the protein, i.e., p22 and p23 (aa 211 to 240) and p27 (aa 261 to 280). Finally, significant levels of IL-2 production were observed in DNA-vaccinated and M. tuberculosis-infected mice but not in BCG-vaccinated mice against p19 and p20 (aa 181 to 210). Spleen cells from BALB/c mice vaccinated with Ag85A DNA demonstrated cross-reactive IL-2 responses against Ag85B as detected by responses against synthetic 18-mer Ag85B peptides spanning aa 64 to 81 and particularly aa 199 to 216 (data not shown). No cross-reactive IL-2 production was found in response to p12 (aa 99 to 116) from Ag85B, which overlapped with the immunodominant p11 region of Ag85A (data not shown). In vitro depletion of CD4+ or CD8+ T cells from total splenocytes of Ag85A DNA-vaccinated mice prior to antigenic or peptide stimulation demonstrated that all IL-2 produced was derived from genuine CD4+ Th1 helper T cells (Table 2).
FIG. 1.
IL-2 production (mean counts per minute) in response to restimulation with synthetic 20-mer peptides (overlapping by 10 aa, spanning the complete mature Ag85A sequence) of spleen cells from BALB/c mice vaccinated with plasmid DNA encoding Ag85A or infected with M. tuberculosis. Pooled spleens from three mice were either unstimulated (control) or stimulated with the peptides (10 μg/ml) or with purified Ag85A (5 μg/ml), and 24-h supernatants were tested by a bioassay on mouse CTLL-2 cells.
TABLE 2.
Contribution of CD4+ and CD8+ T cells to IL-2 and IFN-γ production in Ag85A DNA-vaccinated BALB/c micea
| Supernatantb | IL-2 production (mean cpm)
|
IFN-γ production (pg/ml)
|
||||
|---|---|---|---|---|---|---|
| Total | CD4− | CD8− | Total | CD4− | CD8− | |
| Control | 664a | 127 | 753 | <10b | <10 | <10 |
| CF | 24,148 | 137 | 22,944 | 1,363 | <10 | 2,411 |
| Ag85A | 28,937 | 113 | 23,629 | 2,453 | <10 | 2,708 |
| PHA-P | 205,262 | 56,848 | 159,344 | 8,112 | >9,600 | 1,568 |
| p7 (aa 61 to 80) | 1,515 | 179 | 2,609 | 341 | <10 | 696 |
| p11 (aa 101 to 120) | 6,994 | 162 | 8,316 | 1,376 | 156 | 1,926 |
| p15 (aa 141 to 160) | 950 | 109 | 1,234 | 1,272 | 439 | 407 |
| p20 (aa 191 to 210) | 11,524 | 152 | 19,748 | 894 | <10 | 1,020 |
Cytokine production was determined in total spleen cells or in CD4-depleted (CD4−) or CD8-depleted (CD8−) spleen cells from BALB/c mice vaccinated with DNA encoding secreted Ag85A DNA (pooled spleens of three mice). Cells were assayed for IL-2 by testing 24-h culture supernatants and for IFN-γ by testing 72-h culture supernatants.
Supernatant final concentrations: CF, 25 μg/ml; Ag85A, 5 μg/ml; phytohemagglutin (PHA-P; Wellcome), 4 μg/ml; 20-mer peptides, 10 μg/ml.
IFN-γ secretion in response to synthetic overlapping peptides from Ag85A in BALB/c mice vaccinated with plasmid DNA encoding Ag85A or infected with M. tuberculosis.
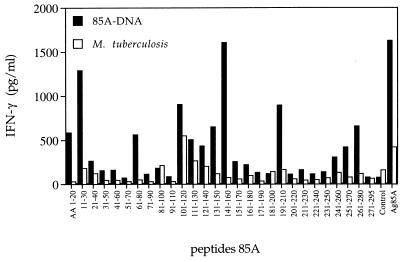
The broadening of the epitopic repertoire following DNA vaccination was even more pronounced for IFN-γ production (Fig. 2). Similar to previous observations for BCG-vaccinated H-2d mice (17), spleen cells from M. tuberculosis-infected BALB/c mice secreted significant levels of IFN-γ almost exclusively in response to p11 (aa 101 to 120). Mice vaccinated with Ag85A DNA, on the other hand, reacted against a wide array of peptides in both the N-terminal and carboxy-terminal parts of Ag85A. Thus, high IFN-γ levels were produced in response to p1 and p2 (aa 1 to 30), p7 (aa 61 to 80), p11 to p15 (aa 101 to 160), p20 (aa 191 to 210), and p25 and p27 (aa 241 to 280). The IFN-γ response towards p15 was mediated partly by CD4+ and partly by CD8+ T cells, whereas all other peptide-stimulated IFN-γ responses could be completely abrogated by CD4+-T-cell depletion (Table 2). The IFN-γ response to whole native Ag85A was completely mediated by CD4+ T cells, whereas CD8+ T cells were the major IFN-γ-producing population in response to the polyclonal T-cell mitogen phytohemagglutinin (Table 2). As for IL-2 production, spleen cells from BALB/c mice vaccinated with Ag85A DNA demonstrated cross-reactive IFN-γ responses against synthetic 18-mer peptides spanning aa 64 to 81 and aa 199 to 216 from the mature Ag85B of M. tuberculosis (data not shown).
FIG. 2.
IFN-γ production (picograms per milliliter) in response to restimulation with synthetic 20-mer peptides (overlapping by 10 aa, spanning the complete Ag85A sequence) of spleen cells from BALB/c mice vaccinated with plasmid DNA encoding Ag85A or infected with M. tuberculosis. Pooled spleens from three mice were unstimulated (control) or stimulated with the peptides (10 μg/ml) or with purified Ag85A (5 μg/ml), and 72-h supernatants were tested by an ELISA.
Generation of cytotoxic CD8+ T cells in BALB/c mice vaccinated with DNA encoding Ag85A or infected with M. tuberculosis H37Rv.
As shown in Table 3, elevated antigen-specific CTL activity could be measured in splenic T-cell cultures from BALB/c mice vaccinated with DNA encoding Ag85A, as assessed in a 51Cr release assay of Ag85A-transfected P815 target cells. No CTL activity could be detected in spleens from BALB/c mice vaccinated with M. bovis BCG or infected with M. tuberculosis 12 weeks previously. Ag85A-specific CTL activity was not detected either 4 weeks following BCG vaccination or 4 weeks after two BCG vaccinations separated by a 2-month interval (data not shown). As previously reported (18), CTL activity induced by Ag85A DNA was mediated exclusively by CD8+ T cells. Cross-reactive CTL responses against P815-Ag85A-transfected cells were also observed in BALB/c mice vaccinated with DNA encoding Ag85B but not Ag85C (data not shown).
TABLE 3.
Cytolytic activity against P815-Ag85A-transfected target cells in BALB/c mice vaccinated with Ag85A DNA or infected with M. tuberculosis or M. bovis BCGa
| E/T ratio | % Specific lysis (mean ± SD) after vaccination witha:
|
||
|---|---|---|---|
| Ag85A DNA | M. tuberculosis | M. bovis BCG | |
| 75 | 97.8 ± 5.3 | 0 | 0 |
| 38 | 93.8 ± 12.8 | 0 | 0 |
| 19 | 91.2 ± 6.6 | 0 | 0 |
| 9.5 | 72.9 ± 8.3 | 0 | 0 |
| 4.75 | 46.8 ± 7.5 | 0 | 0 |
| 2.37 | 21.1 ± 3.6 | 0 | 0 |
Cells were from spleens from three BALB/c mice vaccinated thrice with Ag85A DNA (3 weeks after the third injection) or infected intravenously with M. tuberculosis or M. bovis BCG 12 weeks previously. Spleen cells were amplified with irradiated P815-Ag85A-transfected cells for 6 days and tested for CTL activity by a 51Cr release assay of P815-Ag85A targets.
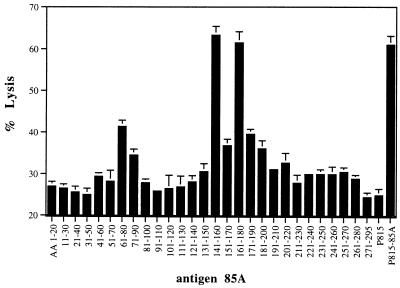
Cytotoxic T-cell activity against peptide-pulsed P815 target cells in BALB/c mice vaccinated with Ag85A DNA.
Strong CTL activity could be detected in spleen cells from Ag85A DNA-vaccinated mice when the cells were tested against P815 target cells pulsed with the following three 85A peptides: p7 (aa 61 to 80), p15 (aa 141 to 160), and p17 (aa 161 to 180) (Fig. 3). Interestingly, p15 was one of the newly defined peptides against which DNA-vaccinated BALB/c mice reacted very strongly but infected mice did not. As already discussed, p7 and p17 induced some IL-2 activity, and p7 and p15 induced significant levels of IFN-γ, which was partly of CD8+ origin for the latter peptide. As shown in Table 4, all three CTL-inducing peptides contained predicted consensus motifs for major histocompatibility complex (MHC) class I binding in accordance with the work of Falk and Rötzchke (11). A search of the Internet by using “HLA peptide motif” (http://bimas.dcrt.nih.gov/molbio/hla_bind/) yielded the following half-time dissociation scores (reflecting affinity for the respective MHC class I molecules): p7, aa 61 to 68 (600 for Kd) and aa 71 to 78 (390 for Ld); p15, aa 145 to 152 (2,000 for Kd); p17, aa 161 to 168 (150 for Ld). Shorter 10-mer peptides spanning only aa 70 to 79 and 144 to 153 were synthesized and could be used for P815 cell pulsing as well, clearly showing their MHC class I-restricted nature (1).
FIG. 3.
CTL activity against P815 target cells loaded with synthetic 20-mer peptides (overlapping by 10 aa, covering the complete Ag85A sequence) of spleen cells from BALB/c mice vaccinated with mature Ag85A DNA. Splenocytes were precultured for 6 days with gamma-irradiated P815-Ag85A-transfected cells, and lytic activity was measured as described in Materials and Methods. Peptide concentration, 5 μg/ml (E/T = 50).
TABLE 4.
MHC class I binding consensus motifs on three Ag85A peptides with H-2d-restricted CTL stimulating activity
| Peptide (aa) | Antigen | Sequencea |
|---|---|---|
| p7(61–80) | 85A | YDQSGLSVVMPVGGQSSFYS |
| 85B | YYQSGLSIVMPVGGQSSFYS | |
| 85C | YYQSGLSVIMPVGGQSSFYT | |
| p15(141–160) | 85A | QQFVYAGAMSGLLDPSQAMG |
| 85B | QQFIYAGSLSALLDPSQGMG | |
| 85C | QQFPYAASLSGFLNPSEGWW | |
| p17(161–180) | 85A | PTLIGLAMGDAGGYKASDMW |
| 85B | PSLIGLAMGDAGGYKAADMW | |
| 85C | PTLIGLAMNDSGGYNANSMW |
Underlined residues are amino acids with predicted Kd binding anchor function. Residues in boldface type are amino acids with predicted Ld binding anchor function.
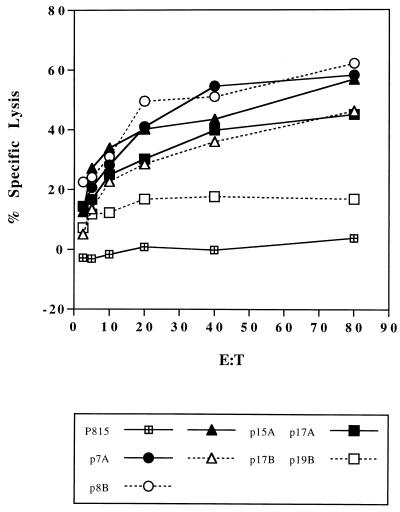
Cross-reactive CTL activity against Ag85B epitopes in BALB/c mice vaccinated with DNA encoding Ag85A.
Spleen cells from Ag85A DNA-vaccinated mice were amplified with P815-Ag85A-transfected cells and tested for CTL activity against P815 targets loaded with 18-mer peptides from Ag85B spanning corresponding regions of three Ag85A-defined CTL epitopes. As shown in Fig. 4, cross-reactive CTL responses were found in response to the p7 region (i.e., against p8 [aa 64 to 81] from Ag85B) and to the p15 region (i.e., p17 [aa 145 to 162] from Ag85B). The Ld predicted regions in p7 are identical for the three Ag85 components, which explains the completely cross-reactive response found for p8 from Ag85B (Table 4). Surprisingly, the Kd binding motif of p15 in Ag85A differs in three amino acids from the Ag85B sequence, but nevertheless, a significant cross-reactive CTL response was observed, suggesting that the two conserved anchor residues (tyrosine at position 145 and leucine at position 152) were essential here. As already mentioned, cross-reactive secretion of IL-2 and IFN-γ was observed in response to the p7 region but not to the p15 region of Ag85B. Analysis of CD4 epitopes predicted according to the TSites program as described previously (17) revealed that a predicted Rothbard motif spanning aa 147 to 154 in Ag85A is lacking in the Ag85B sequence.
FIG. 4.
CTL activity against P815 target cells loaded with p7, p15, and p17, from Ag85A or with p8, p17, and p19 from Ag85B peptides spanning the homologous regions. The experiment was performed as described in the legend to Fig. 3.
DISCUSSION
Protective immunity against mycobacterial infection is mediated by interactions between specifically sensitized CD4+ and CD8+ T lymphocytes and activated macrophage effector cells harboring the intracellular pathogen (19, 20, 26). Activation of Th1 helper T cells and CD8+ CTLs is thought to be essential for protection, whereas activation of Th2 helper T cells is associated with disease progression (34). IFN-γ, a potent activator of macrophages, and tumor necrosis factor alpha (TNF-α) are crucial cytokines in antimycobacterial protection, as demonstrated in knockout mice, which have been genetically altered to eliminate either IFN-γ (5, 7, 13) or TNF-α (14) production.
In the past few years, DNA vaccination has been reported by many authors to be an effective strategy for expression of foreign antigens in vivo leading to immunization against a variety of viruses and protozoa (10). More recently, we and others have reported that DNA vaccination can be used for protection against mycobacterial disease as well (18, 31, 35). More specifically, DNA vaccination with genes encoding Ag85A and Ag85B (but not Ag85C) components of the Ag85 complex from M. tuberculosis resulted in a strong stimulation of a specific Th1-like response and of CTL activity by splenocytes from immunized mice upon in vitro restimulation with native Ag85, leading to protection against mycobacterial challenge in mice. TNF-α and granulocyte-macrophage colony-stimulating factor, but not IL-4 or IL-10, were also detected in such culture supernatants (18, 23).
In this study, we have now analyzed in more detail the Ag85-specific Th1 and CD8+ T-cell response in BALB/c mice vaccinated with an Ag85A DNA vaccine and compared it to the response in mice intravenously infected with M. tuberculosis H37Rv. Whereas robust Ag85A-specific cytolytic activity could be measured in spleen cell cultures from DNA-vaccinated BALB/c mice amplified for 1 week in vitro with Ag85A-transfected, gamma-irradiated, syngeneic P815 mastocytoma cells, we were unable to detect any cytolytic activity in similarly amplified spleen cell cultures from mice infected with M. tuberculosis or vaccinated with M. bovis BCG. Mycobacteria reside almost exclusively in the phagosome, and access of protein antigens to the cytoplasm followed by proteasome cleavage and association of peptides with MHC class I molecules is probably a rare phenomenon. As a consequence of this, the precursor frequency of CD8+ CTLs generated during infection is probably very low as well. DNA vaccination, on the other hand, leads to strong expression of foreign proteins in the host cytoplasm, resulting in genuine MHC class I-restricted CD8+ responses against a number of viral and protozoal antigens (10). Therefore, low-level availability of Ag85 in the infected cytoplasm of antigen-presenting cells may have resulted in antigen presentation to CTL precursors at such a low frequency that they would not have been detected by our protocol with one round of in vitro amplification. In this respect, it is interesting to note that the two studies that previously reported on CD8+ T cells specific for mycobacterial antigens following mycobacterial infection, i.e., the 38-kDa PstS-1 homolog (35) and the 60-kDa heat shock protein (36), precisely worked with established T-cell lines and clones, respectively, amplified by successive rounds of in vitro antigen and IL-2 restimulation, conditions in which the precursor frequency would be of little importance. Moreover, we have previously found that genuine CD8+ CTLs can be generated in BCG-vaccinated mice against a hydrophilic CF antigen(s), but the nature of this particular cross-reactive antigen, also found in CF from M. tuberculosis and Mycobacterium kansasii, remains largely unknown and these CTLs seem to function in an exclusive Db-restricted manner (9).
The generation of Ag85-specific CD8+ CTL responses by DNA vaccination is particularly important for tuberculosis vaccine development, since studies of mice genetically altered so that the β2-microglobulin gene is deleted (and negative for MHC class I expression) have clearly demonstrated the essential role of CD8+ T cells in protection against tuberculosis (12). Studies of perforin and granzyme gene knockout mice and of Fas-receptor-defective mice have shown that the expression of the lytic pathway is not essential for the protective role of these CD8+ T cells (at least in the early phase of infection) but that other types of mechanisms such as cytokine secretion (IFN-γ?) might be involved (6, 21). Three immunodominant H-2d-restricted CTL-inducing peptides, i.e., p7 (aa 61 to 80), p15 (aa 141 to 160), and p17 (aa 161 to 180), were identified on the Ag85A protein by using a 51Cr release assay of peptide-pulsed P815 target cells. These peptides also triggered IFN-γ production, primarily from CD4+ T cells. However, p15 stimulated IFN-γ secretion from both CD4+ and CD8+ T cells. Interestingly, we have previously reported that this peptide was the most dominant and promiscuous epitope on Ag85A recognized by T cells from 90% of healthy humans with primary M. tuberculosis infections with various HLA haplotypes (22). Our results, therefore, could hold promise for potential vaccination of humans with DNA encoding Ag85.
Besides the differential CTL generation, DNA vaccination also differed in another respect from mycobacterial infection. Indeed, DNA vaccination induced IL-2 and particularly IFN-γ responses against a broader spectrum of epitopes than infection with live M. tuberculosis or vaccination with M. bovis BCG did. This broader peptide spectrum was found to be a reflection of specificities recognized not only by Ag85A-specific cytolytic CD8+ T cells but also by Th1 cytokine-secreting CD4+ T cells. All epitopes identified in BCG-vaccinated or M. tuberculosis-infected mice were also recognized by cells from DNA-vaccinated mice, but additional specificities in the amino- and carboxy-terminal parts of the protein were found. Remarkably, two additional T-cell epitopes (p20 and p23) defined for BALB/c mice following Ag85A DNA vaccination that were not identified in our previous BCG vaccination study have been described as promiscuous regions on the Ag85B molecule, again recognized by a majority of tuberculosis patients (28) and healthy PPD-positive human subjects (30). Recently, Ragno et al. have reported on protection of rats against adjuvant arthritis by immunization with DNA encoding mycobacterial hsp65 (27). It is tempting to speculate that DNA vaccination in this model may also have broadened the spectrum of peptides recognized and may have led to preferential amplification of responses to the protective rather than to the arthritogenic peptides on the hsp65 molecule.
In conclusion, our data suggest that fundamental differences exist in the immune response sensitization that occurs following infection with live M. tuberculosis as compared to that after Ag85A DNA vaccination. Specifically, DNA vaccination leads to a stronger Th1-type cytokine response, with a major role for CD4+ T cells as producers of the pivotal cytokine, IFN-γ. In addition, Ag85A-specific MHC class I-restricted CTL responses were found in Ag85 DNA-vaccinated mice exclusively, not in mice infected with M. bovis BCG or M. tuberculosis. Finally, DNA vaccination induced immune responses with a broad epitopic repertoire, directed against peptide regions immunodominant in the natural infection and against other, apparently cryptic peptide regions as well. Given the important roles of cytokine-secreting CD4+ and CD8+ T cells in protection against mycobacterial disease, the facility with which DNA vaccines induce these effector cells bodes well for the development of a DNA vaccine for tuberculosis.
ACKNOWLEDGMENTS
This work was supported in part by grants 3.0020.89 and G.0355.97 from the NFWO (Belgian National Research Foundation) and by the World Health Organization. A.T. holds a fellowship from the Damiaanaktie Belgium.
We thank S. D’Souza for helpful discussions and M. Moser (Université Libre de Bruxelles) for the use of the gamma irradiator.
REFERENCES
- 1.Baldwin, S., et al. Unpublished data.
- 1a.Belisle J T, Vissa V D, Sievert T, Takayama K, Brennan P J, Besra G S. Role of the major antigen of Mycobacterium tuberculosis in cell wall biogenesis. Science. 1997;276:1420–1422. doi: 10.1126/science.276.5317.1420. [DOI] [PubMed] [Google Scholar]
- 2.Bloom B R, Murray C J L. Tuberculosis: commentary on a reemergent killer. Science. 1992;257:1055–1064. doi: 10.1126/science.257.5073.1055. [DOI] [PubMed] [Google Scholar]
- 3.Bloom B R, Fine P E M. The BCG experience: implications for future vaccines against tuberculosis. In: Bloom B R, editor. Tuberculosis: pathogenesis, protection, and control. Washington, D.C: ASM Press; 1994. pp. 531–557. [Google Scholar]
- 4.Borremans M, De Wit L, Volckaert G, Ooms J, De Bruyn J, Huygen K, Van Vooren J-P, Stélandre M, Verhofstadt R, Content J. Cloning, sequence determination, and expression of a 32-kilodalton-protein gene from M. tuberculosis. Infect Immun. 1989;57:3123–3130. doi: 10.1128/iai.57.10.3123-3130.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cooper A M, Dalton D K, Stewart T A, Griffin J P, Russell D G, Orme I M. Disseminated tuberculosis in interferon-gamma gene disrupted mice. J Exp Med. 1993;178:2243–2247. doi: 10.1084/jem.178.6.2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cooper A M, D’Souza C, Frank A A, Orme I M. The course of Mycobacterium tuberculosis infection in the lungs of mice lacking expression of either perforin- or granzyme-mediated cytolytic mechanisms. Infect Immun. 1997;65:1317–1320. doi: 10.1128/iai.65.4.1317-1320.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dalton D K, Pitts-Meek S, Keshav S, Figari I S, Bradley A, Stewart T A. Multiple defects of immune cell function in mice disrupted interferon-γ genes. Science. 1993;259:1739–1742. doi: 10.1126/science.8456300. [DOI] [PubMed] [Google Scholar]
- 8.De Bruyn J, Huygen K, Bosmans R, Fauville M, Lippens R, Van Vooren J-P, Falmagne P, Wiker H G, Harboe M, Turneer M. Purification, characterization and identification of a 32 kDa protein antigen of Mycobacterium bovis BCG. Microb Pathog. 1987;2:351–366. doi: 10.1016/0882-4010(87)90077-5. [DOI] [PubMed] [Google Scholar]
- 9.Denis O, Lozes E, Huygen K. Induction of cytotoxic T-cell responses against culture filtrate antigens in Mycobacterium bovis BCG-infected mice. Infect Immun. 1997;65:676–684. doi: 10.1128/iai.65.2.676-684.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Donnelly J J, Ulmer J B, Shiver J W, Liu M A. DNA vaccines. Annu Rev Immunol. 1997;15:617–648. doi: 10.1146/annurev.immunol.15.1.617. [DOI] [PubMed] [Google Scholar]
- 11.Falk K, Rötzchke O. Consensus motifs and peptide ligands of MHC class I molecules. Semin Immunol. 1993;5:81–94. doi: 10.1006/smim.1993.1012. [DOI] [PubMed] [Google Scholar]
- 12.Flynn J L, Goldstein M A, Treibold K J, Koller B, Bloom B R. Major histocompatibility complex class I-restricted T cells are required for resistance to Mycobacterium tuberculosis. Proc Natl Acad Sci USA. 1992;89:12013–12017. doi: 10.1073/pnas.89.24.12013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Flynn J L, Chan J, Triebold K J, Dalton D K, Stewart T A, Bloom B R. Role for interferon-gamma in resistance to Mycobacterium tuberculosis infection. J Exp Med. 1993;178:2249–2254. doi: 10.1084/jem.178.6.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Flynn J L, Goldstein M M, Chan J, Triebold K J, Pfeffer K, Lowenstein C J, Schreiber R, Mak T W, Bloom B R. Tumor necrosis factor-α is required in the protective immune response against Mycobacterium tuberculosis in mice. Immunity. 1995;2:561–572. doi: 10.1016/1074-7613(95)90001-2. [DOI] [PubMed] [Google Scholar]
- 15.Garbe T R, Hibler N S, Deretic V. Isoniazid induces the expression of the antigen 85 complex in Mycobacterium tuberculosis. Antimicrob Agents Chemother. 1996;40:1754–1756. doi: 10.1128/aac.40.7.1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huygen K, Abramowicz D, Vandenbussche P, Jacobs F, De Bruyn J, Kentos A, Drowart A, Van Vooren J P, Goldman M. Spleen cell cytokine secretion in Mycobacterium bovis BCG-infected mice. Infect Immun. 1992;60:2880–2886. doi: 10.1128/iai.60.7.2880-2886.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huygen K, Lozes E, Gilles B, Drowart A, Palfliet K, Jurion F, Roland I, Art M, Dufaux M, Nyabenda J, De Bruyn J, Van Vooren J-P, DeLeys R. Mapping of TH1 helper T-cell epitopes on major secreted mycobacterial antigen 85A in mice infected with live Mycobacterium bovis BCG. Infect Immun. 1994;62:363–370. doi: 10.1128/iai.62.2.363-370.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huygen K, Content J, Denis O, Montgomery D L, Yawman A M, Deck R R, Dewitt C M, Orme I M, Baldwin S, D’Souza C, Drowart A, Lozes E, Vandenbussche P, Van Vooren J-P, Liu M A, Ulmer J B. Immunogenicity and protective efficacy of a tuberculosis DNA vaccine. Nat Med. 1996;2:893–898. doi: 10.1038/nm0896-893. [DOI] [PubMed] [Google Scholar]
- 19.Kaufmann S H E. Immunity to intracellular bacteria. Annu Rev Immunol. 1993;11:129–190. doi: 10.1146/annurev.iy.11.040193.001021. [DOI] [PubMed] [Google Scholar]
- 20.Ladel C H, Daugelat S, Kaufman S H E. Immune response to Mycobacterium bovis bacille Calmette-Guérin infection in major histocompatibility complex class I- and class II-deficient knock-out mice: contribution of CD4 and CD8 T cells to acquired resistance. Eur J Immunol. 1995;25:377–384. doi: 10.1002/eji.1830250211. [DOI] [PubMed] [Google Scholar]
- 21.Laochumroonvorapong P, Wang J, Liu C-C, Ye W, Moreira A L, Elkon K B, Freedman V H, Kaplan G. Perforin, a cytotoxic molecule which mediates cell necrosis, is not required for the early control of mycobacterial infection in mice. Infect Immun. 1997;65:127–132. doi: 10.1128/iai.65.1.127-132.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Launois P, DeLeys R, N’Diaye Niang M, Drowart A, Andrien M, Dierckx P, Cartel J-L, Sarthou J-L, Van Vooren J-P, Huygen K. T-cell-epitope mapping of the major secreted mycobacterial antigen Ag85A in tuberculosis and leprosy. Infect Immun. 1994;62:3679–3687. doi: 10.1128/iai.62.9.3679-3687.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lozes E, Huygen K, Content J, Denis O, Montgomery D L, Yawman A M, Vandenbussche P, Van Vooren J-P, Drowart A, Ulmer J B, Liu M A. Immunogenicity and efficacy of a tuberculosis DNA vaccine encoding the components of the secreted antigen 85 complex. Vaccine. 1997;15:830–833. doi: 10.1016/s0264-410x(96)00274-5. [DOI] [PubMed] [Google Scholar]
- 24.Montgomery D L, Shiver J W, Leander K R, Perry H C, Friedman A, Martinez D, Ulmer J B, Donnelly J J, Liu M A. Heterologous and homologous protection against influenza A by DNA vaccination: optimization of DNA vectors. DNA Cell Biol. 1993;12:777–783. doi: 10.1089/dna.1993.12.777. [DOI] [PubMed] [Google Scholar]
- 25.Munk M E, De Bruyn J, Gras H, Kaufmann S H E. The Mycobacterium bovis BCG 32-kilodalton protein antigen induces human cytotoxic T-cell responses. Infect Immun. 1994;62:726–728. doi: 10.1128/iai.62.2.726-728.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Orme I M, Roberts A D, Griffin J P, Abrams J S. Cytokine secretion by CD4+ T lymphocytes acquired in response to Mycobacterium tuberculosis infection. J Immunol. 1993;151:518–525. [PubMed] [Google Scholar]
- 27.Ragno S, Colston M J, Lowrie D B, Winrow V R, Blake D R, Tascon R. Protection of rats from adjuvant arthritis by immunization with naked DNA encoding for mycobacterial heat shock protein 65. Arthritis Rheum. 1997;40:277–283. doi: 10.1002/art.1780400212. [DOI] [PubMed] [Google Scholar]
- 28.Roche P W, Peake P W, Billman-Jacobe H, Doran T, Britton W J. T-cell determinants and antibody binding sites on the major mycobacterial secretory protein MPB59 of Mycobacterium bovis. Infect Immun. 1994;62:5319–5326. doi: 10.1128/iai.62.12.5319-5326.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shiver J W, Perry H C, Davies M E, Liu M A. Immune responses to HIV gp120 elicited by DNA vaccination. In: Chanock R M, Brown F, Ginsberg H S, Norrby E, editors. Vaccines 95. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1995. pp. 95–98. [Google Scholar]
- 30.Silver R F, Wallis R S, Ellner J J. Mapping of T cell epitopes of the 30-kDa α antigen of Mycobacterium bovis strain bacillus Calmette-Guérin in purified protein derivative (PPD)-positive individuals. J Immunol. 1995;154:4665–4674. [PubMed] [Google Scholar]
- 31.Tascon R E, Colston M J, Ragno S, Stavropoulos E, Gregory D, Lowrie D B. Vaccination against tuberculosis by DNA injection. Nat Med. 1996;2:888–892. doi: 10.1038/nm0896-888. [DOI] [PubMed] [Google Scholar]
- 32.Ulmer J B, Donnelly J J, Parker S E, et al. Heterologous protection against influenza by injection of DNA encoding a viral protein. Science. 1993;259:1745–1749. doi: 10.1126/science.8456302. [DOI] [PubMed] [Google Scholar]
- 33.Wiker H G, Harboe M. The antigen 85 complex: a major secretion product of Mycobacterium tuberculosis. Microbiol Rev. 1992;56:648–661. doi: 10.1128/mr.56.4.648-661.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yamamura M, Uyemura K, Deans R J, Weinberg K, Rea T H, Bloom B R, Modlin R L. Defining protective responses to pathogens: cytokine profiles in leprosy lesions. Science. 1991;254:277–279. doi: 10.1126/science.254.5029.277. [DOI] [PubMed] [Google Scholar]
- 35.Zhu X, Venkataprasad N, Thangaraj H S, Hill M, Singh M, Ivanyi J, Vordermeier H M. Functions and specificity of T cells following nucleic acid vaccination of mice against Mycobacterium tuberculosis infection. J Immunol. 1997;158:5921–5926. [PubMed] [Google Scholar]
- 36.Zügel U, Kaufmann S H E. Activation of CD8 T cells with specificity for mycobacterial heat shock protein 60 in Mycobacterium bovis bacillus Calmette-Guérin-vaccinated mice. Infect Immun. 1997;65:3947–3950. doi: 10.1128/iai.65.9.3947-3950.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]