Abstract
Leishmania parasites, transmitted by phlebotomine sand flies, are obligate intracellular parasites of macrophages. The sand fly Phlebotomus papatasi is the vector of Leishmania major, a causative agent of cutaneous leishmaniasis in the Old World, and its saliva exacerbates parasite proliferation and lesion growth in experimental cutaneous leishmaniasis. Here we show that P. papatasi saliva contains a potent inhibitor of protein phosphatase 1 and protein phosphatase 2A of murine macrophages. We further demonstrate that P. papatasi saliva down regulates expression of the inducible nitric oxide synthase gene and reduces nitric oxide production in murine macrophages. Partial biochemical characterization of the protein phosphatase and nitric oxide inhibitor indicated that it is a small, ethanol-soluble molecule resistant to boiling, proteolysis, and DNase and RNase treatments. We suggest that the P. papatasi salivary protein phosphatase inhibitor interferes with the ability of activated macrophages to transmit signals to the nucleus, thereby preventing up regulation of the induced nitric oxide synthase gene and inhibiting the production of nitric oxide. Since nitric oxide is toxic to intracellular parasites, the salivary protein phosphatase inhibitor may be the mechanism by which P. papatasi saliva exacerbates cutaneous leishmaniasis.
The leishmaniases are sand fly-borne parasitic diseases that affect large populations in the palaearctic and tropical regions of the world (1). Two major disease types, cutaneous and visceral, are recognized in humans. In cutaneous leishmaniasis, parasites are restricted to dermal lesions that develop at the site of the infectious bite and usually heal spontaneously (29). In the visceral form, parasites invade the spleen, liver, and bone marrow, causing a serious, life-threatening systemic disease (3).
Sand flies become infected with leishmaniae when they ingest blood containing parasitized macrophages (Mφ). In the alimentary canal of the phlebotomine sand fly, leishmaniae transform into, and develop as extracellular, flagellated promastigotes. They reproduce by binary fission and go through a series of developmental stages culminating with the generation of infective-stage metacyclic promastigotes that are inoculated into the vertebrate host’s skin as the female sand fly sucks blood (21). Once in the skin, parasites rapidly invade Mφ and replicate as intracellular amastigotes. Their entry into the Mφ and survival inside the phagolysosome are made possible by a number of strategies that subvert the Mφ’s scavenger functions (reviewed in references 8 and 14). Despite these qualities, experimental inoculations with low doses of promastigotes fail to initiate infections in susceptible mouse strains. However, when similarly small numbers are inoculated by vector sand flies, infections flourish. Higher efficiency of transmission by vectors is a result of parasites being coinoculated with saliva.
Sand fly saliva has been shown to exacerbate experimental cutaneous lesions caused by several different Leishmania species (22, 25, 27, 30). This is probably a result of saliva inhibiting antigen presentation and reducing nitric oxide (NO) production by Leishmania-infected Mφ (9, 26) or enhancing interleukin-4 secretion by T lymphocytes (15). Most of the salivary factor(s) responsible for these phenomena have not been identified. One important molecule is undoubtedly maxadilan, a potent vasodilator that facilitates blood feeding by sand flies (13, 20). Maxadilan was also shown to modulate a number of immune functions in mice (18). However, maxadilan is found in the saliva of only one sand fly species, Lutzomyia longipalpis. Phlebotomus papatasi saliva, which exacerbates cutaneous leishmaniasis and reduces NO production, lacks maxadilan (29a).
The capacity of Mφ to respond to activation signals against intracellular pathogens during the nonimmune early phases of infection is crucial for determining whether the invading organisms proliferate or are eliminated (28). One strategy by which Mφ fight invasive organisms is via the production of the cytotoxic molecule NO (8, 14). In murine Mφ, the signaling process that leads to the activation of the induced nitric oxide synthase (iNOS) gene, and the subsequent production of NO is mediated by protein phosphatase 1 (PP-1) and PP-2A (4, 7). Here we report on the presence of a potent PP-1 and PP-2A inhibitor in the saliva of P. papatasi and its ability to down regulate the iNOS gene expression and inhibit NO production in activated murine Mφ.
MATERIALS AND METHODS
Reagents.
RPMI 1640 medium, fetal bovine serum, mouse recombinant gamma interferon (IFN-γ), okadaic acid (OA), the protein phosphatase (PP) assay kit, and the RNA isolation kit were purchased from GIBCO-BRL, Life Technologies. Ca2+- and Mg2+-free Hanks balanced salt solution (HBSS) and phenol-extracted Escherichia coli lipopolysaccharide (LPS) were purchased from Sigma Chemical Co. (St. Louis, Mo.). [γ-32P]ATP (6,000 Ci/mmol) was purchased from Dupont NEN (Boston, Mass.). The reverse transcriptase (RT)-mediated PCR (RT-PCR) kit, Griess reagent, DNase I, and RNase A were purchased from Promega Corporation.
Sand fly rearing and collection of salivary gland lysate.
P. papatasi was reared as described previously (17). Salivary glands from 3- to 6-day-old sand flies were dissected in Ca2+- and Mg2+-free HBSS and stored at −70°C. Before use, the glands were disrupted by repeated freeze-thawing in liquid nitrogen and centrifugation (10,000 × g for 2 min). Complete disruption was verified microscopically, and the lysate was spun again at 10,000 × g to pellet any debris.
Mice.
Eight- to twelve-week-old C3H/HeN female mice were maintained in a National Institutes of Health-approved sterile pathogen-free animal facility.
Collection and culture of peritoneal exudate Mφ.
Mφ were obtained from LPS-sensitive C3H/HeN inbred mice as described previously (7). Briefly, mice were stimulated with 2.0 ml of 3% thioglycolate injected intraperitoneally. Four days later, Mφ were harvested by peritoneal lavage using 10 ml of RPMI 1640 (GIBCO-BRL), washed in Ca2+- and Mg2+-free HBSS, and resuspended in RPMI 1640 containing 1% fetal bovine serum; 106 cells/well in 1.0 ml were seeded in 24-well plates. Mφ cultures were incubated at 37°C, 5% CO2, and 95% humidity for 90 min. To remove nonadherent cells, cultures were washed with serum-free RPMI 1640. Adherent Mφ were incubated in serum-free RPMI 1640 containing Mφ activators or inhibitors as described below.
For assessing iNOS gene expression, Mφ were activated by incubation for 8 h with LPS (25 ng/ml) and IFN-γ (25 U/ml) with or without saliva. For assessing NO production, activation of Mφ was achieved by incubation for 24 h with LPS (1 μg/ml) alone.
In vitro PP assay.
About 2 × 107 adherent Mφ were washed in Ca2+- and Mg2+-free HBSS and lysed for 5 min on ice in 1 ml of lysis buffer (7). The cytoplasmic and nuclear fractions were separated by centrifugation at 1,000 × g for 2 min. The cytoplasmic fraction was aliquoted and immediately frozen at −70°C. PP activity in the extracts was determined (for 20 min at 34°C) by using a PP assay system (GIBCO) according to the manufacturer’s protocol. To distinguish between release of 32Pi and trichloroacetic acid-soluble 32P-labeled phosphopeptides, the 32Pi was complexed to ammonium molybdate and extracted with organic solvents (23). Various concentrations of sand fly salivary gland lysates or OA were incubated with Mφ extracts for 15 min at room temperature before the addition of 32P-labeled phosphorylase A.
To calculate PP-1 and PP-2A activity, 1.0 nM OA was added to the assay buffer. Since OA at this concentration totally inhibits PP-2A (50% inhibitory concentration [IC50] = 0.2 nM) in dilute Mφ extracts (2), the reduction in PP activity was attributed to PP-2A and the remaining activity was attributed to PP-1 (IC50 = 10 to 15 nM). To measure PP activity of the Mg2+-dependent PP-2C, the assay buffer contained 1 mM EGTA to inhibit PP-2B and contained 10 mM Mg2+ and 1 μM OA to inhibit PP-1 and PP-2A. To measure the activity of the Ca2+/calmodulin-dependent PP-2B, the assay buffer contained 0.1 mM Ca2+ and 0.5 μM OA.
Partial biochemical characterization of the PP-1 and PP-2A inhibitor.
Salivary gland lysates were filtered in succession through membranes with different molecular size cutoffs (Amicon, Beverly, Mass.). The retentates and the filtrates were tested for inhibition of PP-1 and PP-2A activity and NO production as described above. To characterize the PP inhibitor component biochemically, salivary gland lysates were subjected to one of the following treatments: boiling for 10 min, proteolysis with trypsin (0.5 mg/ml) and chymotrypsin (0.5 mg/ml) at pH 7.5 and 37°C for 1 h followed by treatment with soybean trypsin inhibitor, proteolysis with proteinase K (100 μg/ml) for 3 h at 37°C followed by boiling for 10 min to inactivate the enzymes, or treatment with DNase 1 (10 U) and RNase A (10 μg/ml) at 37°C for 1 h. PP-1 and PP-2A inhibition was assayed as described above. Controls comprised all reaction components except the salivary gland lysates.
RNA isolation and RT-PCR.
Total cellular RNA was extracted from control and treated cultures of Mφ by using a Trizol RNA isolation kit (GIBCO). Synthesis of the first-strand cDNA and the subsequent PCR were performed with an Access RT-PCR kit (Promega). Sense and antisense primers for the constitutively expressed hypoxanthine-guanine phosphoribosyltransferase (HPRT) gene and iNOS mRNA were located on different exons to facilitate detection of possible contamination by genomic DNA (19). Two hundred nanograms of total RNA was used in 25 μl of RT-PCR buffer containing 1 mM MgSO4, 0.2 mM deoxynucleoside triphosphates, 50 pmol of sense and antisense primers, 1.25 U of avian myeloblastosis virus RT enzyme, and 1.25 U of Taq DNA polymerase. Reverse transcription was carried out at 48°C for 45 min followed by 40 amplification cycles of 94°C for 2 min, 60°C for 1 min, and 68°C for 2 min in a Biometra Thermocycler. The PCR products were electrophoresed on agarose gels and stained with ethidium bromide, and the UV image was captured and analyzed by using NIH Image computer graphics.
Nitrite analysis.
The concentration of NO2− that accumulated in the Mφ culture medium over 24 h was determined in a microplate assay using Griess reagent. Fifty microliters of the culture supernatant was mixed with an equal amount of 1% sulfanilamide in 5% phosphoric acid and incubated at room temperature for 5 min. Then 50 μl of 0.1% N-1-naphthylethylenediamine dihydrochloride in water was added, and the mixture was incubated for an additional 5 min. The absorbance at 550 nm was read with a microplate reader. NO2− concentrations were determined by using sodium nitrite as a standard.
RESULTS AND DISCUSSION
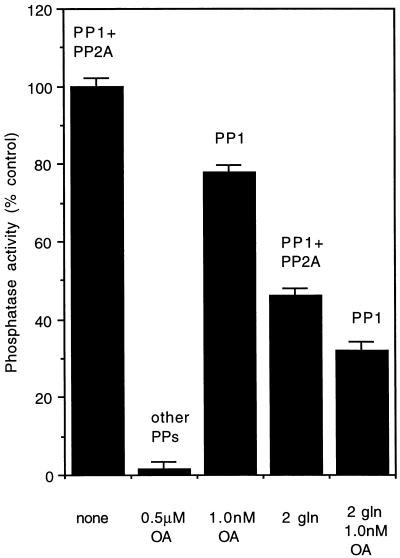
The Mφ cytosolic fraction exhibited high levels of PP activity, and dephosphorylation of 32P-phosphorylase A increased linearly up to a concentration of 40 μg of Mφ protein per ml (Fig. 1, inset). Four enzymes, PP-1, PP-2A, PP-2B, and PP-2C, account for virtually all the phosphatase activity in mammalian cells (11). These enzymes can be differentiated biochemically on the basis of their sensitivity to specific inhibitors and divalent cation dependency (2, 4, 5). For example, OA reduces 50% of the PP-2A at a concentration of 0.2 nM (IC50 = 0.2 nM); complete inhibition is achieved at 1 nM. On the other hand, a 100-fold-higher concentration (10 to 15 nM) is required to inhibit PP-1. PP-2B is a Ca2+-dependent PP and is only weakly affected by OA. PP-2C is completely dependent on Mg2+ for activity and is uninhibited by OA. Therefore, to analyze the contribution of each of these four PPs to the total PP activity, we treated the Mφ cytosolic fraction with various concentrations of OA. At a concentration of 0.5 μM OA, in the presence of Ca2+ or Mg2+, no PP activity was detected, indicating that PP-2B and PP-2C were not significant contributors to overall PP activity. The addition of 1.0 nM OA to the Mφ cytosolic fraction inhibited activity by 21%. Since at this concentration OA inhibits all of the PP-2A activity, the remaining 79% was attributable to PP-1 (Fig. 2).
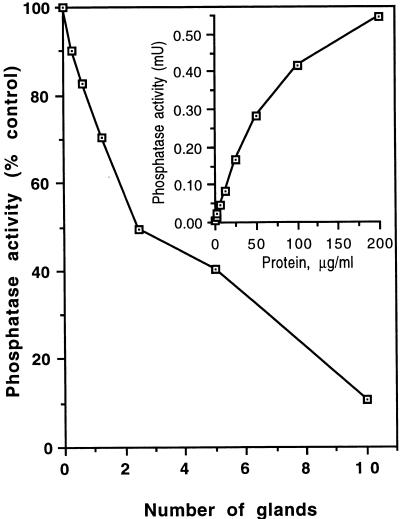
FIG. 1.
Inhibition of PP-1 and PP-2A activity by salivary P. papatasi gland lysates. Mφ cytosolic fraction (12.5 μg/ml) was incubated with different concentrations of salivary gland lysate for 15 min at room temperature, and PP activity was assayed by using [γ-32P]ATP-labeled phosphorylase A as the substrate. Inset, PP activity from the cytosolic fraction of C3H/HeN mice Mφ as a function of Mφ-derived protein (1 mU = 1 nmol of phosphate released from phosphorylase A/min/ml).
FIG. 2.
PP-1 and PP-2A activity in Mφ cytosolic fraction. Column 1 shows total PP activity in 250 ng of untreated Mφ cytosolic fraction; column 2 shows complete PP-1 and PP-2A inhibition by 0.5 μM OA; column 3 shows PP-1 activity after inhibition of PP-2A with 1.0 nm OA; column 4 shows remaining activity after addition of two P. papatasi salivary glands (gln); column 5 shows PP-1 activity after addition of two glands and 1.0 nm OA. Data shown are means ± standard deviations of three experiments.
P. papatasi saliva contains a powerful inhibitor of PP-1 and PP-2A.
P. papatasi salivary gland lysates from male as well as female flies inhibited PP-1 and PP-2A activity in a dose-dependent manner (Fig. 1). An extract of two salivary glands (=1 μg of protein) was as potent as 12.5 nM OA (data not shown). To measure inhibition of PP-1 activity by saliva, assays were conducted in the presence of 1.0 nM OA and any inhibition below that produced by OA was attributed to inhibition of PP-1 (Fig. 2). Controls included sand fly washing solution, HBSS, and material extruded from sand flies during dissection. None had any inhibitory effect on Mφ PP activity.
Protein phosphorylation and dephosphorylation reactions, mediated by protein kinases and PPs, respectively, trigger signal transduction events that control diverse cellular responses to both internal and external signals (4, 10). Here we demonstrate that P. papatasi saliva contains a potent, dose-dependent inhibitor of PP-1 and PP-2A that is distinct from OA. While PP-2A is significantly more sensitive to inhibition by OA (2, 5), it is PP-1 that is more sensitive to P. papatasi saliva (Fig. 2). This is the first report demonstrating the ability of a blood-sucking insect’s saliva to specifically interfere with protein phosphorylation, a key process in eukaryotic cell signal transduction.
P. papatasi saliva inhibits expression of the iNOS gene.
Levels of cellular NO are directly controlled by the production of iNOS enzyme. This is because availability of NO cannot be regulated by storage, release, or uptake (12). Indeed, in our experiments, normal resting Mφ did not express detectable levels of iNOS mRNA (data not shown). However, once activated with LPS, Mφ expressed very high levels of this gene (Fig. 3).
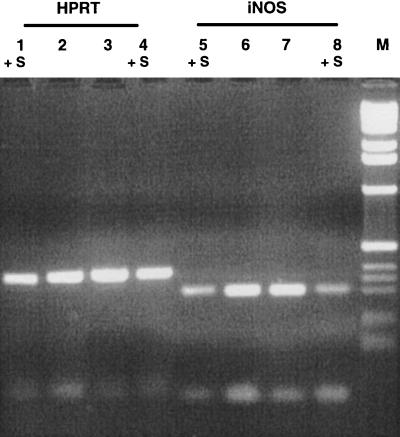
FIG. 3.
Inhibition of iNOS mRNA expression by salivary gland lysates of P. papatasi. Mφ were treated for 8 h with IFN-γ (25 U/ml) and LPS (25 ng/ml) in the presence or absence of saliva. Total RNA was extracted, subjected to RT-PCR, electrophoresed, and analyzed by optical densitometry. Lanes 1 to 4, HPRT; lanes 5 to 8, iNOS. Lanes marked +S include saliva. Results are from one representative experiment of three. M, size markers.
The signaling process for the activation of the iNOS gene and the subsequent production of nitric oxide in murine Mφ is facilitated by PP-1 and PP-2A (7). Since saliva of P. papatasi inhibited PP-1 and PP-2A (Fig. 1 and 2), we used RT-PCR to examine whether expression of the iNOS gene in activated Mφ is inhibited by saliva. Results show that the addition of one P. papatasi salivary gland to 106 Mφ caused a 50% reduction in iNOS mRNA (Fig. 3). The housekeeping gene HPRT was also amplified from each RNA preparation to enable comparisons of the PCR products in different samples.
P. papatasi saliva reduces NO production.
We next examined whether the observed down regulation of the iNOS gene by saliva would result in less NO being produced. Mφ were incubated in serum-free RPMI 1640 containing IFN-γ and LPS with or without saliva. The NO2− concentration was measured after 24 h. As little as 0.5 gland/ml of P. papatasi saliva caused a 30 to 40% reduction in NO2− secretion by Mφ activated with either Leishmania parasites or LPS and IFN-γ (Fig. 4).
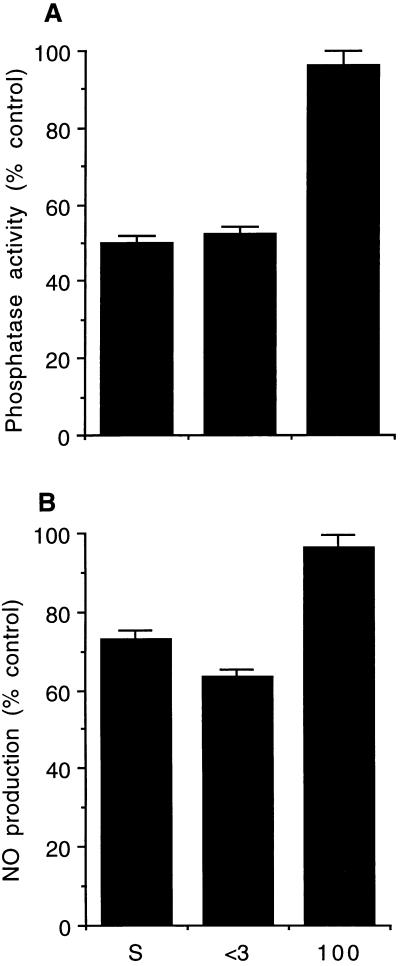
FIG. 4.
Inhibition of PP activity (A) and NO production (B) by whole P. papatasi saliva and by different size fractions obtained by filtration through molecular size cutoff filters. S, whole saliva; <3, <3-kDa filtrate; 100, 100-kDa retentate. (A) PP-1 and PP-2A inhibition assays were performed with 250 ng of untreated Mφ cytosolic fraction and two gland equivalents per assay. (B) For NO analysis, Mφ (106 cells/ml/well) were activated by using LPS in the presence of different fractions for 24 h. NO2− was assayed as described in Materials and Methods. Data shown are means ± standard deviations of triplicate experiments.
While the propensity of sand fly saliva to inhibit NO production has been documented previously (9), our results show that this reduction is a result of saliva interfering with iNOS gene expression. This effect is probably generated via the inhibition of protein dephosphorylation events in mouse Mφ exposed to P. papatasi saliva.
In nature P. papatasi sand flies transmit cutaneous leishmaniasis by inoculating an estimated 10 to 700 Leishmania major promastigotes into the skin (31). However, experimental inoculation of susceptible mice with comparably low numbers of L. major promastigotes (100 to 500) does not cause disease but rather promotes immunity (6, 16). Why do low numbers of fly-transmitted parasites cause disease whereas syringe-inoculated parasites do not? It has been demonstrated that P. papatasi salivary gland lysates enhance the development of cutaneous leishmaniasis lesions (26). One probable means by which saliva promotes parasite survival in Mφ is via the inhibition of NO production. Supportive evidence shows that inhibition of iNOS expression in the skins of chronically infected asymptotic resistant mice reactivates latent L. major infections (24). Therefore, the down regulation of iNOS expression and the resultant reduction in NO production caused by P. papatasi saliva may similarly promote proliferation of amastigotes.
Partial biochemical characterization of the salivary PP-1/2A inhibitor.
For fractionation, 50 glands were lysed and spun filtered through Amicon microconcentrator filters as described in Materials and Methods. The retentate of the different filters and the 3-kDa filtrate were resuspended in HBSS, and two gland equivalents were used for the PP and NO assays. Only the 3-kDa filtrate showed inhibitory activity to PP-1/2A and NO (Fig. 4). The PP inhibition was dose dependent (data not shown). In addition, the PP-1/2A inhibitor was soluble in 100% ethanol. It was resistant to boiling for 10 min and was unaffected by proteases (trypsin and proteinase K) and nucleases (RNase and DNase). Hence, the PP-1/2A and NO inhibitory activities are probably mediated by the same small molecule (<3 kDa) which is neither a polypeptide nor a nucleic acid. Its exact characteristics await further clarification.
The data presented in this report illustrate the intricate nature of the mechanisms by which sand fly saliva enhances disease transmission. Pharmacologically active molecules, such as maxadilan in L. longipalpis or the described PP-1/2A inhibitor in P. papatasi saliva, probably evolved to facilitate blood feeding. However, as with many other biomolecules, salivary factors also exhibit other activities. In this case, Leishmania parasites have capitalized on the immunomodulatory effects of certain salivary factors to facilitate their establishment in the hostile environment of the vertebrate skin.
ACKNOWLEDGMENTS
This research was supported in part by grant 363/96-1 from The Israel Science Foundation and by The Bruno Goldberg Foundation (grants to A.W.). Additional support was provided by National Institutes of Health grant AI36382 (to G. C. Lanzaro, University of Texas Medical Branch, Galveston, Tex.) and AID grant TA-MOU-96-C14-142. J.W. is the recipient of a Golda Meir postdoctoral fellowship.
We thank J. M. C. Ribeiro, J. Shlomai, and M. P. Barrett for many useful suggestions.
REFERENCES
- 1.Ashford R W, Desjeux P, deRaadt P. Estimation of populations at risk of infection and number of cases of leishmaniasis. Parasitol Today. 1992;8:104–105. doi: 10.1016/0169-4758(92)90249-2. [DOI] [PubMed] [Google Scholar]
- 2.Bialojan C, Takai A. Inhibitory effect of a marine-sponge toxin, okadaic acid on protein phosphatases. Biochem J. 1988;256:283–290. doi: 10.1042/bj2560283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bradley D J. Visceral and post kala azar dermal leishmaniasis. In: Peters W, Killick-Kendrick R, editors. The leishmaniases in biology and medicine. II. San Diego, Calif: Academic Press; 1987. pp. 551–582. [Google Scholar]
- 4.Cohen P. The structure and regulation of protein phosphatases. Annu Rev Biochem. 1989;58:435–508. doi: 10.1146/annurev.bi.58.070189.002321. [DOI] [PubMed] [Google Scholar]
- 5.Cohen P, Holmes C F B, Tsukitani Y. Okadaic acid: a new probe for the study of cellular regulation. Trends Biochem Sci. 1990;15:98–102. doi: 10.1016/0968-0004(90)90192-e. [DOI] [PubMed] [Google Scholar]
- 6.Doherty M, Coffman R. Leishmania major: effect of infectious dose on T cell subset development in BALB/c mice. Exp Parasitol. 1996;84:124–135. doi: 10.1006/expr.1996.0098. [DOI] [PubMed] [Google Scholar]
- 7.Dong Z, Yang X, Xie K, Juang S H, Liansa N, Fidler I J. Activation of inducible nitric oxide synthase gene in murine macrophages requires protein phosphatase 1 and 2A. J Leukocyte Biol. 1995;58:725–732. doi: 10.1002/jlb.58.6.725. [DOI] [PubMed] [Google Scholar]
- 8.Green S J, Nacy C A, Meltzer M S. Cytokine-induced synthesis of nitrogen oxide in macrophages: a protective host response to Leishmania and other intracellular pathogens. J Leukocyte Biol. 1991;50:93–103. doi: 10.1002/jlb.50.1.93. [DOI] [PubMed] [Google Scholar]
- 9.Hall L R, Titus T G. Sand fly vector saliva selectively modulates macrophage functions that inhibit killing of Leishmania major and nitric oxide production. J Immunol. 1995;155:3501–3506. [PubMed] [Google Scholar]
- 10.Haystead T J, Sim A T R, Carling D, Honnor R C, Tsukitani Y, Cohen P, Hardie D G. Effects of the tumor promoter okadaic acid on intracellular protein phosphorylation and metabolism. Nature. 1989;337:78–81. doi: 10.1038/337078a0. [DOI] [PubMed] [Google Scholar]
- 11.Ingebritsen S T, Cohen P. Protein phosphatases: properties and role in cellular regulation. Science. 1983;221:331–333. doi: 10.1126/science.6306765. [DOI] [PubMed] [Google Scholar]
- 12.Jaffrey S, Snyder S. Nitric oside: a neural messenger. Annu Rev Cell Dev Biol. 1995;11:417–440. doi: 10.1146/annurev.cb.11.110195.002221. [DOI] [PubMed] [Google Scholar]
- 13.Lerner E A, Ribeiro J M C, Nelson R J, Lerner M. Isolation of Maxadilan, a potent vasodilatory peptide from the salivary glands of the sand fly Lutzomyia longipalpis. J Biol Chem. 1991;266:11234–11236. [PubMed] [Google Scholar]
- 14.Liew F Y, O’Donnel C A. Immunology of leishmaniasis. Adv Parasitol. 1993;32:161–259. doi: 10.1016/s0065-308x(08)60208-0. [DOI] [PubMed] [Google Scholar]
- 15.Lima H, Titus R. Effects of sand fly vector saliva on development of cutaneous lesions and the immune response to Leishmania braziliensis in BALB/c mice. Infect Immun. 1996;64:5442–5445. doi: 10.1128/iai.64.12.5442-5445.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Menon J N, Bretscher P A. Characterization of the immunological memory state generated in mice susceptible to Leishmania major following exposure to low doses of L. major and resulting in resistance to a normally pathogenic challenge. Eur J Immunol. 1996;26:243–249. doi: 10.1002/eji.1830260138. [DOI] [PubMed] [Google Scholar]
- 17.Modi G B, Tesh R B. A simple technique for mass rearing Lutzomyia longipalpis and Phlebotomus papatasi. J Med Entomol. 1983;20:568–569. doi: 10.1093/jmedent/20.5.568. [DOI] [PubMed] [Google Scholar]
- 18.Qureshi A, Asahina A, Ohnuma M, Tajima M, Granstein R, Lerner E. Immunomodulatory properties of maxadilan, the vasodilator peptide from sand fly salivary gland extracts. Am J Trop Med Hyg. 1996;54:665–671. doi: 10.4269/ajtmh.1996.54.665. [DOI] [PubMed] [Google Scholar]
- 19.Reiner S L, Zheng S, Corry D B, Locksley R M. Constructing polycompetitor cDNAs for quantitative PCR. J Immunol Methods. 1993;165:37–46. doi: 10.1016/0022-1759(93)90104-f. [DOI] [PubMed] [Google Scholar]
- 20.Ribeiro J M C, Vachereau A, Modi G B, Tesh R B. A novel vasodilatory peptide from the salivary glands of the sandfly Lutzomyia longipalpis. Science. 1989;243:212–214. doi: 10.1126/science.2783496. [DOI] [PubMed] [Google Scholar]
- 21.Sacks D L, Perkins P V. Development of infective stage Leishmania promastigotes within phlebotomine sand flies. Am J Trop Med Hyg. 1985;34:456–459. doi: 10.4269/ajtmh.1985.34.456. [DOI] [PubMed] [Google Scholar]
- 22.Samuelson J, Lerner E, Tesh R, Titus R. A mouse model of Leishmania braziliensis braziliensis infection produced by coinjection with sand fly saliva. J Exp Med. 1991;173:49–54. doi: 10.1084/jem.173.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shelonikar S, Ingebritsen T S. Protein (serine and threonine) phosphatases. Methods Enzymol. 1984;107:102–107. doi: 10.1016/0076-6879(84)07007-5. [DOI] [PubMed] [Google Scholar]
- 24.Streger S, Donhauser N, Thung H, Rollinghoff M, Bogdan C. Reactivation of latent leishmaniasis by inhibition of inducible nitric oxide synthase. J Exp Med. 1996;183:1501–1514. doi: 10.1084/jem.183.4.1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Theodos C M, Ribeiro J M C, Titus R G. Analysis of enhancing effect of sandfly saliva on Leishmania infection in mice. Infect Immun. 1991;59:1592–1598. doi: 10.1128/iai.59.5.1592-1598.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Theodos C M, Titus R G. Salivary gland material from the sand fly Lutzomyia longipalpis has an inhibitory effect on macrophage function in vitro. Parasite Immunol. 1993;15:481–487. doi: 10.1111/j.1365-3024.1993.tb00634.x. [DOI] [PubMed] [Google Scholar]
- 27.Titus R, Ribeiro J M C. Salivary gland lysates of the sandfly Lutzomyia longipalpis enhance Leishmania infectivity. Science. 1988;239:1306–1308. doi: 10.1126/science.3344436. [DOI] [PubMed] [Google Scholar]
- 28.Vidal S M, Malo D, Vogan K, Skamene E, Gros P. Natural resistance to infection with intracellular parasites: isolation of a candidate for Bcg. Cell. 1993;73:469–485. doi: 10.1016/0092-8674(93)90135-d. [DOI] [PubMed] [Google Scholar]
- 29.Walton B C. American cutaneous and mucocutaneous leishmaniasis. In: Peters W, Killick-Kendrick R, editors. The leishmaniases in biology and medicine. II. San Diego, Calif: Academic Press; 1987. pp. 638–653. [Google Scholar]
- 29a.Warburg, A. Unpublished data.
- 30.Warburg A, Saraiva E, Lanzaro G C, Titus R, Neva F. Saliva of Lutzomyia longipalpis sibling species differs in its composition and propensity to enhance leishmaniasis. Philos Trans R Soc Lond B. 1994;354:223–230. doi: 10.1098/rstb.1994.0097. [DOI] [PubMed] [Google Scholar]
- 31.Warburg A, Schlein Y. The effect of post-bloodmeal nutrition of Phlebotomus papatasi on the transmission of Leishmania major. Am J Trop Med Hyg. 1986;35:926–930. doi: 10.4269/ajtmh.1986.35.926. [DOI] [PubMed] [Google Scholar]