Abstract
Cancer, a leading global cause of mortality, arises from intricate interactions between genetic and environmental factors, fueling uncontrolled cell growth. Amidst existing treatment limitations, vitamins have emerged as promising candidates for cancer prevention and treatment. This review focuses on Vitamins A, C, E, and D because of their protective activity against various types of cancer. They are essential as human metabolic coenzymes. Through a critical exploration of preclinical and clinical studies via PubMed and Google Scholar, the impact of these vitamins on cancer therapy was analyzed, unraveling their complicated mechanisms of action. Interestingly, vitamins impact immune function, antioxidant defense, inflammation, and epigenetic regulation, potentially enhancing outcomes by influencing cell behavior and countering stress and DNA damage. Encouraging clinical trial results have been observed; however, further well-controlled studies are imperative to validate their effectiveness, determine optimal dosages, and formulate comprehensive cancer prevention and treatment strategies. Personalized supplementation strategies, informed by medical expertise, are pivotal for optimal outcomes in both clinical and preclinical contexts. Nevertheless, conclusive evidence regarding the efficacy of vitamins in cancer prevention and treatment is still pending, urging further research and exploration in this compelling area of study.
Keywords: vit-E, vit-A, vit-D, combination anticancer therapy, vit-C
1. Introduction
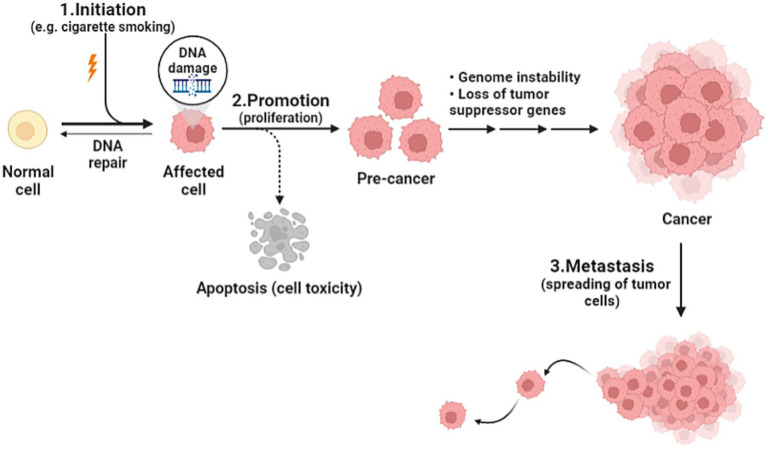
Cancer is the health problem of the century, being the second leading cause of death after heart disease worldwide. As the world population is increasing, and as humanity have made such huge progress against causes of death that once killed people early in life, the number of cancer deaths has increased from around 5.7 million in 1990 to 8.8 million in 2017. It is true, the number of cancer deaths is increasing, but yet, individual death rates are falling (1). Cancer is defined as an unregulated proliferation and spread of cells in the body and can reach trillions in number. In a normal setting, human cells grow and multiply via cell division to form new needed cells. When this order breaks down, abnormal cells start to grow and multiply to develop abnormal tissues (2). The complicated prosperous nature of cancer is affected by various elements including genetic and environmental factors such as tobacco smoking, urbanization, and changing diet patterns. The genetic changes that lead to cancer can happen because of several reasons including errors that occur at cell division, DNA damage that may have been caused by substances in the environment, or genetic predispositions that were inherited from the parents. Each cancer is a unique combination of these genetic changes, and as cancer continues to develop, additional changes will occur (3). Studies devoted to establishing measures that focus on environmental factors have been implemented; nevertheless, little progress in favor of reducing cancer incidence has been made (4). In the domain of cancer biology, cancerous cells develop from healthy cells through a complex process known as malignant transformation, involving several key steps. Firstly, it begins by altering the genetic material of the cells, preparing it to become cancerous. The second step, promotion, is induced by agents known as promoters, which can include substances in the environment or certain medications. Promoters allow cells that were primed to become cancerous, and they do not affect cells that have not undergone initiation. The immune system is usually able to recognize cancer cells and destroy them before they duplicate, this explains why certain cancers are more likely to progress in people whose immune system is impaired. The third step is the spreading of cancer cells through the bloodstream or lymphatic system to nearby or distant locations; it can spread and invade surrounding tissues or organs (Figure 1). As it grows, nutrients are provided by their direct diffusion to cells, this further leads to increased cancer volume, destroying adjacent tissues (5). Understanding cancer’s cellular kinetics gives important insight when it comes to designing dosing schedules and timing intervals of treatment (6). Prevailed anticancer treatments such as chemotherapy, surgery, and radiotherapy have been challenging in terms of their adverse effects and disease progression, hence the humongous number of efforts to establish an alternative treatment regimen (7). The new approach is associated with vitamins and recently their anticipated anticancer effects have been considerably analyzed. Vitamins are essential nutrients for human metabolism, taking part in an essential function as coenzymes or enzymes in many imperative procedures for the regular functioning of the body, and it has been obvious that nutritional vitamins essentially contribute to human disease and healing (7). In a comprehensive review of 65 clinical trials involving cancer patients, the safety of various dietary supplements, particularly vitamins, was assessed. The findings revealed that vitamins, among other supplements, were generally safe for cancer patients, emphasizing the importance of continued research on the impact of vitamins in cancer treatment (8). On the other hand, recent findings suggest a potential benefit of calcium and vitamin D supplementation in cancer prognosis, particularly in reducing colorectal and breast cancer mortality. Similarly, post-diagnosis intake of specific antioxidants (such as vitamins C, D, and E) has shown correlations with decreased mortality among cancer survivors. However, these associations, primarily derived from observational studies, warrant cautious interpretation. Further research, especially randomized controlled trials, is essential to clarify these relationships, focusing on optimal dosages and the timing of supplementation post-diagnosis. Developing evidence-based recommendations for cancer survivors necessitates a comprehensive understanding of dietary supplement use alongside conventional treatments (9). The rationale for our deliberate choice of vitamins A, C, E, and D for inclusion in our comprehensive review is rooted in their remarkable attributes that make them ideal candidates for exploration in the context of therapeutic applications. First and foremost, these vitamins are readily available primarily through dietary consumption. This inherent accessibility renders them mainly practical for potential therapeutic interventions. Vitamin A can be found in numerous common foods such as carrots, sweet potatoes, and spinach. Vitamin C is abundantly present in fruits like oranges, strawberries, and kiwi, while vitamin E is found in nuts, seeds, and vegetable oil. Lastly, vitamin D can be synthesized by the skin when exposed to sunlight, and it is also available in fortified dairy products and fatty fish (10–13). Previous reviews of clinical trials, animal models, and in vitro studies suggest that dietary vitamins and minerals may contribute to the prevention and treatment of a variety of cancers such as those affecting the breast, lungs, liver, cervix, stomach, brain (glioma), lymphatic system (lymphoma), and prostate (14–26). However, further well-controlled studies are needed for a better understanding of their anticancer potential. In this review, we are going to shed light on the association between pharmacological vitamin doses and cancer and discuss the mechanisms of action. Additionally, we will briefly address how some studies have shown a lower incidence of cancer in vitamin-rich diets, which has received considerable attention in recent years.
Figure 1.
Cancer development steps.
2. Methods
Vitamins A, C, E, and D’s cancer treatment and prevention effects were examined in a comprehensive literature review. This investigation examined many human and animal scientific studies. Many petri dish and animal experiments were examined. This study examined how vitamins may treat and prevent cancer by reading many research papers. We searched PubMed, Google Scholar, Scopus, Web of Science, and other scientifically correct databases for the information we needed. With search terms like “vitamins,” “cancer treatment,” “prevention,” “chemotherapy,” and “immunotherapy,” we hoped to find relevant studies and learn from existing research. Researchers looked for English papers on how vitamins A, C, E, and D can treat or prevent cancer. Some studies examined how these vitamins might affect immunotherapy or chemotherapy. After reviewing all literature, 300 studies were found. After that, a strict process selected the best and most relevant studies. After a thorough review, studies that were not directly related to the topic, were not written in English, and did not provide enough experimental details or results were discarded. The best studies to examine and consider were 153. We were able to thoroughly examine the topic because we carefully selected and organized key information.
3. Biological activities of vitamins A, C, D, E and their impact on cancer prevention
Vitamins reflect a concept that involved different groups of bioactive compounds obtaining nutritional benefits for human health (27). Hence, they are one of the main essential nutrients that play a role in enhancing the immune system, improving mental health and the aging process, increasing energy levels, and protecting the cardiovascular system (28). Their biochemical functions in the body are translated by being coenzymes, hormones, antioxidants, promotors of signaling pathways, and modulators of cell and tissue growth and differentiation (29).
Interestingly, vitamin A (VA) showed an indirect impact on regulatory T cell development and cytokines production (30). Due to the chemical structure of VA and carotenoids (the presence of polyene chain), they have exhibited antioxidant activity by protecting cells from the damage of free radicals and singlet oxygen (31, 32). It was reported that dietary VA can improve glutathione peroxidase and superoxide dismutase activities as well as reduce reactive oxygen species (ROS) production (33). Retinol and its derivatives, found in animal-based foods, have essential roles in cell differentiation, proliferation, and apoptosis. They also play a key role in skin and bone growth through retinoic acid. Cytoplasmic binding proteins like CRBP-1 regulate intracellular retinoid levels, affecting retinol uptake and availability. Recent research has linked reduced CRBP-1 levels to increased malignancy in breast, ovarian, and nasopharyngeal cancers. Restoring CRBP-1 could enhance retinol sensitivity and reduce ovarian cancer cell viability, potentially guiding personalized retinoid therapy for cancer patients (34). Moreover, retinoids, when bound to RAR/RXR receptors (RAR: Retinoic Acid Receptor and RXR: Retinoid X Receptor), trigger a series of chromatin structure modifications that can encourage cell differentiation and initiate lasting epigenetic alterations. In the context of cancer cells, these changes hold the potential to induce differentiation toward a less malignant state. Retinoids achieve this by promoting stem cell differentiation and reshaping the gene expression patterns within tumor cells, rendering them more responsive to other therapeutic approaches. Consequently, retinoids are expected to play a significant role in future cancer treatments (35, 36).
On the other hand, Ascorbic acid is a powerful antioxidant in various reactions and metabolic processes. It neutralizes free radicals and toxins, subsequently attenuating oxidative damage and inflammatory responses (37, 38). Acting so, at micromolar concentrations, physiological ascorbate, a fully reduced form of vitamin C (VC), can reduce harmful ROS. Paradoxically, it also functions as a pro-oxidant at millimolar plasma concentrations via intravenous administration of pharmacological ascorbate (39). Besides, several studies have reported the potential of VC in reducing inflammation and modulating inflammatory biomarkers (40–43). A recent study has shown the complicated roles of VC in cancer biology, emphasizing its influence on stem cells, cancer metastasis, and immunotherapy. Vitamin C affects various biochemical reactions in cells, collagen synthesis, and the regulation of hypoxia-inducible factor (HIF), impacting extracellular matrix remodeling and cancer spread. It also displays promise in inhibiting cancer cell glycolysis and enhancing cancer immunotherapy when combined with anti-PD-L1 therapy (44). Beyond its antioxidant properties, VC directly impacts genomic stability, making it relevant in cancer prevention, stem cell therapy, and the treatment of hematological malignancies where TET mutations and aberrant DNA methylation are common (45). In recent years, the role of VC in cancer treatment has gained attention due to its influence on epigenetic regulation. Vitamin C, acting as an antioxidant and a co-factor for crucial enzymes like TET methylcytosine dioxygenases, plays a significant role in cancer therapy. Epigenetic abnormalities are common in cancer, and VC has consistently been shown to enhance DNA demethylation by TET enzymes when combined with DNA methyltransferase inhibitors (46).
As well, strong antioxidant properties are present in all vitamin E (VE) isomers (47). Because their chromanol ring contains phenolic hydrogen, they can scavenge reactive oxygen species (ROS). Vitamin E’s ability to scavenge reactive oxygen species (ROS) and prevent oxidative damage to cells and tissues is due to its phenolic hydrogen (48). Oxidative stress can cause free radical chain reactions that lead to lipid peroxidation in both people and animals used in experiments. Vitamin E is very important for stopping these free radical chain reactions, which stops lipid peroxidation and protects the integrity of biological membranes. As a strong antioxidant, vitamin E helps keep cells and tissues safe from the damage caused by oxidative stress (48). Many cancers, including skin and gastrointestinal cancers, may have oxidative stress as an underlying cause. A transcription factor known as Nrf2 regulates the induction of antioxidant enzymes. Research has demonstrated that specific forms of vitamin E, particularly γ-tocopherol and, to a lesser degree, α-tocopherol, can activate Nrf2. This, in turn, enhances the production of different antioxidant enzymes, such as SOD, catalase, glutathione peroxidase, and phase II detoxifying enzymes. In response to oxidative stress, cells launch an antioxidant response, which aids in cellular homeostasis maintenance (49). One reason why γ-tocopherol and δ-tocopherol are more effective than other tocopherol isoforms is because their chromanol rings have an unmethylated C-5 position. These isoforms are able to effectively combat reactive nitrogen species, such as NO2 and peroxynitrite, due to this structural feature. They can detoxify these reactive nitrogen species more effectively by producing 5-nitro-γ-tocopherol (50). Research has shown that vitamin E metabolites, like CEHCs (carboxyethyl hydroxychromans), can inhibit lipid peroxidation and have strong antioxidant capabilities. The free radical scavenging capabilities of these metabolites are even greater than those of the original vitamin E isoforms. Out of all the metabolites, γ-tocopheryl quinone is the one that triggers the antioxidant response the most effectively. This is accomplished by increasing glutathione levels and activating transcription factor 4 transcription. As a defense mechanism against oxidative stress, this antioxidant response shields cells from harm (51). Tocotrienols, like tocopherols, work as antioxidants by encouraging the production of different antioxidant enzymes, such as catalase and superoxide dismutase (SOD) (52). Tocopherols and tocotrienols are both very good at removing free radicals from membranes and lipoproteins. They can stop fatty acid peroxyl radicals from forming and turn them into tocopheroxyl radicals. These radicals are then broken down to make vitamin E again. This cycle of antioxidants helps keep membranes and lipoproteins intact and protects cell parts from oxidative damage (53, 54). It is widely recognized that eicosanoids, which are generated through the COX-2 and 5-LOX pathways, play a role in the advancement of colon cancer. Inhibiting these pathways is one mechanism by which vitamin E prevents carcinogenesis and reduces inflammation in the colon (55). It is well-established that eicosanoids produced by the COX-2 and 5-LOX pathways contribute to the progression of colon cancer. Therefore, vitamin E’s ability to inhibit these pathways contributes to its ability to inhibit colon inflammation and prevent carcinogenesis (55). A group of transcription factors known as peroxisome proliferator-activated receptors (PPARs) include PPAR-α, γ, and δ. These receptors control inflammatory pathways by blocking COX-2. In addition to PI3k/Akt and NF-κB, PPARs are linked to other signaling pathways. The ability of δ-tocopherol to activate PPARγ in various cell lines has been proven in studies conducted in both laboratory and living organism settings. Research has shown that δ-tocopherol inhibits inflammation, slows down cell cycle progression, and triggers cell death by activating PPARγ (56). In addition, research has shown that vitamin E (VE) can inhibit inflammatory markers through various pathways. Important inflammatory factors such as cyclooxygenase 2 (COX-2), tumor necrosis factor (TNF-α), and interleukin-6 (IL-6) are downregulated when prostaglandin E2 (PGE2) is inhibited (57).
Many studies have shown the correlation between vitamin D deficiency and the high incidence of infectious diseases and inflammatory autoimmune diseases (58, 59). Vitamin D reduces inflammation by mediating different pathways such as inhibition of NF-kB, increasing MKP5 expression, and blocking the prostaglandins pathway (60, 61). Regarding antioxidant activity, vitamin D was able to activate the Nrf2-Keap1 antioxidant pathway and improved nephropathy in a diabetic animal model (62). As well, VD supplementation can modulate oxidative stress parameters by reducing nitric oxide (NO), increasing glutathione (GSH) expression, and improving the total antioxidant capacity (63). Moreover, experimental findings indicate that the preventive effects of VD in cancer primarily result from its ability to influence critical biological processes, including cell proliferation, cell differentiation, the expression of growth factor genes, signal transduction, and apoptosis regulation (64). Additionally, VD has been found to interfere with the action of growth factors like insulin-like growth factors (IGF) by promoting the release of IGF binding protein 3 (IGFBP3), which limits the pro-proliferative effects of IGF (65). Vitamin D plays a crucial role in preventing early neoplastic processes through its anti-inflammatory, antioxidant, DNA repair, and cell regulation mechanisms. It also influences the intestinal microbiota, linked to inflammatory bowel diseases and colon cancer (66). It has shown promise in preventing and improving outcomes in colorectal cancer (CRC). Acting as a regulatory prohormone through its widespread vitamin D receptor (VDR) binding, it influences immune modulation and microbial composition in the colonic mucosa, affecting key mechanisms in CRC development (67). Calcitriol, known as bioactive vitamin D, plays a role in regulating numerous biological pathways. Its various actions, such as DNA damage repair and protecting against oxidative stress, can contribute to preservation of somatic stem cells. Additionally, calcitriol may inhibit the growth of cancer stem cells by inducing cell cycle arrest and promoting apoptosis (68). In summary, these vitamins collectively contribute to cancer defense and hold promise for improving cancer-related outcomes.
4. Vitamins as an anticancer agent: preclinical studies
4.1. Vitamin A
Vitamin A is a collection of life-essential, fat-soluble substances with an unsaturated isoprenoid main chain that is derived from both plants and animals. All derivatives of vitamin A share similar structural and physiological functions within the human body. Substances with a common structure of four isoprenoid subunits, whether of synthetic or natural origin, are also categorized as retinoids (69). Nevertheless, the core vitamin A sequence is concealed in their structures, and their activation of retinoid receptors is comparable to most of the other retinoids. Each of these substances is fat-soluble and, unlike water-soluble vitamins, accumulates rapidly in the body, particularly in the adipose tissue and liver. This is advantageous since temporary vitamin A deficiency is not linked with clinical symptoms, but on the other side, this can lead to accumulation as well as toxic effects (69). Vitamin A can be obtained from the diet either as provitamin A (carotenoids) through plants or as vitamin A (retinol and its near derivatives) through animal-based foods. The most common type of retinoid in the body is retinol. Furthermore, all-trans retinoic acid and 11-cis-retinal are the most active substances within the body (69).
Multiple mechanisms have been studied and proven for vitamin A, including more than gene signaling pathways in terms of anticancer effects (70), the effect of retinoids on mitochondrial functions (71), and other mechanisms and pathways that have been studied and proven (72, 73). All-trans retinoic acid, an endogenous retinoid, has been shown to inhibit the expression of PPARγ and to enhance the expression of DOK1 as it regulates the DOK1/PPARγ pathways thus inhibiting cell proliferation in breast cancer (MCF7 cell line) (74). As well, the synthetic retinoid (ST1926) had a greater effect on MDA-MB-231 and MCF7 cell lines represented by binding to β-catenin and cyclin D1, which regulates the Wnt/−catenin signaling pathway (75). Peretinoin, a synthetic retinoid, revealed a potential to inhibit hepatocarcinogenesis by downregulation of sphingosine kinase 1, which resulted in hindering cancer progression (76). It is also believed that the regulation of microRNAs is linked to retinoid anti-cancer mechanisms. An in vitro and in vivo study has shown the synergistic effect of all-trans-RA when combined with paclitaxel in A549 cell line. The difference in IC50 values between the combination and all-trans-RA alone was almost three times more efficient, which means that the cytotoxicity of the combination is higher. The researchers went further by testing this combination on solid tumors in mice, and it was proven that the treatment prolonged their survival rate and effectively prevented tumor progression (76). As well, all-trans-RA with a γ-secretase inhibitor (DAPT) as a combination therapy has shown better results than monotherapy in gastric cancer cells (MKN45 cells). Using MTT assay, a significant decrease in cell viability was reported. Additionally, it induced apoptosis by increasing caspases expression (77). An in vitro study demonstrated the antiproliferative activity of all-trans-RA-Podophyllotoxin conjugate against human gastric cancer cells (MKN-45 and BGC-823 cell lines). It inhibited cell growth by promoting apoptosis and blocking the ERK1/2 and AKT signaling pathways (78). In addition, all-trans-RA effectively reduced myeloid-derived suppressor cell (MDSC) accumulation and increased the frequency of CD8+ T cells in mice with cervical tumors. Notably, combining all-trans-RA with anti-PD-L1 antibody treatment resulted in further delayed tumor growth, along with an increased proportion of activated T cells and elevated levels of immune-stimulating cytokines in tumors (79). As well, all-trans-RA significantly inhibited hepatocellular carcinoma progression, reduced MDSCs and tumor-associated macrophages, and suppressed the expression of immunosuppressive molecules in the tumor microenvironment. In particular, it reduced the intratumoral infiltration G-MDSCs and the expression of protumor immunosuppressive molecules including arginase 1, iNOS, IDO and S100A8 + A9, which was accompanied by increased cytotoxic cell infiltration (80). Moreover, in a 4 T1 mouse model of breast cancer, researchers employed a combination of cryo-thermal therapy and all-trans-RA to address myeloid-derived suppressor cells (MDSCs). The combination significantly increased mouse long-term survival rates by promoting MDSC maturation, reducing suppressive molecule expression, and inhibiting metabolic pathways. Early after treatment, Th2 and Treg subsets decreased, improving Th1-dominant CD4+ T-cell differentiation, while later stages showed enhanced cytotoxicity of CD8+ T cells and natural killer cells. This approach offers promise for breast cancer therapy to overcome MDSC-induced immunosuppression (81). Chuang et al. suggested a synergistic therapeutic effect of polyinosinic-polycytidylic acid combined with 13-cis-retinoic acid in neuroblastoma (82). Using this combination in a mouse xenograft model led to suppressing tumor development through stimulating retinoic acid receptors beta (RAR-β), and preventing vessel formation (82).
4.2. Vitamin C
Vitamin C (VC) is a water-soluble natural compound, that has shown multiple mechanisms to target cancer. VC is being widely studied and this may be attributed to its high safety profile. It cannot be synthesized by the human body, and strictly and easily obtained from dietary intake to achieve a daily allowance of 75–90 mg per day. It can be obtained from fruits, like citrus fruits and berries, and vegetables like raw sweet pepper and broccoli, to achieve a plasma ascorbate concentration of 30–80 μM (12). Ascorbic acid is a powerful antioxidant in various reactions and metabolic processes. It neutralizes free radicals and toxins, subsequently attenuating oxidative damage and inflammatory responses (12, 83). VC has shown both preventive and anticancer capacities. As for its preventive capacity, studies have suggested a possible protective role of high VC intake with reduced colorectal cancer (84, 85), stomach cancer (86, 87), and pancreatic cancer (88). In addition, a recent in vivo study suggests that oral VC may have a preventive effect on the development of leukemia (89). Determining the adequate amount of oral VC is essential for optimizing human health and preventing chronic diseases including cancer.
Several studies have aimed to demonstrate the anticancer activity of VC alone or as adjunct therapies with conventional treatment methods. A study experimented VC in combination with each of eribulin mesylate, tamoxifen, fulvestrant, and trastuzumab on a variety of breast cancer cell lines (MCF7, SK-BR3, MDA-MB-231, and breast epithelial cells MCF10A), have shown that high-dose VC with each of those anti-cancer drugs had decreased the cell viability of cancer cells more comprehensively than high-dose of VC or chemotherapy alone (90). VC’s ability to reduce oxidative stress in order to manage the side effects of different cancer treatments has been explored in multiple studies (91, 92). An in vitro study has examined VC along with methotrexate (MTX) on their effectiveness against human glioblastoma multiforme (Human glioblastoma DBTRG cell line) (93). The results showed that VC alone (5 μM) is not cytotoxic to either DBTRG or HK-2 cells; however, in combination with 0.01 μM MTX was significantly able to reduce cell viability. Another in vitro study explored VC effects on oral squamous cell carcinoma (OSCC) using human OSCC-derived cell lines CAL27, SCC9, and SCC25 (94). Their finding suggested various mechanisms by which VC can induce anticancer effects. Initially, VC led to a significant rise in the ROS levels, inducing DNA damage and ATP depletion stresses. It also inhibited Bcl-2 expression and promoted Bax expression and caspase-3 cleavage. Additionally, VC induced cell cycle arrest at the G0/G1 phase in OSCC cells. Their data demonstrated a significant inhibitory impact of VC on the colony-forming ability of OSCC cells, dependent on concentration and time (94).
Another aspect of VC is its ability to modulate anticancer immune responses (39, 95). A study, using several mouse cancer models including colorectal, breast, melanoma, and pancreatic, explored the impact of daily intravenous high-dose VC (4 g/kg) (96). They observed that, in most cases, tumor growth was delayed only in the presence of a fully competent immune system, which suggests that VC antitumor activity is dependent on some immunomodulatory functions and not only on its pro-oxidant effects. Despite this being mostly an uncharted area, this study is evidence of VC’s capacity to modulate infiltration of the tumor microenvironment by cells of the immune system, delaying cancer growth in a T-cell-dependent manner. VC enhances the cytotoxic activity of adoptively transferred CD8 T cells, and also co-operates with immune checkpoint therapy (ICT) in many cancer types, proving that a combination of VC and ICT can be curative in models of mismatch repair deficient tumors with high mutational burden. To optimize the antiproliferative impacts of VC in murine tumors such as breast, colorectal, melanoma, and pancreatic cancers, a fully functional immune system is required (96).
Recent studies have provided a better mechanistic understanding of VC anti-cancer effects. VC has been proven to have anti-tumorigenic activity by increasing the reactive oxygen species in cancer cells without major toxicities. It can address three common vulnerabilities shared by many cancer cell, including redox imbalance, epigenetic programming, and oxygen-sensing regulation (39). Cancer cells tend to depict compensation mechanisms to survive. Antioxidants can be both destructive and accelerative of tumor progression. So, alone, it cannot prevent or suppress cancer. Hence the employment of pro-oxidant anticancer therapies, such as radiation, but serious collateral damage can occur causing a narrow therapeutic window. Ascorbate can overcome this issue via two common features of cancer cells: elevated levels of labile iron both extracellular and/or intracellular, and heightened dependence on glucose uptake and glycolysis. In the presence of redox-active transition metals, VC exhibits pro-oxidant effects, inducing oxidative stress through the formation of ROS or by inhibiting the antioxidant system (39, 97). In the process of cellular iron metabolism, the labile iron pool is chelatable and redox-active. When ferrous ion reacts with peroxide in those pools, they can produce the damaging hydroxyl radical •OH via the Fenton reaction. Ascorbate then comes to sustain this reaction by donating electrons to ferric ions generating redox active ferrous ions and perpetuating the generation of ROS in continuation that contributes to cell death. It was shown that Asc•− and peroxide were generated in vivo following intravenous ascorbate injections in rats (0.5 g/kg), and the production was ascorbate-dose-dependent (98). In an animal model study, daily high-dose ascorbate (4 g/kg) inhibited neuroblastoma growth, and tumors had increased activity of checkpoint kinase 2 (CHK2) and histone 2AX (H2AX). This implies that pharmacological ascorbate can induce DNA damage in tumors implanted in animals (99). Its selectivity toward cancer cells can be explained by different potential reasons. First, it has been shown in various types of cancer such as breast, prostate, and lymphoma that iron metabolism can be reprogrammed either by the upregulation of several iron intake pathways or the downregulation of iron export and storage pathways (100, 101). For instance, in breast cancer cells, the intracellular pool of labile ions is twice as elevated as in normal breast epithelial cells. Additionally, tumor cells may exhibit greater vulnerability to high-dose ascorbate compared to normal cells as they can generate higher levels of peroxide and •OH than their normal counterparts (39).
Oncogenic KRAS or BRAF mutations are contributors to the Warburg effect by upregulating GLUT1. This exploitation by cancer could serve as a potential therapeutic strategy for targeting the disease. When ascorbate is administered in high doses, it gets oxidized to DHA (Docosahexaenoic acid). DHA is structurally similar to glucose and thus, efficiently taken up via GLUT1 in KRAS or BRAF mutant cells. DHA is rapidly reduced back to ascorbate inside the cell. The decrease in intracellular antioxidants and subsequent rise in ROS levels interferes with glycolytic pathways, resulting in an energy crisis. For instance, recent studies demonstrated the selective elimination of gastric cancers and von Hippel–Lindau-null renal cancers, characterized by high GLUT1 expression, through high-dose ascorbate treatment (102, 103). Ascorbate has a significantly long half-life. It efficiently and continuously generates DHA in the tumors’ oxidative microenvironment. In addition, extracellular peroxide generated via the oxidation of ascorbate contributes to increased levels of DHA, as DHA is the main oxidized form of ascorbate. Normal erythrocytes are protected from high-dose ascorbate due to their increased level of antioxidant enzymes like GSH and have an enhanced glucose flux into the pentose phosphate (PP) pathway to generate more NADPH, which is a crucial molecule for the recovery of GSH. Patients who are deficient in G6PD, which is the rate-limiting enzyme in PP pathway, have a lagging in NADPH production, hindering those cells vulnerable to VC-induced oxidative stress, subsequently leading to erythrocytes death, thereby causing anemia (39).
Another way that VC acts to hinder cancer is its ability to activate 10-11 translocation (TET) proteins. Those proteins belong to the αKGDD enzyme family and they demethylate DNA and therefore, liberate the abnormal epigenetic reprogrammed methylated DNA seen in cancer. Ascorbate can donate an electron to a ferric ion and convert it to a ferrous ion, which is critical for TET activity. Ascorbate treatment in vitro increased TET activity, which led to DNA methylation, and subsequently liberated the expression of tumor suppressor genes, which increased chemosensitivity (104). An in vivo study has shown that daily injection of high-dose ascorbate (4 g/kg) in a leukemic mouse model, promoted DNA demethylation and the expression of genes critical for myeloid cell differentiation, and subsequently recapitulated the TET2 restoration phenotypes (105). Other αKGDDs such as JHDM and ALKB have yet an unclear role in cancer development and to what extent ascorbate influences their activity.
Usually, solid tumors obstruct and compress surrounding blood vessels, resulting in a hypoxic encounter. Tumor cells in their striving to survive, adapt to this hypoxic microenvironment. One of those oxygen-sensing regulations is the tumor cell’s activation of HIF1, an evolutionarily conserved transcription factor. HIF1 consists of two subunits, the O2-regulated HIF1α and a constitutively expressed HIF1β. Oxygen regulates HIF1α activity through “Proline hydroxylase domain proteins” (PHD) and HIF hydroxylases. Under normal oxygen concentration, HIF1 transcription is inhibited. Under hypoxic conditions, PHDs and HIF hydroxylases are inactive, leading to HIF1 activation. So, cells that lack adequate amounts of ascorbate can have increased HIF1α function, which may potentially play a role in tumor progression. HIF1 is an important target in cancer therapy via HIF hydroxylase that is enhanced by ascorbate treatment, thus suppressing tumor growth. Two studies support this hypothesis, indicating a mounting body of evidence that suggests inhibition of HIF1α-dependent tumor growth by ascorbate (106, 107). An in vitro study demonstrated that pharmacological ascorbate led to a reduction in the expression of HIF1α and its downstream target GLUT1 in thyroid cancer cells (108).
The previously mentioned findings have renewed people’s scope and vision of the pharmacological antitumor effects of VC, putting it in the spotlight for further elucidation of its function and mechanism.
4.3. Vitamin E
The compound known as α-tocopherol, often termed the “classic” form of vitamin E, has shown significant efficacy in traditional fertility restoration tests compared to other tocopherols. It is primarily present in wheat germ, almonds, and sunflower oil. On the other hand, γ-tocopherol is more commonly found in the American diet and is frequently present in vegetable oils like soybean, corn, and cottonseed (109). δ-Tocopherol is predominantly present in soybean and castor oils, with a comparatively lower concentration in wheat germ oil (52). The production of β-tocopherol (γ-TmT) frequently occurs as an incidental outcome in the course of vegetable oil refinement (110). Tocotrienols are commonly consumed in diets prevalent in East-South Asian regions, with palm and annatto oils serving as their primary sources (111).
A plethora of epidemiological research have investigated the association between the risk of cancer and vitamin E. Emerging studies indicate that vitamin E exhibits potential as a viable contender for cancer adjuvant therapy, primarily due to its anticarcinogenic characteristics. The initial hypothesis regarding the chemopreventive effects of vitamin E emerged due to the observation that individuals residing in the Mediterranean region, whose diets are rich in various isoforms of vitamin E, demonstrate a decreased susceptibility to colon cancer in comparison to individuals residing in Northern Europe and the United States (49, 112). Vitamin E exhibits an inhibitory effect on cell proliferation through the induction of apoptosis and cell cycle arrest. Apoptosis occurs via two distinct pathways: the extrinsic pathway, which is mediated by death receptor signaling, and the intrinsic pathway, which is impacted by mitochondrial rupture leading to the release of cytochrome c into the cytosol. In the end, these routes converge to trigger the activation of execution caspases, particularly caspase-3, resulting in the fragmentation of poly-ADP-ribose-polymerase (PARP) (52). Angiogenesis plays a crucial role in both the growth and spread of tumors, involving the rapid proliferation and migration of endothelial cells. Studies conducted in both laboratory settings (in vitro) and living organisms (in vivo) have provided evidence that certain isoforms of vitamin E, particularly tocotrienols, possess the capability to hinder the angiogenesis process (113). Additionally, it has been found that tocotrienols have the ability to decrease the expression of VEGF receptors. In a study conducted by Shibata et al., it was observed that tocotrienol administration led to the suppression of hypoxia-inducible factor (HIF-1), a transcription factor known to induce the expression of vascular endothelial growth factor (VEGF) and initiate angiogenesis in the presence of low oxygen levels. The study focused on human colorectal adenocarcinoma cells in an in vitro setting. The suppression of HIF-1 resulted in the blocking of the release of angiogenic factors (114). The proangiogenic cytokines interleukin-6 and interleukin-8 are well-known to elevate VEFG levels. Tocotrienol therapy resulted in a reduction of IL-6 and IL-8 levels in human umbilical vein endothelial cells (HUVEC cells) (115). Tocotrienols have demonstrated inhibitory effects on the phosphorylation of the PI3k/Akt signaling pathway, resulting in the downregulation of key signaling molecules including endothelial nitric oxide synthase (eNOS), glycogen synthase kinase 3 (GSK3), and extracellular signal-regulated kinase (ERK). This pathway plays a role in angiogenesis by promoting the binding interaction between vascular endothelial growth factor (VEGF) and its corresponding receptor (116).
In a comprehensive study investigating the anticancer effects of vitamin E variants, including natural (αT) and synthetic (γT) forms, on breast cancer models, γT emerged as particularly promising. In vitro experiments demonstrated γT’s efficacy in inhibiting colony formation and inducing apoptosis in various breast cancer cell types. The underlying mechanisms involved activation of caspases-8 and -9, coupled with downregulation of c-FLIP and survivin. Concurrently, assessments in a BALB/c mouse mammary cancer model revealed that both all-rac-αT and all-rac-αTAc significantly reduced tumor development and lung metastasis, highlighting their potential as anti-tumor and anti-metastatic agents in breast cancer. The study also compared αT, all-rac-αT, and all-rac-αTAc, with αT and all-rac-αT exhibiting lesser efficacy. The findings emphasize γT’s persistent and robust anticancer effects across in vitro and in vivo models, positioning it as a noteworthy candidate for breast cancer treatment. Further research is needed to unravel the molecular mechanisms and assess efficacy in clinical settings (117).
Research on β-tocotrienol (β-T3) showed its ability to lower PD-L1, a key immune checkpoint ligand, in both in vitro and in vivo settings. This decrease was caused by JAK2/STAT3 pathway inactivation. Results showed improved immune response and reduced PD-L1-induced tumor-intrinsic signaling, highlighting β-T3’s anticancer potential (118).
δ-Tocotrienol (δ-T3) has been widely recognized for its remarkable anticancer properties (119). In accordance with prior investigations, it has been observed that the administration of the antioxidant δ-tocotrienol (δ-T3) elicits apoptotic, paraptotic, and autophagic responses in castration-resistant prostate cancer (CRPC) cells (120). Recent findings show that δ-T3, a vitamin E variation, increases ROS and mitochondrial Ca2+ levels in PC3 and DU145 cell lines. This study aims to understand the mechanisms behind δ-T3’s anticancer effects on PC3 cells, focusing on the role of Ca2+ and ROS. The study examines how δ-T3 affects biological processes such as cell viability, apoptosis, paraptosis, autophagy, and mitophagy (121). Consistent with our earlier hypothesis, an elevated concentration of Ca2+ ions was identified as a contributor to the pro-death effects triggered by δ-T3. Interestingly, this excess Ca2+ did not influence autophagy and mitophagy processes in autophagy-defective DU145 cells. Additionally, our study reveals that δ-T3 retains its anticancer properties in this cell line, and notably, these properties persist despite an excessive accumulation of reactive oxygen species (ROS). This observation highlights the limited vulnerability of these cells to oxidative stress induced by ROS (121). Collectively, these discoveries demonstrate that δ-T3 induces structural and functional abnormalities in the mitochondria of CRPC cells, leading to cell death. These insights provide novel mechanistic understanding into the anticancer effects of this compound (121).
In a previous investigation, the utilization of mice as experimental subjects was undertaken to examine the levels of tocotrienol (T3) in plasma. T3, a member of the vitamin E family renowned for its anti-cancer attributes, was analyzed alongside a recently developed succinate ether derivative of T3 known as 6-O-carboxypropyl-α-tocotrienol (T3E). The primary objective of this study was to discern any potential disparities in pharmacokinetics between these two compounds (122). A mouse xenograft model was employed to evaluate the impact of oral administration of T3E on tumor growth in human malignant mesothelioma MM cells. Specifically, the H2052 cell line was utilized for this assessment (122). Tumor volume was significantly decreased in mice given 150 mg/kg T3E orally once every 2 days for 10 days without any reduction in body weight, indicating that T3E might have anti-MM effects (122). An in vitro study was undertaken to investigate the anticancer effects and mechanisms of action of methotrexate (MTX) in conjunction with vitamin E variants (tocopherol) and derivatives (tocopherol succinate) on triple-negative breast cancer (TNBC) (123). The study evaluated cell survival rates and protein levels using the MTT test and western blot analysis. Results indicated that combined therapy with MTX and tocopherol inhibited the growth of triple-negative breast cancer (TNBC) cells. Interestingly, varying doses of MTX exhibited distinct lethal effects on cells treated with tocopherol succinate. Higher MTX doses enhanced the anticancer activity of tocopherol succinate, while lower doses impeded it. Furthermore, the combined treatment demonstrated caspase-3 activation and poly (adenosine diphosphate ribose) polymerase cleavage in the treated cells (123). Against chondrosarcoma cells, a form of cartilage cell malignant primary bone tumor (124), Annatto tocotrienol (AnTT), -T3, and -T3 have anticancer properties. AnTT, -T3 and -T3 produced G1 arrest in SW1353 cells after 24 h of treatment, which escalated to apoptosis after 48 h. Furthermore, tocotrienol treatment caused considerable cytoplasmic vacuolation in SW1353 cells. The transcriptome analysis after tocotrienol therapy revealed enhanced signaling pathways in the endoplasmic reticulum stress, unfolded protein response, autophagy, and transcription (125).
4.4. Vitamin D
Vitamin D (VD) is a lipid-soluble vitamin that belongs to steroidal hormones (126). Vitamin D is found in two forms: vitamin D2 (ergocalciferol) and vitamin D3 (cholecalciferol) (127). Yeast and plants can produce ergocalciferol while humans obtain it from their diet. The human body can make vitamin D endogenously by exposing the skin to UV radiation from the sun and then converting cholesterol to vitamin D (128). Vitamin D can undergo different metabolism processes in the liver and kidney through the hydroxylation process. Vitamin D3 25-Hydroxyvitamin is produced by hydroxylation in the liver, whereas calcitriol (1,25-dihydroxycholecalciferol), the active form of vitamin D, is produced in the kidney (129). Calcitriol can bind to a special receptor called vitamin D receptor (VDR) and bind to DNA promoter sequences then control the gene expression. In addition, Calcitriol can play a major role in triggering Intracellular signaling such as Ca2+ and Cl− channels (130). In recent years, scientists conducted many studies and research to show either the effect of VD on cancer or enhancing the chemotherapy response. Vitamin D low levels significantly increased tumor growth in solid and non-solid types (131).
Most studies have found that the status of VD in serum can be a risk factor in various kinds of tumors for instance gastric, breast, colon, and prostate tumor (132). Furthermore, VD is important in controlling the tumorigenesis pathway, from admission to metastasis and microenvironment responses (64). Through a variety of mechanisms, including the regulation of cell behaviors like proliferation, differentiation, increased maturation and apoptosis, autophagy, and epithelial-mesenchymal transition (EMT), as well as the modulation of interactions between cells and their microenvironment, such as the suppression of inflammation, the immune system, angiogenesis, and anti-oxidants, vitamin D can control tumor cells (64). Furthermore, VD had been showed to activate apoptosis in cancer cells such as breast and carcinomas of the colon but not in a variety of prostate carcinoma cells, showing that various tumor cells react differently to vitamin D (133).
The animal model preclinical studies revealed that VD treatment is essential in controlling tumor development (134). A significant variable linked to tumor aggression in prostatic cancer (PCa) is the expression of Lysine-specific demethylase 1A (LSD1), which controls the expression of both estrogen and androgen receptors (ER) (135). Lysine-specific demethylase 1A (LSD1) regulates the transcriptional process of vitamin D receptors (VDR). They evaluated the role of LSD1 in VDR-mediated gene transcription for Cdkn1a, E2f1, Cyp24a1, and S100g using CWR22 cells transplanted in athymic nude mice via qRT-PCR-TaqMan. The cancer progression in xenograft mouse models correlated to the elevation of LSD1 and VDR protein levels (135). The findings showed that lowering LSD1 lowers PCa survival, and information on gene expression suggests that LSD1 has a dual co-regulatory function for VDR. LSD1 is regulating the 1, 25(OH)2D effects brought on by target genes by functioning as a coactivator and corepressor of VDR action. Translation (135).
In an in vivo examination, they used a non-immunodeficient MMTV-PyMT mice model for metastatic breast cancer to examine the effect of VD expression on breast tumor development. The study demonstrated that using exogenic of 25(OH)D slow down cancer growth with no hypercalcemic was observed (136). When compared to a normal diet (1,000 IU/kg), the non-immunodeficient MMTV-PyMT (mouse mammary tumor virus-polyoma middle tumor-antigen) mouse model of metastatic breast cancer with a low VD diet (25 IU/kg) demonstrated a significant advancement of mammary neoplasia (136). Moreover, systemic perfusion containing 1,25(OH)2D or 25(OH)D delayed the development of tumors and reduced lung metastasis. Equally metabolites decreased Ki-67, cyclin D1, and ErbB2 levels in cancer. In constant, the profusion of D 25(OH)obtained increasing levels of vitamin D up to 50% in tumor cells, which indicates effective activation and good cancer invasion. The obtained results in this study revealed that neoplasia, tumor growth, and metastasis can be delayed by vitamin D (136).
To determine if vitamin D-facilitated reduction of cell growth is associated with JNK1 (c-Jun NH2-terminal kinases), an in vitro study was carried out utilizing colorectal cells (137). The HT29 and Caco-2 human colon cancer cell lines as well as the HEK 293 T human embryonic kidney cell line were treated with calcitriol (1,25(OH)2-D3). Calcitriol was able to reduce cell creation in HT29 colon tumors and was linked with cell cycle stop. The phosphorylation of JNK1 and elevated expression of the cell cycle regulators p21 and p27 may be connected to the inhibitory effects of calcitriol (137). It has been shown unequivocally that JNK1 interacted with VDR both physically and functionally, gradually regulating both transcriptional and translational levels of VDR expression. The JNK1/VDR connection generated calcitriol-mediated suppression of cancer cell growth through JNK1 activation (137).
1,25-dihydroxyvitamin D (1,25(OH)2D) was discovered to predominantly modulate ROS and mitochondrial activity in an in vitro investigation employing osteosarcoma cells, which led to anticancer effects. The treatment led to reduced mitochondrial ROS and altered mitochondrial dynamics, while enhancing mitophagy and ROS defense mechanisms. Key molecular changes included the activation of tumor-suppressing pathways and the relocalization of the mTORC1 inhibitor DDIT4/REDD1, indicating a shift toward non-oxidative energy metabolism and reduce of tumor cell growth. This highlights the role of 1,25(OH)2D in controlling osteosarcoma cell creation through mitochondrial and redox balance regulation (138).
Moreover, it was found that effective vitamin D [(1,25(OH)2D3)] significantly hindered the growth of CD44-expressing human gastric cancer cells. Researchers linked the vitamin D impact to the Wnt/β-catenin signaling pathway and CD44 downregulation. Positive correlations were observed between CD44 and Vitamin D Receptor gene expressions in gastric tumor cells and patient tissues. In mouse model, both oral vitamin D intake and injections of 1,25(OH)2D3 overcame gastric cancer growing and CD44 expression, suggesting a potential therapeutic role for vitamin D in gastric cancer management and prevention (139).
Table 1 summarizes the preclinical studies of the mentioned vitamins.
Table 1.
Preclinical studies of vitamins A, C, E, and D.
| Form of the vitamin | Type of cancer | Model of the study | Mechanism of action | References | ||
|---|---|---|---|---|---|---|
| Vitamin A | All-trans retinoic acid | Breast cancer | In vitro (MCF-7 cells) | ↓expression of PPARγ | (74) | |
| ↑stimulation of DOK1/PPARγ pathways | ||||||
| ↓ cell proliferation | ||||||
| Synthetic retinoid | Breast cancer | In vitro (MCF-7 and MDA-MB-231) | ↓ Wnt/−catenin signaling pathway | (75) | ||
| ↓ cell proliferation and differentiation | ||||||
| All-trans retinoic acid | Lung cancer | In vitro (Huh-7 cells) | ↑ survival rate | (76) | ||
| In vivo (SPHK1 knockout mice and SPHK1 transgenic mice) | ↓ tumor progression | |||||
| All-trans retinoic acid | Gastric cancer | In vitro (Human GC cell lines, AGS and MKN-45) | ↓ cell viability | (77) | ||
| ↑ caspases | ||||||
| ↑ apoptosis | ||||||
| All-trans-RA-Podophyllotoxin | Gastric cancer | In vitro (MKN-45 and BGC-823) | ↓ cell growth | (78) | ||
| ↓ ERK1/2 and AKT signaling pathways | ||||||
| ↑ apoptosis | ||||||
| Polyinosinic-polycytidylic acid/ 13-cis-retinoic acid | Neuroblastoma | In vitro [(SK-N-AS) (CRL-2137), SK-N-FI (CRL-2142), SK-N-DZ (CRL-2149), and BE(2)-M17 (CRL-2267)] | ↑ stimulation of retinoic acid receptors beta (RAR-β) | (82) | ||
| In vivo (nonobese diabetic/SCID) (NOD/SCID, NOD.CB17-Prkdcscid/NcrCrl) mice | ↓ vessel formation | |||||
| all-trans-RA | Cervical cancer | In vivo (BALB/c mice model) | ↓ MDSC | (79) | ||
| ↑ CD + 8 T cells | ||||||
| ↓ tumor growth | ||||||
| all-trans-RA | Hepatocellular carcinoma | In vitro (HepG2, Hepa1-6 and H22 hepatoma carcinoma cell line) | ↓ MDSC | (80) | ||
| In vivo (C57BL/6 N mice with Hepa1-6 tumor) | ↓ tumor-associated macrophages | |||||
| ↓ arginase 1, iNOS, IDO, S100A8 + A9 | ||||||
| all-trans-RA | Breast cancer | In vivo (4 T1 mouse model of breast cancer) | ↑ mouse long-term survival rates | (81) | ||
| ↑ cytotoxicity of CD8+ T cells and natural killer cells | ||||||
| Vitamin C | Breast cancer | In vitro (MCF10A, MDA-MB-231, MCF-7, and SK-BR-3) | ↓ cell viability | (90) | ||
| Brain cancer | In vitro [human glioblastoma DBTRG and human renal tubular epithelial (HK-2)] | ↓ cell viability | (93) | |||
| Oral cancer | In vitro (human OSCC-derived cell lines CAL27, SCC9, and SCC25) | ↑ ROS level | (94) | |||
| ↓ Bcl-2 | ||||||
| ↑ Bax expression, caspases | ||||||
| ↑ cell cycle arrest at the G0/G1 phase | ||||||
| Colorectal cancer, breast cancer, melanoma, pancreatic cancer | In vivo (C57BL/6 J, BALB/c, FVB, and NOD-SCID mice) | ↓ tumor growth | (96) | |||
| ↑ CD8 T cells activity | ||||||
| Ascorbate | Lymphoma | In vitro (DLBCL cell line OC-LY1) | ↑ TET activity | (104) | ||
| ↑ expression of tumor suppressor genes | ||||||
| ↑ chemosensitivity | ||||||
| Ascorbate | Leukemia | In vivo (Vav-tTA (VTA) transgenic mice) | ↑ DNA demethylation | (105) | ||
| ↑ TET2 restoration phenotypes | ||||||
| Ascorbate | Thyroid cancer | In vitro (FTC133 and 8305c) | ↓ expression of HIF1α and GLUT1 | (108) | ||
| Vitamin E | β-tocotrienol | Prostate cancer and lung cancer | In vitro (Mouse Lewis lung cancer (LLC) cells, DU145 human prostate cancer cells and Jurkat T cells) | ↓ PD-L1 expression | (118) | |
| In vivo | ↓ JAK2/STAT3 pathway | |||||
| δ-tocotrienol | Prostate cancer | In vitro (DU145 and PC3) | ↑ ROS | (120) | ||
| ↑ apoptosis | ||||||
| Tocotrienol/6-O-carboxypropyl-α-tocotrienol | Malignant mesothelioma |
In vitro (H2052 cell line) In vivo (ICR mice) |
↓ tumor size | (122) | ||
| Tocopherol/ tocopherol succinate | Breast cancer | In vitro (MDA-MB-231 and MDA-MB-468) | ↓ cell growth | (123) | ||
| Annatto tocotrienol/γ-tocotrinol/ δ-tocotrienol | Chondrosarcoma | In vitro (Human chondrosarcoma SW1353 cells) | ↑ G1 arrest | (125) | ||
| ↑ apoptosis | ||||||
| (RRR-α-tocopherol [αT], RRR-γ-tocopherol [γT]) | Breast cancer | In vitro (human MDA-MB-435, MCF-7, and murine 66 cL-4 mammary cancer cells) | ↑ caspases-8 and -9 | (117) | ||
| ↓ c-FLIP and surviving | ||||||
| ↑ apoptosis | ||||||
| ↓ colony formation | ||||||
| Synthetic forms (all-rac-α-tocopherol [all-rac-αT], all-rac-α-tocopheryl acetate [all-rac-αTAc]) | In vivo (BALB/c mouse mammary cancer model) | ↓ tumor growth | ||||
| ↓ metastasis | ||||||
| Vitamin D | 1,25(OH)2-D3 | Prostate cancer | In vitro (C4-2 cells and CWR22 cells) | ↑ tumor regression | (135) | |
| in vivo (athymic nude (Hsd:Athymic Nude-Foxn1nu) mice) | ↓ LSD1 | |||||
| 1,25-dihydroxycholecalciferol | Breast cancer | In vitro | ↑ tumor regression | (136) | ||
| In vivo (MMTV-polyoma middle T antigen (PyMT) transgenic mice) | ↓ lung metastasis | |||||
| ↓ Ki-67, cyclin D1, and ErbB2 levels | ||||||
| Calcitriol | Colon cancer | In vitro (HT29 and Caco-2 cells) | ↑ cell cycle arrest | (137) | ||
| ↑ expression of cell cycle regulators p21 and p27 | ||||||
| ↑ activation of JNK1 | ||||||
| 1,25-dihydroxyvitamin D (1,25(OH)2D) | Bone cancer | In vitro (Human MG-63 osteosarcoma cells) | ↓ mTORC1 | (138) | ||
| ↓ ROS | ||||||
| 1,25(OH)2D3 | Gastric cancer | In vitro (Human GC cells MKN45, MKN28, and KATO III) | ↓ CD44 | (139) | ||
| In vivo (orthotopic GC nude mice model) | ↓ Wnt/β-catenin signaling pathway |
5. Clinical trials
Numerous in vitro studies determined the importance of vitamins in preventing cancer initiation and empowering the immune system to defeat tumorigenesis. To support the preclinical results several clinical trials have been conducted using vitamins as adjuvant treatment with chemotherapy or immunotherapy.
5.1. Vitamin A
In a randomized, controlled, phase 3 trial, arsenic acid, and all-trans retinoic acid (45 mg/m2) treatment was applied in 235 patients with acute promyelocytic leukemia (140). The outcomes of this trial have shown that using this combination led to an increase in the cure rate and reduce relapse chances (140).
Another clinical trial demonstrated the effect of oral vitamin A (6,000 IU/day) in patients (n = 32) with low-risk gestational trophoblastic neoplasia who receive methotrexate chemotherapy (141). The intervention group exhibited a reduction of chemoresistance as well as a downregulation of the tumor marker β-hCG level (141). It was reported in a randomized double-blind study that using vitamin A supplementation (8,000 IU/8 h) with neoadjuvant chemotherapy (64 weeks) in 15 patients (total sample size is 30) has significantly improved the therapeutic response in the treatment of an advanced cervical carcinoma (142).
5.2. Vitamin C
Vitamin C is well-supported with studies that prove its direct potential to hinder cancer cell growth in preclinical models, yet there is limited clinical evidence regarding its anti-tumoral efficacy. Many studies have scrutinized the effects of pharmacological VC’s mechanisms on targeting vulnerable survival utilities of cancer cells that are essential for their perpetuating growth; however, the outcomes of high-dose intravenous VC in terms of its anti-cancer effects remain controversial. In the 1970s, clinical trials on patients with terminal cancer showed that intravenous high-dose ascorbate treatment, that achieved a peak plasma concentration of 6 mM, extended their survival (143, 144), whereas in the 1980s they failed to confirm these findings because ascorbate was given at the same dose orally and the peak plasma concentration was of less than 200 μM (145, 146). This was revealed with follow-up studies which concluded that the route of administration strongly affects VC pharmacokinetics, hence the discrepant results (147). A recent clinical study evaluated the addition of pharmacological ascorbate to platinum-based chemotherapy in patients with advanced-stage non-small cell lung cancer (NSCLC). This study was conducted on 38 patients who were administered 75 g ascorbate twice per week intravenously with carboplatin and paclitaxel every 3 weeks for four cycles. Reported results have shown that the addition of pharmacological ascorbate to platinum-based chemotherapy significantly improves tumor deterioration in the advanced stage of NSCLC, by meeting its primary endpoint with an objective response rate of 34.2% and reaching a disease-control rate of 84.2%. cytokine and chemokine levels propose that the studied combination induces an immune response by altering the host immune defenses (148). Another clinical study investigated the maximum tolerated dose (MTD) and recommended phase 2 dose (RP2D) of pharmacological ascorbic acid when it is combined with mFOLFOX6 or FOLFIRI with or without bevacizumab regimens in patients with metastatic colorectal cancer or gastric cancer. Thirty-six patients received 0.2–1.5 g/kg ascorbic acid infusion, in the dose-escalation phase, once daily (days 1–3) with mFOLFOX6 or FOLFIRI in a 14-day cycle until the MTD was reached. Followed by the speed-expansion phase in which pharmacological ascorbic acid was administered at the MTD, but the MTD was not reached and the RP2D was established to be 1.5 g/kg/day, days 1–3, with mFOLFOX6 or FOLFIRI. Results have reported 58.3% objective response rate, and 95.8% disease-control rate. This study has shown that combining pharmacological ascorbic acid with mFOLFOX6 or FOLFIRI has a significant decrease effect in all-grade and grade ≥ 3 bone marrow and gastrointestinal toxic effects (149).
5.3. Vitamin E
Furthermore, within the confines of a randomized controlled study, a total of 89 individuals who had been clinically diagnosed with differentiated thyroid cancer were subjected to I-131 radiation therapy. In the subsequent phase of the study, participants were assigned to receive various interventions, namely vitamin E (n = 30), vitamin C (n = 30), and supragingival scaling with vitamin C (n = 29). The present study aimed to investigate the potential protective effect of vitamin E on salivary glands, with a specific focus on enhancing parotid excretion function. The results obtained from the experimental analysis provide evidence supporting the notion that vitamin E indeed possesses a beneficial impact on salivary glands, particularly in terms of augmenting parotid excretion function (150). Vitamin E’s impact on lowering prostate cancer incidence has been demonstrated by two randomized controlled trials’ use of serum metabolomics analysis (151). Serum C22 lactone sulfate, a molecule strongly linked to the modulation of androgenic steroid metabolites, was found to be substantially reduced by a high-dose of vitamin E (400 IU/day) (151). On the other hand, the utilization of α-tocopherol and β-carotene as dietary supplements did not demonstrate a significant reduction in the likelihood of developing liver cancer or chronic liver disease (152).
5.4. Vitamin D
High serum levels of 25(OH)D were found to be associated with improved early-stage survival in non-small-cell lung cancer (NSCLC) in a randomized, double-blinded trial (153). The outcomes were classified into RFS and OS as primary and secondary, respectively. Referred to RFS was the period of time between the start date of the supplement and the earlier date of the cancer relapse or death from any cause. They referred to this period of time as OS, which is the start date of the supplement and the date of death from any cause. The overall results were no improvements in RFS and OS in the total study population, better OS in patients with high Vitamin D before taking the supplement (≥20 ng/mL) than the low level (<20 ng/mL) (153). When the study was limited to patients with early-stage adenocarcinoma and low 25(OH)D, the vitamin D group had significantly better 5-year RFS (86% vs. 50%, p = 0.04) and OS (91% vs. 48%, p = 0.02) than the placebo group. Among the polymorphisms studied, vitamin D binding protein (DBP1-rs7041) TT and CDX2 (rs11568820) AA/AG genetic constitution were associated with a well prediction, even after multivariable adjustment. Finally, vitamin D dietary supplementation may improve the prognosis of patients with lower 25(OH)D levels who have early-stage lung adenocarcinoma (153). Table 2 reviews the clinical studies of the mentioned vitamins.
Table 2.
Clinical studies of vitamins A, C, E, and D.
| Vitamins | Cancer type | Sample size | Study type | Type of vitamins and doses | The outcome of the study | References |
|---|---|---|---|---|---|---|
| Vitamin A | Acute promyelocytic leukemia | 235 | A randomized, controlled, open-label multicenter trial, phase 3 trial | All-trans retinoic acid (45 mg/m2) | Using all-trans retinoic acid with arsenic acid has increased the cure rate and reduced relapse chances | (140) |
| Gestational trophoblastic neoplasia | 32 | A randomized, controlled clinical trial | oral vitamin A (6,000 IU/day) | The intervention group exhibited a reduction of chemoresistance as well as a downregulation of the tumor marker β-hCG level | (141) | |
| Advanced cervical carcinoma | 30 | A randomized double-blind study | Vitamin A supplementation (8,000 IU/8 h) | Improved therapeutic response of the chemotherapy | (142) | |
| Vitamin C | Advanced-stage non-small cell lung cancer | 38 | Open-label, single-arm, non-randomized phase II study | Ascorbate (75 g twice per week) intravenously | Improved tumor deterioration and enhance immune response | (148) |
| Metastatic colorectal cancer or gastric cancer | 36 | Phase 1 open-label, single-center, dose-escalation, and speed-expansion study | Ascorbic acid (0.2–1.5 g/kg) infusion | Improved therapeutic response of the chemotherapy and significantly decreased the side effects | (149) | |
| Vitamin E | Thyroid carcinoma | 89 | A randomized, controlled clinical trial | NA | The results revealed the protective impact of vitamin E on salivary glands by enhancing parotid excretion function | (150) |
| Prostate cancer | 154 | A randomized, placebo-controlled, double-blinded clinical trial | Vitamin E (400 IU/day) | Significantly decreased serum C22 lactone sulfate which is highly associated with modulating androgenic steroid metabolites | (151) | |
| Vitamin D | non-small-cell lung cancer | 155 | A randomized, double-blind, placebo-controlled, parallel group trial | Vitamin D supplements (1,200 IU/day) | Patients with lower 25(OH)D levels who have early-stage lung adenocarcinoma may have a better chance of surviving if they take vitamin D supplements. | (153) |
6. Critical assessment of vitamins safety and potential toxicity
In this section, we will assess the safety and potential toxicity of vitamins A, C, E, and D. By examining their toxicity profiles, we aim to provide a holistic perspective on their applications and shed light on the fine balance between their potential anticancer effects and the risk of toxicity when taken in excess.
Hypervitaminosis is a rapid-onset pathological condition caused by the excessive accumulation of any vitamin in the body. Specifically concerning vitamin A, hypervitaminosis is considered when the plasma concentration of retinol surpasses 2.09 μM. Toxicity is commonly associated with the improper use of dietary supplements but may also manifest following an elevated consumption of foods rich in preformed vitamin A, such as liver and eggs (154, 155). Hypervitaminosis A can occur in two forms: acute and chronic. Acute toxicity manifests within days to weeks after ingesting a few very high doses, typically more than 100 times the recommended dietary allowance (RDA). Symptoms include severe headache, blurred vision, nausea, dizziness, muscle aches, and coordination problems. Chronic hypervitaminosis A can result from regular consumption of high doses and may lead to dry skin, joint pain, fatigue, depression, and abnormal liver test results (10). Beta-carotene, a provitamin A, is generally regarded as safe when consumed through a balanced diet rich in fruits and vegetables. However, taking high-dose beta-carotene supplements may pose certain risks (156). Long-term, excessive beta-carotene intake, particularly in supplement form, has been associated with a higher risk of adverse health effects. Some studies suggest that high-dose beta-carotene supplements may be linked to an increased risk of lung cancer, particularly in individuals who smoke (157). Additionally, excessive beta-carotene intake can cause a harmless but noticeable condition called carotenemia, characterized by yellowish or orange discoloration of the skin (155).
Vitamin C has low toxicity, and excessive consumption is unlikely to cause serious adverse effects. The most commonly reported adverse effects include diarrhea, nausea, abdominal cramps, and other gastrointestinal disturbances due to the osmotic effect of unabsorbed vitamin C in the gastrointestinal tract. There is a theoretical concern that high vitamin C intake might lead to excess iron absorption, as vitamin C enhances nonheme iron absorption. Additionally, there is a possibility that vitamin C could act as a pro-oxidant, contributing to oxidative damage. Long-term intakes of vitamin C exceeding the tolerable upper intake level (UL) of 2,000 mg may increase the risk of adverse health effects (12). High doses of intravenous vitamin C or oral vitamin C can trigger hemolysis in individuals with glucose-6-phosphate deficiency. For those with paroxysmal nocturnal hemoglobinuria, oral ascorbic acid can exacerbate hemolysis. In vitro studies show that the impact of vitamin C on red blood cell lysis is concentration-dependent, worsening at low concentrations and inhibiting at high concentrations (158).
There is no evidence that high consumption of vitamin E in food can cause any adverse effects. However, it has been shown in animals that high doses of vitamin E supplements can cause hemorrhage and interrupt blood coagulation, and platelet aggregation inhibition. Consumption of 1,000 mg/day in adults appears to be safe, but yet, data are limited and based on small groups of people for short periods of time (11).
Excess consumption of vitamin D can be toxic, leading to marked hypercalcemia, hypercalciuria, and elevated serum 25(OH)D levels. Symptoms of vitamin D toxicity include nausea, vomiting, muscle weakness, neuropsychiatric disturbances, pain, loss of appetite, dehydration, polyuria, excessive thirst, and kidney stones. Toxicity is unlikely at daily intakes below 250 mcg, but even intakes below the UL may have adverse health effects over time. It is recommended to avoid serum 25(OH)D levels above 125–150 nmol/L (13).
7. Conclusion
In conclusion, the potential responsibility of vitamins A, C, E, and D in preventing and treating tumor is a subject of growing interest, supported by a combination of preclinical, clinical studies, and considerations of safety. Vitamins play complex roles in cancer prevention, influencing immune job, antioxidant resistance, inflammatory modulation, and epigenetic directive, which could enhance outcomes by affecting cell differentiation, proliferation, and apoptosis, and combating oxidative stress and DNA damage. Particularly, vitamin A with its regulatory role in cell growth and differentiation, impacts several signaling pathways linked to cancer. Vitamin C functions as an antioxidant, reducing oxidative stress and possibly enhancing immune responses against cancer cells. High-dose intravenous applications of vitamin C have shown potential in selectively targeting cancer cells. The antioxidative properties of vitamin E protect cell membranes from damage, with certain forms exhibiting anti-inflammatory effects. Vitamin D, through its role in cell differentiation and immune function, shows links to reduced risks in certain cancers like colorectal cancer. Interestingly, combining vitamins A, C, D, and E with chemotherapy and radiotherapy offers a customized strategy for cancer treatment. These vitamins enhance immune response, alleviate oxidative stress, inhibit angiogenesis, and induce apoptosis. The synergy between vitamins and conventional treatments provides promising paths for more effective and personalized therapies. Further research is essential to unlock their complete potential in cancer treatments.
Nevertheless, the current studies present limitations that must be acknowledged. Using different dosage regimens in human and animal studies may impact the mechanisms underlying the medicinal prospective of vitamins in tumors, often with complex, unpredictable outcomes. Although animal models offer preliminary insights, they do not always replicate human metabolic processes, potentially leading to different bioavailability and efficacy in humans. Additionally, in vitro findings, such as the observed toxicity of vitamins A and E to cancer cells, may not translate directly into therapeutic benefits in vivo, where complex biological systems can alter the vitamins’ impact on cancer cells.
The integration of preclinical and clinical research highlights the need for particular approaches based on cancer type, stage, and patient characteristics. However, there are significant gaps in our understanding, including determining optimal dosages for efficacy and safety, elucidating precise mechanisms of action, establishing clinical efficacy through more large-scale trials, developing personalized treatment approaches, and studying the longstanding effects of vitamin supplementation in cancer patients. Addressing these gaps through comprehensive, multidisciplinary research is crucial for fully realizing the potential of these vitamins in oncology.
Author contributions
WT: Conceptualization, Project administration, Supervision, Validation, Visualization, Writing – review & editing. DA: Data curation, Software, Writing – original draft. ZA: Data curation, Methodology, Software, Writing – original draft. MJ: Data curation, Investigation, Software, Writing – original draft. LA: Conceptualization, Project administration, Writing – review & editing. RH: Data curation, Methodology, Software, Visualization, Writing – original draft. AM: Formal analysis, Investigation, Methodology, Software, Writing – original draft, Writing – review & editing.
Acknowledgments
The authors are grateful to the applied science private university, Amman, Jordan for the full support granted to this review.
Funding Statement
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Glossary
| DNA | Deoxyribonucleic acid |
| VA | Vitamin A |
| VC | Vitamin C |
| VE | Vitamin E |
| VD | Vitamin D |
| SOD | Superoxide dismutase |
| ROS | Reactive oxygen species |
| PCa | Prostatic cancer |
| LSD1 | Lysine-specific demethylase 1A |
| VDR | Vitamin D receptors |
| AR | Androgen receptor |
| ER | Estrogen receptors |
| JNK1 | c-Jun NH2-terminal kinases |
| HEK | Human embryonic kidney |
| DOK1 | Docking protein 1 |
| PPARγ | Peroxisome proliferator-activated receptor gamma |
| ERK1/2 | The extracellular signal-regulated kinase 1/2 |
| akt | Protein kinase B |
| RAR-beta | Retinoic acid receptor beta |
| HIF-1-alpha | Hypoxia-inducible factor 1-alpha |
| GLUT1 | Glucose transporter 1, |
| PD-L1 | Programmed death-ligand 1 |
| JAK2 | Janus kinase 2 |
| STAT3 | Signal transducer and activator of transcription 3 |
| Ki-67 | Antigen Kiel 67 |
| MTD | Maximum tolerated dose |
| RP2D | Recommended phase 2 dose |
| OS | Overall survival |
| RFS | Recurrence-free survival |
| CRBP-1 | Cellular Retinol-Binding Protein 1 |
| RAR | Retinoic Acid Receptor |
| RXR | Retinoid X Receptor |
| IGF | Insulin-like growth factors |
| IGFBP3 | Insulin-like growth factors binding protein 3 |
| MDSCs | Myeloid-derived suppressor cell |
| RDA | Recommended dietary allowance |
| UL | Upper intake level |
References
- 1.Roser M, Ritchie H. Cancer. Our World in Data (2015); Available at: https://ourworldindata.org/cancer.
- 2.MSD , Overview of Cancer. Merck Sharp & Dohme Corp. (MSD) (2022).
- 3.MSD , Risk factors for Cancer. Merck Sharp & Dohme Corp. (MSD) (2022).
- 4.You W, Henneberg M. Cancer incidence increasing globally: the role of relaxed natural selection. Evol Appl. (2018) 11:140–52. doi: 10.1111/eva.12523, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.MSD , Development and spread of Cancer. Merck Sharp & Dohme Corp. (MSD) (2022).
- 6.Manuals . TM Cellular and molecular basis of Cancer. Rahway, NJ, USA: Merck & Co, Inc. (2022).
- 7.Behranvand N, Nasri F, Emameh RZ, Khani P, Hosseini A, Garssen J, et al. Correction to: chemotherapy: a double-edged sword in cancer treatment. Cancer Immunol Immunother. (2021):1. doi: 10.1007/s00262-021-03013-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zeng Z, Mishuk AU, Qian J. Safety of dietary supplements use among patients with cancer: a systematic review. Crit Rev Oncol Hematol. (2020) 152:103013. doi: 10.1016/j.critrevonc.2020.103013, PMID: [DOI] [PubMed] [Google Scholar]
- 9.Kanellopoulou A, Riza E, Samoli E, Benetou V. Dietary supplement use after cancer diagnosis in relation to total mortality, cancer mortality and recurrence: a systematic review and meta-analysis. Nutr Cancer. (2021) 73:16–30. doi: 10.1080/01635581.2020.1734215, PMID: [DOI] [PubMed] [Google Scholar]
- 10.National Institute of Health . Vitamin A and carotenoids: Fact sheet for health professionals. (2022); Available at: https://ods.od.nih.gov/factsheets/VitaminA-HealthProfessional/.
- 11.National Institute of Health . Vitamin E: Fact sheet for health professionals. (2021); Available at: https://ods.od.nih.gov/factsheets/VitaminE-HealthProfessional/
- 12.National Institute of Health , Vitamin C: Fact sheet for health professionals. (2021). Available at: https://ods.od.nih.gov/factsheets/VitaminC-HealthProfessional/
- 13.National Institute of Health , Vitamin D: Fact sheet for health professionals. (2023). Available at: (https://ods.od.nih.gov/factsheets/VitaminD-HealthProfessional/)
- 14.O’Connor EA, Evans CV, Ivlev I, Rushkin MC, Thomas RG, Martin A, et al. Vitamin and mineral supplements for the primary prevention of cardiovascular disease and Cancer: updated evidence report and systematic review for the US preventive services task force. JAMA. (2022) 327:2334–47. doi: 10.1001/jama.2021.15650, PMID: [DOI] [PubMed] [Google Scholar]
- 15.Cheema HA, Fatima M, Shahid A, Bouaddi O, Elgenidy A, Rehman AU, et al. Vitamin D supplementation for the prevention of total cancer incidence and mortality: an updated systematic review and meta-analysis. Heliyon. (2022) 8:e11290. doi: 10.1016/j.heliyon.2022.e11290, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mohseni S, Tabatabaei-Malazy O, Ejtahed HS, Qorbani M, Azadbakht L, Khashayar P, et al. Effect of vitamins C and E on cancer survival; a systematic review. DARU J Pharmaceut Sci. (2022) 30:427–41. doi: 10.1007/s40199-022-00451-x, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barnhart AS, Anthony AL, Conaway KR, Sibbitt BG, Delaney E, Haluschak J, et al. Safety and efficacy of Vitamin C, Vitamin E, and selenium supplementation in the oncology setting: a systematic review. J Oncol Pharm Pract. (2023) 15:10781552231182362. doi: 10.1177/10781552231182362, PMID: [DOI] [PubMed] [Google Scholar]
- 18.de Oliveira VA, Oliveira IKF, Pereira IC, Mendes LKF, Carneiro da Silva FC, Torres–Leal FL, et al. Consumption and supplementation of Vitamin E in breast cancer risk, treatment, and outcomes: a systematic review with meta-analysis. Clin Nutri ESPEN. (2023) 54:215–26. doi: 10.1016/j.clnesp.2023.01.032 [DOI] [PubMed] [Google Scholar]
- 19.Goulão B, Stewart F, Ford JA, MacLennan G, Avenell A. Cancer and vitamin D supplementation: a systematic review and meta-analysis. Am J Clin Nutr. (2018) 107:652–63. doi: 10.1093/ajcn/nqx047 [DOI] [PubMed] [Google Scholar]
- 20.Leelakanok N, D’Cunha RR, Sutamtewagul G, Schweizer ML. A systematic review and meta-analysis of the association between vitamin a intake, serum vitamin a, and risk of liver cancer. Nutr Health. (2018) 24:121–31. doi: 10.1177/0260106018777170, PMID: [DOI] [PubMed] [Google Scholar]
- 21.Alsharairi NA. Dietary Antioxidants and Lung Cancer Risk in Smokers and Non-Smokers. Healthcare (Basel) (2022) 10:2501. doi: 10.3390/healthcare10122501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.He J, Gu Y, Zhang S. Vitamin a and breast cancer survival: a systematic review and meta-analysis. Clin Breast Cancer. (2018) 18:e1389–400. doi: 10.1016/j.clbc.2018.07.025, PMID: [DOI] [PubMed] [Google Scholar]
- 23.van Gorkom GNY, Lookermans EL, van Elssen CHMJ, Bos GMJ. The effect of vitamin C (ascorbic acid) in the treatment of patients with cancer: a systematic review. Nutrients. (2019) 11:977. doi: 10.3390/nu11050977, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hossain S, Beydoun MA, Beydoun HA, Chen X, Zonderman AB, Wood RJ. Vitamin D and breast cancer: a systematic review and meta-analysis of observational studies. Clin Nutr ESPEN. (2019) 30:170–84. doi: 10.1016/j.clnesp.2018.12.085, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen Z, Huang Y, Cao D, Qiu S, Chen B, Li J, et al. Vitamin C intake and cancers: an umbrella review. Front Nutr. (2022) 8:812394. doi: 10.3389/fnut.2021.812394, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu Y, Qian M, Hong J, Ng DM, Yang T, Xu L, et al. The effect of vitamin D on the occurrence and development of colorectal cancer: a systematic review and meta-analysis. Int J Color Dis. (2021) 36:1329–44. doi: 10.1007/s00384-021-03879-w, PMID: [DOI] [PubMed] [Google Scholar]
- 27.Kraemer K, Semba RD, Eggersdorfer M, Schaumberg DA. Introduction: the diverse and essential biological functions of vitamins. Ann Nutr Metab. (2012) 61:185–91. doi: 10.1159/000343103, PMID: [DOI] [PubMed] [Google Scholar]
- 28.Zhang FF, Barr SI, McNulty H, Li D, Blumberg JB. Health effects of vitamin and mineral supplements. BMJ. (2020) 369:m2511. doi: 10.1136/bmj.m2511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bender DA. Nutritional biochemistry of the vitamins. New York: Cambridge University Press; (2003). [Google Scholar]
- 30.Benson MJ, Pino-Lagos K, Rosemblatt M, Noelle RJ. All-trans retinoic acid mediates enhanced T reg cell growth, differentiation, and gut homing in the face of high levels of co-stimulation. J Exp Med. (2007) 204:1765–74. doi: 10.1084/jem.20070719, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xavier AA, Pérez-Gálvez A. Carotenoids as a source of antioxidants in the diet. Subcell Biochem. (2016) 79:359–75. doi: 10.1007/978-3-319-39126-7_14 [DOI] [PubMed] [Google Scholar]
- 32.Palace VP, Khaper N, Qin Q, Singal PK. Antioxidant potentials of vitamin a and carotenoids and their relevance to heart disease. Free Radic Biol Med. (1999) 26:746–61. doi: 10.1016/S0891-5849(98)00266-4, PMID: [DOI] [PubMed] [Google Scholar]
- 33.Zhang T, Wang Z, Wang X, Sun W, Cui X, Li R, et al. Effects of vitamin a on antioxidant functions, immune functions and production performance in male sika deer (Cervus nippon) during the first antler growth period. Ital J Anim Sci. (2019) 18:98–104. doi: 10.1080/1828051X.2018.1456978 [DOI] [Google Scholar]
- 34.Doldo E, Costanza G, Agostinelli S, Tarquini C, Ferlosio A, Arcuri G, et al. Vitamin a, cancer treatment and prevention: the new role of cellular retinol binding proteins. Biomed Res Int. (2015) 2015:1–14. doi: 10.1155/2015/624627, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gudas LJ, Wagner JA. Retinoids regulate stem cell differentiation. J Cell Physiol. (2011) 226:322–30. doi: 10.1002/jcp.22417, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bar-El Dadon S, Reifen R. Vitamin a and the epigenome. Crit Rev Food Sci Nutr. (2017) 57:2404–11. doi: 10.1080/10408398.2015.1060940 [DOI] [PubMed] [Google Scholar]
- 37.Abdullah M, Jamil RT, Attia FN. Vitamin C (ascorbic acid) In: StatPearls. NA, Treasure Island (FL): StatPearls Publishing; (2022) [PubMed] [Google Scholar]
- 38.Vitamin D , Fact sheet for health professionals. National Institutes of Health. Office of Dietary Supplements. Available at: https://ods.od.nih.gov/factsheets/VitaminC-HealthProfessional, (2017).
- 39.Ngo B, van Riper JM, Cantley LC, Yun J. Targeting cancer vulnerabilities with high-dose vitamin C. Nat Rev Cancer. (2019) 19:271–82. doi: 10.1038/s41568-019-0135-7, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ellulu MS, Rahmat A, Patimah I, Khaza'ai H, Abed Y. Effect of vitamin C on inflammation and metabolic markers in hypertensive and/or diabetic obese adults: a randomized controlled trial. Drug Des Devel Ther. (2015) 9:3405–12. doi: 10.2147/DDDT.S83144, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yussif NM, Abdul Aziz MA, Abdel Rahman AR. Evaluation of the anti-inflammatory effect of locally delivered Vitamin C in the treatment of persistent gingival inflammation: clinical and histopathological study. J Nutr Metab. (2016) 2016:2978741. doi: 10.1155/2016/2978741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kwak SG, Choo YJ, Chang MC. The effectiveness of high-dose intravenous vitamin C for patients with coronavirus disease 2019: a systematic review and meta-analysis. Complement Ther Med. (2022) 64:102797. doi: 10.1016/j.ctim.2021.102797, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.du YT, Long Y, Tang W, Liu XF, Dai F, Zhou B. Prooxidative inhibition against NF-κB-mediated inflammation by pharmacological vitamin C. Free Radic Biol Med. (2022) 180:85–94. doi: 10.1016/j.freeradbiomed.2022.01.007, PMID: [DOI] [PubMed] [Google Scholar]
- 44.Fu J, Wu Z, Liu J, Wu T. Vitamin C: a stem cell promoter in cancer metastasis and immunotherapy. Biomed Pharmacother. (2020) 131:110588. doi: 10.1016/j.biopha.2020.110588, PMID: [DOI] [PubMed] [Google Scholar]
- 45.Brabson JP, Leesang T, Mohammad S, Cimmino L. Epigenetic regulation of genomic stability by vitamin C. Front Genet. (2021) 12:675780. doi: 10.3389/fgene.2021.675780, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mikkelsen SU, Gillberg L, Lykkesfeldt J, Grønbæk K. The role of vitamin C in epigenetic cancer therapy. Free Radic Biol Med. (2021) 170:179–93. doi: 10.1016/j.freeradbiomed.2021.03.017, PMID: [DOI] [PubMed] [Google Scholar]
- 47.Khan Z, Ahmed S, Marya F, Ullah H, Khan H. Vitamin E (tocopherols and tocotrienols)(natural-occurring antioxidant; bright and dark side) In: Nabavi SM, Silva AS. editors. Antioxidants effects in health. United States, Cambridge: Elsevier; (2022). 547–60. [Google Scholar]
- 48.Jiang Q. Natural forms of vitamin E: metabolism, antioxidant, and anti-inflammatory activities and their role in disease prevention and therapy. Free Radic Biol Med. (2014) 72:76–90. doi: 10.1016/j.freeradbiomed.2014.03.035, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Smolarek AK, So JY, Thomas PE, Lee HJ, Paul S, Dombrowski A, et al. Dietary tocopherols inhibit cell proliferation, regulate expression of ERα, PPARγ, and Nrf2, and decrease serum inflammatory markers during the development of mammary hyperplasia. Mol Carcinog. (2013) 52:514–25. doi: 10.1002/mc.21886, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Smolarek AK, Suh N. Chemopreventive activity of vitamin E in breast cancer: a focus on γ-and δ-tocopherol. Nutrients. (2011) 3:962–86. doi: 10.3390/nu3110962, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cornwell DG, Williams MV, Wani AA, Wani G, Shen E, Jones KH. Mutagenicity of tocopheryl quinones: evolutionary advantage of selective accumulation of dietary a-tocopherol. Nutr Cancer. (2002) 43:111–8. doi: 10.1207/S15327914NC431_13, PMID: [DOI] [PubMed] [Google Scholar]
- 52.Aggarwal BB, Sundaram C, Prasad S, Kannappan R. Tocotrienols, the vitamin E of the 21st century: its potential against cancer and other chronic diseases. Biochem Pharmacol. (2010) 80:1613–31. doi: 10.1016/j.bcp.2010.07.043, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Powers SK, Sollanek KJ, Wiggs MP. Endurance exercise and antioxidant supplementation: sense or nonsense?-Part 1. Sport Sci Exch. (2014) 27:1–4. [Google Scholar]
- 54.Higgins MR, Izadi A, Kaviani M. Antioxidants and exercise performance: with a focus on vitamin E and C supplementation. Int J Environ Res Public Health. (2020) 17:8452. doi: 10.3390/ijerph17228452, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ju J, Picinich SC, Yang Z, Zhao Y, Suh N, Kong AN, et al. Cancer-preventive activities of tocopherols and tocotrienols. Carcinogenesis. (2010) 31:533–42. doi: 10.1093/carcin/bgp205, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Campbell SE, Stone WL, Whaley SG, Qui M, Krishnan K. Gamma (γ) tocopherol upregulates peroxisome proliferator activated receptor (PPAR) gamma (γ) expression in SW 480 human colon cancer cell lines. BMC Cancer. (2003) 3:1–13. doi: 10.1186/1471-2407-3-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lewis ED, Meydani SN, Wu D. Regulatory role of vitamin E in the immune system and inflammation. IUBMB Life. (2019) 71:487–94. doi: 10.1002/iub.1976, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ao T, Kikuta J, Ishii M. The effects of vitamin D on immune system and inflammatory diseases. Biomol Ther. (2021) 11:1624. doi: 10.3390/biom11111624, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.L Bishop E, Ismailova A, Dimeloe S, Hewison M, White JH. Vitamin D and immune regulation: antibacterial, antiviral, anti-inflammatory. JBMR plus. (2021) 5:e10405. doi: 10.1002/jbm4.10405, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Raymond E, Dalgleish A, Damber JE, Smith M, Pili R. Mechanisms of action of tasquinimod on the tumour microenvironment. Cancer Chemother Pharmacol. (2014) 73:1–8. doi: 10.1007/s00280-013-2321-8, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Liu W, Zhang L, Xu HJ, Li Y, Hu CM, Yang JY, et al. The anti-inflammatory effects of vitamin D in tumorigenesis. Int J Mol Sci. (2018) 19:2736. doi: 10.3390/ijms19092736, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nakai K, Fujii H, Kono K, Goto S, Kitazawa R, Kitazawa S, et al. Vitamin D activates the Nrf2-Keap1 antioxidant pathway and ameliorates nephropathy in diabetic rats. Am J Hypertens. (2014) 27:586–95. doi: 10.1093/ajh/hpt160, PMID: [DOI] [PubMed] [Google Scholar]
- 63.Sepidarkish M, Farsi F, Akbari-Fakhrabadi M, Namazi N, Almasi-Hashiani A, Maleki Hagiagha A, et al. The effect of vitamin D supplementation on oxidative stress parameters: a systematic review and meta-analysis of clinical trials. Pharmacol Res. (2019) 139:141–52. doi: 10.1016/j.phrs.2018.11.011, PMID: [DOI] [PubMed] [Google Scholar]
- 64.Giammanco M, di Majo D, la Guardia M, Aiello S, Crescimannno M, Flandina C, et al. Vitamin D in cancer chemoprevention. Pharm Biol. (2015) 53:1399–434. doi: 10.3109/13880209.2014.988274 [DOI] [PubMed] [Google Scholar]
- 65.Fleet JC. Molecular actions of vitamin D contributing to cancer prevention. Mol Asp Med. (2008) 29:388–96. doi: 10.1016/j.mam.2008.07.003, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Antonio V, Francesca F, Grazia BM, Federica G, Anna C, Giovanni S, et al. Vitamin D in the prevention, development and therapy of oncological diseases. Pharmacologyonline. (2021) 2:267–76. [Google Scholar]
- 67.Rinninella E, Mele MC, Raoul P, Cintoni M, Gasbarrini A. Vitamin D and colorectal cancer: Chemopreventive perspectives through the gut microbiota and the immune system. Biofactors. (2022) 48:285–93. doi: 10.1002/biof.1786, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Marigoudar JB, Sarkar D, Yuguda YM, Abutayeh RF, Kaur A, Pati A, et al. Role of vitamin D in targeting cancer and cancer stem cell populations and its therapeutic implications. Med Oncol. (2022) 40:2. doi: 10.1007/s12032-022-01855-0, PMID: [DOI] [PubMed] [Google Scholar]
- 69.Sajovic J, Meglič A, Glavač D, Markelj Š, Hawlina M, Fakin A. The role of vitamin a in retinal diseases. Int J Mol Sci. (2022) 23:1014. doi: 10.3390/ijms23031014, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gui Y, Duan S, Xiao L, Tang J, Li A. Bexarotent attenuated chronic constriction injury-induced spinal neuroinflammation and neuropathic pain by targeting mitogen-activated protein kinase phosphatase-1. J Pain. (2020) 21:1149–59. doi: 10.1016/j.jpain.2019.01.007, PMID: [DOI] [PubMed] [Google Scholar]
- 71.Terao M, Goracci L, Celestini V, Kurosaki M, Bolis M, di Veroli A, et al. Role of mitochondria and cardiolipins in growth inhibition of breast cancer cells by retinoic acid. J Exp Clin Cancer Res. (2019) 38:1–20. doi: 10.1186/s13046-019-1438-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cui J, Gong M, Fang S, Hu C, Wang Y, Zhang J, et al. All-trans retinoic acid reverses malignant biological behavior of hepatocarcinoma cells by regulating miR-200 family members. Genes Dis. (2021) 8:509–20. doi: 10.1016/j.gendis.2019.12.012, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nguyen PH, Giraud J, Staedel C, Chambonnier L, Dubus P, Chevret E, et al. All-trans retinoic acid targets gastric cancer stem cells and inhibits patient-derived gastric carcinoma tumor growth. Oncogene. (2016) 35:5619–28. doi: 10.1038/onc.2016.87, PMID: [DOI] [PubMed] [Google Scholar]
- 74.Ding X, Wang W, Wang M, Wu J, Yao F. DOK1/PPARgamma pathway mediates anti-tumor ability of all-trans retinoic acid in breast cancer MCF-7 cells. Biochem Biophys Res Commun. (2017) 487:189–93. doi: 10.1016/j.bbrc.2017.04.018, PMID: [DOI] [PubMed] [Google Scholar]
- 75.Aouad P, Saikali M, Abdel-Samad R, Fostok S, el-Houjeiri L, Pisano C, et al. Antitumor activities of the synthetic retinoid ST1926 in two-dimensional and three-dimensional human breast cancer models. Anti-Cancer Drugs. (2017) 28:757–70. doi: 10.1097/CAD.0000000000000511, PMID: [DOI] [PubMed] [Google Scholar]
- 76.Funaki M, Kitabayashi J, Shimakami T, Nagata N, Sakai Y, Takegoshi K, et al. Peretinoin, an acyclic retinoid, inhibits hepatocarcinogenesis by suppressing sphingosine kinase 1 expression in vitro and in vivo. Sci Rep. (2017) 7:16978. doi: 10.1038/s41598-017-17285-2, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Patrad E, Niapour A, Farassati F, Amani M. Combination treatment of all-trans retinoic acid (ATRA) and γ-secretase inhibitor (DAPT) cause growth inhibition and apoptosis induction in the human gastric cancer cell line. Cytotechnology. (2018) 70:865–77. doi: 10.1007/s10616-018-0199-3, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhang L, Wang J, Liu L, Zheng C, Wang Y. Synthesis and antiproliferative activity of novel all-trans-retinoic acid-podophyllotoxin conjugate towards human gastric cancer cells. Molecules. (2017) 22:628. doi: 10.3390/molecules22040628, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Liang Y, Wang W, Zhu X, Yu M, Zhou C. Inhibition of myeloid-derived suppressive cell function with all-trans retinoic acid enhanced anti-PD-L1 efficacy in cervical cancer. Sci Rep. (2022) 12:9619. doi: 10.1038/s41598-022-13855-1, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Li X, Luo X, Chen S, Chen J, Deng X, Zhong J, et al. All-trans-retinoic acid inhibits hepatocellular carcinoma progression by targeting myeloid-derived suppressor cells and inhibiting angiogenesis. Int Immunopharmacol. (2023) 121:110413. doi: 10.1016/j.intimp.2023.110413, PMID: [DOI] [PubMed] [Google Scholar]
- 81.Lou Y, Peng P, Wang S, Wang J, du P, Zhang Z, et al. Combining all-trans retinoid acid treatment targeting myeloid-derived suppressive cells with cryo-thermal therapy enhances antitumor immunity in breast cancer. Front Immunol. (2022) 13:1016776. doi: 10.3389/fimmu.2022.1016776, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chuang H-C, Lin HY, Liao PL, Huang CC, Lin LL, Hsu WM, et al. Immunomodulator polyinosinic-polycytidylic acid enhances the inhibitory effect of 13-cis-retinoic acid on neuroblastoma through a TLR3-related immunogenic-apoptotic response. Lab Investig. (2020) 100:606–18. doi: 10.1038/s41374-019-0356-0, PMID: [DOI] [PubMed] [Google Scholar]
- 83.Abdullah M, Jamil RT, Attia FN. Vitamin C (Ascorbic Acid). Treasure Island (FL): National Library of Medicine NIH; (2022). [Google Scholar]
- 84.Fu Y, Xu F, Jiang L, Miao Z, Liang X, Yang J, et al. Circulating vitamin C concentration and risk of cancers: a mendelian randomization study. BMC Med. (2021) 19:1–14. doi: 10.1186/s12916-021-02041-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Park Y, Spiegelman D, Hunter DJ, Albanes D, Bergkvist L, Buring JE, et al. Intakes of vitamins a, C, and E and use of multiple vitamin supplements and risk of colon cancer: a pooled analysis of prospective cohort studies. Cancer Causes Control. (2010) 21:1745–57. doi: 10.1007/s10552-010-9549-y, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jacobs EJ, Connell CJ, McCullough ML, Chao A, Jonas CR, Rodriguez C, et al. Vitamin C, vitamin E, and multivitamin supplement use and stomach cancer mortality in the Cancer prevention study II cohort. Cancer Epidemiol Biomark Prev. (2002) 11:35–41. [PubMed] [Google Scholar]
- 87.Kong P, Cai Q, Geng Q, Wang J, Lan Y, Zhan Y, et al. Vitamin intake reduce the risk of gastric cancer: meta-analysis and systematic review of randomized and observational studies. PLoS One. (2014) 9:e116060. doi: 10.1371/journal.pone.0116060, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hua Y-F, Wang GQ, Jiang W, Huang J, Chen GC, Lu CD. Vitamin C intake and pancreatic cancer risk: a meta-analysis of published case-control and cohort studies. PLoS One. (2016) 11:e0148816. doi: 10.1371/journal.pone.0148816, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Agathocleous M, Meacham CE, Burgess RJ, Piskounova E, Zhao Z, Crane GM, et al. Ascorbate regulates haematopoietic stem cell function and leukaemogenesis. Nature. (2017) 549:476–81. doi: 10.1038/nature23876, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.LEE SJ, JEONG JH, LEE IH, LEE J, JUNG JH, PARK HY, et al. Effect of high-dose vitamin C combined with anti-cancer treatment on breast cancer cells. Anticancer Res. (2019) 39:751–8. doi: 10.21873/anticanres.13172, PMID: [DOI] [PubMed] [Google Scholar]
- 91.Jafari E, Alavi M, Zal F. The evaluation of protective and mitigating effects of vitamin C against side effects induced by radioiodine therapy. Radiat Environ Biophys. (2018) 57:233–40. doi: 10.1007/s00411-018-0744-7, PMID: [DOI] [PubMed] [Google Scholar]
- 92.Klimant E, Wright H, Rubin D, Seely D, Markman M. Intravenous vitamin C in the supportive care of cancer patients: a review and rational approach. Curr Oncol. (2018) 25:139–48. doi: 10.3747/co.25.3790, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yiang GT, Chen TY, Chen C, Hung YT, Hsueh KC, Wu TK, et al. Antioxidant vitamins promote anticancer effects on low-concentration methotrexate-treated glioblastoma cells via enhancing the caspase-3 death pathway. Food Sci Nutr. (2021) 9:3308–16. doi: 10.1002/fsn3.2298, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhou J, Chen C, Chen X, Fei Y, Jiang L, Wang G. Vitamin C promotes apoptosis and cell cycle arrest in oral squamous cell carcinoma. Front Oncol. (2020) 10:976. doi: 10.3389/fonc.2020.00976, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Shenoy N, Creagan E, Witzig T, Levine M. Ascorbic acid in cancer treatment: let the phoenix fly. Cancer Cell. (2018) 34:700–6. doi: 10.1016/j.ccell.2018.07.014, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Magrì A, Germano G, Lorenzato A, Lamba S, Chilà R, Montone M, et al. High-dose vitamin C enhances cancer immunotherapy. Sci Transl Med. (2020) 12:eaay8707. doi: 10.1126/scitranslmed.aay8707 [DOI] [PubMed] [Google Scholar]
- 97.Sotler R, Poljšak B, Dahmane R, Jukić T, Pavan Jukić D, Rotim C, et al. Prooxidant activities of antioxidants and their impact on health. Acta Clin Croat. (2019) 58:726–36. doi: 10.20471/acc.2019.58.04.20, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chen Q, Espey MG, Sun AY, Lee JH, Krishna MC, Shacter E, et al. Ascorbate in pharmacologic concentrations selectively generates ascorbate radical and hydrogen peroxide in extracellular fluid in vivo. Proc Natl Acad Sci. (2007) 104:8749–54. doi: 10.1073/pnas.0702854104, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ma E, Chen P, Wilkins HM, Wang T, Swerdlow RH, Chen Q. Pharmacologic ascorbate induces neuroblastoma cell death by hydrogen peroxide mediated DNA damage and reduction in cancer cell glycolysis. Free Radic Biol Med. (2017) 113:36–47. doi: 10.1016/j.freeradbiomed.2017.09.008, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kiessling MK, Klemke CD, Kamiński MM, Galani IE, Krammer PH, Gülow K. Inhibition of constitutively activated nuclear factor-κB induces reactive oxygen species-and iron-dependent cell death in cutaneous T-cell lymphoma. Cancer Res. (2009) 69:2365–74. doi: 10.1158/0008-5472.CAN-08-3221, PMID: [DOI] [PubMed] [Google Scholar]
- 101.Rychtarcikova Z, Lettlova S, Tomkova V, Korenkova V, Langerova L, Simonova E, et al. Tumor-initiating cells of breast and prostate origin show alterations in the expression of genes related to iron metabolism. Oncotarget. (2017) 8:6376–98. doi: 10.18632/oncotarget.14093, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lu Y-X, Wu QN, Chen DL, Chen LZ, Wang ZX, Ren C, et al. Pharmacological ascorbate suppresses growth of gastric cancer cells with GLUT1 overexpression and enhances the efficacy of oxaliplatin through redox modulation. Theranostics. (2018) 8:1312–26. doi: 10.7150/thno.21745, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Tian W, Wang Y, Xu Y, Guo X, Wang B, Sun L, et al. The hypoxia-inducible factor renders cancer cells more sensitive to vitamin C-induced toxicity. J Biol Chem. (2014) 289:3339–51. doi: 10.1074/jbc.M113.538157, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Shenoy N, Bhagat T, Nieves E, Stenson M, Lawson J, Choudhary GS, et al. Upregulation of TET activity with ascorbic acid induces epigenetic modulation of lymphoma cells. Blood Cancer J. (2017) 7:e587–7. doi: 10.1038/bcj.2017.65, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Cimmino L, Dolgalev I, Wang Y, Yoshimi A, Martin GH, Wang J, et al. Restoration of TET2 function blocks aberrant self-renewal and leukemia progression. Cells. (2017) 170:1079–1095.e20. e20. doi: 10.1016/j.cell.2017.07.032, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Gao P, Zhang H, Dinavahi R, Li F, Xiang Y, Raman V, et al. HIF-dependent antitumorigenic effect of antioxidants in vivo. Cancer Cell. (2007) 12:230–8. doi: 10.1016/j.ccr.2007.08.004, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wilkes JG, O’Leary BR, du J, Klinger AR, Sibenaller ZA, Doskey CM, et al. Pharmacologic ascorbate (P-AscH−) suppresses hypoxia-inducible factor-1α (HIF-1α) in pancreatic adenocarcinoma. Clin Exp Metastasis. (2018) 35:37–51. doi: 10.1007/s10585-018-9876-z, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Jóźwiak P, Ciesielski P, Zaczek A, Lipińska A, Pomorski L, Wieczorek M, et al. Expression of hypoxia inducible factor 1α and 2α and its association with vitamin C level in thyroid lesions. J Biomed Sci. (2017) 24:1–10. doi: 10.1186/s12929-017-0388-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Traber MG. Vitamin E regulatory mechanisms. Annu Rev Nutr. (2007) 27:347–62. doi: 10.1146/annurev.nutr.27.061406.093819 [DOI] [PubMed] [Google Scholar]
- 110.Lee HJ, Ju J, Paul S, So JY, DeCastro A, Smolarek A, et al. Mixed tocopherols prevent mammary tumorigenesis by inhibiting estrogen action and activating PPAR-γ. Clin Cancer Res. (2009) 15:4242–9. doi: 10.1158/1078-0432.CCR-08-3028, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sen CK, Khanna S, Roy S. Tocotrienols in health and disease: the other half of the natural vitamin E family. Mol Asp Med. (2007) 28:692–728. doi: 10.1016/j.mam.2007.03.001, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Meganathan P, Fu J-Y. Biological properties of tocotrienols: evidence in human studies. Int J Mol Sci. (2016) 17:1682. doi: 10.3390/ijms17111682, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Samant G, Sylvester P. γ-Tocotrienol inhibits ErbB3-dependent PI3K/Akt mitogenic signalling in neoplastic mammary epithelial cells. Cell Prolif. (2006) 39:563–74. doi: 10.1111/j.1365-2184.2006.00412.x, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Shibata A, Nakagawa K, Sookwong P, Tsuduki T, Tomita S, Shirakawa H, et al. Tocotrienol inhibits secretion of angiogenic factors from human colorectal adenocarcinoma cells by suppressing hypoxia-inducible Factor-1 α. J Nutr. (2008) 138:2136–42. doi: 10.3945/jn.108.093237, PMID: [DOI] [PubMed] [Google Scholar]
- 115.Selvaduray KR, Radhakrishnan AK, Kutty MK, Nesaretnam K. Palm tocotrienols decrease levels of pro-angiogenic markers in human umbilical vein endothelial cells (HUVEC) and murine mammary cancer cells. Genes Nutr. (2012) 7:53–61. doi: 10.1007/s12263-011-0223-0, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Liu H-K, Wang Q, Li Y, Sun WG, Liu JR, Yang YM, et al. Inhibitory effects of γ-tocotrienol on invasion and metastasis of human gastric adenocarcinoma SGC-7901 cells. J Nutr Biochem. (2010) 21:206–13. doi: 10.1016/j.jnutbio.2008.11.004, PMID: [DOI] [PubMed] [Google Scholar]
- 117.Yu W, Jia L, Wang P, Lawson KA, Simmons-Menchaca M, Park SK, et al. In vitro and in vivo evaluation of anticancer actions of natural and synthetic vitamin E forms. Mol Nutr Food Res. (2008) 52:447–56. doi: 10.1002/mnfr.200700254, PMID: [DOI] [PubMed] [Google Scholar]
- 118.Sun Z, Yin S, Zhao C, Fan L, Hu H. Inhibition of PD-L1-mediated tumor-promoting signaling is involved in the anti-cancer activity of β-tocotrienol. Biochem Biophys Res Commun. (2022) 617:33–40. doi: 10.1016/j.bbrc.2022.05.082, PMID: [DOI] [PubMed] [Google Scholar]
- 119.Montagnani Marelli M, Marzagalli M, Fontana F, Raimondi M, Moretti RM, Limonta P. Anticancer properties of tocotrienols: a review of cellular mechanisms and molecular targets. J Cell Physiol. (2019) 234:1147–64. doi: 10.1002/jcp.27075, PMID: [DOI] [PubMed] [Google Scholar]
- 120.Fontana F, Moretti RM, Raimondi M, Marzagalli M, Beretta G, Procacci P, et al. δ-Tocotrienol induces apoptosis, involving endoplasmic reticulum stress and autophagy, and paraptosis in prostate cancer cells. Cell Prolif. (2019) 52:e12576. doi: 10.1111/cpr.12576, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Fontana F, Raimondi M, Marzagalli M, Audano M, Beretta G, Procacci P, et al. Mitochondrial functional and structural impairment is involved in the antitumor activity of δ-tocotrienol in prostate cancer cells. Free Radic Biol Med. (2020) 160:376–90. doi: 10.1016/j.freeradbiomed.2020.07.009, PMID: [DOI] [PubMed] [Google Scholar]
- 122.Sato A, Arai T, Fusegi M, Ando A, Yano T. A redox-inactive derivative of tocotrienol suppresses tumor growth of mesothelioma cells in a xenograft model. Biol Pharm Bull. (2019) 42:1034–7. doi: 10.1248/bpb.b18-00924, PMID: [DOI] [PubMed] [Google Scholar]
- 123.Wei CW, Yu YL, Chen YH, Hung YT, Yiang GT. Anticancer effects of methotrexate in combination with α-tocopherol and α-tocopherol succinate on triple-negative breast cancer. Oncol Rep. (2019) 41:2060–6. doi: 10.3892/or.2019.6958, PMID: [DOI] [PubMed] [Google Scholar]
- 124.Leddy LR, Holmes RE. Chondrosarcoma of bone. Orthopaed Oncol. (2014) 2014:117–30. doi: 10.1007/978-3-319-07323-1_6 [DOI] [PubMed] [Google Scholar]
- 125.Pang K-L, Foong LC, Abd Ghafar N, Soelaiman IN, Law JX, Leong LM, et al. Transcriptomic analysis of the anticancer effects of annatto tocotrienol, Delta-tocotrienol and gamma-tocotrienol on chondrosarcoma cells. Nutrients. (2022) 14:4277. doi: 10.3390/nu14204277, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zhang R, Naughton DP. Vitamin D in health and disease: current perspectives. Nutr J. (2010) 9:1–13. doi: 10.1186/1475-2891-9-65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Bikle DD. Vitamin D: production, metabolism and mechanisms of action. South Dartmouth, Massachusetts, USA: Endotext; (2021). [Google Scholar]
- 128.Bikle DD. Vitamin D metabolism, mechanism of action, and clinical applications. Chem Biol. (2014) 21:319–29. doi: 10.1016/j.chembiol.2013.12.016, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Trump DL, Aragon-Ching JB. Vitamin D in prostate cancer. Asian J Androl. (2018) 20:244–52. doi: 10.4103/aja.aja_14_18, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Hii CS, Ferrante A. The non-genomic actions of vitamin D. Nutrients. (2016) 8:135. doi: 10.3390/nu8030135, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Jiang L, Zhang X, Chen Y, Huo X, Deng S, Yang X, et al. Alteration of serum 25 (OH) Vitamin D, Vitamin D binding protein, and C-reactive protein levels in acute leukemia patients. Clin Lab. (2018) 64:1553–9. doi: 10.7754/Clin.Lab.2018.180412, PMID: [DOI] [PubMed] [Google Scholar]
- 132.Wu X, Hu W, Lu L, Zhao Y, Zhou Y, Xiao Z, et al. Repurposing vitamin D for treatment of human malignancies via targeting tumor microenvironment. Acta Pharm Sin B. (2019) 9:203–19. doi: 10.1016/j.apsb.2018.09.002, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Nagpal S, Na S, Rathnachalam R. Noncalcemic actions of vitamin D receptor ligands. Endocr Rev. (2005) 26:662–87. doi: 10.1210/er.2004-0002, PMID: [DOI] [PubMed] [Google Scholar]
- 134.Negri M, Gentile A, de Angelis C, Montò T, Patalano R, Colao A, et al. Vitamin D-induced molecular mechanisms to potentiate cancer therapy and to reverse drug-resistance in cancer cells. Nutrients. (2020) 12:1798. doi: 10.3390/nu12061798, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Battaglia S, Karasik E, Gillard B, Williams J, Winchester T, Moser MT, et al. LSD1 dual function in mediating epigenetic corruption of the vitamin D signaling in prostate cancer. Clin Epigenetics. (2017) 9:1–15. doi: 10.1186/s13148-017-0382-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Rossdeutscher L, Li J, Luco AL, Fadhil I, Ochietti B, Camirand A, et al. Chemoprevention activity of 25-hydroxyvitamin D in the MMTV-PyMT mouse model of breast CancerChemopreventive action of 25 (OH) Vitamin D in breast Cancer. Cancer Prev Res. (2015) 8:120–8. doi: 10.1158/1940-6207.CAPR-14-0110, PMID: [DOI] [PubMed] [Google Scholar]
- 137.Bi X, Shi Q, Zhang H, Bao Y, Hu D, Pohl N, et al. C-Jun NH2-teminal kinase 1 interacts with vitamin D receptor and affects vitamin D-mediated inhibition of cancer cell proliferation. J Steroid Biochem Mol Biol. (2016) 163:164–72. doi: 10.1016/j.jsbmb.2016.05.009, PMID: [DOI] [PubMed] [Google Scholar]
- 138.Quigley M, Rieger S, Capobianco E, Wang Z, Zhao H, Hewison M, et al. Vitamin d modulation of mitochondrial oxidative metabolism and mTOR enforces stress adaptations and anticancer responses. JBMR plus. (2022) 6:e10572. doi: 10.1002/jbm4.10572, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Li Q, Li Y, Jiang H, Xiao Z, Wu X, Zhang H, et al. Vitamin D suppressed gastric cancer cell growth through downregulating CD44 expression in vitro and in vivo. Nutrition. (2021) 91:111413. doi: 10.1016/j.nut.2021.111413 [DOI] [PubMed] [Google Scholar]
- 140.Burnett AK, Russell NH, Hills RK, Bowen D, Kell J, Knapper S, et al. Arsenic trioxide and all-trans retinoic acid treatment for acute promyelocytic leukaemia in all risk groups (AML17): results of a randomised, controlled, phase 3 trial. Lancet Oncol. (2015) 16:1295–305. doi: 10.1016/S1470-2045(15)00193-X, PMID: [DOI] [PubMed] [Google Scholar]
- 141.Hidayat YM, Darmadi E, Rachmayati S, Kusumah WP, Djuwantono T, Pramatirta AY, et al. Efficacy of Oral Vitamin a in reducing β-hCG levels in low-risk gestational trophoblastic neoplasia patients. Asian Pac J Cancer Prev. (2020) 21:3325–9. doi: 10.31557/APJCP.2020.21.11.3325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Sanusi RS. Outcome of combined neoadjuvant chemotherapy and Vitamin a in advanced cervical carcinoma: a randomized double-blind clinical trial. Asian Pac J Cancer Prev. (2019) 20:2213–8. doi: 10.31557/APJCP.2019.20.7.2213, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Cameron E. Ascorbic acid and cancer: a review. Proc Natl Acad Sci USA. (1987) 75:4538–42. doi: 10.1073/pnas.75.9.4538 [DOI] [Google Scholar]
- 144.CameronPauling E. Supplemental ascorbate in the supportive treatment of cancer: prolongation of survival times in terminal human cancer. Proc Natl Acad Sci U S A. (1976) 73:3685–9. doi: 10.1073/pnas.73.10.3685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Creagan ET, Moertel CG, O'Fallon JR, Schutt AJ, O'Connell MJ, Rubin J, et al. Failure of high-dose vitamin C (ascorbic acid) therapy to benefit patients with advanced cancer. N Engl J Med. (1979) 301:687–90. doi: 10.1056/NEJM197909273011303, PMID: [DOI] [PubMed] [Google Scholar]
- 146.Moertel CG, Fleming TR, Creagan ET, Rubin J, O'Connell MJ, Ames MM. High-dose vitamin C versus placebo in the treatment of patients with advanced cancer who have had no prior chemotherapy: a randomized double-blind comparison. N Engl J Med. (1985) 312:137–41. doi: 10.1056/NEJM198501173120301, PMID: [DOI] [PubMed] [Google Scholar]
- 147.Padayatty SJ, Sun AY, Chen Q, Espey MG, Drisko J, Levine M. Vitamin C: intravenous use by complementary and alternative medicine practitioners and adverse effects. PLoS One. (2010) 5:e11414. doi: 10.1371/journal.pone.0011414, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Furqan M, Abu-Hejleh T, Stephens LM, Hartwig SM, Mott SL, Pulliam CF, et al. Pharmacological ascorbate improves the response to platinum-based chemotherapy in advanced stage non-small cell lung cancer. Redox Biol. (2022) 53:102318. doi: 10.1016/j.redox.2022.102318, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Wang F, He MM, Wang ZX, Li S, Jin Y, Ren C, et al. Phase I study of high-dose ascorbic acid with mFOLFOX6 or FOLFIRI in patients with metastatic colorectal cancer or gastric cancer. BMC Cancer. (2019) 19:460–10. doi: 10.1186/s12885-019-5696-z, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Cheng Y, Tong H, Li X, Zhang X, Fang J, Yue R, et al. Effect of vitamin E and supragingival scaling on salivary gland function in patients with differentiated thyroid cancer treated with 131I. Nucl Med Commun. (2022) 43:995–1003. doi: 10.1097/MNM.0000000000001605, PMID: [DOI] [PubMed] [Google Scholar]
- 151.Huang J, Hodis HN, Weinstein SJ, Mack WJ, Sampson JN, Mondul AM, et al. Serum metabolomic response to low-and high-dose Vitamin E supplementation in two randomized controlled TrialsSerum metabolomic response to Vitamin E supplementation. Cancer Epidemiol Biomark Prev. (2020) 29:1329–34. doi: 10.1158/1055-9965.EPI-20-0187, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Lai GY, Weinstein SJ, Taylor PR, McGlynn KA, Virtamo J, Gail MH, et al. Effects of α-tocopherol and β-carotene supplementation on liver cancer incidence and chronic liver disease mortality in the ATBC study. Br J Cancer. (2014) 111:2220–3. doi: 10.1038/bjc.2014.514, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Akiba T, Morikawa T, Odaka M, Nakada T, Kamiya N, Yamashita M, et al. Vitamin D supplementation and survival of patients with non–small cell lung Cancer: a randomized, double-blind, placebo-controlled TrialRCT of Vitamin D for lung Cancer. Clin Cancer Res. (2018) 24:4089–97. doi: 10.1158/1078-0432.CCR-18-0483, PMID: [DOI] [PubMed] [Google Scholar]
- 154.Beltrán-de-Miguel B, Estévez-Santiago R, Olmedilla-Alonso B. Assessment of dietary vitamin a intake (retinol, α-carotene, β-carotene, β-cryptoxanthin) and its sources in the National Survey of dietary intake in Spain (2009–2010). Int J Food Sci Nutr. (2015) 66:706–12. doi: 10.3109/09637486.2015.1077787, PMID: [DOI] [PubMed] [Google Scholar]
- 155.Carazo A, Macáková K, Matoušová K, Krčmová LK, Protti M, Mladěnka P. Vitamin a update: forms, sources, kinetics, detection, function, deficiency, therapeutic use and toxicity. Nutrients. (2021) 13:1703. doi: 10.3390/nu13051703, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Roop JK. Hypervitaminosis-an emerging pathological condition. Int J Heal Sci Res. (2018) 8:280. [Google Scholar]
- 157.Yang J, Zhang Y, Na X. β-Carotene supplementation and risk of cardiovascular disease: a systematic review and Meta-analysis of randomized controlled trials. Nutrients. (2022) 14:1284. doi: 10.3390/nu14061284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Doseděl M, Jirkovský E, Macáková K, Krčmová L, Javorská L, Pourová J, et al. Vitamin C—sources, physiological role, kinetics, deficiency, use, toxicity, and determination. Nutrients. (2021) 13:615. doi: 10.3390/nu13020615 [DOI] [PMC free article] [PubMed] [Google Scholar]